Abstract
Composite tissue allotransplantation holds great promise for upper extremity reconstruction but is limited by donor part availability. Cryopreservation may increase the availability of donor parts and even reduce antigenicity. The purpose of the study was to evaluate the viability of cryopreserved composite tissues and to demonstrate the feasibility of microvascular isotransplantation of cryopreserved composite flaps. Twenty epigastric flaps were harvested from Lewis rats. Ten flaps were analyzed fresh. Ten flaps were perfused with dimethyl sulfoxide (DMSO)/trehelose cryoprotectant agent (CPA), frozen by controlled cooling to −140°C, and stored for 2 weeks. Flaps were evaluated by factor VIII endothelial staining and MTT tetrazolium salt assay. For the in vivo phase, 30 flaps were harvested. Ten were transplanted fresh to isogenetic recipient animals, ten were perfused with CPA and transplanted, and ten were cryopreserved for 2 weeks, thawed, and transplanted. All cryopreserved samples displayed intact vascular endothelia on factor VIII staining. On MTT analysis, the epithelial viability index for the cryopreserved samples was not significantly different from fresh controls (p = 0.12). All freshly transplanted flaps (10/10) were viable at 60 days. Nine of ten flaps in the perfused/transplanted group were viable at 60 days. Survival of cryopreserved/transplanted flaps ranged from 5 to 60 days. The skin and vascular endothelial components of composite tissue flaps appear to retain their viability after cryopreservation. The in vivo studies demonstrate that the long-term survival of cryopreserved composite tissue transplants is feasible and support an indirect injury, rather than direct injury from freezing or cryoprotectant agents, as the mechanism of flap loss.
Keywords: Cryopreservation, Composite tissue transplantation, Epigastric flap
Introduction
Composite tissue allotransplantation is a technique which holds great promise for the restoration of severe upper extremity defects, but is limited by the scarcity of suitable donor parts. To date, more than 20 successful hand transplantation procedures have been performed [7, 9, 16, 19], as well as allotransplantation of the larynx [3, 24, 31], knee and femur joint [13, 14, 24], abdominal wall [20, 27], and face [6]. Strategies are being developed to induce donor-specific tolerance to allotransplanted tissues, thus, reducing or eliminating the need for long-term immunosuppression [1, 8, 23, 28–30]. This is expected to greatly broaden the indications for composite tissue allotransplantation. Today’s indications for free composite flaps may be tomorrow’s indications for composite tissue allotransplantation, placing an enormous demand on the already-limited donor pool.
One way to improve the availability of donor composite parts would be to devise successful strategies for the long-term preservation of these tissues. Clinicians could harvest composite tissues when a donor is available, preserve these parts in “tissue banks”, and transplant them at a later date when there is a need. Recent advances in solid organ cryopreservation suggest that this goal is achievable. The past decade has seen the successful cryopreservation of blood vessels, knee joints, and skin in animal models [11, 12, 18, 21]. In 2003, the successful allotransplantation of a cryopreserved trachea was reported in the rabbit model [32], as well as the first successful microvascular transfer of cryopreserved testes and ovaries in the rat model [34].
The experience with blood vessels and solid organs suggests that the cryopreservation process reduces the antigenicity of parts [11, 18, 32]. If this observation holds true for composite tissues, then cryopreservation may actually improve the survival rate of allotransplanted composite parts and increase their availability. However, to date, no reliable protocols for the cryopreservation of composite tissues have been developed. The purpose of this study is to evaluate the viability of composite tissues after cryopreservation, storage, and thawing, and to demonstrate the feasibility of the microvascular isotransplantation of cryopreserved composite tissue flaps.
Materials and Methods
Experimental Design
Phase 1 The purpose of this phase was to evaluate the viability of the component tissues of composite tissue flaps after cryopreservation. Twenty epigastric flaps were harvested from Lewis rats. Ten flaps were perfused with dimethyl sulfoxide (DMSO)/trehelose cryoprotectant agent (CPA), frozen by controlled cooling to −140°C, and placed in liquid nitrogen for 2 weeks. Ten flaps were analyzed fresh, immediately after surgical harvest. Skin samples from each flap were analyzed with the MTT tetrazolium salt assay [2, 4] and an epithelial viability index was calculated. The epithelial viability index of the cryopreserved flap skin was compared to that of fresh specimens. All flaps were sectioned and examined by light microscopy with H/E and factor VIII staining, to determine vascular endothelial architecture and viability.
Phase 2 The purpose of this phase was to determine the optimal technical parameters for the cryopreservation of composite flaps. The parameters addressed included the cooling rate, seeding time, CPA perfusing system and perfusion time, choice of CPA, concentration of CPA, and warming rate. With each change in the protocol, four to six samples were cryopreserved, thawed, and analyzed for cell viability and histological features. To determine the optimal CPA choice, 18 flaps were divided into three groups (six recipient rats each), with each group receiving a different CPA protocol. The final concentrations of CPA in the three groups were: 1.5 M glycerol, 2.0 M DMSO, and a mixture of both 1.0 M glycerol and 1.0 M DMSO, respectively. The flaps were cryopreserved for 2 weeks and transplanted to recipient isogenetic animals. Due to superior viability in the 2.0-M DMSO group, this cryoprotective agent was adopted for subsequent studies.
Phase 3 The purpose of this phase was to demonstrate the feasibility of the microvascular isotransplantation of cryopreserved composite tissue flaps. Thirty flaps were harvested from male Lewis rats. Ten flaps were transplanted immediately using microvascular technique to the corresponding anatomic site of isogenetic recipient animals (fresh group). Ten flaps were perfused with CPA and transplanted to recipient animals (perfused group). Ten flaps were perfused with CPA, cryopreserved for 2 weeks, thawed, and transplanted to recipient animals (cryopreserved/thawed group). The animals were examined daily for evidence of partial or total flap loss. Animals displaying evidence of rejection or flap loss were euthanized. At 24 h and 7 days after surgery, the vascular anastomotic patency was assessed by a noninvasive surface Doppler probe. At 60 days postoperatively, any surviving animals were euthanized. The flaps were removed and studied histologically with hematoxylin–eosin (H&E) and factor VIII staining for signs of inflammation or vascular endothelial damage.
Animal Care
Adult male Lewis rats weighing between 300 and 400 g were used as flap donors and recipients. Two epigastric flaps were harvested from each donor animal. Animals were housed in the Animal Care Facility and were inspected daily by study personnel before the surgical procedure. After surgery, rats were placed on a heating pad and observed carefully until fully recovered and mobile. Rats were individually housed after surgery to minimize interference with surgical sites. They were monitored daily by study personnel for signs of pain, lethargy, weight loss, dehydration, or wound complications. Animals displaying evidence of rejection or flap loss, such as softening of the surface of the skin such that it could be wiped away with a light touch, dry gangrene of the flap, or total flap hair loss, were euthanized. At 60 days postoperatively, any surviving animals were euthanized. The experimental protocol was approved by the Institutional Animal Care and Use Committee, and institutional guidelines were followed.
Surgery
For all animals, induction of anesthesia was accomplished using isofluorane gas (5% in O2) in an induction chamber. The animals were placed on the operating platform and maintained throughout the procedure with 2–3% isoflurane gas via nose cone.
For the donor procedure, the animal was placed in dorsal recumbency, and two epigastric flaps, each measuring 4-cm wide by 6-cm long, were marked as previously described [10, 25, 33]. The flap was raised in a distal to proximal direction, exposing the vascular pedicle. The pedicle was dissected back to its origin from the femoral vessels. Proximal and distal control was obtained. The flap was harvested with its vascular pedicle and a cuff of femoral artery and vein (Fig. 1). The second flap was harvested in an identical fashion and the animal euthanized. Flaps designated for cryopreservation were cannulated with a 24-g angiocatheter and flushed sequentially with heparinized Ringer’s lactate solution and Wisconsin fluid, in which they were immersed at 4°C until cryopreserved.
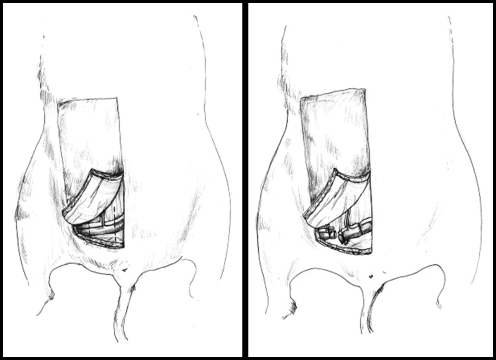
Figure 1.
(Left) Schematic of donor surgical procedure, showing flap design. (Right) Showing method of epigastric flap harvest, including a cuff of the femoral vessels.
For the transplant surgery, the recipient animal was anesthetized as above. A 4 × 6 cm defect was created by excising the skin and subcutaneous tissue corresponding to one epigastric flap. The femoral vessels were exposed under the operating microscope and proximal and distal control obtained with microsurgical clamps. The femoral vessels were divided, and the femoral vessel cuff on the composite tissue transplant was anastomosed to the recipient artery and vein in an interpositional fashion using 10-0 nylon microsuture (Fig. 2). Once arterial and venous flow in the flap had been established, the borders of the flap were sutured to the recipient site using 5-0 chromic catgut suture. Animals were closely monitored during emergence from anesthesia and given postoperative analgesia.
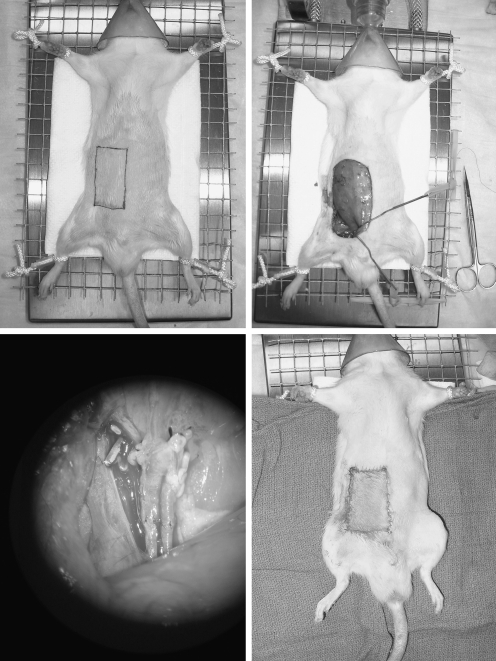
Figure 2.
(Above, Left) Isotransplantation recipient animal under anesthesia with outline of defect marked. (Above, Right) After creation of abdominal defect and preparation of recipient vessels. (Below, Left) Microscopic view of vascular anastomosis. (Below, Right) Immediately after isotransplantation of a cryopreserved and thawed epigastric flap.
Cryopreservation
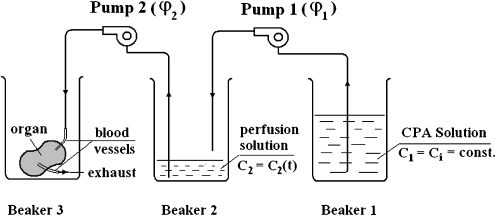
The initial cryopreservation protocol chosen was one which had previously been used in our laboratory for rat ovary and testis preservation. Flaps were prepared for cryopreservation by slow perfusion with ice-cold M2 medium (Sigma, USA) containing 0.1 M trehalose and a gradient of DMSO rising from 0 to 2.0 mol/l at a rate of 0.35 ml/min over 30 min. The controlled rise in DMSO gradient was achieved with a two-pump system (Fig. 3). The flaps were prepared in cryobags and placed in the chamber of a controlled rate freezer programmed to cool from 0 to −7°C at 2°C/min. The flaps were held at this temperature for 5 min, cooled at 0.3°C/min to −40°C, and then cooled further at 10°C/min to −140°C. They were then immersed in liquid nitrogen and stored for 2 weeks. They were thawed rapidly by swirling in a water bath at 40°C. The cryoprotectant was washed out by reversing the concentration gradient by perfusion using the two-pump system.
Figure 3.
Schematic of the two-pump system for providing graded perfusion of cryoprotective agent.
Histological Evaluation
For the histological evaluation, each flap was mounted in paraffin, sectioned, and stained with H&E. Preservation of the cellular architecture of the epithelial and adipose components was assessed qualitatively, as was the presence or absence of thrombosis, inflammation, or necrosis. The epigastric artery and vein were fixed, sectioned, and stained with factor VIII, whereby intact endothelial cells stain brown [35]. Under light microscopy, ten sequential sections of the vessels were examined, and an assessment of endothelial integrity was made by a blinded pathologist.
MTT Quantitative Assay
Using a 6-mm biopsy punch (Owens & Minor, Richmond, VA), three uniform skin samples were taken from each flap [2, 4]. One skin biopsy was taken from the proximal edge (closest to the vascular pedicle), one from the center, and one from the distal edge. Skin specimens were weighed and placed dermis side down in a 24-well culture plate. Two negative controls were prepared by harvesting five fresh skin specimens of similar weight to those being tested and placing them in a microwave for 20 min at maximum temperature. These were placed in the culture plate as well. MTT salts (0.5 mg/ml) were added to the transport medium, and the samples were incubated at 37°C in an atmosphere of 5% CO2/air. After 3 h of incubation, the precipitated salts were solubilized for 3 h with 2-methoxyethanol. The solution was read on a spectrophotometer at 570 nm. The mean optical density of the negative controls was subtracted from each sample. The epithelial viability index (V.I.) was defined as the optical density at 570 nm of the skin sample divided by its weight in grams. Epithelial viability in the cryopreserved/thawed specimens was compared to fresh specimens using the two tailed t test. The data were expressed as mean ± SD, and statistical significance was defined as p ≤ 0.05. A percentage viability index (PVI) was calculated for the cryopreserved/thawed samples. The PVI was defined as the ratio of the viability of the specimen after cryopreservation to the value recorded in fresh samples [4].
Results
Phase 1 On light microscopy with H&E staining, the cellular architecture in the cryopreserved/thawed specimens was well preserved, both in the epithelial and adipose components (Fig. 4). In addition, the blood vessels showed no histological evidence of thrombosis, inflammation, or necrosis. All cryopreserved/thawed and fresh specimens displayed a high degree of vascular endothelial preservation upon factor VIII staining, and a blinded pathologist was unable to distinguish ten cryopreserved/thawed specimens from equivalent fresh samples. On MTT analysis, the mean optical density of the heat-denatured negative controls (n = 5) was 0.371 ± 0.01. The mean epithelial viability index of the fresh skin samples (n = 10) was 12.15 ± 1.32, whereas that of the cryopreserved/thawed samples (n = 10) was 10.80 ± 2.00. This difference did not reach statistical significance by the two-tailed Student’s t test (p = 0.21). The calculated percentage of viability index (PVI) of the cryopreserved/thawed specimens was 91.9%. This value compares favorably to published values of PVI for cryopreserved skin in skin banks (54 to 60%) [2, 4].
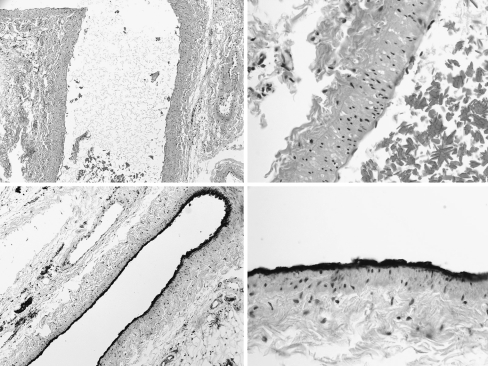
Figure 4.
(Above, Left) Histologic section of a cryopreserved/thawed flap vessel and surrounding connective tissue with H&E staining, showing preservation of cellular architecture. (Above, Right) High-power view of the perivascular region of a cryopreserved/thawed flap with H&E staining. (Below, Left) Histologic section of cryopreserved epigastric artery with factor VIII staining, showing intact vascular endothelium. (Below, Right) High-power view of the cryopreserved epigastric artery, with factor VII endothelial staining.
Phase 2 The result of varying the parameters of cooling and warming were assessed in three ways: MTT tetrazolium salt assay, histology, and transplanting to recipient animals. Five rates of cooling and warming were assessed: 0.5, 1, 1.5, 2, and 2.5°C/min. Of these, 2°C/min was found to be the optimal cooling rate. No significant difference was observed upon varying warming rate.Three cryoprotective agent protocols were assessed: 1.5 M glycerol, 2.0 M DMSO, and a mixture of both 1.0 M glycerol and 1.0 M DMSO. There were six flaps in each group. None of the transplanted flaps in the 1.5-M glycerol group or mixed glycerol and DMSO group displayed flap survival beyond the seventh postoperative day. The mean flap survival time in the 1.5-M glycerol group was 3.5 ± 1.0 days. The mean flap survival time in the mixed glycerol and DMSO group was 4.0 ± 1.8 days. In the 2.0-M DMSO group, three flaps survived beyond the seventh day, and the mean flap survival time was 17 ± 21.6 days. The 2.0 M DMSO CPA protocol was adopted for all subsequent studies.
Phase 3 All of the freshly transplanted flaps (10/10) were viable at 60 days, with normal color and hair growth (Table 1). Nine of the ten flaps in the perfused/transplanted group were fully viable at 60 days; one flap exhibited partial loss. Survival of the ten flaps in the cryopreserved group ranged from 5 to 60 days (mean survival 15.4 ± 16.3 days). One flap survived the full length of the study period, and the animal was euthanized on day 60 (Fig. 5). Histologic analysis of this flap revealed pristine cellular architecture and no evidence of inflammation, thrombosis, or necrosis. Upon evaluation of the nine flaps, which failed before the 60-day study end-point, vascular thrombosis was observed.
Table 1.
Survival following isotransplantation of flaps (Phase 3).
| Group | Treatment | n | Flap Survival (days) |
|---|---|---|---|
| 1 | Fresh | 10 | 60,60,60,60,60,60,60,60,60,60 |
| 2 | Perfused | 10 | 60,60,60,7,60,60,60,60,60,60 |
| 3 | Cryopreserved/Thawed | 10 | 8,15,5,19,7,7,9,14,60,10 |
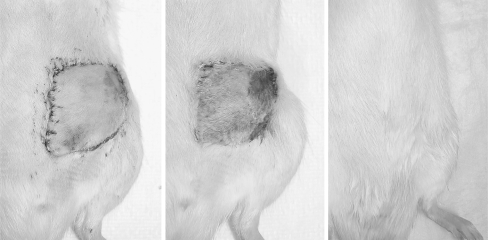
Figure 5.
(Left) Recipient animal on postoperative day 1 after isotransplantation of a cryopreserved and thawed epigastric flap. (Center) Appearance of transplant on postoperative day 7, with a small area of peripheral necrosis. (Right) Appearance of transplant on postoperative day 60, with normal color and hair growth.
Discussion
The successful cryopreservation of human embryos was reported by Wilmut and Whittingham in 1972 [1, 23, 30], and since that time, there has been steady progress in the field of cryopreservation. Over the past decade, improved programmed cooling devices have allowed more precise control of intracellular crystal formation. In addition, refinements in the composition and application of cryoprotectant solutions have allowed more reliable survival of single cell lines and tissues. Improved techniques in the cryopreservation of human hepatocytes may soon help hepatocyte transplant to become a viable alternative to liver allotransplantation for many patients with chronic hepatic failure [17, 22]. Ovarian tissue cryopreservation has already become a clinical reality for women undergoing aggressive regimens of chemical or radiological therapy. The first live human birth after ovarian tissue autotransplantation was reported in 2002 [15]. In recent years, whole organs have been successfully cryopreserved and transplanted in animal models [32, 34].
If the cryopreservation of composite tissues becomes a clinical reality, it has the potential to revolutionize organ and composite tissue transplantation. One may envision “tissue banks” where organs, limbs, and other composite parts could be stored from the time they are harvested to when a need arises. This raises the possibility of an immediate hand transplantation, performed immediately following a devastating injury to replace the lost part. This would help avoid the problems of incomplete nerve regeneration and musculotendinous atrophy and fibrosis, which complicate delayed hand transplantations.
The cryopreservation of composite tissues poses technical challenges beyond those seen in the preservation of single tissue types or organs. The parameters of cryopreservation, such as freezing and thawing rates, concentration of cryoprotective agent (CPA), or type of CPA which are ideal for one component tissue of a composite flap may not be suitable for another component. Before the cryopreservation of composite tissue transplants can become a clinical reality, protocols must be developed, which are capable of maintaining viability in all of the component tissues. In the present study, the protocols used successfully in our laboratory for the cryopreservation of ovaries and testes were selected as initial parameters for composite tissue preservation. The rat epigastric flap was selected as the model of composite tissue, as it is well-described in the literature [10, 25, 33], technically easy to harvest, and small enough for uniform cryopreservation. The epigastric flap consists of three tissue types: vascular, adipose, and epithelial, and the viability of each tissue type can be individually assessed.
The in vitro studies (phase 1) demonstrated the viability of the epithelial, adipose, and vascular endothelial components of the flaps following cryopreservation and thawing. In the in vivo studies, however, these flaps exhibited variable survival once transplanted to recipient animals. Whereas long-term survival was observed, this result was not reliably achieved. Excellent viability was observed in the flaps which were transplanted fresh or perfused with CPA and transplanted. Therefore, the flap losses were not likely the result of technical factors during surgery or direct osmotic injury from the CPA.
Intact cellular architecture was seen upon histological examination in all three tissue types after cryopreservation and thawing. This suggests that one of the primary goals of the cryoprotective regimen, namely, the prevention of cell lysis secondary to intracellular ice formation, was achieved. However, damage may be occurring on a subcellular level during cryopreservation, which may not be apparent upon light microscopy, but may lead to cell death upon reperfusion. For example, during periods of ischemia, irreversible membrane depolarization and an extended period of anaerobic metabolism may occur, leading to fatally low intracellular pH. Depolarization during ischemia also causes the entry into cells of calcium ions, which activate phospholipase enzymes which attack cell membrane phospholipids, causing the release of arachidonic acid. Calcium-activated nuclear endonucleases have been shown to cleave chromatin and begin the process of apoptosis [26]. Cryopreserved tissues are particularly vulnerable to this type of injury during cooling and thawing [18].
Upon reperfusion, accumulated NADH within the mitochondria reacts with newly introduced oxygen to produce superoxide radicals, which mediate injury to cellular membranes. Superoxide radicals and eicosanoids generated by arachidonic acid have been shown to increase the adhesion of leucocytes to vessel walls. They also mediate increased capillary permeability leading to perivascular edema and capillary narrowing. These effects quickly become pronounced enough in reperfusion to block capillaries entirely, the no reflow phenomenon [5]. Vascular endothelium seems to be particularly sensitive to this type of injury [35], and reperfusion injury was likely the mechanism of flap failure in the cryopreserved/thawed flaps in the present study.
Based on these observations, future studies will focus on further refining the techniques of cryopreservation to reduce the injury to vascular endothelium. These techniques could then be applied to the allotransplantation of cryopreserved flaps and limbs, to examine whether cryopreserved flaps display decreased antigenicity. In animal models, cryopreservation has been shown to decrease antigenicity in a variety of tissues. This may confer a survival benefit to cryopreserved allotransplants over their fresh counterparts [11, 18, 32]. As the present study involved isotransplanation alone, the effects of cryopreservation upon antigenicity could not be examined.
Composite tissue allotransplantation is being performed today in multiple centers around the world and will continue in increasing numbers. However, its applicability is limited by the need for long-term immunosuppression and scarcity of donor parts. New strategies for donor-specific tolerance are being developed which may well eliminate the former problem. Cryopreservation is a technique with great potential to help solve the latter.
Acknowledgement
This work is supported by an American Society for Surgery of the Hand Basic Science Research Grant.
Footnotes
Presented at the Annual Meeting of the American Association for Hand Surgery, Rio Grande, Puerto Rico, January, 2007.
References
- 1.Akst LM, Siemionow M, Dan O, Izycki D, Strome M. Induction of tolerance in a rat model of laryngeal transplantation. Transplantation 2003;76:1763–70. [DOI] [PubMed]
- 2.Alotto D, Ariotti S, Graziano S, Verrua R, Stella M, Magliacani G, et al. The role of quality control in a skin bank: tissue viability determination. Cell and Tissue Banking 2002;3:3–10. [DOI] [PubMed]
- 3.Birchall MA, Lorenz RR, Berke GS, Genden EM, Haughey BH, Siemionow M, et al. Laryngeal transplantation in 2005: a review. Am J Transplant 2006;6:20–6. [DOI] [PubMed]
- 4.Castagnoli C, Alotto D, Cambieri I, Casimiri R, Aluffi M, Stella M, et al. Evaluation of donor skin viability: fresh and cryopreserved skin using tetrazolium salt assay. Burns 2003;29:759–67. [DOI] [PubMed]
- 5.Dawson DA, Ruetzler CA, Hallenbeck JM. Temporal impairment of microcirculatory perfusion following focal cerebral ischemia in the spontaneously hypertensive rat. Brain Res 1997;28:200–8. [DOI] [PubMed]
- 6.Devauchelle B, Badet L, Lengele B, Morelon E, Testelin S, Michallet M, et al. First human face allograft: early report. Lancet 2006;368:203–9. [DOI] [PubMed]
- 7.Dubernard J-M, Owen E, Herzberg G, Lanzetta M, Martin X, Kapila H, et al. Human hand allograft: report on the first six months. Lancet 1999;353:1315–20. [DOI] [PubMed]
- 8.Foster RD, Fan L, Niepp M, Kaufman C, McCalmont T, Ascher N, et al. Donor-specific tolerance induction in composite tissue allografts. Am J Surg 1998;176:418–21. [DOI] [PubMed]
- 9.Francois CG, Briedenbach WC, Maldonado C, Kakoulidis TP, Hodges A, Dubernard JM, et al. Hand transplantation: comparisons and observations of the first four clinical cases. Microsurgery 2000;20:360–71. [DOI] [PubMed]
- 10.Frost-Arner L, Spotnitz WD, Rodeheaver GT, Drake DB. Comparison of the thrombogenicity of internationally available fibrin sealants in an established microsurgical model. Plast Reconstr Surg 2001;108:1655–60. [DOI] [PubMed]
- 11.Gu S, Liu CJ, Qiao T, Sun XM, Chen JH. Abdominal aorta transplantation after programmed cryopreservation. World J Gastroenterol 2004;15:555–9. [DOI] [PMC free article] [PubMed]
- 12.Hirase Y, Kojima T, Takeishi M, Hwang KH, Tanaka M. Transplantation of long-term cryopreserved allocutaneous tissue by skin graft or microsurgical anastomosis: experimental studies in the rat. Plast Reconstr Surg 1993;91:492–501. [DOI] [PubMed]
- 13.Hofmann GO, Kirschner MH, Wagner FD, Brauns L, Gonschorek O, Buhren V. Allogeneic vascularized grafting of human knee joints under postoperative immunosuppression of the recipient. World J Surg 1998;22:818–23. [DOI] [PubMed]
- 14.Hofmann GO, Kirschner MH. Clinical experience in allogeneic vascularized bone and joint allografting. Microsurgery 2000;20:375–83. [DOI] [PubMed]
- 15.Jakimiuk AJ, Grzybowski W. Ovarian tissue preservation, present and clinical perspectives. Gynecol Endocrinol 2007;23:87–93. [DOI] [PubMed]
- 16.Jones JW, Gruber SA, Barker JH, Breidenbach WC. Successful hand transplantation. One year follow up. N Engl J Med 2000;17:468–73. [DOI] [PubMed]
- 17.Katenz E, Vondran FW, Schwartlander R, Pless G, Gong X, Cheng X, et al. Cryopreservation of primary human hepatocytes: the benefit of trehalose as an additional cryoprotective agent. Liver Transpl 2007;13:38–45. [DOI] [PubMed]
- 18.Komorowska-Timek, E, Zhang F, Shi D-Y, Lineaweaver WC, Buncke HJ. Effect of cryopreservation on patency and histological changes of arterial isogeneic and allogeneic grafts in the rat model. Ann Plast Surg 2002;49:404–9. [DOI] [PubMed]
- 19.Lee WPA, Nguyen VT. Perspectives on hand transplantation. Clin Plast Surg 2005;32:463–70. [DOI] [PubMed]
- 20.Levi DM, Tzakis AG, Kato T, Madariaga J, Mittal NK, Nery J, et al. Transplantation of the abdominal wall. Lancet 2003;361:2173–6. [DOI] [PubMed]
- 21.Meuli-Simmen C, Kehrer S, Eiman T, Schiestl R, Griffey S, Placik O, et al. Microvascular transplantation of cryopreserved knee joints. Ann Plast Surg 1995;35:184–90. [DOI] [PubMed]
- 22.Miyamoto Y, Suzuki S, Nomura K, Enosawa S. Improvement of hepatocyte viability after cryopreservation by supplementation of long-chain oligosaccharide in the freezing medium in rats and humans. Cell Transplant 2006;15:911–9. [DOI] [PubMed]
- 23.Ozer K, Gurunluoglu R, Zielinski M, Izycki D, Unsal M, Siemionow M. Extension of composite tissue allograft survival across major histocompatibility barrier under short course of anti-lymphocyte serum and cyclosporine a therapy. J Reconstr Microsurg 2003;19:249–56. [DOI] [PubMed]
- 24.Petit F, Minns AB, Dubernard JM, Hettiaratchy S, Lee WP. Composite tissue allotransplantation and reconstructive surgery: first clinical applications. Ann Surg 2003;237(1):19–25, Jan. [DOI] [PMC free article] [PubMed]
- 25.Petry JJ, Wortham KA. The anatomy of the epigastric flap in the experimental rat. Plast Reconstr Surg 1984;74:410–3. [DOI] [PubMed]
- 26.Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neural proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A 1993;90:2628–32. [DOI] [PMC free article] [PubMed]
- 27.Selvaggi G, Levi DM, Kato T, Madariaga J, Moon J, Nishida S, et al. Expanded use of transplantation techniques: abdominal wall transplantation and intestinal autotransplantation. Transplant Proc 2004;36:1561–3. [DOI] [PubMed]
- 28.Siemionow M, Ozer K. Advances in composite tissue allograft transplantation as related to the hand and upper extremity. J Hand Surg 2002;27A:565–80. [DOI] [PubMed]
- 29.Siemionow M, Ortak T, Izycki D, Oke R, Cunningham B, Prajapati R, et al. Induction of tolerance in composite-tissue allografts. Transplantation 2002;74:1211–7. [DOI] [PubMed]
- 30.Siemionow M, Demir Y, Mukherjee A, Klimczak A. Development and maintenance of donor-specific chimerism in semi-allogenic and fully major histocompatibility complex mismatched facial allograft transplants. Transplantation 2005;79:558–67. [DOI] [PubMed]
- 31.Strome M, Stein J, Esclamado R, Hicks D, Lorenz RR, Braun W, et al. Laryngeal transplantation and 40-month follow-up. N Engl J Med 2001;344:1676–9. [DOI] [PubMed]
- 32.Tanaka H, Maeda K, Okita Y. Transplantation of the cryopreserved tracheal allograft in growing rabbits. J Pediatr Surg 2003;38:1707–11. [DOI] [PubMed]
- 33.Taub PJ, Marmur JD, Zhang WX, Senderoff D, Nhat PD, Phelps R, et al. Locally administered vascular endothelial growth factor cDNA increases survival of ischemic experimental skin flaps. Plast Reconstr Surg 1998;102:2033–9. [DOI] [PubMed]
- 34.Yin H, Wang X, Kim SS, Chen H, Tan SL, Gosden RG. Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischaemia, and genotype. Hum Reprod 2003;18:1165–72. [DOI] [PubMed]
- 35.Zhang F, Attkis KJ, Walker M, Buncke HJ. Effect of cryopreservation on survival of composite tissue grafts. J Reconstr Microsurg 1998;14:559–64. [DOI] [PubMed]