Abstract
In this pilot study, hypertonic dextrose solution was used to induce fibrosis of the subsynovial connective tissue (SSCT) and create an animal model of potential use in the study of carpal tunnel syndrome (CTS). The SSCT of the carpal tunnel in 15 New Zealand white rabbits were injected with 0.05 ml of 10% dextrose solution in 1 paw and 0.05 ml of saline in the contralateral paw, to serve as a control. The animals were killed at 1, 2, 4, 8, or 12 weeks. While the saline side showed minimal changes at any time period, the hypertonic dextrose side showed progressive noninflammatory SSCT fibrosis, with vascular proliferation and thickening of collagen bundles. Demyelination of the median nerve developed at 12 weeks after the injection on the dextrose side. These findings are similar to the progression of pathology noted in humans with CTS.
Keywords: Carpal tunnel, Subsynovial connective tissue, Dextrose, Rabbit, Animal model
Introduction
Carpal tunnel syndrome (CTS), compression neuropathy of the median nerve at the wrist, is the most common and best known of the compression neuropathies of the upper extremity. More than 200,000 carpal tunnel releases are performed each year in the United States, which makes it the most common surgical procedure performed on the hand. Each year, close to 1,000,000 people require medical care, or are temporarily disabled by CTS [31].
Increased pressure within the carpal tunnel is the presumed immediate cause of the neuropathy [13, 33]. In some cases, the cause of this pressure elevation is clear, as with fractures that deform the carpal canal [1, 2, 18, 28], or localized inflammation that results in synovial hypertrophy [4, 29], as in rheumatoid arthritis or infection. Although in most cases, the cause of this localized pressure elevation is unknown.For such cases of idiopathic CTS, clinical studies [13] and clinical observation suggest that the pressure elevations are first intermittent, resulting in transient symptoms, only later becoming continuous and resulting in neurophysiological changes consistent with demyelination. To explain this clinical picture, microtrauma has been commonly implicated as an etiological factor [12], as well physiological abnormalities, especially diabetes mellitus [3, 27, 30].
Noninflammatory fibrosis of the subsynovial connective tissue (SSCT) within the carpal tunnel is the most characteristic histopathological finding in patients with idiopathic carpal tunnel syndrome [6, 17]. While it is reasonable to presume that a progressive noninflammatory fibrosis of the SSCT might lead to an increased volume within the carpal tunnel and thus increased pressure on the nerve [22], an experimental model that can replicate this progression has yet to be established.
We have been intrigued by the superficial similarity in the pathological changes of progressive fibrosis and vascular changes induced by prolotherapy [10, 14] and those seen in the SSCT of patients with CTS [6, 19]. We hypothesized that prolotherapy could be adapted to induce a progressive change in the SSCT of an experimental animal that would, over time, lead to morphological changes in the median nerve, in essence reproducing the clinical course seen in patients with idiopathic CTS. If this hypothesis was supported, a new animal model would be available to study the cascade of events leading to CTS, including, possibly, therapies to abort the process before the neuropathy became established. We preferred to avoid compounds such as phenol, which have a direct toxic effect on nerve, and were intrigued by the possibility of using a physiological substance such as glucose, which has also been shown to have a prolotherapy effect when administered in hypertonic concentrations. The purpose of this study was to evaluate, in a pilot study, the effect over time of a single injection of 10% dextrose solution in the SSCT of the rabbit carpal tunnel on the morphology of the SSCT and median nerve.
Materials and Methods
Fifteen adult New Zealand White rabbits, 14 male and 1 female, with a weight between 4–4.5 kg, were used for this study. Our Institutional Animal Care and Use Committee approved this study.
The rabbits were anesthetized by an intramuscular injection of ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg). Following the induction of satisfactory anesthesia, both forepaws were prepared and draped. One paw was randomly selected to receive a 0.5 ml injection of 10% glucose solution, while the contralateral paw received a similar volume of saline solution as a control. The paw selected to receive the glucose solution was alternated between the right and left side among the animals. In each paw, the limb was exsanguinated with an elastic bandage, which was then used as a tourniquet. A small incision was made in the paw 1 cm proximal to the carpal tunnel. Localization of the carpal tunnel is facilitated in the rabbit, as the flexor retinaculum contains an easily palpated fibrocartilaginous disc (Fig. 1). Dissection was carried out under 3.5 power loupe magnification. The flexor tendons were identified, and the middle digit flexor was identified by moving that digit passively. The injection was then made into the synovium around the middle digit flexor digitorum superficialis tendon, using a 30 gauge needle to minimize trauma. Care was taken to avoid any injection into the median nerve. The tourniquet was then removed. Hemostasis was achieved with local pressure, the wound closed with sutures of 5-0 Vicryl (Johnson and Johnson, New Jersey USA), and a sterile dressing was applied. Upon awakening, the rabbits were allowed full cage activity until the time of sacrifice. Three animals each were sacrificed at 1, 2, 4, 8, and 12 weeks after the injections. After sacrifice, the front paws were harvested and the total contents of the carpal tunnel were divided and prepared for light and scanning electron microscopy (SEM).
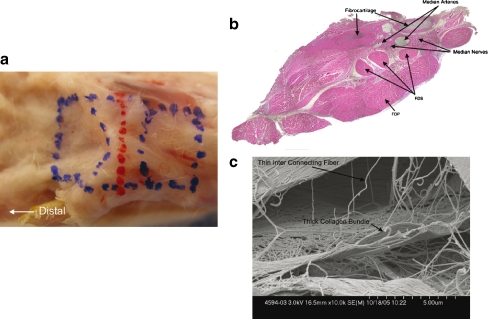
Figure 1.
a Normal rabbit carpal tunnel outlined in blue in this dissected specimen. b Cross-section through area marked in red in a. Note fibrocartilaginous disc within the flexor retinaculum, three separate flexor digitorum superficialis (FDS) tendons, and a single large flexor digitorum profundus tendon (FDP). Bundles of the median nerve, and the median arteries are noted. Hematoyxlin and eosin (HE) staining (×40). c SEM (×10,000) of rabbit carpal tunnel. Note thicker collagen bundles and thinner interconnecting fibers.
The contents of the carpal tunnel were marked with permanent ink to orient the specimen proximal to distal and superficial to deep (Fig. 1). The biopsies for SEM were fixed in Trump’s fixative (1% glutaraldehyde and 4% formaldehyde in 0.1 M phosphate buffer, pH 7.2 [15]), and dehydrated through a graded series of ethanol solutions in a critical point dryer. Tissues were then rinsed for 30 min in two changes of 0.1 M phosphate buffer, pH 7.2, and dehydrated in progressive concentrations of ethanol. The specimens were mounted on aluminum stubs and sputter coated with gold-palladium. Images were captured on a Hitachi S4700 cold field emission scanning electron microscope operating at 2 KV (Hitachi S-4700, Hitachi High Technologies America, Pleasanton, CA, USA). Pictures were taken with the palmar side of the tissue up and at different levels from proximal to distal throughout the harvested specimen. The specimens were evaluated qualitatively for collagen fiber organization and thickness.
The tissue for histology was formalin fixed and paraffin embedded. Five-micrometer sections were made and stained with standard hematoxylin and eosin or Luxol Fast Blue. The specimens were evaluated qualitatively for cellularity, neovascularization, fibrosis, and inflammation, as well as for median nerve appearance.
Based on the findings in the initial animal cohort, we subsequently studied an additional rabbit, followed for 16 weeks. This additional animal was also approved by our Institutional Animal Care and Use Committee and followed the same surgical and postoperative protocol. Immediately before killing, compound muscle action potential was measured for each median nerve. The muscle compound action potential of median supplied paw muscles was measured using stainless steel near-nerve stimulating and recording electrodes, recording from the ventral aspect of the forepaw while stimulating just above the wrist. Recordings were done at 35°C and amplified ×1,000, stored on computer disk, and analyzed off-line using a digital oscilloscope (Nicolet Instruments, Madison, WI). These studies were performed in the laboratory of our colleague, Philip A. Low, MD.
Immediately after killing, the median nerve and carpal tunnel contents of this additional animal were fixed for transmission electron microscopy and prepared in our institutional electron microscopy core laboratory.
Results
Postoperatively, all the animals recovered without difficulty and the wounds healed uneventfully. The rabbits then resumed normal behavior and skin wound healing proceeded uneventfully until the time of sacrifice, except for two of the three animals sacrificed at 12 weeks, who developed ulcerations on the dextrose injected paw in the week before killing. One rabbit showed a 5 × 5 mm superficial ulceration just radial to the fibrocartilage disc and the other showed a 3 × 5 mm size superficial ulceration also just radial to the fibrocartilage disc. There were no ulnar sided ulcerations. These small ulcerations did not connect to the carpal tunnel itself.
For the first 2 weeks following the dextrose injection, the SSCT appeared to be somewhat hypercellular, but otherwise, the collagen organization and vascularity appeared to be similar to the saline injected paw (Fig. 2). There was no evidence of neutrophil invasion or any other histological evidence of inflammation. Nerve histology was similar comparing the two sides, with no evidence of changes compared to the normal rabbit median nerve [7].
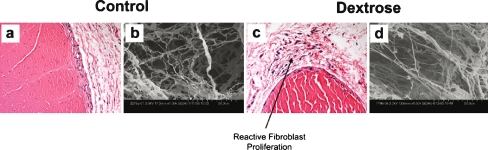
Figure 2.
a 1 week saline injection HE (×400); b 1 week saline SEM (×1,000); c 1 week dextrose HE (×400); d 1 week dextrose SEM (×1,000). Early interstitial fibrosis with reactive fibroblast proliferation is identified in the specimen removed 1 week after dextrose injection. However, there is no associated inflammation. In contrast, the saline injected specimen shows no conspicuous changes compared to the normal control.
At 4 weeks after the dextrose injection, the cellularity appeared to increase further, and evidence of vascular proliferation was seen along with collagen remodeling (Fig. 3). In contrast, the saline injected paws at 4 weeks appeared to be similar to the normal histology. Again, there was no evidence of neutrophil invasion or any other histological evidence of inflammation. Nerve histology was unchanged from the 1- and 2-week findings.
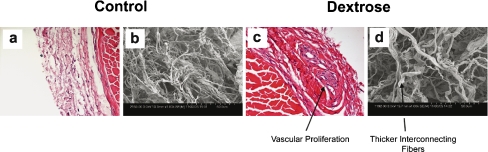
Figure 3.
a 4 week saline HE (×400); b 4 week saline SEM (×1,000); c 4 week dexrose HE (×400); d 4 week dextrose SEM (×1,000). Interstitial, somewhat disorganized, collagenation with fibrosis is identified in association with vascular proliferation in the specimen removed 4 weeks after dextrose injection. On SEM, these findings correspond to thickened interconnecting fibers. In contrast, the saline injected specimen shows no conspicuous changes compared to the normal control.
By 8 weeks after the dextrose injection, more angiogenesis and thicker collagen bundles were observed, without evidence of inflammation, whereas again the saline injected paws’ histological appearance was unremarkable (Fig. 4). Nerve histology was unchanged from the 1-, 2-, and 4-week findings.
Figure 4.
a 8-week saline HE (×400); b 8-week saline SEM (×1,000); c 8 week saline HE (×400); d 8 week saline SEM(×1,000). Dense collagenation and vascular proliferation become more prominent in the dextrose injected specimen compared to earlier specimens. In contrast, the saline injected specimen shows no conspicuous changes compared to the normal control. On SEM these findings correspond to thickened interconnecting fibers.
Twelve weeks after the dextrose injection we observed vascular proliferation and thicker collagen bundles in the SSCT (Fig. 5). We also observed changes suggestive of demyelination in all the median nerves after dextrose injection (Fig. 5f). The saline injected paws were histologically normal.
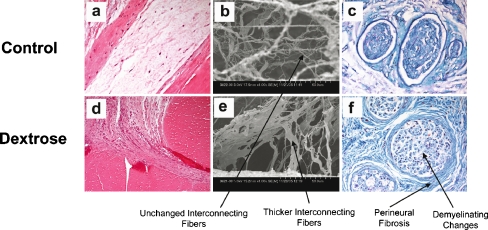
Figure 5.
a 12-week saline HE (×400); b 12 -week saline SEM (×1,000); c 12-week saline Luxol fast blue staining (×400); d 12-week dextrose HE (×400); e 12 week dextrose SEM (×1,000); f 12-week dextrose Luxol fast blue staining (×400). Demyelination is identified in association with interstitial organization fibrosis with collagenation and perineural fibrosis in the specimen removed 12 weeks after dextrose injection. In contrast, the saline injected specimen shows no conspicuous changes compared to the normal control.
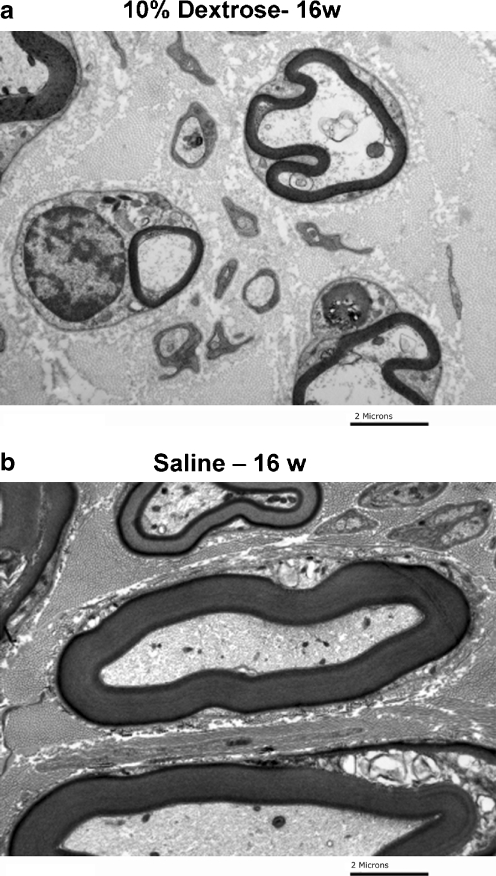
To verify these findings, we subsequently studied an additional rabbit, followed for 16 weeks, as noted above. This rabbit showed evidence of demyelination on transmission electron microscopy (Fig. 6), and both a reduction in motor compound action potential and slowing of motor nerve conduction (Fig. 7).
Figure 6.
aTransmission electron microscopy of median nerve 16 weeks after dextrose injection and b 16 weeks after saline injection.
Figure 7.
Motor nerve conduction 16 weeks after a dextrose injection and b saline injection. Note slowing on dextrose side.
Discussion
Previous experimental studies of CTS using animal models have focused on the pathology of the median nerve compression, induced by direct balloon catheter compression [5, 8, 9], surgically tightening the flexor retinaculum [12], or application of a tourniquet [13]. Although progressive demyelination of the median nerve has been observed in those experiments, such models only replicate those cases of CTS caused by acute space occupying lesions within the carpal tunnel, such as hematoma or abscess, or acute alterations in carpal canal anatomy or perfusion, as may occur after wrist fracture or dislocation [18]. In essence, these are excellent models to study the intraneural pathology of compression neuropathy, while being less well suited to investigate the chain of events proceeding and leading up to the neuropathy in patients with idiopathic CTS.
Experimental methods of inducing gradual, noninflammatory fibrosis are poorly defined. Recently, the concept of prolotherapy has been put forth in the alternative medicine literature. Prolotherapy, or proliferation therapy, is based on the premise that damaged soft tissues, such as tendons and ligaments, can be treated by injecting into them a solution that stimulates cellular proliferation and neovascularization. Phenol, sodium morrhuate, glycerin, or hypertonic glucose are most commonly used. While some clinical studies have shown conflicting conclusions about the effectiveness of prolotherapy in treating musculoskeletal pain [20, 34], other clinical studies have demonstrated more promising effects, especially in osteoarthritis and chronic tendon injuries [23–25, 32]. There are also experimental data to support the effectiveness of common prolotherapy agents for inducing cellular proliferation and vascular changes [14].
The rabbit has been a commonly used animal model of CTS because the rabbit’s carpal tunnel anatomy is similar to that of the human [11]. In the rabbit, the carpal bones and the flexor retinaculum form a rigid passageway at the wrist through which the flexor tendons and the median nerve travel [11]. The rabbit’s median nerve, flexor digitorum profundus, and flexor digitorum superficialis tendons all lay inside the carpal tunnel. The rabbit’s carpal bones are composed of three proximal bones, the radial, intermedium, and ulnare, and a small accessory carpal bone on the lateral side of the wrist [16]. The rabbit carpal tunnel also contains a SSCT, which is histologically and ultrastructurally similar to that of the human carpal tunnel [7]. Most importantly, the rabbit is a common model to study nerve compression, so there are extensive data available on the histopathology of the rabbit median nerve [21, 26].
The role of this pilot study was to determine if there was any evidence that local hypertonic dextrose injection could induce progressive morphological changes in the SSCT of an animal model, which might mimic the changes seen in carpal tunnel syndrome. The results showed evidence of such changes, which appeared to progress throughout the 12 weeks of observation. Although we have no direct evidence in the form of nerve conduction studies of the affected animals in this pilot data, we suspect that a loss of sensibility may be responsible for the ulcerations, which we observed in the dextrose injected paws of these animals. However, we have shown in one additional animal that nerve conduction is affected at 16 weeks post injection.
We do not know the cellular mechanism responsible for the effects that were observed. The putative mechanism of hypertonic dextrose prolotherapy is osmotic injury. It is possible that ischemia-reperfusion or other effects may also be involved. Further study in a larger experiment will be needed to clarify these issues.
Our findings in this small study are only qualitative. The analysis was not blinded. Nevertheless, we believe that the results are sufficiently suggestive of an effect from the dextrose injection to warrant a larger study with a more formal quantitative analysis. Potential study parameters could include histology, electrophysiology, and biological markers such as cytokines, matrix macromolecules, proteases, protease inhibitors, and markers for apoptosis and cellular proliferation. These could then be compared with similar studies in tissue from humans with carpal tunnel syndrome, to establish whether this model possesses more than superficial similarity with human CTS.
The strength of this study is the ability to document sequential structural changes in the SSCT and median nerve in this rabbit model. The limitations relate to the small sample size, lack of blinding, and the absence of any quantitative analyses or comprehensive electrophysiological studies.
Whether this model truly mimics the characteristics of human CTS remains to be shown. It was important to perform a pilot study with minimal animals to establish if 0.05 ml of 10% dextrose would show at least some general effects similar to human CTS, and to discover which tissues were most affected. The data we have collected here will allow us to design a far more comprehensive study of the effect of hypertonic dextrose on the rabbit carpal tunnel than would have been possible without this pilot data. We hope that others will also consider investigating and refining this new model, which may prove useful in the study of the causes and prevention of carpal tunnel syndrome.
Acknowledgement
This study was funded by grants from NIH (AR49823) and Mayo Foundation.
References
- 1.Bienek T, Kusz D, Cielinski L. Peripheral nerve compression neuropathy after fractures of the distal radius. J Hand Surg [Br] 2006;31:256–60. [DOI] [PubMed]
- 2.Bruske J, Niedzwiedz Z, Bednarski M, Zyluk A. Acute carpal tunnel syndrome after distal radius fractures-long term results of surgical treatment with decompression and external fixator application. Chir Narzadow Ruchu Ortop Pol 2002;67:47–53. [PubMed]
- 3.Cagliero E, Apruzzese W, Perlmutter GS, Nathan DM. Musculoskeletal disorders of the hand and shoulder in patients with diabetes mellitus. Acad Med 2002;112:487–90. [DOI] [PubMed]
- 4.De Smet L, Wouters C. Severe carpal tunnel syndrome in a patient with juvenile idiopathic arthritis due to proximal migration of hypertrophic lumbrical muscles. Clin Rheumatol 2004;23:552–4. [DOI] [PubMed]
- 5.Diao E, Shao F, Liebenberg E, Rempel D, Lotz JC. Carpal tunnel pressure alters median nerve function in a dose-dependent manner: a rabbit model for carpal tunnel syndrome. J Orthop Res 2005;23:218–23. [DOI] [PubMed]
- 6.Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg [Am] 2004;86:1458–66. [DOI] [PubMed]
- 7.Ettema AM, Zhao C, An KN, Anke M, Amadio PC. Comparative anatomy of the subsynovial connective tissue in the carpal tunnel of the rat, rabbit, dog, baboon, and human. HAND 2006;1:78–84. [DOI] [PMC free article] [PubMed]
- 8.Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol 2004;187:500–8. [DOI] [PubMed]
- 9.Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurolol 2003;461:174–86. [DOI] [PubMed]
- 10.Kim SR, Stitik TP, Foye PM, Greenwald BD, Campagnolo DI. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: a physiatric perspective. Am J Phys Med Rehabil 2004;83:379–89. [DOI] [PubMed]
- 11.Kornek GV, Rosen HR, Mohr W, Firbas W. Topography of carpal bone—An experimental model for carpal tunnel syndrome. Acta Anat 1990;139:1–4. [PubMed]
- 12.Lluch AL. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg [Br] 1992;17:209–12. [DOI] [PubMed]
- 13.Lundborg G, Gelberman RH, Minteer-Convery M, Lee YF, Hargens AR. Median nerve compression in the carpal tunnel-functional response to experimentally induced controlled pressure. J Hand Surg [Am] 1982;7:252–9. [DOI] [PubMed]
- 14.Maynard JA, Pedrini VA, Pedrini-Mille A, Romanus B, Ohlerking F. Morphological and biochemical effects of sodium morrhuate on tendons. J Orthop Res 1985;3:236–48. [DOI] [PubMed]
- 15.McDowell EM, Trump BF. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med 1976;100:405–14. [PubMed]
- 16.McLaughlin CA, Chiasson RB. External Anatomy and Skin. In: ed. Laboratory anatomy of the rabbit. 3rd ed. Dubuque, IA: Wm. C. Brown; 1990. pp. 16–93.
- 17.Neal NC, McManners J, Stirling GA. Pathology of the flexor tendon sheath in the spontaneous carpal tunnel syndrome. J Hand Surg [Br] 1987;12:229–32. [DOI] [PubMed]
- 18.Nishikawa T, Kurosaka M, Mitani M, Matsubara N, Harada T, Mizuno K. Ulnar bursa distention following volar subluxation of the distal radioulnar joint after distal radial fracture: a rare cause of carpal tunnel syndrome. J Orthop Trauma 2001;15:450–2. [DOI] [PubMed]
- 19.Jinrok O, Zhao C, Amadio PC, An KN, Zobitz ME, Wold LE. Vascular pathologic changes in the flexor tenosynovium (subsynovial connective tissue) in idiopathic carpal tunnel syndrome. J Orthop Res 2004;22:1310–5. [DOI] [PubMed]
- 20.Ongley MJ, Klein RG, Dorman TA, Eek, BC, Hubert LJ. A new approach to the treatment of chronic low back pain. Lancet 1987;2:143–6. [DOI] [PubMed]
- 21.Paik NJ, Cho SH, Han TR. Ultrasound therapy facilitates the recovery of acute pressure-induced conduction block of the median nerve in rabbits. Muscle Nerve 2002;26:356–61. [DOI] [PubMed]
- 22.Phalen GS. The carpal-tunnel syndrome. Seventeen years’ experience in diagnosis and treatment of six hundred fifty-four hands. J Bone Joint Surg [Am] 1966;48:211–28. [PubMed]
- 23.Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med 2000;6:68–74. [PubMed]
- 24.Reeves KD, Hassanein K. Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP, PIP, and trapeziometacarpal) joints: evidence of clinical efficacy. J Altern Complement Med 2000;6:311–20. [DOI] [PubMed]
- 25.Reeves KD, Hassanein KM. Long-term effects of dextrose prolotherapy for anterior cruciate ligament laxity. Altern Ther Health Med 2003;9:58–62. [PubMed]
- 26.Rosen HR, Ammer K, Mohr W, Böck P, Korneck GV, Firbas W. Chemically-induced chronic nerve compression in rabbits—a new experimental model for the carpal tunnel syndrome. Langenbecks Arch Chir 1992;377:216–21. [DOI] [PubMed]
- 27.Schreiber JE, Foran MP, Schreiber DJ, Wilgis EF. Common risk factors seen in secondary carpal tunnel surgery. Ann Plast Surg 2005;55:262–5. [DOI] [PubMed]
- 28.Seiler JG, 3rd, Havig M, Carpenter W. Acute carpal tunnel syndrome complicating chronic palmar subluxation of the distal ulna. J South Orthop Assoc 1996;5:108–10. [PubMed]
- 29.Serafin-Krol M, Ciechomska A, Tlustochowicz W, Jakubowski W, Cholewa M. Ultrasonography of the hand in rheumatoid arthritis. Pol Merkuriusz Lek 2003;15:491–4. [PubMed]
- 30.Singh R, Gamble G, Cundy T. Lifetime risk of symptomatic carpal tunnel syndrome in Type 1 diabetes. Diabet Med 2005;22:625–30. [DOI] [PubMed]
- 31.Tanaka S, Wild DK, Seligman PJ, Behrens V, Cameron L, Putz-Anderson V. The US prevalence of self-reported carpal tunnel syndrome: 1988 National Health Interview Survey data. Am J Public Health 1994;84:1846–8. [DOI] [PMC free article] [PubMed]
- 32.Topol GA, Reeves KD, Hassanein KM. Efficacy of dextrose prolotherapy in elite male kicking-sport athletes with chronic groin pain. Arch Phys Med Rehabil 2005;86:697–702. [DOI] [PubMed]
- 33.Tucci M, Sud V, Freeland A. Compression of the median nerve in CTS is mediated by periods of acute synovial swelling. Biomed Sci Instrum 2001;37:299–303. [PubMed]
- 34.Yelland MJ, Mar C, Pirozzo S, Schoene ML, Vercoe P. Prolotherapy injections for chronic low-back pain. Cochrane Database Syst Rev 2004;CD004059. [DOI] [PubMed]