Abstract
TAK1 (transforming growth factor-β-activated kinase 1), a mitogen-activated protein kinase kinase kinase, is activated by various cytokines, including interleukin-1 (IL-1). However, the precise regulation for TAK1 activation at the molecular level is still not fully understood. Here we report that dual phosphorylation of Thr-178 and Thr-184 residues within the kinase activation loop of TAK1 is essential for TAK1-mediated NFκB and AP-1 activation. Once co-overexpressed with TAB1, TAK1 mutant with alanine substitution of these two residues fails to activate IKKβ-mediated NFκB and JNK-mediated AP-1, whereas TAK1 mutant with replacement of these two sites with acidic residues acts like the TAK1 wild type. Consistently, TAK1 mutant with alanine substitution of these two residues severely inhibits IL-1-induced NFκB and AP-1 activities, whereas TAK1 mutant with replacement of these two sites with acidic residues slightly enhances IL-1-induced NFκB and AP-1 activities compared with the TAK1 wild-type. IL-1 induces the phosphorylation of endogenous TAK1 at Thr-178 and Thr-184. Reconstitution of TAK1-deficient mouse embryo fibroblast cells with wild-type TAK1 or a TAK1 mutant containing threonine 178 and 184 to alanine mutations revealed the importance of these two sites in IL-1-mediated IKK-NFκB and JNK-AP-1 activation as well as IL-1-induced IL-6 gene expression. Our finding is the first report that substitution of key serine/threonine residues with acidic residues mimics the phosphorylated state of TAK1 and renders TAK1 active during its induced activation.
Interleukin-1 (IL-1)4 is a proinflammatory cytokine and plays a crucial role in inflammation, stress, and disease in many cell types(1–4). Cellular responses to IL-1 are mediated by intracellular signaling pathways that activate nuclear transcription factor κB (NFκB) and AP-1 (activator protein 1) (1, 5).
TAK1 (transforming growth factor-β-activated kinase 1), a member of the evolutionarily conserved mitogen-activated protein kinase kinase kinase family, was originally found to function in signaling of the transforming growth factor-β (6). TAK1 is activated by various cellular stresses, including IL-1, tumor necrosis factor-α, lipopolysaccharide, osmotic stress, and latent membrane protein 1 from Epstein-Barr virus (7–10). TAK1 has been demonstrated to be essential in IL-1-mediated NFκB, JNK, and p38 activation (11–14). Upon binding of IL-1 to the extracellular part of the IL-1 receptor, the adaptor protein MyD88 (myeloid differentiation factor 88) is recruited to the IL-1·IL-1R complex, which then recruits the IL-1R-associated kinases and TRAF6 (tumor necrosis factor receptor-associated factor 6) to activate TAK1 (3, 4, 15). Once activated, TAK1 translocates from the membrane to the cytosol along with TRAF6 and its association partners, TAB1 (TAK1-binding protein 1), TAB2, and TAB3 (16–19). TAK1 activation subsequently leads to the activations of IκB kinase (IKK) and c-Jun NH2-terminal kinase (JNK) as well as p38. Activated IKK phosphorylates IκB proteins, and phosphorylated IκB proteins are degraded by the ubiquitin-mediated proteosome pathway (20). Degradation of IκB proteins leads to NFκB translocation into the nucleus and activation of NFκB-dependent gene transcription in the nucleus (20, 21). Activated JNKs phosphorylate specific sites on the amino-terminal trans-activation domain of transcription factor c-Jun, an important component of transcriptional activator AP-1. Phosphorylation of these sites stimulates the ability of c-Jun to activate AP-1-dependent gene expression (22–25). However, the precise mechanism of TAK1-mediated IKK and JNK activation is still not fully understood.
TAB1, a regulatory subunit of the TAK1 complex, was isolated as a TAK1-interacting protein in a yeast two-hybrid screening. TAB1 interacts constitutively with TAK1 and induces TAK1 kinase activity when overexpressed (18). TAB1 is an inactive pseudophosphatase structurally related to members of the PPM family of protein serine/threonine phosphatases (26). The 67 amino acids at the COOH-terminal end of TAB1 were demonstrated to be sufficient for full TAK1 activation (27). This claim was supported by a crystal structure study of an TAK1-TAB1 chimeric protein (28).
Phosphorylation and dephosphorylation of critical serine and threonine residues in the activation loop of serine/threonine protein kinases are essential for kinase activation and inactivation (29–33). Many studies have shown that substitution of these critical residues with acidic residues to mimic the phosphorylation state renders the kinase constitutively active (34, 35). In the case of TAK1, several studies have been carried out to identify the phosphorylation sites and examine the effect of phosphorylation on TAK1 activity. For example, phosphorylation of Thr-187 and Ser-192 within the activation loop of TAK1 has been shown to be promoted by cytokine tumor necrosis factor-α. However, instead of rendering the kinase constitutively active, replacement of these residues with acidic residues leads to inactivation of TAK1 kinase (36–39).
In this report, we further examine the potential phosphorylation sites in the activation loop of TAK1 responsible for TAK1-mediated NFκB and AP-1 activations. Using mutational analysis and reporter assays, we identified two key amino acids at positions Thr-178 and Thr-184 that are additional regulatory phosphorylation sites required for TAK1-mediated optimal NFκB and AP-1 activation. We confirmed the phosphorylation of both Thr-178 and Thr-184 residues on TAK1 by using a specific antibody that recognizes the dual phosphorylation of these two sites. These two residues are located within the TAK1 kinase subdomains VII and VIII. Interestingly, phosphorylation of these two residues can be induced by IL-1 stimulation and are required for IL-1-induced optimal IKK-NFκB and JNK-AP-1 activation as well as IL-6 gene expression.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection—TAK1-deficient MEF cells have been described before (40–42). HeLa, HEK293T, and MEF-TAK1 knock-out cells were maintained in Dulbecco's modified Eagle's medium (high glucose) supplemented with 10% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in 5% CO2. HeLa, HEK293T, and MEF-TAK1 knock-out cells were transfected with expression plasmids using Fugene6 (Roche Applied Science) and Lipofectamine 2000 reagents (Invitrogen), respectively.
Expression Vectors—A cDNA construct containing the full-length open reading frame of the wild-type human TAK1 was subcloned into the 3FLAG-tagged mammalian expression vector pcDNA3.1. Point mutations were made by site-directed PCR mutagenesis and verified by DNA sequencing analysis. The NFκB-dependent firefly luciferase reporter plasmid and pCMV promoter-dependent Renilla luciferase reporter were purchased from Clontech. The retroviral expression vectors were constructed by subcloning the TAK1-wild type or TAK1-T178A/T184A cDNA fragment into the pBabe-puro vector. Mammalian expression vectors for TAB1, TRAF6, and IKKβ were constructed by subcloning cDNAs encoding the full-length wild-type human proteins into the pcDNA3.1 vector.
Antibody and Reagents—A specific anti-phospho-TAK1 (pT178/pT184) antibody was generated by immunizing rabbits with the synthetic phosphopeptide corresponding to amino acids VLKICDFGpTACDIQpTHM (where pT represents phosphothreonine) of human TAK1 by Genemed Synthesis, Inc. (South San Francisco, CA). Antibodies against TAK1, phospho-TAK1 (Thr-184), p38, phospho-p38, JNK, phospho-JNK, IκBα, phospho-IκBα, phospho-IKKα/β, phospho-p65, and secondary antibodies conjugated to horseradish peroxidase were purchased from Cell Signaling Technology (Beverly, MA). Antibodies for HA epitope, proliferating cell nuclear antigen (PC-10), and NFκB-p65 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The anti-FLAG antibody (M5) and anti-actin antibodies were obtained from Sigma. Recombinant human IL-1β and mouse IL-1β were obtained from R & D Systems (Minneapolis, MN).
Establishment of Stable TAK1-deficient MEF Cell Lines Expressing TAK1 Wild Type and TAK1-T178A/T184A—The pBabe-TAK1 wild type or pBabe-TAK1-T178A/T184A or pBabe empty vector were co-transfected with retrovirus packing vector Pegpam 3e and PLC-ECO in HEK293T cells to obtain retroviral supernatants. Viral supernatants were collected after 48 and 72 h. MEF-TAK1 knock-out cells were incubated with virus-containing medium in the presence of 4 μg/ml Polybrene (hexadimethrine bromide; Sigma). Stable cell lines were established after 10 days of puromycin (2 μg/ml) selection.
Immunoprecipitation and Immunoblotting—To prepare total cell lysates, cells were placed on ice and washed three times with ice-cold phosphate-buffered saline. Cells were lysed by adding lysis buffer (25 mm HEPES (pH 7.7), 135 mm NaCl, 3 mm EDTA, 1% Triton X-100, 25 mm β-glycerophosphate, 0.1 mm sodium orthovanadate, 0.5 mm phenylmethylsulfonyl fluoride, 1 mm dithiothreitol, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mm benzamidine, 20 mm disodium p-nitrophenyl phosphate, 1 mm phenylmethylsulfonyl fluoride, phosphatase inhibitor mixture 1 and 2 (Sigma)). After centrifuging the lysate at 13,000 × g for 15 min at 4 °C, antibody was added to the supernatant and incubated with rotation for 3 h at 4 °C. After adding a protein A-agarose bead suspension (protein A; Santa Cruz Biotechnology), the mixture was further incubated with rotation for 3 h at 4 °C. After three washes with the washing buffer (20 mm HEPES (pH 7.7), 50 mm NaCl, 2.5 mm MgCl2, 0.1 mm EDTA, and 0.05% Triton X-100), the beads were resuspended in Laemmli sample buffer and boiled for 5 min. The immunoprecipitates or the whole cell lysates were resolved by SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). The membranes were probed with appropriate antibodies. The IgG horseradish peroxidase-conjugated antibodies were used as the secondary antibodies. The proteins were detected using the ECL-Plus Western blotting detection system (Amersham Biosciences) and visualized by autography.
Preparation of Cytoplasmic and Nuclear Extracts—Cytoplasmic and nuclear extracts were obtained as described by Singhirunnusorn et al. (39). Cytoplasmic extracts were prepared by adding Buffer A (10 mm HEPES, pH 7.9, 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 20 mm glycerophosphate, 0.1 mm sodium orthovanadate, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) to cell pellets. The cells were suspended and chilled on ice for 15 min. Next, 25 μl of 10% Nonidet P-40 was added, and the suspension was vortexed vigorously for 10 s. Cytoplasmic extracts were collected after centrifugation at 15,000 rpm for 5 min. To prepare nuclear extracts, the nuclear pellets were washed twice using Buffer A. Then Buffer B (20 mm HEPES, pH 7.9, 0.4 m NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 20 mm glycerophosphate, 1 mm sodium orthovanadate, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) was added to the nuclear pellets. The resuspension was put on ice for 15 min, followed by centrifugation at 15,000 rpm for 5 min. The supernatants were collected as nuclear extracts.
Reverse Transcription-Polymerase Chain Reaction—Mouse IL-6 expression in MEF cells was analyzed by reverse transcription-PCR. Total RNA was isolated with TRIzol reagent (Invitrogen), and cDNA was prepared using SuperScript III gene expression tools (Invitrogen) according to the manufacturer's protocol. The primers used were as follows: mouse IL-6/F442, 5′-caaagccagagtccttcagag-3′; mouse IL-6/R593, 5′-tggtccttagccactccttc-3′; mouse Actb/F305, 5′-tgttaccaactgggacgac-3′; mouse mActb/R711, 5′-cacgcacgatttccctctc-3′. The PCR products were subjected to electrophoresis on a 2% agarose gel.
Enzyme-linked Immunosorbent Assay—Supernatants of stable MEF-TAK1 knock-out cell lines expressing TAK1 wild type and TAK1-T178A/T184A, which were treated with or without mouse IL-1β (10 ng/ml), were collected at different time points. Mouse IL-6 concentrations in medium were determined by enzyme-linked immunosorbent assay using a kit (BD Biosciences) according to the manufacturer's instructions.
In Vitro Phosphatase Treatment—HEK293T cells were transfected with FLAG-TAK1 and TAB1 expression vectors, and cell extracts were prepared in protein lysis buffer. Epitope-tagged TAK1 was immunoprecipitated with anti-FLAG antibody and washed three times with washing buffer and then two times with 1× phosphatase reaction buffer (50 mm HEPES, 100 mm NaCl, 2 mm dithiothreitol, 0.1 mm EGTA, 0.025% Tween 20). Then 40 μl of 1× phosphatase reaction buffer with 1 μl of λ-protein phosphatase (New England Biolabs, Ipswich, MA) was added to the immunoprecipitates at 30 °C for 30 min.
Luciferase Reporter Assay—MEF-TAK1 cells were seeded at a concentration of 3 × 105 cells/well and cultured overnight in 6-well plates. NFκB-dependent firefly luciferase reporter and effector plasmids were co-transfected along with the Renilla luciferase plasmid. Thirty-six hours after transfection, cells were harvested in lysis buffer (Promega, Madison, WI), and luciferase assays were performed using the Dual-Luciferase reporter assay system (Promega). The relative luciferase activity was calculated by dividing the firefly luciferase activity by the Renilla luciferase activity. Data represent three independent experiments performed in duplicate.
RESULTS
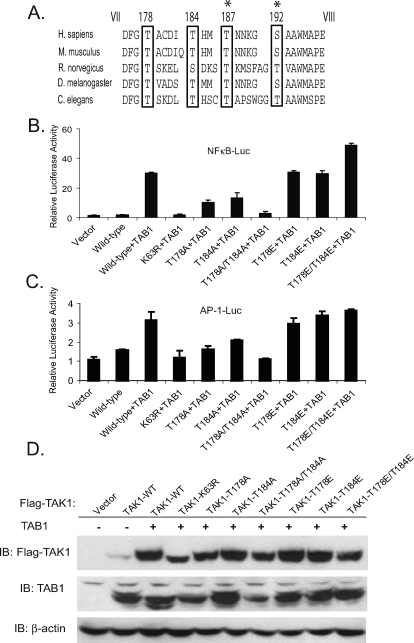
Thr-178 and Thr-184 Residues in the Activation Loop of Human TAK1 Are Two Potential Phosphorylation Sites Essential for TAK1/TAB1-mediated NFκB and AP-1 Activation—Phosphorylation of serine and/or threonine residue(s) between kinase subdomains VII and VIII is essential for the activation of many kinases (29–33). Furthermore, substitution of these critical serine or threonine residues with acidic residues mimics the phosphorylation and renders these kinases constitutively active (34, 35). TAK1 has previously been identified to play a critical role in the cytokine-mediated NFκB and JNK signal transduction pathways (9, 43–45). To investigate which serine or threonine residue within the activation loop is essential for TAK1-mediated NFκB and AP-1 activation, we aligned amino acid sequences in a region of the kinase activation loop between domains DFG and M(A/S)PE of TAK1 from various species (Fig. 1A). Four conserved Ser/Thr sites (Thr-178, Thr-184, Thr-187, and Ser-192) were found in this region. Phosphorylation of Thr-187 and Ser-192 residues has been shown to be involved in the regulation of TAK1 kinase activity (36, 39). However, replacement of these two sites with acidic residues inactivates TAK1 kinase activity. These studies suggest that Thr-178 and Thr-184 residues might also be the potential phosphorylation sites that are involved in the regulation of TAK1 activity.
FIGURE 1.
Thr-178 and Thr-184 residues within the activation loop of human TAK1 are potential phosphorylation sites required for the TAK1/TAB1-induced NFκB and AP-1 activation. A, sequence alignment of kinase activation loop of TAK1 from different species. The potential phosphorylation sites are marked with an open box. Thr-187 and Ser-192 have been reported previously and are marked with a star. B and C, the effect of alanine or glutamic acid substitution at the indicated residue within the activation loop on the TAK1-induced NFκB(B) and AP-1 (C) activities. One microgram of NFκBor AP-1 luciferase reporter plasmid and 20 ng of Renilla luciferase plasmid were cotransfected into TAK1-deficient cells with 1 μg of empty vector control or different TAK1 expression plasmids. The relative luciferase activity was measured 36 h later and normalized with the Renilla activity. Error bars, ±S.D. in triplicate experiments. D, expression levels of FLAG-TAK1 and TAB1 in the transfected cells were detected by immunoblotting, respectively. β-Actin was detected as a loading control.
To characterize the physiological role of Thr-178 and Thr-184 on human TAK1, NFκB and AP-1-dependent luciferase reporter assays were used to assess the effect of point mutations (threonine to alanine or glutamic acid) at Thr-178 and/or Thr-184 on TAK1-induced NFκB and AP-1 activities. In these assays, the expression plasmid encoding the wild-type, active site lysine to arginine mutant (K63R) or each point mutant of TAK1 was transfected into TAK1-deficient cells, along with plasmids containing TAB1-, NFκB-, and AP-1-dependent firefly and control pCMV Renilla luciferase reporter genes. As shown in Fig. 1, B–D, TAK1 T178A and T184A single mutation partially impaired TAK1-induced NFκB and AP-1-dependent luciferase activities compared with the wild-type TAK1, whereas the TAK1 T178A/T184A double mutation as well as the K63R kinase-inactive mutation completely abrogated TAK1/TAB1-induced NFκB and AP-1 activities. In contrast, T178E and T184E single or double mutant TAK1 induced NFκB and AP-1 activation in the reporter assays at a similar or slightly higher levels compared with the wild type. These results strongly suggest that the TAK1 T178E/T184E mutant is active in the assays, and the phosphorylation of both Thr-178 and Thr-184 residues is required for the TAK1/TAB1-induced NFκB and AP-1 activities.
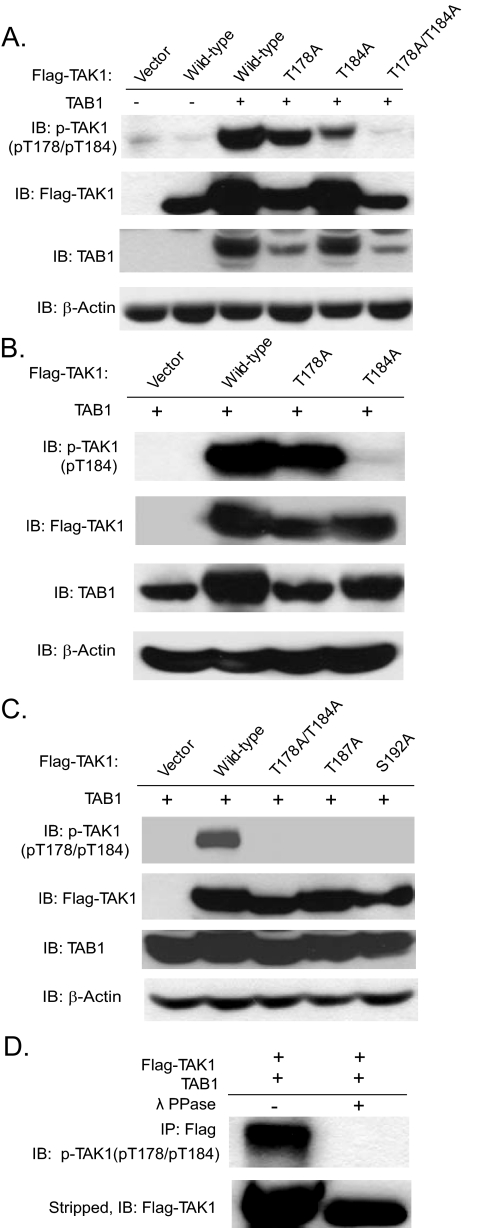
Overexpression of TAK1 and TAB1 Induces Phosphorylation of TAK1 at Thr-178 and Thr-184—To examine directly whether Thr-178 and Thr-184 are phosphorylated during TAK1 activation, we generated a new phospho-specific antibody recognizing human TAK1 phosphorylated at Thr-178 and Thr-184 (pT178/pT184) by immunizing rabbits with a synthetic phosphopeptide (KLH-coupled) corresponding to residues surrounding Thr-178 and Thr-184 of human TAK1. Antibodies were purified by peptide affinity chromatography (Genemed Synthesis, Inc., South San Francisco, CA). As shown in Fig. 2A, our antibody specific for phospho-TAK1 (pT178/pT184) recognized the wild type, T178A, and T184A in a TAB1-dependent manner but not T178A/T184A double mutant TAK1 proteins.
FIGURE 2.
Generation and characterization of an antibody specific for phospho-TAK1 at Thr-178 and Thr-184. A, the anti-phospho-TAK1 (pT178/pT184) antibody is specific for human TAK1 phospho-Thr-178/Thr-184. Cell extracts were prepared from HEK293T cells transfected with expression vectors for TAB1 as well as the FLAG-TAK1 and its derived mutants as indicated and analyzed by immunoblotting with the anti-phospho-TAK1 antibody (pT178/pT184) (top) and reprobed with an anti-FLAG and anti-TAB1 antibodies to detect the expression level of FLAG-TAK1 and TAB1, respectively. β-Actin was detected as a loading control (bottom). B, the anti-phospho-TAK1 (pT184) antibody detects the phosphorylated form of human TAK1 at Thr-184. Cell extracts were prepared from HEK293T cells transfected with an expression vector for TAB1 as well as the FLAG-TAK1 and its derived mutants as indicated and analyzed by immunoblotting with the anti-phospho-TAK1 antibody (pT184) and reprobed with anti-FLAG and anti-TAB1 antibodies to detect the expression level of FLAG-TAK1 and TAB1, respectively. β-Actin was detected as a loading control. C, Ala substitution at Thr-187 and Ser-192 abolished TAK1/TAB1 overexpression-induced TAK1 phosphorylation at Thr-178 and Thr-184. Cell extracts were prepared from HEK293T cells transfected with an expression vector for TAB1 as well as the FLAG-TAK1, as indicated, and analyzed by immunoblotting with the anti-phospho-TAK1 antibody (pT178/pT184) and reprobed with anti-FLAG and anti-TAB1 antibodies to detect the expression level of FLAG-TAK1 and TAB1, respectively. β-Actin was detected as a loading control. D, the anti-phospho-TAK1(pT178/pT184) antibody detects the phosphorylated form of human TAK1 at Thr-178 and Thr-184. Cell extracts were prepared from HEK293T cells transfected with an expression vector for FLAG-TAK1 and TAB1 and were immunoprecipitated with anti-FLAG antibody and then treated with or without λ-protein phosphatase (λ-PPase) for 30 min before being analyzed by immunoblotting with the anti-phospho-TAK1 antibody and reprobed with anti-FLAG antibody.
To further test the specificity of our antibody, another phosphoantibody recognizing the phospho-Thr-184 of TAK1 was used to detect the phosphorylation of the two sites. As shown in Fig. 2B, anti-phospho-TAK1 (pT184) only recognized TAK1 T178A and not the T184A mutant. These results demonstrate that our antibody can recognize both TAK1 T178A and T184A single mutant and suggest that Ala substitution at Thr-178 had no effect on the phosphorylation of Thr-184 on the activated TAK1. However, Ala substitution at Thr-187 and Ser-192 abolished TAK1/TAB1 overexpression-induced TAK1 phosphorylation at Thr-178 and Thr-184 (Fig. 2C). To further validate the specificity of our antibody to the phosphorylation at these two sites, we coexpressed FLAG-tagged wild-type TAK1 and TAB1 in HEK293T cells. TAK1 immunoprecipitates were treated in vitro with λ-protein phosphatase. This treatment, whose efficacy was demonstrated by extinction of the anti-phospho-TAK1 (pT178/pT184) signal, resulted in complete abrogation of the phosphorylation of the two sites (Fig. 2D). Taken together, these results demonstrate that our antibody specifically detects TAK1 only when phosphorylated at threonine 178 and/or 184 residues. Phosphorylation at these two sites could be induced by TAK1 and TAB1 co-overexpression in the cells.
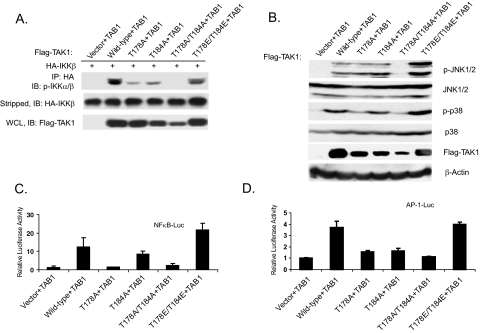
Phosphorylation at Thr-178 and Thr-184 Is Required for TAK1/TAB1-induced IKK-NFκB and JNK-AP-1 Activation—IKKβ plays a critical role in the activation of NFκB, and the phosphorylation of Ser-176 and Ser-180 residues at the activation loop is essential for IKKβ activation by tumor necrosis factor-α and IL-1 (34). Overexpression of TAK1 and TAB1 results in IKKβ activation (18, 36). To test whether dual phosphorylation of both Thr-178 and Thr-184 is required for TAK1/TAB1-mediated phosphorylation of IKKβ at Ser-176 and Ser-180 residues, we transfected the full-length wild-type and mutant TAK1 expression plasmids with HA-tagged IKKβ and TAB1 into TAK1-deficient mouse embryonic fibroblasts, immunoprecipitated HA-tagged IKKβ from the cell lysates with an anti-HA antibody, and immunoblotted with an antibody specific for phospho-IKKα/β. As shown in Fig. 3A, overexpression of wild-type and T178E/T184E double mutant TAK1 along with TAB1 induced a higher level of IKKβ phosphorylation at the Ser-176 and Ser-180 residues compared with T178A or T184A single mutant, whereas T178A/T184A double mutant completely failed to induce IKKβ phosphorylation.
FIGURE 3.
Phosphorylation of TAK1 at Thr-178 and Thr-184 residues is required for the TAK1-mediated IKK-NFκB and JNK-AP-1 activations. A, phosphorylation of both Thr-178 and Thr-184 residues is essential for TAK1-mediated phosphorylation of IKKβ. TAK1-deficient (–/–) MEF cells were cotransfected with empty vector or FLAG-TAK1 wild-type expression vector or derived mutants in the presence of HA-IKKβ and TAB1 using Lipofectamine 2000. After 36 h, cell extracts were immunoprecipitated (IP) with anti-HA and immunoblotted (IB) with anti-phospho-IKKα/β (p-IKKβ). HA-IKKβ proteins present in the immunoprecipitates were detected by stripping the blots and reprobing with anti-HA antibody. The expression level of FLAG-TAK1 wild-type and derived mutated proteins were detected by anti-FLAG antibody. B, HEK293T cells were transiently transfected with empty vector, TAK1 wild type, and the indicated mutants. Cells were collected 36 h after transfection and lysed with protein lysis buffer, and the same amount of protein was subjected to SDS-PAGE followed by immunoblotting analysis to detect the phospho-JNK and phospho-p38 proteins in the cell lysates using antibodies specific for the phosphorylated form of JNK and p38, respectively. The same membranes were stripped and reprobed with anti-JNK and anti-p38 antibodies to detect the level of total JNK and p38 expression. The expression of TAK1 was analyzed by immunoblotting with the antibodies indicated. β-Actin was detected as a loading control. C and D, phosphorylation of both Thr-178 and Thr-184 residues is required for TAK1-induced NFκB(C) and AP-1 (D) luciferase activities in the TAK1-deficient MEF cells. One microgram of NFκB or AP-1 luciferase reporter plasmid and 20 ng of Renilla luciferase plasmid as well as TAB1 expression plasmid were transfected into the deficient MEF cells, along with 1 μg of empty vector or expression plasmid encoding the wild type or the indicated TAK1 mutants. The relative luciferase activity was measured 36 h later and normalized with the Renilla activity. Error bars, ±S.D. in triplicate experiments.
Co-overexpression of TAK1 and TAB1 results in the activation of JNK and p38 MAP kinases (18). To determine whether dual phosphorylation of the Thr-178 and Thr-184 residues is required for TAK1/TAB1-induced JNK and p38 activation, the wild-type, catalytically inactive K63R and Thr-178 or/and Thr-184 TAK1 mutants were overexpressed in HEK-293T cells along with TAB1. The transfected cells were then lysed and immunoblotted for phosphorylated JNK and p38. As shown in Fig. 3B, overexpression of the TAK1 T178A/T184A double mutant along with TAB1 completely failed to induce JNK and p38 phosphorylation, whereas the T178E/T184E mutant induced a higher level of JNK and p38 phosphorylation comparable with the wild-type and other single mutants. These results suggest that phosphorylation at Thr-178 and Thr-184 is required for the TAK1/TAB1-induced activation of JNK and p38 MAPK.
Consistent with these results, luciferase analysis with NFκB and AP-1-responsive reporters showed that the TAK1 T178A/T184A mutant failed to induce the luciferase reporter gene expression, whereas TAK1 wild type and T178E/T184E mutant induced a high level of reporter gene expression when co-overexpressed with TAB1 in TAK1-deficient MEF cells (Fig. 3, C and D). Taken together, these results suggest that phosphorylation at Thr-178 and Thr-184 is required for the TAK1/TAB1-induced IKK-NFκB and JNK-AP-1 activation.
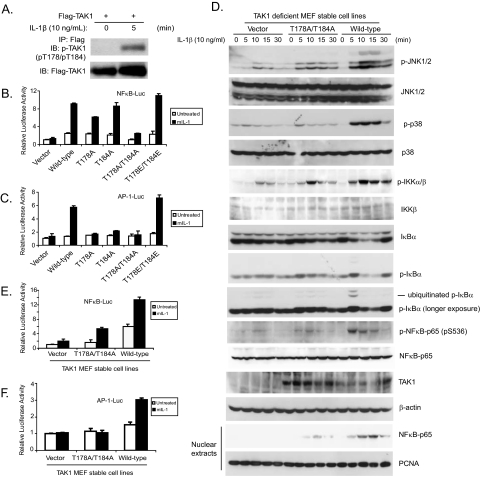
Phosphorylation of TAK1 at Thr-178 and Thr-184 Is Required for IL-1-induced Optimal IKK-NFκB and JNK-AP-1 Activation—TAK1 is required in the IL-1-mediated NFκB and AP-1 activation (9, 11–13, 41). Our overexpression experiments showed that phosphorylation at Thr-178 and Thr-184 is required for TAK1/TAB1-induced NFκB and AP-1 activations. These results strongly suggest that phosphorylation of both Thr-178 and Thr-184 on TAK1 is involved in the IL-1-induced IKK-NFκB and AP-1 activation. To characterize the physiological role of TAK1 phosphorylation at Thr-178 and Thr-184 sites in the IL-1 signaling pathway, we first determined whether IL-1 could induce the phosphorylation of TAK1 at Thr-178 and Thr-184 in vivo. In this assay, HeLa cells were transiently transfected with FLAG-tagged wild-type TAK1 expression vectors. The transfected cells were then treated with IL-1 for 5 min and lysed. FLAG-tagged TAK1 was immunoprecipitated with anti-FLAG antibody from the cell extracts and subsequently immunoblotted with antibody specific for phospho-TAK1 (Thr-178/Thr-184) to detect the phosphorylation of TAK1 at Thr-178 and Thr-184 sites. As shown in Fig. 4A, IL-1 induced the phosphorylation of TAK1 at the Thr-178 and Thr-184 sites in the cells within 5 min of treatment.
FIGURE 4.
Phosphorylation of TAK1 at both Thr-178 and Thr-184 residues is required for IL-1-mediated IKK-NFκB and JNK-AP-1 activations. A, IL-1 induces the phosphorylation of TAK1 at Thr-178 and Thr-184 residues. HeLa cells were transfected with the expression plasmid encoding TAK1. 48 h after transfection, cells were either untreated or treated with IL-1 (10 ng/ml) before being harvested and lysed. FLAG-tagged TAK1 protein in the cell lysates was immunoprecipitated (IP) with antibody specific for FLAG tag and subsequently subjected to immunoblotting analysis (IB) with the anti-phospho-TAK1 (pT178/pT184) antibody to detect the level of phosphorylated TAK1 (top). The same blot was stripped and reprobed with an anti-FLAG antibody to detect the level of immunoprecipitated total TAK1 protein (bottom). B and C, expression of the TAK1 T178A/T184A mutant inhibits IL-1-induced NFκB and AP-1 reporter activities. One μg of NFκB (NFκB-Luc) or AP-1 (AP-1-Luc) reporter and 20 ng of Renilla-Luc plasmid were cotransfected into TAK1-deficient MEF cells with control vector or TAK1 wild-type expression vector or derived mutants for 24 h followed by the addition of IL-1 (10 ng/ml) for 12 h. Cell extracts were collected to determine the relative luciferase activity. Error bars, ±S.D. in triplicate experiments. D, expression of the TAK1 T178A/T184A mutant inhibits the IL-1-induced JNK, p38, IKK, IκBα, and NFκB-p65 phosphorylation as well as IκBα degradation and NFκB nuclear translocation. TAK1-deficient MEF cells were transduced with the retrovirus encoding the vector control, TAK1 wild type, or TAK1 T178A/T184A mutant and subsequently selected with puromycin (2 μg/ml) to establish the TAK1-deficient MEF cell lines with the stable expression of either TAK1 wild type or T178A/T184A mutant. TAK1-deficient, reconstituted wild-type and T178A/T184A MEF cells were untreated or treated with IL-1 (10 ng/ml) for the time points indicated and subsequently harvested. Whole cell extracts and nuclear extracts were subjected to SDS-PAGE and immunoblotted with the antibodies indicated. β-Actin was detected as a loading control for whole cell extracts, and proliferating cell nuclear antigen was used as a loading control for nuclear extracts. E and F, expression of the TAK1 T178A/T184A mutant inhibits IL-1-induced NFκB and AP-1 reporter activities. One μg of NFκB (E) or AP-1 (F) reporter and 20 ng of Renilla-Luc plasmid were cotransfected into TAK1-deficient MEF stable cell lines with control or TAK1 wild-type or T178A/T184A mutant expression vectors for 24 h followed by the addition of IL-1 (10 ng/ml) for 12 h. The relative luciferase activity was measured and normalized with the Renilla activity. Error bars, ±S.D. in triplicate experiments.
To test the role of phosphorylation of TAK1 at Thr-178 and Thr-184 in IL-1-induced NFκB and AP-1 activities, we performed luciferase analysis in TAK1-deficient MEF cells transfected with TAK1 wild type and various mutants with NFκB and AP-1-responsive reporters. In these assays, the TAK1 T178A/T184A mutant severely blocked the IL-1-induced NFκB and AP-1-dependent luciferase activities, whereas the TAK1 T178E/T184E mutant mediated a higher level of reporter gene expression compared with the wild-type (Fig. 4, B and C). Taken together, these results suggest that phosphorylation of TAK1 at Thr-178 and Thr-184 is required for the IL-1-induced NFκB and AP-1 activation.
To further study the role of phosphorylation of TAK1 at Thr-178 and Thr-184 residues in IL-1-induced NFκB and AP-1 activation, the TAK1 wild-type and T178A/T184A mutant expression vectors were stably introduced back into the TAK1-deficient MEF cells by a retroviral transduction system. The stable MEF cell lines with the expression of TAK1 wild type and T178A/T184A double mutant were established, and the expression of TAK1 was verified by immunoblotting (Fig. 4D). To determine the role of TAK1 phosphorylation at Thr-178 and Thr-184 in the IL-1-mediated NFκB and AP-1 activation, MEF cell lines with the expression of vector control, wild-type, and T178A/T184A double mutant TAK1 were treated with IL-1 at different time points, and then the cell lysates were immunoblotted with the indicated antibodies to examine the IL-1-induced JNK and p38 MAPK phosphorylation, IKK phosphorylation, IκBα phosphorylation and degradation, and NFκB-p65 phosphorylation on Ser-536. In this assay, IL-1-induced-JNK2 and p38 phosphorylation were completely blocked in the TAK1-deficient and T178A/T184A double mutant MEF cells, whereas IL-1-induced-JNK1 activation was only partially inhibited in the T178A/T184A double mutant MEF cells. Similarly, IL-1-induced phosphorylations of IKK and NFκB-p65 Ser-536 as well as IκBα phosphorylation and degradation were partially impaired in the TAK1 T178A/T184A double mutant MEF cells compared with the cells expressing the wild-type TAK1. We also isolated nuclear extracts from the above cells treated with IL-1 at the indicated time points and found that IL-1-induced NFκB nuclear translocation was also significantly impaired in TAK1-T178A/T184A double mutant MEF cells (Fig. 4D).
Consistent with the above results, IL-1 induced a much higher NFκB and AP-1-dependent luciferase activities in MEF cells expressing the TAK1 wild type but not the TAK1-deficient or T178A/T184A double mutant (Fig. 4, E and F). Taken together, these results indicate that phosphorylation of TAK1 at Thr-178 and Thr-184 sites is required for the IL-1-induced optimal IKK-NFκB and JNK-AP-1 activation.
Phosphorylation of Thr-187 and Ser-192 residues has been shown to be critical for TAK1 kinase activity (36, 39). To further understand the role of the four suggested phosphorylation sites in the regulation of TAK1-mediated NFκB activation, an NFκB-dependent luciferase reporter assay was used to assess the effect of the different combination of point mutations (threonine or serine to alanine or glutamic acid) at Thr-178, Thr-184, Thr-187, and Ser-192 on TAK1/TAB1-induced NFκB activity. In this assay, only the TAK1 T178E/T184E mutant induced a relatively higher NFκB-dependent luciferase activity compared with the TAK1 wild type, whereas the rest of the TAK1 mutants failed to induce NFκB reporter activity (data not shown). These results indicate that Thr-187 and Ser-192 residues cannot be replaced with alanine and/or glutamic acid even on the background of TAK1 T178E/T184E mutant to mimic the active nature of the TAK1 T178E/T184E mutant in the assay.
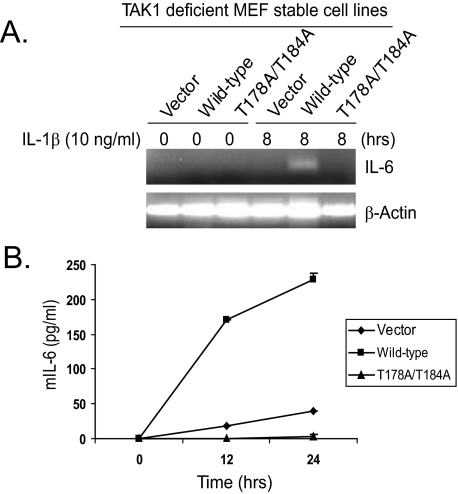
Phosphorylation of TAK1 at Thr-178 and Thr-184 Is Required for IL-1-induced IL-6 Expression—TAK1 is involved in the IL-1-mediated NFκB and AP-1 activation and IL-6 gene expression (1, 3). To determine the role of TAK1 phosphorylation at Thr-178 and Thr-184 in IL-1-induced IL-6 gene expression, total RNA was extracted from the control, wild-type, and T178A/T184A double mutant TAK1-stable MEF cell lines treated with or without IL-1β. Then reverse transcription-PCR was carried out to examine the IL-1-induced IL-6 transcription level in the cells. As shown in Fig. 5A, the IL-1-induced IL-6 transcription was detected in the cells expressing the wild-type TAK1 but not in the TAK1-deficient and T178A/T184A mutant cells. Consistent with this result, IL-6 presence in the cell medium was significantly induced by IL-1 only in cells expressing the wild-type TAK1 and not in the TAK1-deficient and T178A/T184A mutant cells (Fig. 5B). These results demonstrate that phosphorylation of TAK1 at Thr-178 and Thr-184 is required for the IL-1-induced IL-6 production in the cells.
FIGURE 5.
Phosphorylation of TAK1 at Thr-178 and Thr-184 residues is required for IL-1-induced IL-6 gene expression. A, expression of the TAK1 T178A/T184A mutant inhibits IL-1-induced IL-6 gene transcription. TAK1-deficient, wild-type, and T178A/T184A MEF cells were untreated or treated with IL-1 (10 ng/ml) for 8 h and subsequently harvested for extraction of total RNA using Trizol reagent. One μg of total RNA was used to synthesize first-strand cDNA using a reverse transcription kit according to the manufacturer's instructions. These synthesized cDNAs were used as templates for mouse IL-6 PCR amplification. The PCR products were resolved in 2% agarose gel. B, expression of the TAK1 T178A/T184A mutant inhibits IL-1-induced IL-6 production. TAK1-deficient, wild-type, and T178A/T184A MEF cells were untreated or treated with IL-1 (10 ng/ml) for the times indicated. The supernatants from these cultures were collected and subjected to mouse IL-6 enzyme-linked immunosorbent assay analysis according to the manufacturer's instructions.
DISCUSSION
In this study, we obtained novel insights into the regulatory mechanism of the rapid and transient activation of TAK1 in IL-1-mediated NFκB and AP-1 activation by analyzing the phosphorylation status of serine and threonine residues in the kinase activation loop of TAK1. Phosphorylation of serine or threonine residues in the kinase activation loop between the kinase subdomains VII and VIII is essential for the activation of many serine/threonine kinases. In addition, substitution of these critical serine or threonine residues with alanine abolishes the ability of these kinases to activate downstream signal transduction pathways, whereas replacement of these sites with acidic residues renders the kinase constitutively active (29–33). So far, there are two serine/threonine sites (Thr-187 and Ser-192) in the kinase activation loop that have been reported to be critical for TAK1 activation. However, substitution of these critical serine or threonine residues with alanine or acidic residues both ablated the kinase activation (36, 39). It remains unclear whether other serine or threonine sites within the activation loop of TAK1 are required for its activation of NFκB and AP-1. In this study, we further examined the mechanism of phosphorylation responsible for TAK1-mediated NFκB and AP-1 activation. Here we report that both Thr-178 and Thr-184 residues within the kinase activation loop are also essential regulatory phosphorylation sites for TAK1-mediated NFκB and AP-1 activation. We propose that phosphorylation of these two residues within the kinase activation loop is a general mechanism for TAK1-mediated NFκB and AP-1 activation. Interestingly, it seems that four conserved serine/threonine residues within the activation loop are phosphorylated during the TAK1 activation process. However, substitution of Thr-178 and Thr-184 but not Thr-187 and Ser-192 with acidic residues renders TAK1 active in a TAB1-dependent manner in the co-overexpression assay (Fig. 1). In addition, substitution of Thr-187 and Ser-192 with alanine abolished TAK1/TAB1 overexpression-induced TAK1 Thr-178 and Thr-184 phosphorylation (Fig. 2C).
We also mutated Thr-187 and Ser-192 to either A or E on the background of TAK1 T178E/T184E mutant in order to further understand the relationship of these four phosphorylation residues in their regulation of TAK1 kinase activation. Similar to the Thr-187 or Ser-192 single mutant, TAK1 T178E/T184E/T187A(E)/S192A(E) mutants failed to induce NFκB activation when overexpressed with TAB1 and to mediate IL-1-induced NFκB activation (data not shown). These data indicate that Thr-187 and Ser-192 cannot be mutated to mimic the active state of TAK1 due to their position within the kinase activation loop. Taken together, these results suggest that the TAK1 activation process is tightly controlled by the phosphorylation of these four serine/threonine residues.
The impairment of IL-1-mediated IκBα phosphorylation, degradation, and NFκB nuclear translocation in the TAK1 T178A/T184A reconstituted MEF cells was not as severe as in the TAK1-deficient cells (Fig. 4D). The simplest explanation to account for this observation is that the TAK1 T178A/T184A mutant in the cells retains residual activity in response to IL-1 stimulation. However, we did not observe any discrepency between TAK1-deficient and TAK1 T178A/T184A reconstituted MEF cells in IL-1-induced JNK phosphorylation and AP-1 reporter activity (Fig. 4, D and F). These results suggest that the defect of the TAK1 T178A/T184A mutant is more severe in its ability to activate JNK-mediated AP-1 activity. Together, IL-1-induced IL-6 gene expression was completely inhibited in TAK1 T178A/T184A mutant cells due to the combinatorial effect of defective IKK and JNK activation.
TAK1 is a central signal transducer to mediate the IL-1-induced IKK-NFκB, JNK, and p38 activation (11–14). Our current finding that the TAK1 T178E/T184E mutant acts like TAK1 wild type and mediates slightly stronger IL-1-induced NFκB and AP-1 activities suggests that IL-1-induced TAK1 T178E/T184E activation may be resistant to TAK1 phosphatase-mediated TAK1 inactivation due to T178E/T184E mutations and that the phosphorylated Thr-178 and Thr-184 are the target sites for TAK1 phosphatase in the cells to prevent prolonged TAK1 activation.
In our studies, overexpression of TAK1 and its regulatory subunit, TAB1, leads to the phosphorylation of TAK1 at the Thr-178 and Thr-184 residues within the kinase activation loop. However, the mechanism of TAB1 and TAK1 co-overexpression-induced TAK1 activation remains unknown. One of the possibilities could be that the amount of endogenous negative regulator protein for TAK1 is not sufficient to inhibit TAK1 activation in TAB1/TAK1-overexpressed cells (37). However, the exact role of TAB1 in IL-1-mediated TAK1 activation remains unclear due to the controversial reports that TAB1 is dispensable in IL-1-mediated NFκB and AP-1 activation (13) and TAB1 is required for IL-1-induced TAK1 activation (46). In our studies, overexpression of the TAK1 T178E/T184E mutant induces NFκB and AP-1 activities in a TAB1-dependent manner, and the TAK1 178E/184E mutant is able to mediate IL-1-induced NFκB and AP-1 activation (Fig. 3, C and D), These results suggest that TAB1 may be an essential regulatory subunit for the activation of the TAK1 holoenzyme and that IL-1-induced association of TAK1 with TAB1 in the cells is an important step for the phosphorylation and activation of TAK1. Recently, it has been shown that IL-1 induces phosphorylation of TAK1 regulatory subunits, including TAB1, TAB2, and TAB3 (16–19). Further studies will be needed to determine whether the phosphorylation of TAB1, TAB2, and TAB3 are required for IL-1-mediated TAK1 activation.
Mass spectrometry is a powerful tool in the identification of protein post-translational modification, such as protein phosphorylation (47, 48). We have overexpressed FLAG-tagged TAK1 with TAB1 in HEK293T cells and purified FLAG-tagged TAK1 for the identification of phosphorylation sites on TAK1 using mass spectrometry. However, mass spectrometry analysis failed to identify any of the phosphorylation sites, including the reported Thr-187 and Ser-192 on TAK1. This result suggests that only a small portion of the purified TAK1 proteins was phosphorylated at these serine/threonine sites, and mass spectrometry cannot detect the phosphorylated peptides when their percentage is too low. Thus, we have to combine different approaches in our effort to identify protein post-translational modifications.
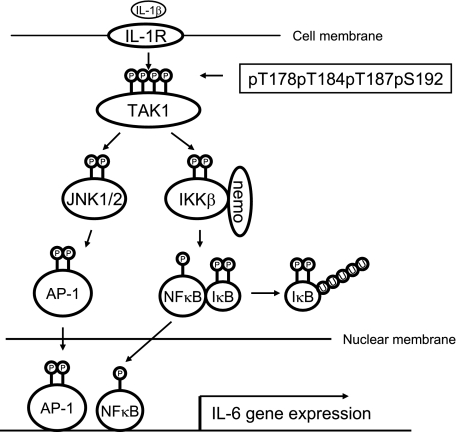
In summary, our study revealed that phosphorylation of Thr-178 and Thr-184 in the kinase activation loop of TAK1 is involved in the regulation of IL-1-mediated NFκB and AP-1 activation. We successfully generated the antibody specific for the phosphorylated TAK1 at these two sites. Importantly, our finding in this study is the first report that substitution of key serine/threonine residues with acidic residues mimics the phosphorylated state of TAK1 and renders TAK1 active during its induced activation. In view of the data presented here and in previous reports, we propose a working model (Fig. 6), in which, upon IL-1β binding to IL-1R, IL-1R-mediated signaling events lead to the phosphorylation of TAK1 at Thr-178/Thr-184/Thr-187/Ser-192 and subsequent TAK1 activation that in turn triggers the activation of IKK-NFκB and JNK-AP-1 as well as NFκB and AP-1-dependent IL-6 gene expression in the cells.
FIGURE 6.
A working model for TAK1 action in the IL-1-mediated IKK-NFκB and JNK-AP-1 activation. IL-1β induces TAK1 phosphorylation at four sites within the kinase activation loop and subsequent activation in the cells to mediate optimal IKK-NFκB and JNK-AP-1 activation as well as NFκB- and AP-1-dependent IL-6 gene expression.
Acknowledgments
We greatly appreciate the gift of the TAB1 expression construct from Dr. Xin Lin and the mouse retrovirus generation system from Dr. Biao Zheng.
This work was supported, in whole or in part, by National Institutes of Health Grant 1R21CA106513-01A2 (to J. Y.). This work was also supported by American Cancer Society Grant RSG-06-070-01-TBE (to J. Y.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: IL, interleukin; IKK, IκB kinase; JNK, c-Jun-NH2-terminal kinase; NFκB, nuclear transcription factor κB; IL-1R, IL-1 receptor; MEF, mouse embryo fibroblast; HA, hemagglutinin.
References
- 1.Dinarello, C. A. (1997) Cytokine Growth Factor Rev. 8 253–265 [DOI] [PubMed] [Google Scholar]
- 2.O'Neill, L. A., and Greene, C. (1998) J. Leukocyte Biol. 63 650–657 [PubMed] [Google Scholar]
- 3.Arend, W. P. (2002) Cytokine Growth Factor Rev. 13 323–340 [DOI] [PubMed] [Google Scholar]
- 4.Beutler, B. (2004) Nature 430 257–263 [DOI] [PubMed] [Google Scholar]
- 5.Baud, V., and Karin, M. (2001) Trends Cell Biol. 11 372–377 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi, K., Shirakabe, K., Shibuya, H., Irie, K., Oishi, I., Ueno, N., Taniguchi, T., Nishida, E., and Matsumoto, K. (1995) Science 270 2008–2011 [DOI] [PubMed] [Google Scholar]
- 7.Irie, T., Muta, T., and Takeshige, K. (2000) FEBS Lett. 467 160–164 [DOI] [PubMed] [Google Scholar]
- 8.Takaesu, G., Surabhi, R. M., Park, K. J., Ninomiya-Tsuji, J., Matsumoto, K., and Gaynor, R. B. (2003) J. Mol. Biol. 326 105–115 [DOI] [PubMed] [Google Scholar]
- 9.Vidal, S., Khush, R. S., Leulier, F., Tzou, P., Nakamura, M., and Lemaitre, B. (2001) Genes Dev. 15 1900–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan, J., Sun, L., Mendoza, J. W., Chui, Y. L., Huang, D. P., Chen, Z. J., Suzuki, N., Suzuki, S., Yeh, W. C., Akira, S., Matsumoto, K., Liu, Z. G., and Wu, Z. (2004) Mol. Cell. Biol. 24 192–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ninomiya-Tsuji, J., Kishimoto, K., Hiyama, A., Inoue, J., Cao, Z., and Matsumoto, K. (1999) Nature 398 252–256 [DOI] [PubMed] [Google Scholar]
- 12.Sato, S., Sanjo, H., Takeda, K., Ninomiya-Tsuji, J., Yamamoto, M., Kawai, T., Matsumoto, K., Takeuchi, O., and Akira, S. (2005) Nat. Immunol. 6 1087–1095 [DOI] [PubMed] [Google Scholar]
- 13.Shim, J. H., Xiao, C., Paschal, A. E., Bailey, S. T., Rao, P., Hayden, M. S., Lee, K. Y., Bussey, C., Steckel, M., Tanaka, N., Yamada, G., Akira, S., Matsumoto, K., and Ghosh, S. (2005) Genes Dev. 19 2668–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, C., Deng, L., Hong, M., Akkaraju, G. R., Inoue, J., and Chen, Z. J. (2001) Nature 412 346–351 [DOI] [PubMed] [Google Scholar]
- 15.Qin, J., Jiang, Z., Qian, Y., Casanova, J. L., and Li, X. (2004) J. Biol. Chem. 279 26748–26753 [DOI] [PubMed] [Google Scholar]
- 16.Cheung, P. C., Nebreda, A. R., and Cohen, P. (2004) Biochem. J. 378 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishitani, T., Takaesu, G., Ninomiya-Tsuji, J., Shibuya, H., Gaynor, R. B., and Matsumoto, K. (2003) EMBO J. 22 6277–6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibuya, H., Yamaguchi, K., Shirakabe, K., Tonegawa, A., Gotoh, Y., Ueno, N., Irie, K., Nishida, E., and Matsumoto, K. (1996) Science 272 1179–1182 [DOI] [PubMed] [Google Scholar]
- 19.Takaesu, G., Kishida, S., Hiyama, A., Yamaguchi, K., Shibuya, H., Irie, K., Ninomiya-Tsuji, J., and Matsumoto, K. (2000) Mol. Cell 5 649–658 [DOI] [PubMed] [Google Scholar]
- 20.Karin, M., and Ben-Neriah, Y. (2000) Annu. Rev. Immunol. 18 621–663 [DOI] [PubMed] [Google Scholar]
- 21.Baeuerle, P. A., and Henkel, T. (1994) Annu. Rev. Immunol. 12 141–179 [DOI] [PubMed] [Google Scholar]
- 22.Davis, R. J. (1999) Biochem. Soc. Symp. 64 1–12 [DOI] [PubMed] [Google Scholar]
- 23.Davis, R. J. (2000) Cell 103 239–252 [DOI] [PubMed] [Google Scholar]
- 24.Karin, M., Liu, Z., and Zandi, E. (1997) Curr. Opin. Cell Biol. 9 240–246 [DOI] [PubMed] [Google Scholar]
- 25.Weston, C. R., Lambright, D. G., and Davis, R. J. (2002) Science 296 2345–2347 [DOI] [PubMed] [Google Scholar]
- 26.Conner, S. H., Kular, G., Peggie, M., Shepherd, S., Schuttelkopf, A. W., Cohen, P., and Van Aalten, D. M. (2006) Biochem. J. 399 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakurai, H., Miyoshi, H., Toriumi, W., and Sugita, T. (1999) J. Biol. Chem. 274 10641–10648 [DOI] [PubMed] [Google Scholar]
- 28.Brown, K., Vial, S. C., Dedi, N., Long, J. M., Dunster, N. J., and Cheetham, G. M. (2005) J. Mol. Biol. 354 1013–1020 [DOI] [PubMed] [Google Scholar]
- 29.Alessi, D. R., Saito, Y., Campbell, D. G., Cohen, P., Sithanandam, G., Rapp, U., Ashworth, A., Marshall, C. J., and Cowley, S. (1994) EMBO J. 13 1610–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, L. N., Noble, M. E., and Owen, D. J. (1996) Cell 85 149–158 [DOI] [PubMed] [Google Scholar]
- 31.Payne, D. M., Rossomando, A. J., Martino, P., Erickson, A. K., Her, J. H., Shabanowitz, J., Hunt, D. F., Weber, M. J., and Sturgill, T. W. (1991) EMBO J. 10 885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resing, K. A., Mansour, S. J., Hermann, A. S., Johnson, R. S., Candia, J. M., Fukasawa, K., Vande Woude, G. F., and Ahn, N. G. (1995) Biochemistry 34 2610–2620 [DOI] [PubMed] [Google Scholar]
- 33.Zheng, C. F., and Guan, K. L. (1994) EMBO J. 13 1123–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delhase, M., Hayakawa, M., Chen, Y., and Karin, M. (1999) Science 284 309–313 [DOI] [PubMed] [Google Scholar]
- 35.Mercurio, F., Zhu, H., Murray, B. W., Shevchenko, A., Bennett, B. L., Li, J., Young, D. B., Barbosa, M., Mann, M., Manning, A., and Rao, A. (1997) Science 278 860–866 [DOI] [PubMed] [Google Scholar]
- 36.Kishimoto, K., Matsumoto, K., and Ninomiya-Tsuji, J. (2000) J. Biol. Chem. 275 7359–7364 [DOI] [PubMed] [Google Scholar]
- 37.Sakurai, H., Miyoshi, H., Mizukami, J., and Sugita, T. (2000) FEBS Lett. 474 141–145 [DOI] [PubMed] [Google Scholar]
- 38.Sakurai, H., Nishi, A., Sato, N., Mizukami, J., Miyoshi, H., and Sugita, T. (2002) Biochem. Biophys. Res. Commun. 297 1277–1281 [DOI] [PubMed] [Google Scholar]
- 39.Singhirunnusorn, P., Suzuki, S., Kawasaki, N., Saiki, I., and Sakurai, H. (2005) J. Biol. Chem. 280 7359–7368 [DOI] [PubMed] [Google Scholar]
- 40.Liu, H. H., Xie, M., Schneider, M. D., and Chen, Z. J. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 11677–11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan, Y. Y., Chi, H., Xie, M., Schneider, M. D., and Flavell, R. A. (2006) Nat. Immunol. 7 851–858 [DOI] [PubMed] [Google Scholar]
- 42.Xie, M., Zhang, D., Dyck, J. R., Li, Y., Zhang, H., Morishima, M., Mann, D. L., Taffet, G. E., Baldini, A., Khoury, D. S., and Schneider, M. D. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 17378–17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boutros, M., Agaisse, H., and Perrimon, N. (2002) Dev. Cell 3 711–722 [DOI] [PubMed] [Google Scholar]
- 44.Park, J. M., Brady, H., Ruocco, M. G., Sun, H., Williams, D., Lee, S. J., Kato, T., Jr., Richards, N., Chan, K., Mercurio, F., Karin, M., and Wasserman, S. A. (2004) Genes Dev. 18 584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silverman, N., Zhou, R., Erlich, R. L., Hunter, M., Bernstein, E., Schneider, D., and Maniatis, T. (2003) J. Biol. Chem. 278 48928–48934 [DOI] [PubMed] [Google Scholar]
- 46.Mendoza, H., Campbell, D. G., Burness, K., Hastie, J., Ronkina, N., Shim, J. H., Arthur, J. S., Davis, R. J., Gaestel, M., Johnson, G. L., Ghosh, S., and Cohen, P. (2008) Biochem. J. 409 711–722 [DOI] [PubMed] [Google Scholar]
- 47.Gafken, P. R., and Lampe, P. D. (2006) Cell Commun. Adhes. 13 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goshe, M. B. (2006) Brief. Funct. Genomics Proteomics 4 363–376 [DOI] [PubMed] [Google Scholar]