Abstract
The calcium-sensing receptor (CaR) is a G-protein-coupled receptor that signals in response to extracellular calcium and regulates parathyroid hormone secretion. The CaR is also expressed on normal mammary epithelial cells (MMECs), where it has been shown to inhibit secretion of parathyroid hormone-related protein (PTHrP) and participate in the regulation of calcium and bone metabolism during lactation. In contrast to normal breast cells, the CaR has been reported to stimulate PTHrP production by breast cancer cells. In this study, we confirmed that the CaR inhibits PTHrP production by MMECs but stimulates PTHrP production by Comma-D cells (immortalized murine mammary cells) and MCF-7 human breast cancer cells. We found that changes in intracellular cAMP, but not phospholipase C or MAPK signaling, correlated with the opposing effects of the CaR on PTHrP production. Pharmacologic stimulation of cAMP accumulation increased PTHrP production by normal and transformed breast cells. Inhibition of protein kinase A activity mimicked the effects of CaR activation on inhibiting PTHrP secretion by MMECs and blocked the effects of the CaR on stimulating PTHrP production in Comma-D and MCF-7 cells. We found that the CaR coupled to Gαi in MMECs but coupled to Gαs in Comma-D and MCF-7 cells. Thus, the opposing effects of the CaR on PTHrP production are because of alternate G-protein coupling of the receptor in normal versus transformed breast cells. Because PTHrP contributes to hypercalcemia and bone metastases, switching of G-protein usage by the CaR may contribute to the pathogenesis of breast cancer.
The extracellular calcium-sensing receptor (CaR)2 is a 7-transmembrane-spanning, G-protein-coupled cell-surface receptor (GPCR) that binds calcium ions and allows cells to react to changes in the extracellular calcium concentration (Ca2+o) (1). It was initially discovered as a parathyroid calcium sensor and has subsequently been documented to be a critical component in the regulation of systemic calcium metabolism (2–4). The CaR is expressed prominently in the parathyroid gland and in the kidney, and activation of the receptor by increased Ca2+o suppresses PTH secretion and increases renal calcium excretion (3, 5). The CaR is also expressed at lower levels in many other organs and has been implicated in the regulation of ion and fluid transport, proliferation, differentiation, and apoptosis in a variety of cell types (1, 6–13).
The CaR exists on the cell surface as a homodimer and binds ionized calcium and other cations or cationic compounds such as magnesium, gadolinium, and neomycin (type I calcimimetics) to initiate signaling (1, 14). The activity of the receptor can also be modulated by allosteric activators or inhibitors, so-called type II calcimimetics or calcilytics, which are compounds that cannot initiate signaling alone but instead modulate the signaling response to calcium or other ligands (14, 15). As a prototypical GPCR, signaling is initiated by activation of heterotrimeric G-proteins. The CaR has been shown to couple to Gαi/0 and Gαq/11, and some data suggest that it may also interact with Gα12/13 (1, 16, 17). The signaling events put in motion by these G-proteins are complex and appear to vary considerably from one cell type to another. In parathyroid cells and in HEK cells transfected with the CaR, coupling to Gαi/0 and/or Gαq/11 is associated with activation of phospholipase C (PLC) and the generation of IP3 and intracellular calcium transients (1, 16, 17). This, in turn, is associated with the initiation of MAPK cascades, including the extracellular signal-regulated kinases (ERK) 1 and 2, p38, and the stress-activated, c-Jun N-terminal kinase (JNK) (16, 18, 19). Activation of ERK1 and -2 through a PLC, intracellular calcium transient, protein kinase C (PKC) pathway has been suggested to be important for CaR-mediated suppression of PTH secretion in dispersed parathyroid cells (16, 18, 19). Activation of the CaR also decreases cAMP concentrations in these cells through a Gαi-mediated mechanism, although this has been thought to be of secondary importance for regulating PTH secretion (16, 17). In other cell types, MAPK activation downstream of the CaR appears to be independent of PLC and/or PKC (20–22). Finally, the CaR has also been show to stimulate arachidonic acid metabolism, the Ras-Raf pathway, and the phosphatidylinositol 3-kinase/Akt pathway (1, 16, 17). Thus, like with many other GPCRs, signaling from the CaR is varied and cell-specific.
Parathyroid hormone-related protein (PTHrP) is a peptide growth factor that was originally described as the cause of humoral hypercalcemia of malignancy (23, 24). PTHrP is expressed by many different cells and has important local functions in diverse sites such as the skeleton, vasculature, teeth, and mammary glands (23, 24). It is produced by mammary epithelial cells and is necessary for the development of embryonic mammary glands in both mice and humans (25, 26). It is made by the lactating breast as well and is secreted into the circulation and into milk in large quantities (27). Lactation appears to be the only instance, other than humoral hypercalcemia of malignancy, in which PTHrP acts in a classical endocrine fashion to up-regulate osteoclastic bone resorption and liberate skeletal calcium stores for milk production. Because of its ability to increase osteoclast activity, PTHrP can also contribute to the pathogenesis of breast cancer by promoting osteolytic bone destruction surrounding metastatic deposits in the skeleton (27, 28).
The CaR has been shown to regulate PTHrP production in a variety of cell types. Astrocytes, osteoblasts, cytotrophoblasts, ovarian surface epithelial cells, and HEK 293 cells transfected with the CaR all secrete more PTHrP in response to increased Ca2+o or treatment with calcimimetics (29). Likewise, in malignancies such as human prostate cancer, human breast cancer, and rat Leydig cell tumor cell lines, CaR signaling increases PTHrP production (29). The CaR was first described in normal human breast tissue and in breast cancers by Cheng et al. (30) and was shown to stimulate PTHrP production by breast cancer cell lines by Sanders et al. (31). Subsequently, the CaR was shown to be expressed by mammary epithelial cells during lactation in mice (11). However, unlike breast cancer cells, normal mammary epithelial cells suppress PTHrP gene expression and secretion in response to activation of the CaR (11, 32). In fact, negative regulation of PTHrP in response to calcium delivery appears to be an important component of an endocrine feedback circuit between the lactating breast and bone, which acts to maintain a steady flow of skeletal calcium for milk production (33, 34).
We were intrigued by the discrepancy between the suppression of PTHrP secretion by calcium in normal breast cells and the stimulation of PTHrP secretion by calcium in breast cancer cells. We hypothesized that these opposite responses might result from alterations in signaling downstream of the CaR in normal versus malignant cells. As expected, we found that the CaR coupled to Gαi in MMEC, but surprisingly, it coupled to Gαs in Comma-D and MCF-7 cells. The data presented in this study suggest that malignant transformation of breast epithelial cells can lead to a switch in G-protein coupling that reverses the normal suppression of PTHrP by calcium.
EXPERIMENTAL PROCEDURES
Materials
Forskolin, H-89, N6,2′-O-dibutyryl adenosine 3′,5′-cyclic monophosphate sodium salt (Bt2cAMP), 3-isobutyl-1-methylxanthine (IBMX), pertussis toxin (PTX), cholera toxin (CTX), guanosine 5′-diphosphate sodium salt (GDP), and guanosine 5′-triphosphate sodium salt hydrate (GTP) were purchased from Sigma. DMEM/F-12, DMEM, calcium-free DMEM, gentamycin, dispase, NuPAGE LDS sample buffer (4×), CaR siRNAs, and TRIzol were obtained from Invitrogen. Fetal bovine serum was obtained from Atlanta Biologicals (Atlanta, GA). Collagenase type III was purchased from Worthington and [35S]GTPγS from PerkinElmer Life Sciences. Insulin, hydrocortisone, prolactin, and recombinant human epidermal growth factor were purchased from Cambrex Corp. (Walkersville, MD). PTHrP-IRMA kits were from Diagnostic Systems Laboratories (Webster, TX) and Correlate-EIA direct cAMP assay kits were from Assay Designs (Ann Arbor, MI). Polyvinylidene difluoride membrane came from Fisher, and protease inhibitors were from Pierce. Antibodies recognizing phospho-ERK1/2, phospho-p38, phospho-JNK, ERK1/2, p-38, JNK, α-actin, and Gαs were purchased from Cell Signaling Technologies (Danvers, MA). An antibody recognizing all three Gαi isoforms as well as isoform-specific antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-mouse and goat anti-rabbit secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). The Message Clean system was obtained from Gen Hunter (Nashville, TN), and Brilliant SYBR-Green QRT-PCR Master Mix kit was purchased from Stratagene (La Jolla, CA). The enhanced chemiluminescence kit, employing the SuperSignal West Pico substrate, was purchased from Pierce.
Methods
Cell Culture—Primary cultures of mouse mammary epithelial cells (MMECs) were prepared from pregnant CD1 mice as described previously (11). Organoids were filtered, plated, and grown in DMEM/F-12 medium supplemented with 5% fetal bovine serum containing insulin (5 μg/ml), hydrocortisone (1 μg/ml), and recombinant human epidermal growth factor (3 ng/ml), amphotericin B (2.5 μg/ml), and gentamycin (50 μg/ml). Experiments were performed on cells after the first passage. The media were switched to DMEM containing 0.1 mm calcium, 0.2% bovine serum albumin, 5 μg/ml insulin, 1 μg/ml hydrocortisone, 3 μg/ml prolactin, and antibiotics. The final calcium content of the media was adjusted with the addition of calcium chloride. Comma-D cells were maintained in DMEM/F-12 medium as mentioned for MMECs, whereas MCF-7 cells were cultured in DMEM with 10% fetal bovine serum and antibiotics.
Measurement of PTHrP Secretion—MMECs, Comma-D cells, or MCF-7 cells were seeded into 12-well plates at a density of 106 cells per well. After 48 h, the cells were placed into 0.5 ml of media containing 0.1 mm calcium with either additional CaCl2 (to a final concentration of 0.5, 1.0, 2.5, and 5 mm calcium) or the polycationic agonists neomycin (300 μm) or gadolinium (100 μm) (11, 12). Conditioned medium was removed after 16 h and PTHrP-(1–86) was measured using a two-site immunoradiometric assay following the manufacturer's instructions. PTHrP levels were normalized to the total cellular protein from each well. In other experiments, cells were preincubated with forskolin (10 μm), Bt2cAMP (1 mm), IBMX (1 mm), CTX (100 nm), PTX (100 nm), or PKA inhibitor H89 (10 μm) for 1–2 h before the addition of CaCl2 (5 mm), neomycin, or gadolinium. Conditioned medium was then collected after 16 h, and PTHrP was measured as above.
Analysis of Total Inositol Phosphates—Total inositol phosphates were measured in cell lysates as described in detail previously (35, 36). Briefly, cells were grown in 12-well plates at a density of 100,000 cells per well for 3 days and then labeled overnight with 10 μCi/ml [2-3H]inositol in inositol-free medium (MEM). Subsequently, the cells were washed with LiCl (10 mm)-containing medium. They were stimulated for 5 min with calcium chloride (5 mm), gadolinium (100 μm), or the known phospholipase C agonists, carbachol (100 μm) and 30 mm potassium, in 0.4 ml of basal medium supplemented with 10 mm LiCl. CaCl2 (0.1 mm) was included in the medium when carbachol/potassium and gadolinium were used as stimulants. Stimulation was terminated by the addition of 0.4 ml of perchloric acid (20%) to yield a final concentration of 10%, and cells were incubated on ice for 20 min. The inositol phosphates were collected in the supernatants after precipitating the cell contents by the addition of 270 μl of 6 n KOH and 5 ml of ice-cold water. Analysis of total inositol phosphates was done by subjecting the supernatants to anion exchange chromatography using AG1-X8 resin (200–400 mesh) as described previously (35, 36). The columns were washed with 10 ml of ice-cold water, 5 ml of borax (5 mm), and NaF (60 mm) solution, and total inositol phosphates were eluted with two times 5 ml of formic acid (0.1 m) and ammonium formate (1 m) solution. Radioactivity was measured in a scintillation counter (Wallac 1409DSA liquid scintillation counter, PerkinElmer Life Sciences).
Western Blot Analysis—For the determination of ERK1/2, p38, and JNK phosphorylation, cells were grown to 70–80% confluence on 6-well plates, incubated at 0.1 mm calcium for 1 h, and then stimulated by adding CaCl2 (to a final concentration of 0.5, 1.0, 2.5, and 5.0 mm) for 5 min. The stimulation was terminated by washing the cells with ice-cold phosphate-buffered saline, and then cells were lysed in 400 μl of Nonidet P-40 lysis buffer (20 mm Tris base, pH 8.0, 150 mm NaCl, 1% Nonidet P-40 with protease and phosphatase inhibitors). Cell lysates were centrifuged at 14,000 × g to pellet cell debris. Equal amounts of cellular protein (25 μg) were subjected to 12% SDS-PAGE and transferred electrophoretically to polyvinylidene difluoride membranes. Activation of MAPKs was detected by immunoblotting overnight at 4 °C with specified dilutions (according to manufacturer's protocol) of rabbit/mouse monoclonal/polyclonal antiserum specific for phospho-ERK1/2, phospho-p38 and phospho-JNK. Blots were then washed in TBST (20 mm Tris-HCl, 137 mm NaCl, and 0.05% Tween 20, pH 8.0) four times for 5 min each at room temperature and then incubated for 1 h with a 1:6000 dilution of horseradish peroxidase-conjugated secondary antibodies in 5% nonfat milk in TBST. After washing, bands were visualized by chemiluminescence using the SuperSignal West Pico detection kit according to the manufacturer's protocol. After stripping, the same membranes were used for the determination of total ERK1/2, p38 JNK, as well as α-actin, which served as a loading control. For the determination of G-protein expression in different cell types, cell lysates were prepared from MMEC, Comma-D, and MCF-7 cells (without CaR stimulation), and Western blots as described above were performed with anti-Gαi (1:200) and anti-Gαs (1:1000) antibodies.
cAMP Assay—MMEC, Comma-D, or MCF-7 cells were seeded into 60-mm dishes. After reaching 70–80% confluence, 2 ml of media containing 0.1 mm calcium were added to the cells, and additional CaCl2 (to a final concentration of 0.5, 1.0, 2.5, and 5 mm) or the polycationic agonists neomycin (300 μm) or gadolinium (100 μm) were also added. Cells were incubated at 37 °C for 30 min and washed with cold phosphate-buffered saline, and cell lysates were prepared with 0.1 m HCl. cAMP was measured in cell lysates by enzyme immunoassay as per the manufacturer's instructions. In additional experiments, cells were preincubated with forskolin (10 μm) for 30 min, cholera toxin (100 ng) for 2 h, or pertussis toxin (100 ng) for 90 min, before additional calcium or calcimimetics were added. The amount of cAMP was corrected for the protein content of each individual lysate.
Quantitative Real Time RT-PCR—Total RNA was isolated from MMEC and Comma-D cells treated with varying amounts of calcium or calcimimetics, using TRIzol as per the manufacturer's instructions. Any contaminating DNA was removed using the Message Clean system. The Brilliant SYBR-Green QRT-PCR Master Mix kit was used to perform one-step QRT-PCRs, in triplicate, using 20 ng of RNA with a reaction volume of 20 μl on the Opticon 2 DNA Engine (MJR, Waltham, MA). Reverse transcription was carried out for 30 min at 50 °C, followed by 40 cycles consisting of 30 s at 95 °C and 1 min at 60 °C. The glyceraldehyde-3-phosphate dehydrogenase primers were as follows: forward, 5′-CGTCCCGTAGACAAAATGGT-3′, and reverse, 5′-TCAATGAAGGGGTCGTTGAT-3′, and the PTHrP primers were forward, 5′-TTCAGCAGTGGAGTGTCCTG-3′, and reverse, 5′-TTGCCCTTGTCATGCAGTAG-3′. For each experimental sample, a control without reverse transcriptase was run to verify that the amplification product arose from cDNA and not from genomic DNA. The relative expression levels, normalized to glyceraldehyde-3-phosphate dehydrogenase, were determined using the comparative CT method (also known as the 2ΔΔCT method) (32, 37). PTHrP mRNA levels were expressed as the fold stimulation or suppression relative to basal expression in unstimulated cells.
[35S]GTPγS Binding Assay—For the determination of G-protein coupling to the CaR, we carried out [35S]GTPγS binding assays according to the method described by Barr and Manning (38). Briefly, activation of specific Gα subunits was determined by immunoprecipitating either Gαi or Gαs from membrane preparations exposed to [35S]GTPγS in the presence or absence of ligands for the CaR. The degree of incorporation of 35S into the immunoprecipitate served as an index of receptor usage of that particular G-protein subunit (38, 39). Binding of [35S]GTPγS to G-proteins was carried out in a 50-μl reaction mixture containing membrane protein (500 μg for MMEC and Comma-D cells, 1 mg for MCF-7 cells) and 360 nm [35S]GTPγS in 50 mm Tris-HCl, pH 7.4, 2.8 mm MgCl2, 100 mm NaCl, and 1 μm GDP. The reaction was initiated by adding 5 mm CaCl2 or 300 μm of neomycin for 30 min at 30 °C, and the reaction was terminated by adding 600 μl of ice-cold immunoprecipitation buffer (50 mm Tris-HCl, pH 7.5, 20 mm MgCl2, 150 mm NaCl, 0.5% Nonidet P-40, 20 μg/ml aprotinin, 100 μm GDP, and 100 μm GTP), followed by a 30-min incubation on an orbital shaker at 4 °C. After incubation, the reaction contents were centrifuged at 14,000 × g, and the supernatant was transferred to 110 μl of a previously prepared antisera-protein A-Sepharose mixture. The resulting mixtures were incubated for 1 h at 4 °C on an orbital shaker, and immunoprecipitates were pelleted by centrifugation at 4,000 × g for 2 min at 4 °C. The pellet was washed three times, boiled for 1 min in 500 μl of 0.5% SDS, and transferred to scintillation vials containing 4 ml of scintillation liquid. Samples were analyzed directly in triplicate on a scintillation counter (Beckman Coulter, LS5000 TA and TD liquid scintillation system, Fullerton, CA). Nonimmune serum was used to control for nonspecific binding of [35S]GTPγS.
Inhibition of CaR Expression by siRNA in Comma-D Cells—Comma-D cells were grown to 70% confluence and then transfected with Casr Stealth Select RNAi (MSS202652 or MSS202654, 260 pmol/well) using Lipofectamine™-2000 (7.8 μl/well) according to the manufacturer's protocol. The Stealth RNAi Negative Control Med GC (Invitrogen) was transfected in parallel as a control in all experiments. To verify knockdown of CaR mRNA, total cellular RNA was isolated from cells 48 h post-transfection and subjected to RT-PCR as described above. cAMP and PTHrP levels were assayed after treating transfected cells with calcium and calcimimetics as described previously. Membranes were prepared from cells 48 hours after transfection and used for [35S]GTPγS binding assays as described previously.
Statistical Analysis—Results are presented as the mean ± S.E. To compare the basal levels of PTHrP secretion and cAMP to the CaR-stimulated levels, the two-tailed Student's t test was used. A paired, two-tailed Student's t test was used when comparing PTHrP secretion or cAMP accumulation between the two groups across different concentrations of calcium or pharmacological agents. One-way analysis of variance with the Newman-Keuls post test was used to compare all pairs of columns. Gene expression levels in MMEC, Comma-D, and MCF-7 as assayed by real time RT-PCR were analyzed using a one-sample t test with a hypothetical mean of 1. All statistical analyses were carried out using Graph Pad Prism 4.00 for Macintosh (Graph-Pad Software for Science Inc., San Diego).
RESULTS
Effect of Calcium and Calcimimetics on PTHrP Secretion and Expression—To understand how CaR signaling might regulate PTHrP secretion in an opposite fashion in normal versus transformed breast cells, we first sought a murine cell line to study in parallel with MMECs to avoid any confounding species differences between human breast cancer cells and normal mouse breast cells. We chose to study Comma-D cells, which are an immortalized but not fully transformed murine mammary epithelial cell line that has been used extensively in prior studies (40, 41). As reported previously, increasing concentrations of Ca2+o led to a dose-dependent decrease in PTHrP secretion from MMECs (Fig. 1A). The effects of high Ca2+o were reproduced by neomycin and gadolinium, two different calcimimetic compounds, demonstrating that the CaR mediated this response. In contrast, although base-line PTHrP secretion was similar in MMECs and Comma-D cells, increasing doses of calcium or treatment with calcimimetics led to a progressive increase in PTHrP secretion in the Comma-D cells (Fig. 1B). These data show that activation of the CaR modulates PTHrP secretion in exactly the opposite fashion in MMECs as compared with Comma-D cells. This was also the case for PTHrP gene expression. As shown in Fig. 1, C and D, as assessed by real time RT-PCR, treatment of the cells with 5 mm Ca2+o, neomycin, or gadolinium for 16 h decreased PTHrP mRNA levels in MMECs but increased PTHrP levels in Comma-D cells.
FIGURE 1.
CaR signaling has opposing effects on PTHrP production in MMEC and Comma-D cells. PTHrP concentrations were measured in conditioned media harvested from MMEC (A) and Comma-D (B) cells exposed to the indicated concentrations of Ca2+o, neomycin (300 μm), and gadolinium (100 μm). PTHrP mRNA levels were assessed by real time RT-PCR from MMEC (C) and Comma-D cells (D) exposed to 0.1 mm Ca2+o, 5 mm Ca2+o, neomycin, and gadolinium. The graphs present the mean of three separate experiments; error bars represent the S.E. * denotes a statistically significant difference (p < 0.01) as compared with base-line values at 0.1 mm Ca2+o.
Effects of the CaR on Inositol Phosphate Turnover and MAPK Activation in MMECs and Comma-D Cells—In a variety of cell types, stimulation of the CaR has been described to activate phospholipase C, to generate IP3, to increase intracellular calcium, and to activate MAPK cascades (1, 16). MAPK signaling has, in turn, been suggested to contribute to the regulation of both PTH and PTHrP production by the CaR (1, 16). Therefore, we wondered if differences in PLC and/or MAPK activation in response to the CaR might explain the differences in PTHrP production in MMECs versus Comma-D cells. To assess PLC activation, we examined total inositol phosphate generation in response to 5 mm Ca2+o and gadolinium in MMEC and Comma-D cells (Fig. 2, A and B). Neither calcium nor gadolinium increased total IP production in either cell line suggesting that stimulation of the CaR does not activate PLC in breast cells. We next examined activation of the MAPKs, ERK1 and ERK2, because they have been implicated in mediating the effects of CaR on PTHrP secretion in several cell types (16, 29). As shown in Fig. 2, C and D, treatment of both cell lines with 5 mm Ca2+o caused transient activation of ERK1/2 that peaked at 5 min, receded by 10 min, and which had largely returned to base line by 30 min. Activation of ERK1/2 by extracellular calcium was dose-dependent, and treatment of both cell lines with calcimimetics led to ERK phosphorylation (data not shown). Finally, we also found that 5 mm Ca2+o activated both p38 MAPK and JNK phosphorylation in both cell lines (data not shown). Therefore, as in vascular smooth muscle cells (20), engagement of the CaR led to PLC-independent activation of MAPK signaling in breast cells. However, there were no obvious differences in MAPK activation between the cell types that might explain the very different effects of CaR signaling on PTHrP gene expression and secretion.
FIGURE 2.
Activation of the CaR has opposing effects on cAMP production in MMEC and Comma-D cells. A and B, activation of the CaR failed to trigger accumulation of [3H]inositol phosphate (IPs) in either MMEC (A) or Comma-D cells (B), demonstrating that neither cell line activates PLC in response to calcium or calcimimetics. Bars indicate the mean ± S.E. for three separate experiments, each carried out in triplicate. In contrast, total IPs were significantly (p < 0.01) higher when cells were treated with carbachol (100 μm) plus high potassium (30 mm), known activators of PLC in other cell types. * denotes a statistically significant difference as compared with base-line values at 0.1 mm Ca2+o. C and D, stimulation of the CaR activates ERK1 and ERK2 in both MMECs (C) and Comma-D (D) cells. Cells were incubated with 5 mm Ca2+o for the times indicated. Activation of the CaR led to similar transient ERK1/2 phosphorylation in both cell types. Levels of p-ERK1/2 peaked at 5 min and returned to base line by 30–60 min. E and F, intracellular cAMP concentrations in response to CaR activation. MMECs (E) and Comma-D cells (F) were treated with varying concentrations of Ca2+o, neomycin, or gadolinium as described under “Experimental Procedures.” CaR activation led to a decrease in cAMP concentration in MMECs but an increase in cAMP concentration in Comma-D cells. Bars represent the mean ± S.E. of four independent experiments, each performed in triplicate. * denotes a statistically significant difference (p < 0.001) as compared with base-line values at 0.1 mm Ca2+o.
Extracellular Calcium Inhibits cAMP Production in MMEC and Stimulates cAMP Production in Comma-D Cells—Activation of the CaR has been shown to decrease intracellular cAMP levels, presumably through Gαi-mediated inhibition of adenylyl cyclase activity (1, 16). Therefore, we next examined how changes in extracellular calcium and treatment with calcimimetics affected intracellular cAMP levels in MMECs and Comma-D cells. As expected, calcium, neomycin, and gadolinium all decreased cAMP levels in MMECs (Fig. 2E). In the continued presence of CaR agonists, the responses lasted at least 60 min (data not shown). By contrast, treatment of Comma-D cells with increasing doses of calcium, neomycin, or gadolinium had exactly the opposite effect on cAMP levels. As shown in Fig. 2F, base-line cAMP levels in Comma-D cells were similar to those in MMECs. However, increasing concentrations of extracellular calcium led to a progressive increase in cAMP levels as did treatment with calcimimetic agents. Like in MMECs, increases in cAMP concentration in response to CaR activation persisted for up to 1 h (data not shown). These data demonstrate that activation of the CaR has opposing effects on cAMP levels in MMECs and Comma-D cells that mirror the differing effects of calcium on PTHrP production by these two cell lines.
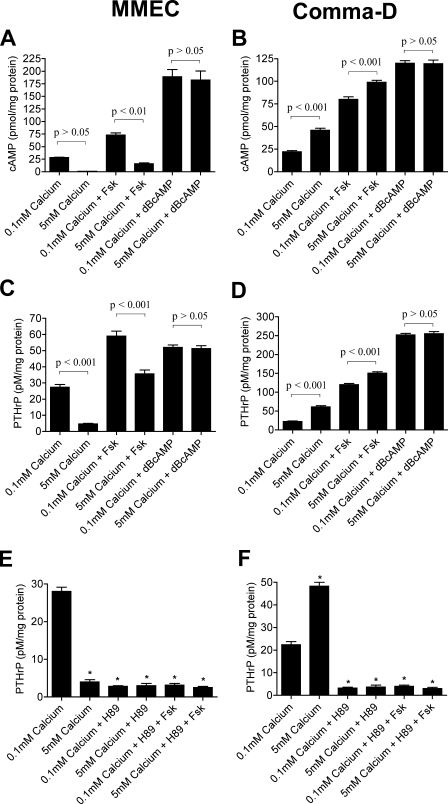
Manipulation of cAMP/PKA Signaling Affects PTHrP Production—The data presented above suggested that alterations in cAMP might be an important regulator of PTHrP production in breast cells. To test if this was the case, we manipulated cAMP levels pharmacologically and examined PTHrP production. Forskolin is a selective activator of adenylyl cyclase and stimulates cAMP production, whereas dibutyryl cAMP is a cell-permeable analog of cAMP that activates protein kinase A (PKA) and is resistant to degradation by phosphodiesterases (42, 43).
As shown in Fig. 3, A and B, treatment of both cell lines with either forskolin or Bt2cAMP increased intracellular cAMP levels. Interestingly, the response to high extracellular calcium (5 mm Ca2+o) remained intact when both cell lines were treated with forskolin but was lost when they were treated with Bt2cAMP. Importantly, in both MMECs and Comma-D cells, changes in PTHrP production tracked the changes in intracellular cAMP concentrations. As shown in Fig. 3, C and D, treatment of both MMECs and Comma-D cells with forskolin and Bt2cAMP increased PTHrP secretion. As with the changes in intracellular cAMP, although overall PTHrP secretion was higher in MMECs treated with forskolin, high calcium continued to suppress PTHrP production. However, high calcium no longer had any effect on PTHrP secretion in cells treated with Bt2cAMP. Likewise, in Comma-D cells treated with forskolin, 5 mm Ca2+o increased PTHrP secretion further, but high calcium had no additional effect in cells exposed to Bt2cAMP. Manipulating cAMP levels also affected PTHrP gene expression. Treatment of both cell lines with forskolin and Bt2cAMP increased PTHrP mRNA levels (data not shown). These data demonstrate that intracellular cAMP levels regulate PTHrP production in both cell lines.
FIGURE 3.
Manipulation of cAMP levels alters PTHrP secretion in MMECs and Comma-D cells. A and B, intracellular cAMP levels from MMECs (A) or Comma-D cells (B) treated with forskolin (Fsk) (10 μm) and Bt2cAMP (dbcAMP) (1 mm) in the presence of 0.1 mm Ca2+ o or 5 mm Ca2+ o. As expected, treatment with forskolin and Bt2cAMP increased intracellular cAMP levels in both cell types. The high levels of cAMP measured in cells treated with Bt2cAMP represent cross-reactivity of the assay for Bt2 cAMP. 5 mm Ca2+ o decreased cAMP levels in MMECs treated with forskolin but not in cells treated with Bt2cAMP. In contrast, 5 mm Ca2+o increased cAMP levels in Comma-D cells treated with forskolin but not in cells treated with Bt2cAMP. C and D, PTHrP levels from conditioned media harvested from MMECs (C) and Comma-D cells (D) treated with forskolin and Bt2cAMP as in A and B. Note that the changes in PTHrP secretion exactly mirror the changes in intracellular cAMP. E and F, PTHrP levels in conditioned media harvested from MMECs (E) or Comma-D cells (F) pretreated with the PKA inhibitor, H89 (10 μm), before being exposed to forskolin (10 μm) or 5 mm Ca2+o. H89 mimicked the inhibition of PTHrP secretion by high calcium in MMECs (E) and blocked the stimulation of PTHrP secretion by high calcium in Comma-D cells (F). In both cell types, H89 blocked the effects of forskolin. In all panels bars represent the mean ± S.E. of three individual experiments. Statistical significance is denoted on the graphs. Although the decrease in cAMP in response to 5 mm Ca2+o in MMECs was not significant by analysis of variance, it was significant (p < 0.001) by a paired t test.
Many of the actions of cAMP are mediated by PKA (44). Therefore, we treated both MMECs and Comma-D cells with the selective PKA inhibitor, H89, and measured PTHrP production (45). Inhibition of PKA activity lowered overall PTHrP secretion from both cell lines (Fig. 3, E and F). Treatment with H89 mimicked the suppressive effects of high calcium on PTHrP secretion in MMECs and blocked the stimulatory effects of high calcium on PTHrP secretion by Comma-D cells. As expected, in the presence of H89, forskolin failed to stimulate PTHrP secretion in either cell line. These data confirm that the cAMP/PKA pathway regulates PTHrP production in these cells and suggest that CaR activation modulates PTHrP production by controlling cAMP levels and PKA activity.
CaR Regulates cAMP Production, Not Degradation—In intestinal epithelial cells, activation of the CaR lowers cAMP levels by stimulating phosphodiesterase activity (7). To determine whether the CaR might regulate PTHrP production by breast cells in a similar fashion, we treated cells with the isoform-nonspecific phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (IBMX), to see if this would block the ability of high calcium to modulate cAMP and PTHrP production in MMECs and Comma-D cells (7). As expected (Fig. 4, A–D), IBMX treatment increased basal cAMP levels and PTHrP production in both cell lines. However, despite the presence of IBMX, treatment with 5 mm Ca2+o continued to decrease cAMP and to suppress PTHrP production in MMECs. Similarly, high calcium increased cAMP levels and PTHrP secretion further in Comma-D cells treated with IBMX. The failure of IBMX to abrogate the effects of calcium suggested that the CaR did not regulate intracellular cAMP concentrations in these cells by controlling its degradation. Instead, we hypothesized that activation of the CaR altered cAMP production. If this were the case, we reasoned that maximal stimulation of adenylyl cyclase activity with CTX (7, 46) should block the ability of high calcium to change cAMP levels or PTHrP production in either cell type. As shown in Fig. 4, E–H, treatment with CTX increased cAMP levels and PTHrP secretion in both MMECs and Comma-D cells. Furthermore, treatment with 5 mm Ca2+o no longer had an effect on these parameters in either cell type. In summary, when adenylyl cyclase activity was irreversibly and maximally stimulated with CTX or bypassed with Bt2cAMP, the CaR lost its ability to regulate PTHrP production. However, calcium continued to modulate PTHrP production when adenylyl cyclase activity was submaximally stimulated with forskolin or when cAMP degradation was blocked with IBMX. Together, these pharmacological data suggest that the CaR regulates PTHrP production by modulating cAMP production by adenylyl cyclase.
FIGURE 4.
Effects of IBMX and CTX on cAMP and PTHrP levels in MMECs and Comma-D cells. A and B represent intracellular cAMP levels, and C and D represent PTHrP levels in conditioned media from MMECs (A and C) and Comma-D cells (B and D) treated with IBMX in the presence of 0.1 mm Ca2+o or 5 mm Ca2+o. IBMX treatment elevated both cAMP and PTHrP levels in both cell types. However, in MMEC, 5 mm Ca2+o continued to inhibit cAMP accumulation and PTHrP secretion (A and C). In IBMX-treated Comma-D cells (B and D), 5 mm Ca2+o further stimulated cAMP levels and PTHrP secretion. E and F represent intracellular cAMP levels, and G and H represent PTHrP levels in conditioned media from MMECs (E and G) and Comma-D cells (F and H) treated with CTX in the presence of 0.1 mm Ca2+ o or 5 mm Ca2+ o. CTX stimulated both cAMP levels and PTHrP secretion in both cell types and abrogated any effects of 5 mm Ca2+o. In all panels, bars represent the mean ± S.E. for three separate experiments. Statistical significance is noted on the graphs.
The CaR Decreases cAMP and PTHrP Levels in MMEC through a Pertussis Toxin-sensitive Mechanism—Activation of Gαi is known to decrease intracellular cAMP levels by inhibiting adenylyl cyclase activity (16). Therefore, we asked if blocking Gαi activity in MMECs using pertussis toxin would interfere with the ability of the CaR to inhibit cAMP and PTHrP. Pertussis holotoxin (PTX) is a heterohexameric protein well known to disrupt the function of the Gαi subunit (47). As shown in Fig. 5, A and C, treatment with PTX abolished the CaR-mediated inhibition of cAMP and PTHrP secretion in MMECs, without affecting base-line cAMP or PTHrP levels at 0.1 mm Ca2+o. In contrast, in Comma-D cells, PTX had no effect either on cAMP levels or on PTHrP secretion in response to CaR activation by 5 mm Ca2+o (Fig. 5, B and D). Therefore, the CaR likely suppresses PTHrP secretion in MMECs through coupling to Gαi, but Gαi does not appear to mediate the effects of the CaR on PTHrP production in Comma-D cells.
FIGURE 5.
Inhibition of PTHrP production by CaR signaling in MMECs is pertussis toxin-sensitive. A and B represent intracellular cAMP levels, and C and D represent PTHrP levels in conditioned media from MMECs (A and C) and Comma-D cells (B and D) treated with PTX in the presence of 0.1 mm Ca2+o or 5 mm Ca2+o. PTX blocked the inhibition of cAMP accumulation and the decrease in PTHrP secretion triggered by 5 mm Ca2+o in MMECs (A and C). PTX had no effect on cAMP levels or PTHrP secretion in Comma-D cells. The bars in each graph represent the mean ± S.E. of three individual experiments. Statistical significance is noted on the graphs.
The CaR Couples to Gαi in MMECs and to Gαs in Comma-D Cells—Our data to this point indicate that, in breast cells, the CaR regulates PTHrP secretion by controlling the production of cAMP. The PTX-sensitive inhibition of cAMP production suggests that the CaR couples to Gαi in MMECs. However, in Comma-D cells, the CaR appears to increase adenylyl cyclase activity in a PTX-insensitive fashion. The simplest explanation for these findings would be a switch in coupling of the receptor to Gαs in these cells. To test this idea, we performed [35S]GTPγS binding assays using membranes prepared from MMECs and Comma-D cells. This assay relies on the activation of the CaR in vitro in the presence of a 35S-labeled, nondegradable form of GTP (GTPγS). Specific G-proteins are subsequently immunoprecipitated, and the radioactivity associated with each G-protein is an index of its activation in response to CaR activation (38, 39). Before performing this experiment, however, we first assessed if Gαi and Gαs were expressed in MMECs and Comma-D cells. As shown in Fig. 6, A and B, we found both G-proteins to be expressed in both cell lines. The Gαs subunit was expressed equally in both MMEC and Comma-D cells (Fig. 6A). There are three different isoforms of the Gαi subunit, Gαi1, Gαi2, and Gαi3 (48). Using an antibody that recognizes all three isoforms, we found that both cell lines expressed Gαi but that Comma-D cells expressed these subunits more abundantly than MMECs (Fig. 6B). Using isoform-specific antibodies, it appeared that Gαi2 was the dominant type in both cell lines, although Gαi3 was also expressed in both cell types at lower levels. Gαi1 was not expressed in either cell line (data not shown).
FIGURE 6.
The CaR couples to Gαi in MMEC and Gαs in Comma-D cells. A and B, Western blots showing the expression of Gαs (A) and Gαi (B) in MMECs and Comma-D cells. The antibody used for Gαi recognized all three isoforms. Anti-α-actin was used to control for equivalent protein loading. C and D, results of [35S]GTPγS binding assays in MMECs (C) and Comma-D cells (D) using antibodies specific for Gαs and all three isoforms of Gαi. As can be seen, activation of the CaR with 5 mm Ca2+o or neomycin triggered [35S]GTPγS binding to Gαi in MMECs (C) and [35S]GTPγS binding to Gαs in Comma-D cells. Nonspecific binding of [35S]GTPγS was assessed by using nonimmune serum (N.I. Serum) in control immunoprecipitations. Each bar represents the mean ± S.E. for data from three individual experiments, each of which was performed in triplicate. * denotes statistical significance (p < 0.001) as compared with nonimmune serum controls.
As expected, in MMECs we found that stimulation of the CaR increased the association of [35S]GTPγS with Gαi (Fig. 6C). There was little incorporation of GTPγS into anti-Gαi immunoprecipitates in the presence of 0.1 mm Ca2+o, but incorporation increased ∼25-fold in the presence of 5 mm Ca2+o and 20-fold in the presence of neomycin. In comparison, MMECs showed no incorporation of [35S]GTPγS into anti-Gαs immunoprecipitates in response to 5 mm Ca2+o or neomycin. The incorporation of [35S]GTPγS into immunoprecipitates using nonimmune serum was used to control for nonspecific binding of GTPγS in these experiments.
We carried out identical experiments with membrane preparations from Comma-D cells and found the opposite pattern. As illustrated in Fig. 6D, there was little incorporation of [35S]GTPγS into anti-Gαi immunoprecipitates from Comma-D membranes treated with either 5 mm Ca2+o or neomycin. Likewise, there was little binding of GTPγS to Gαs at 0.1 mm Ca2+o. However, activation of the CaR with 5 mm Ca2+o or neomycin resulted in a 15- and 13-fold increase, respectively, in the binding of [35S]GTPγS to Gαs. This result revealed that the CaR couples to Gαs in Comma-D cells thereby enhancing cAMP levels and PTHrP production when activated by calcium or calcimimetics.
As opposed to the treatment of intact cells, it is possible that high concentrations of calcium, neomycin, and/or gadolinium could affect G-protein activation in membrane preparations in a nonspecific manner. To control for this possibility, we used an mRNA “knockdown” approach to ensure that the G-protein activation measured in the GTPγS binding assay was caused by activation of the CaR. These experiments were limited to Comma-D cells, as MMECs transfect poorly. As shown in Fig. 7A, two different CaR-specific siRNA oligonucleotides reduced CaR mRNA levels in Comma-D cells by over 90% as compared with cells transfected with a random control siRNA. Knockdown of CaR expression in this manner resulted in loss of the cAMP and PTHrP responses to 5 mm Ca2+o, gadolinium, and neomycin (Fig. 7, B and C), as well as a significant reduction in Gαs activation in response to calcium or calcimimetics (Fig. 7D). These results demonstrate that the association of [35S]GTPγS with Gαs in response to calcium or calcimimetics requires the presence of the CaR.
FIGURE 7.
Knockdown of CaR expression inhibits the effects of calcium and calcimimetics on cAMP, PTHrP, and Gαs activation in Comma-D cells. A, real time RT-PCR analysis of CaR mRNA in Comma-D cells transfected with two different specific siRNAs (CaR-siRNA-A and CaR-siRNA-B) demonstrates approximately a 90% reduction in CaR mRNA levels as compared with the nonspecific control siRNA. Bars represent the mean ± S.E. of three experiments. * denotes a significant difference between CaR-siRNAs A and B versus control siRNA, p < 0.001. B and C show the effects of these siRNAs on intracellular cAMP and PTHrP levels in conditioned media, respectively, in response to CaR activation by calcium, gadolinium, and neomycin. CaR activation failed to increase cAMP and PTHrP levels in cells transfected with specific CaR siRNAs, whereas in cells transfected with nonspecific siRNA (control-siRNA), cAMP and PTHrP levels were elevated following CaR activation by 5 mm calcium, gadolinium, and neomycin. Bars represent the mean ± S.E. of three experiments; * denotes a significant difference between CaR-siRNA-A and -B versus control-siRNA (p < 0.05). D shows the results for [35S]GTPγS binding to Gαs in response to CaR activation in vitro. Depletion of the CaR by transfection of Comma-D cells with CaR-siRNA-A and -B led to a substantial reduction in [35S]GTPγS binding to Gαs in response to 5 mm calcium, gadolinium, or neomycin. However, CaR activation triggered [35S]GTPγS binding to Gαs in cell membranes prepared from cells transfected with nonspecific siRNA. Bars represent the mean ± S.E. of three experiments. * denotes a significant difference compared with control siRNA at 0.1 mm calcium, p < 0.001, whereas # denotes a significant difference between control siRNA and CaR siRNA-A and -B, p < 0.001.
CaR Stimulates PTHrP Production through Gαs Signaling in MCF-7 Cells—To determine whether stimulation of PTHrP production by the CaR in breast cancer cells was also the result of Gαs-mediated stimulation of cAMP production, we examined MCF-7 cells, a well established human breast cancer cell line that had previously been shown to secrete more PTHrP in response to calcium or calcimimetics (31). As with Comma-D cells, treatment of MCF-7 cells with 5 mm Ca2+o and forskolin increased intracellular cAMP levels (data not shown) and PTHrP secretion (Fig. 8A). As before, inhibition of PKA activity with H89 decreased basal PTHrP secretion and blocked the stimulatory effects of calcium and forskolin. Thus, in MCF-7 cells, cAMP/PKA signaling regulates PTHrP production. We also performed the [35S]GTPγS binding assay using membranes prepared from MCF-7 cells. As shown in Fig. 8B, the CaR coupled to Gαs in these cells. [35S]GTPγS incorporation was negligible in immunoprecipitates pulled down with anti-Gαi antiserum. However, compared with 0.1 mm Ca2+o, activation of the CaR increased [35S]GTPγS binding to Gαs by 85-fold in response to 5 mm Ca2+o and 96-fold in response to neomycin. These data demonstrate that in MCF-7 breast cancer cells, the CaR couples to Gαs and, as a result, stimulates cAMP and PTHrP production when activated.
FIGURE 8.
The CaR couples to Gαs and stimulates PTHrP production through a cAMP/PKA pathway in MCF-7 breast cancer cells. A, PTHrP concentrations in conditioned media harvested from MCF-7 cells treated with various combinations of 0.1 mm Ca2+ o, 5 mm Ca2+ o, forskolin, or H89. As with Comma-D cells, 5 mm calcium and forskolin both stimulated PTHrP secretion. Pretreatment of MCF-7 cells with H89 lowered base-line PTHrP secretion at 0.1 mm Ca2+o and blocked the stimulatory effects of 5 mm Ca2+o, forskolin, or the combination on PTHrP secretion. Each bar represents the mean ± S.E. of three individual experiments. * denotes significant difference from 0.1 mm Ca2+o p < 0.05. ** denotes a significant difference with 5 mm Ca2+o, p < 0.001. *** denotes a significant difference with 0.1 mm Ca2+o and forskolin, p < 0.001. **** denotes a significant difference with 5 mm Ca2+o and forskolin, p < 0.001. B, [35S]GTPγS binding activity in response to CaR activation in MCF-7 cells. As can be seen, 5 mm Ca2+o and neomycin stimulated binding of [35S]GTPγS to Gαs but not to Gαi. Reactions were performed in triplicate, and the experiment was repeated three times. Bars represent the mean ± S.E.
DISCUSSION
Our studies demonstrate opposite effects of CaR signaling on PTHrP production in primary cultures of normal mouse mammary epithelial cells as compared with either an immortalized but not fully transformed murine mammary cell line, Comma-D cells, or the human breast cancer cell line, MCF-7. Exposure of MMECs to increasing concentrations of extracellular calcium or to calcimimetics inhibited PTHrP gene expression and decreased the secretion of PTHrP into the media (11, 32). In contrast, activation of the CaR in Comma-D or MCF-7 cells stimulated PTHrP gene expression and increased the secretion of PTHrP into the media. These effects of the CaR on PTHrP expression and secretion did not appear to be mediated by differences in signaling pathways that had previously been described to mediate the effects of Ca2+o on PTHrP secretion in other cell types (29). Activation of the CaR did not stimulate phosphoinositide turnover and thus did not appear to activate phospholipase C in either MMECs or Comma-D cells. Nonetheless, both cell types did stimulate MAPK signaling, as evidenced by the transient activation/phosphorylation of ERK1 and ERK2. Despite the opposite effects of CaR signaling on PTHrP secretion, the activation of MAPK in response to the CaR appeared identical in MMECs as compared with Comma-D cells. In contrast, we found that CaR activation had opposing effects on cAMP levels in the different cell lines. Pharmacological data suggested that activation of the CaR inhibited adenylyl cyclase activity in MMECs but stimulated adenylyl cyclase activity in Comma-D and MCF-7 cells. We found that these opposing effects of the CaR on cAMP production were related to a change in its G-protein coupling. In MMECs, the receptor couples to Gαi; in Comma-D cells and in MCF-7 cells it switches to Gαs. Thus, our data suggest that the opposite effect of extracellular calcium on PTHrP secretion in normal versus transformed breast cells is the result of opposing effects on adenylyl cyclase activity associated with alternative G-protein usage.
We found that both PTHrP gene expression and PTHrP secretion are regulated by cAMP/PKA in breast cells. There is precedence for this, as stimulation of cAMP production has been shown to increase PTHrP gene expression and/or secretion in human lung cancer cells, myometrial cells, and human T-cell lymphotrophic virus, type I-infected T cells (49–52). Regulation of PTHrP production by cAMP in these cells has been shown to be mediated by a cAMP-response element located within exon 4 of the human gene, a site that is conserved in both the rat and mouse PTHrP genes as well (49, 52). Interestingly, activation of the CaR has been shown to stimulate cAMP production in some pituitary cell lines, and it has also been suggested that PTHrP production may be under the control of the CaR in fish pituitary glands (1, 53). It will be interesting to see if the CaR regulates PTHrP production by regulating cAMP production in pituitary or other cells in addition to breast cells.
The CaR has previously only been suggested to couple to Gαi, Gαq/11, and Gα12/13 (16, 54). However, our results suggest that the receptor can also utilize Gαs. Furthermore, the change in G-protein usage between normal and transformed breast cells is not simply a matter of G-protein availability, because both Gαi and Gαs are expressed abundantly in both MMECs and Comma-D cells. It is well established that GPCRs often utilize different heterotrimeric G-proteins, but stable coupling of the same receptor to Gαi and Gαs is not a commonly reported pattern (55, 56). Dynamic feedback switching from Gαs to Gαi usage has been documented to occur in response to ligand binding for several receptors, including the β1- and β2-adrenergic receptors, the prostacyclin receptor, and the vasoactive intestinal polypeptide receptor (45, 57–59). This particular phenomenon of G-protein switching has been most intensively studied for the β2-adrenergic receptor (57, 60, 61). Binding of ligand to this receptor leads to activation of Gαs, generation of cAMP, and PKA-mediated phosphorylation of sites within the third intracellular loop and C-terminal tail of the receptor (57). These phosphorylation events, in turn, reduce the affinity of the receptor for Gαs and increase its affinity for Gαi. As a result of this switch in G-protein usage, downstream signaling transitions from cAMP/PKA pathways to MAPK activation. G-protein switching has been noted for the β2-adrenergic receptor both in vitro and in vivo and has been suggested to contribute to the development of cardiac hypertrophy in states of chronic catecholamine excess (61, 62). Recent data have also demonstrated that association of the β2-adrenergic receptor with β-arrestin can modulate the magnitude of G-protein switching (63).
We can only speculate about the mechanisms through which malignant transformation potentially causes the CaR to switch from Gαi to Gαs in breast cells. It is certainly possible that alterations in receptor phosphorylation and/or changes in the scaffolding proteins interacting with the CaR in normal versus transformed mammary cells might contribute to this phenomenon. For instance, PKC-mediated phosphorylation of the CaR has been shown to inhibit its ability to activate PLC and MAPK activity perhaps by altering interactions of the intracellular domain of the receptor with Gαi and Gαq (16, 64–66). PKC activity has also been shown to be increased in many breast tumors and breast cancer cell lines (67). Thus, phosphorylation of PKC sites on the CaR theoretically could impair coupling of the CaR to Gαi in breast cancers. Proper activation of MAPK signaling by the CaR has also been shown to require interactions with filamin A (68–70). In addition, the CaR has been found to localize in caveolae, and interaction of the CaR with caveolin 1 has been suggested to be important for proper calcium-mediated suppression of PTH secretion in parathyroid cells (70–73). Loss of caveolin 1 in mice has been shown to foster malignant changes in the mammary gland, and loss-of-function mutations in caveolin 1 have been associated with human breast cancer (74, 75). Hence, it is also possible that altered expression or function of specific scaffolding proteins, such as caveolin 1 or filamin A, in malignant breast cells could either impair Gαi coupling and/or enhance Gαs coupling to the CaR.
The shift from inhibition of PTHrP production by the CaR in normal mammary cells to stimulation of PTHrP production by the CaR in transformed breast cells may contribute to the pathophysiology of breast cancer. Normally, PTHrP is produced by the lactating breast, is secreted into the circulation, and stimulates osteoclastic bone resorption to liberate calcium from the maternal skeleton (32–34). Calcium, in turn, interacts with the CaR on mammary epithelial cells to inhibit PTHrP gene expression and secretion, thus defining a classic negative feedback loop that helps to maintain the delivery of calcium to the breast to be used for milk production (32–34). Breast cancer cells also interact with the skeleton resulting in two very common complications, osteolytic bone metastases and hypercalcemia (27, 28, 76). In both instances, tumor cells secrete PTHrP, leading to pathologic bone resorption that either causes localized bone destruction around metastatic tumor deposits in the skeleton or floods the systemic circulation with enough calcium to overwhelm homeostatic mechanisms and rapidly raise systemic calcium concentrations. If tumor cells suppressed PTHrP production in response to calcium as do normal cells, then osteolysis and/or increased systemic calcium concentrations would inhibit further PTHrP secretion, which might be expected to limit the size of osteolytic lesions or the development of hypercalcemia. However, if tumor cells instead responded like Comma-D or MCF-7 cells, then the normal negative feedback loop would be converted to a positive or feed-forward loop, resulting in amplified PTHrP secretion and worsening of hypercalcemia and osteolysis. Switching from Gαi to Gαs usage by the CaR may therefore mark an important transition during the development of breast tumors, one that enhances their ability to cause bone metastases and/or hypercalcemia. This may also affect the growth of the metastatic tumor cells directly for it has been suggested that growth factors released from the bone matrix during bone resorption can support the proliferation and survival of breast cancer cells in the skeleton (27, 28, 76). A clone of cells with the ability to secrete more PTHrP in response to the high local calcium concentrations in the bone microenvironment might then be more likely to survive and grow within the skeleton. The ability of the CaR to modulate tumor cell growth in the skeleton has recently been demonstrated in prostate cancer cell lines, which also stimulate PTHrP production in response to extracellular calcium (77). Given the results of our study, we believe a better understanding of the molecular mechanisms altering G-protein coupling of the CaR in transformed breast cells may suggest new targets for the treatment of osteolytic bone metastases and/or hypercalcemia in patients with breast cancer.
Acknowledgments
We thank Drs. John Geibel and Arthur Broadus for valuable discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants DK077565, DK69542, and DK41230 from the NIDDK. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: CaR, calcium-sensing receptor; PKA, protein kinase A; MMEC, mammary epithelial cell; PTHrP, parathyroid hormone-related protein; MAPK, mitogen-activated protein kinase; GTPγS, guanosine 5′-3-O-(thio)triphosphate; IBMX, 3-isobutyl-1-methylxanthine; PTX, pertussis toxin; CTX, cholera toxin; Bt2cAMP, dibutyryl cyclic AMP; DMEM, Dulbecco's modified Eagle's medium; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; siRNA, small interfering RNA; GPCR, G-protein-coupled, cell-surface receptor; RT, reverse transcription; PLC, phospholipase C; IP3, inositol 1,4,5-trisphosphate.
References
- 1.Brown, E. M., and MacLeod, R. J. (2001) Physiol. Rev. 81 239–297 [DOI] [PubMed] [Google Scholar]
- 2.Brown, E. M., Gamba, G., Riccardi, D., Lombardi, M., Butters, R., Kifor, O., Sun, A., Hediger, M. A., Lytton, J., and Hebert, S. C. (1993) Nature 366 575–580 [DOI] [PubMed] [Google Scholar]
- 3.Chattopadhyay, N., and Brown, E. M. (2006) Mol. Genet. Metab. 89 189–202 [DOI] [PubMed] [Google Scholar]
- 4.Tfelt-Hansen, J., and Brown, E. M. (2005) Crit. Rev. Clin. Lab. Sci. 42 35–70 [DOI] [PubMed] [Google Scholar]
- 5.Quarles, L. D. (2003) Curr. Opin. Nephrol. Hypertens. 12 349–355 [DOI] [PubMed] [Google Scholar]
- 6.Dvorak, M. M., Chen, T. H., Orwoll, B., Garvey, C., Chang, W., Bikle, D. D., and Shoback, D. M. (2007) Endocrinology 148 3156–3163 [DOI] [PubMed] [Google Scholar]
- 7.Geibel, J., Sritharan, K., Geibel, R., Geibel, P., Persing, J. S., Seeger, A., Roepke, T. K., Deichstetter, M., Prinz, C., Cheng, S. X., Martin, D., and Hebert, S. C. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 9390–9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacs, C. S., Ho-Pao, C. L., Hunzelman, J. L., Lanske, B., Fox, J., Seidman, J. G., Seidman, C. E., and Kronenberg, H. M. (1998) J. Clin. Investig. 101 2812–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeil, L., Hobson, S., Nipper, V., and Rodland, K. D. (1998) Am. J. Obstet. Gynecol. 178 305–313 [DOI] [PubMed] [Google Scholar]
- 10.Tu, C. L., Chang, W., Xie, Z., and Bikle, D. D. (2008) J. Biol. Chem. 283 3519–3528 [DOI] [PubMed] [Google Scholar]
- 11.VanHouten, J., Dann, P., McGeoch, G., Brown, E. M., Krapcho, K., Neville, M., and Wysolmerski, J. J. (2004) J. Clin. Investig. 113 598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.VanHouten, J. N., Neville, M. C., and Wysolmerski, J. J. (2007) Endocrinology 148 5943–5954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu, J., Wang, C., Han, R., Pavlos, N., Phan, T., Steer, J. H., Bakker, A. J., Joyce, D. A., and Zheng, M. H. (2005) J. Cell. Physiol. 202 554–562 [DOI] [PubMed] [Google Scholar]
- 14.Hu, J., and Spiegel, A. M. (2007) J. Cell. Mol. Med. 11 908–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown, E. M. (2007) Subcell Biochem. 45 139–167 [DOI] [PubMed] [Google Scholar]
- 16.Ward, D. T. (2004) Cell Calcium 35 217–228 [DOI] [PubMed] [Google Scholar]
- 17.Hofer, A. M., and Brown, E. M. (2003) Nat. Rev. Mol. Cell Biol. 4 530–538 [DOI] [PubMed] [Google Scholar]
- 18.Corbetta, S., Lania, A., Filopanti, M., Vicentini, L., Ballare, E., and Spada, A. (2002) J. Clin. Endocrinol. Metab. 87 2201–2205 [DOI] [PubMed] [Google Scholar]
- 19.Kifor, O., MacLeod, R. J., Diaz, R., Bai, M., Yamaguchi, T., Yao, T., Kifor, I., and Brown, E. M. (2001) Am. J. Physiol. 280 F291–F302 [DOI] [PubMed] [Google Scholar]
- 20.Smajilovic, S., Hansen, J. L., Christoffersen, T. E., Lewin, E., Sheikh, S. P., Terwilliger, E. F., Brown, E. M., Haunso, S., and Tfelt-Hansen, J. (2006) Biochem. Biophys. Res. Commun. 348 1215–1223 [DOI] [PubMed] [Google Scholar]
- 21.Wang, W., Lu, M., Balazy, M., and Hebert, S. C. (1997) Am. J. Physiol. 273 F421–F429 [DOI] [PubMed] [Google Scholar]
- 22.Ward, D. T., McLarnon, S. J., and Riccardi, D. (2002) J. Am. Soc. Nephrol. 13 1481–1489 [DOI] [PubMed] [Google Scholar]
- 23.Philbrick, W. M., Wysolmerski, J. J., Galbraith, S., Holt, E. H., Orloff, J. J., Yang, K. H., Vasavada, R., Weir, E. C., Broadus, A. E., and Stewart, A. F. (1996) Physiol. Rev. 76 127–173 [DOI] [PubMed] [Google Scholar]
- 24.Strewler, G. J. (2000) N. Engl. J. Med. 342 177–185 [DOI] [PubMed] [Google Scholar]
- 25.Hens, J. R., and Wysolmerski, J. J. (2005) Breast Cancer Res. 7 220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson, G. W. (2007) Nat. Rev. Genet. 8 963–972 [DOI] [PubMed] [Google Scholar]
- 27.DeMauro, S., and Wysolmerski, J. (2005) J. Mammary Gland Biol. Neoplasia 10 157–167 [DOI] [PubMed] [Google Scholar]
- 28.Brown, S. A., Clines, G. A., and Guise, T. A. (2007) Curr. Opin. Endocrinol. Diabetes Obes. 14 436–441 [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhyay, N. (2006) Am. J. Physiol. 290 E761–E770 [DOI] [PubMed] [Google Scholar]
- 30.Cheng, I., Klingensmith, M. E., Chattopadhyay, N., Kifor, O., Butters, R. R., Soybel, D. I., and Brown, E. M. (1998) J. Clin. Endocrinol. Metab. 83 703–707 [DOI] [PubMed] [Google Scholar]
- 31.Sanders, J. L., Chattopadhyay, N., Kifor, O., Yamaguchi, T., Butters, R. R., and Brown, E. M. (2000) Endocrinology 141 4357–4364 [DOI] [PubMed] [Google Scholar]
- 32.Ardeshirpour, L., Dann, P., Pollak, M., Wysolmerski, J., and VanHouten, J. (2006) Bone (San Diego, CA) 38 787–793 [DOI] [PubMed] [Google Scholar]
- 33.VanHouten, J. (2005) J. Mammary Gland Biol. Neoplasia 12 477–482 [DOI] [PubMed] [Google Scholar]
- 34.Wysolmerski, J. J. (2007) BoneKEy 4 209–225 [Google Scholar]
- 35.Zawalich, W., Takuwa, N., Takuwa, Y., Diaz, V. A., and Rasmussen, H. (1987) Diabetes 36 426–433 [DOI] [PubMed] [Google Scholar]
- 36.Zawalich, W. S., Zawalich, K. C., and Kelley, G. G. (1995) Endocrinology 136 4903–4909 [DOI] [PubMed] [Google Scholar]
- 37.Applied Biosystems (Foster City, CA) (1997) ABI Prism 7700, User Bulletin 2
- 38.Barr, A., and Manning, D. (1999) G Proteins Techniques of Analysis, (Manning, D. R., ed) pp. 227–245 CRC Press, Inc., Boca Raton, FL
- 39.Harrison, C., and Traynor, J. R. (2003) Life Sci. 74 489–508 [DOI] [PubMed] [Google Scholar]
- 40.Jahnke, G. D., Trempus, C. S., Kari, F. W., and DiAugustine, R. P. (1996) J. Mol. Endocrinol. 17 247–256 [DOI] [PubMed] [Google Scholar]
- 41.O'Rourke, J., Yuan, R., and DeWille, J. (1997) J. Biol. Chem. 272 6291–6296 [DOI] [PubMed] [Google Scholar]
- 42.Insel, P. A., and Ostrom, R. S. (2003) Cell. Mol. Neurobiol. 23 305–314 [DOI] [PubMed] [Google Scholar]
- 43.Ryan, W. L., and Heidrick, M. L. (1974) Adv. Cyclic Nucleotide Res. 4 81–116 [PubMed] [Google Scholar]
- 44.Taylor, S. S., Kim, C., Vigil, D., Haste, N. M., Yang, J., Wu, J., and Anand, G. S. (2005) Biochim. Biophys. Acta 1754 25–37 [DOI] [PubMed] [Google Scholar]
- 45.Martin, N. P., Whalen, E. J., Zamah, M. A., Pierce, K. L., and Lefkowitz, R. J. (2004) Cell. Signal. 16 1397–1403 [DOI] [PubMed] [Google Scholar]
- 46.Banwell, J. G., Pierce, N. F., Mitra, R. C., Brigham, K. L., Caranasos, G. J., Keimowitz, R. I., Fedson, D. S., Thomas, J., Gorbach, S. L., Sack, R. B., and Mondal, A. (1970) J. Clin. Investig. 49 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaslow, H. R., and Burns, D. L. (1992) FASEB J. 6 2684–2690 [DOI] [PubMed] [Google Scholar]
- 48.Birnbaumer, L. (2007) Biochim. Biophys. Acta 1768 772–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chilco, P. J., Leopold, V., and Zajac, J. D. (1998) Mol. Cell. Endocrinol. 138 173–184 [DOI] [PubMed] [Google Scholar]
- 50.Ikeda, K., Okazaki, R., Inoue, D., Ogata, E., and Matsumoto, T. (1993) J. Biol. Chem. 268 1174–1179 [PubMed] [Google Scholar]
- 51.Morimoto, T., Devora, G. A., Mibe, M., Casey, M. L., and MacDonald, P. C. (1997) Mol. Cell. Endocrinol. 129 91–99 [DOI] [PubMed] [Google Scholar]
- 52.Zajac, J. D., Callaghan, J., Eldridge, C., Diefenbach-Jagger, H., Suva, L. J., Hudson, P., Moseley, J. M., Michelangeli, V. P., and Pasquini, G. (1989) Mol. Cell. Endocrinol. 67 107–112 [DOI] [PubMed] [Google Scholar]
- 53.Abbink, W., Bevelander, G. S., Hang, X., Lu, W., Guerreiro, P. M., Spanings, T., Canario, A. V., and Flik, G. (2006) J. Exp. Biol. 209 3550–3557 [DOI] [PubMed] [Google Scholar]
- 54.Arthur, J. M., Collinsworth, G. P., Gettys, T. W., Quarles, L. D., and Raymond, J. R. (1997) Am. J. Physiol. 273 F129–F135 [DOI] [PubMed] [Google Scholar]
- 55.Cruciani, R. A., Dvorkin, B., Morris, S. A., Crain, S. M., and Makman, M. H. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 3019–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sidhu, A., Sullivan, M., Kohout, T., Balen, P., and Fishman, P. H. (1991) J. Neurochem. 57 1445–1451 [DOI] [PubMed] [Google Scholar]
- 57.Daaka, Y., Luttrell, L. M., and Lefkowitz, R. J. (1997) Nature 390 88–91 [DOI] [PubMed] [Google Scholar]
- 58.Lawler, O. A., Miggin, S. M., and Kinsella, B. T. (2001) J. Biol. Chem. 276 33596–33607 [DOI] [PubMed] [Google Scholar]
- 59.Luo, X., Zeng, W., Xu, X., Popov, S., Davignon, I., Wilkie, T. M., Mumby, S. M., and Muallem, S. (1999) J. Biol. Chem. 274 17684–17690 [DOI] [PubMed] [Google Scholar]
- 60.Hill, S. J., and Baker, J. G. (2003) Br. J. Pharmacol. 138 1188–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith, N. J., and Luttrell, L. M. (2006) Hypertension 48 173–179 [DOI] [PubMed] [Google Scholar]
- 62.Hasseldine, A. R., Harper, E. A., and Black, J. W. (2003) Br. J. Pharmacol. 138 1358–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baillie, G. S., Sood, A., McPhee, I., Gall, I., Perry, S. J., Lefkowitz, R. J., and Houslay, M. D. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Davies, S. L., Ozawa, A., McCormick, W. D., Dvorak, M. M., and Ward, D. T. (2007) J. Biol. Chem. 282 15048–15056 [DOI] [PubMed] [Google Scholar]
- 65.Jiang, Y. F., Zhang, Z., Kifor, O., Lane, C. R., Quinn, S. J., and Bai, M. (2002) J. Biol. Chem. 277 50543–50549 [DOI] [PubMed] [Google Scholar]
- 66.Lorenz, S., Frenzel, R., Paschke, R., Breitwieser, G. E., and Miedlich, S. U. (2007) Endocrinology 148 2398–2404 [DOI] [PubMed] [Google Scholar]
- 67.Mackay, H. J., and Twelves, C. J. (2003) Endocr.-Relat. Cancer 10 389–396 [DOI] [PubMed] [Google Scholar]
- 68.Awata, H., Huang, C., Handlogten, M. E., and Miller, R. T. (2001) J. Biol. Chem. 276 34871–34879 [DOI] [PubMed] [Google Scholar]
- 69.Hjalm, G., MacLeod, R. J., Kifor, O., Chattopadhyay, N., and Brown, E. M. (2001) J. Biol. Chem. 276 34880–34887 [DOI] [PubMed] [Google Scholar]
- 70.Huang, C., and Miller, R. T. (2007) J. Cell. Mol. Med. 11 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kifor, O., Diaz, R., Butters, R., Kifor, I., and Brown, E. M. (1998) J. Biol. Chem. 273 21708–21713 [DOI] [PubMed] [Google Scholar]
- 72.Kifor, O., Kifor, I., Moore, F. D., Jr., Butters, R. R., Jr., Cantor, T., Gao, P., and Brown, E. M. (2003) J. Clin. Endocrinol. Metab. 88 4455–4464 [DOI] [PubMed] [Google Scholar]
- 73.Quinn, S. J., Kifor, O., Kifor, I., Butters, R. R., Jr., and Brown, E. M. (2007) Biochem. Biophys. Res. Commun. 354 8–13 [DOI] [PubMed] [Google Scholar]
- 74.Sotgia, F., Rui, H., Bonuccelli, G., Mercier, I., Pestell, R. G., and Lisanti, M. P. (2006) Cancer Res. 66 10647–10651 [DOI] [PubMed] [Google Scholar]
- 75.Williams, T. M., Medina, F., Badano, I., Hazan, R. B., Hutchinson, J., Muller, W. J., Chopra, N. G., Scherer, P. E., Pestell, R. G., and Lisanti, M. P. (2004) J. Biol. Chem. 279 51630–51646 [DOI] [PubMed] [Google Scholar]
- 76.Siclari, V. A., Guise, T. A., and Chirgwin, J. M. (2006) Cancer Metastasis Rev. 25 621–633 [DOI] [PubMed] [Google Scholar]
- 77.Liao, J., Schneider, A., Datta, N. S., and McCauley, L. K. (2006) Cancer Res. 66 9065–9073 [DOI] [PubMed] [Google Scholar]