Abstract
Arachidonic acid is an essential constituent of cell membranes that is esterified to the sn-2-position of glycerophospholipids and is released from selected lipid pools by phospholipase cleavage. The released arachidonic acid can be metabolized by three enzymatic pathways: the cyclooxygenase pathway forming prostaglandins and thromboxanes, the lipoxygenase pathway generating leukotrienes and lipoxins, and the cytochrome P450 (cP450) pathway producing epoxyeicosatrienoic acids and hydroxyeicosatetraenoic acids. The present study describes a novel group of cP450 epoxygenase-dependent metabolites of arachidonic acid, termed 2-epoxyeicosatrienoylglycerols (2-EG), including two regioisomers, 2-(11,12-epoxyeicosatrienoyl)glycerol (2-11,12-EG) and 2-(14,15-epoxyeicosatrienoyl)glycerol (2-14,15-EG), which are both produced in the kidney and spleen, whereas 2-11,12-EG is also detected in the brain. Both 2-11,12-EG and 2-14,15-EG activated the two cannabinoid (CB) receptor subtypes, CB1 and CB2, with high affinity and elicited biological responses in cultured cells expressing CB receptors and in intact animals. In contrast, the parental arachidonic acid and epoxyeicosatrienoic acids failed to activate CB1 or CB2 receptors. Thus, these cP450 epoxygenase-dependent metabolites are a novel class of endogenously produced, biologically active lipid mediators with the characteristics of endocannabinoids. This is the first evidence of a cytochrome P450-dependent arachidonate metabolite that can activate G-protein-coupled cell membrane receptors and suggests a functional link between the cytochrome P450 enzyme system and the endocannabinoid system.
When cells are stimulated by relevant growth factors or hormones, the essential constituent of cell membrane, arachidonic acid, is hydrolyzed from the sn-2-position of selected glycerophospholipids by activated phospholipases (1). We and others (2–4) have previously documented that metabolism of the released arachidonic acid by the cytochrome P450 enzyme system (cP450)2 produces biologically active lipid mediators, epoxyeicosatrienoic acids (EETs) and hydroxyeicosatetraenoic acids. Subsequent studies revealed that EETs can serve as intracellular second messengers (5, 6), activate Ca2+ signaling (7), and promote cell proliferation (8, 9). EETs also act as endothelium-derived hyperpolarizing factors by opening Ca2+-activated K+ channels (10, 11) and play an anti-inflammatory role by inhibiting NF-κB-mediated vascular cell adhesion molecule 1 expression (12). Recent studies indicate that EETs are downstream effectors of anandamide that activate TRPV4 (13). Since cP450 arachidonate metabolites have been demonstrated to play important roles in regulating salt and fluid balance, vascular tone, systemic blood pressure, and local blood supply in vital organs such as the brain, heart, and kidney, the cP450-dependent metabolic pathway has emerged as a potential target for treatment of hypertension and ischemic organ damage (14–17).
Cell surface receptors that mediate the biological effects of the arachidonic acid metabolites derived from cyclooxygenase and lipoxygenase pathways have been well characterized (18, 19). However, no study to date has identified any cP450-dependent arachidonate metabolites that exert their biological effects through cell membrane receptor-mediated mechanisms. In the present study, using ESI/LC/MS/MS analysis, we identified two novel cP450-dependent metabolites of arachidonic acid, termed 2-(11,12-epoxyeicosatrienoyl)glycerol (2-11,12-EG) and 2-(14,15-epoxyeicosatrienoyl)glycerol (2-14,15-EG), and determined that these compounds are potent cannabinoid receptor agonists. This report provides the first direct evidence that cP450-dependent arachidonate metabolites can exert their biological effects by directly binding to and activating cell surface receptors coupled to Gi/o proteins.
EXPERIMENTAL PROCEDURES
Chemicals and Antibodies—Arachidonic acid was purchased from Nu Chek Prep (Elysian, MN). [3H]CP55940 (specific activity 168 Ci/mmol) was from PerkinElmer Life Sciences. 2-Arachidonylglycerol (2-AG), WIN55212-2, AM251, and AM630 were from Tocris Cookson Inc. (Ellisville, MO). A deuterated standard of 2-arachidonoylglycerol was from Cayman Chemical (Ann Arbor, MI). Wortmannin, LY294002, H89, MDL12,330A, and U73122 were purchased from EMD Biosciences/Calbiochem. Polyclonal antibodies to total and phospho-ERKs were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Other chemicals were from Sigma.
Synthesis of EET, 2-EG, and ACU—11,12-EET and 14,15-EET were synthesized as we described previously (8). 2-11,12-EG and 2-14,15-EG were synthesized as we have described recently (20). 1-Adamantyl-3-cyclohexylurea was synthesized as described (21, 22).
Analysis of Endogenous 2-EG by ESI/LC/MS/MS Analysis—Synthetic mixtures of glycerol 1- and 2-positional isomers of 11,12- and 14,15-EG were injected onto a reversed phase HPLC column (Kromasil 100 C18, 3 μm, 150 × 2.1 mm) and eluted using a solvent gradient from initially 30% H2O, 70% CH3OH containing 25 μm AgBF4 to 100% CH3OH containing 25 μm AgBF4 in 10 min and at a flow of 0.2 ml/min. Column eluents were monitored by ESI/MS/MS using a TSQ Quantum 700 triple quadrupole mass spectrometer. Freshly isolated samples of rat spleen, kidney, or brain (1 g each) were homogenized in CHCl3/CH3OH (2:1) containing half a volume of aqueous 0.15 m KCl and a mixture of the 1- and 2-positional isomers of synthetic 13C3-labeled 11,12- and 14,15-EG (30 ng each) as internal standards. After low speed centrifugation, the organic phases were collected and evaporated under argon. The dry extracts were suspended in a minimal volume of 30% ethyl ether, 49.5% hexane, 0.5% HOAc and loaded onto a SiO2 column (0.5 × 5 cm), and the column was washed with the same solvent to remove free fatty acids and nonesterified eicosanoids. The sample EGs were then eluted using a mixture of 92.5% hexane, 7% EtOH, 0.5% HOAc and, after solvent evaporation, purified by reversed phase HPLC on a Dynamax C18 column (150 × 4.6 mm; 5 μm), using a liner solvent gradient from 49.9% H2O, 50% CHCN3, 0.1% HOAc to 99.9% CHCN3, 0.1% HOAc in 40 min at 1 ml/min. Fractions with the retention times of authentic 11,12- and 14,15-EG (between 19 and 21 min) were collected and analyzed by ESI/LC/MS/MS-selected ion monitoring of fragmentation product ions from the 13C3-labeled 11,12- and 14,15-EG at m/z 504.2 or M + 3, from the 11,12- and 14,15-EG of biological origin at m/z 501.2 as well as m/z 426.5–427.5, 302.5–303.5, and 342.5–343.5.
To compare the levels of endogenous 2-11,12- and 2-14,15-EG with that of 2-AG in the fresh tissues, including kidney, spleen, and brain, both 2-EGs and 2-AG were measured in the same batch of lipid extract samples by ESI/LC/MS/MS analysis and calculated based on their internal standards, respectively.
Cell Culture—Chinese hamster ovary (CHO) cells were cultured in F-12K medium. Human promyelocytic leukemia HL-60 cells were grown in RPMI 1640 medium and N18TG2 neuroblastoma cells in Dulbecco's modified Eagle's medium supplemented with 6-thioguanine, as described previously (23).
cDNA Constructs and Transfection—Expression plasmids carrying the entire coding region of human cannabinoid receptor 1 (hCB1) were kindly provided by Dr. Tom Bonner (NIMH, National Institutes of Health, Bethesda, MD), whereas human CB2 expression cDNA construct (hCB2) was kindly provided by Dr. Ken Mackie (University of Washington, Seattle, WA). The hCB1 expression plasmids, hCB2 expression plasmids, or empty vector alone were transfected into CHO cells, respectively, using Lipofectamine (Invitrogen), as we described previously (6, 24). The cells were deprived of serum overnight before being used for signaling studies or isolation of plasma membranes for radioligand binding assays.
Radioligand Binding Assay—Sprague-Dawley rat cerebellum, spleen, or CHO cells transfected with human CB1 or CB2 receptor were Dounce-homogenized in a freshly prepared, ice-cold buffer containing 50 mm Tris-HCl, pH 7.4, 3 mm MgCl2, 1 mm EDTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 μg/ml pepstatin A, 1 mm benzamidine, 1 mm phenylmethylsulfonyl fluoride, and the membranes were pelleted from the post-nuclear supernatant at 90,000 × g for 20 min, washed, and then resuspended in a freshly prepared, ice-cold binding buffer containing 50 mm Tris-HCl, pH 7.4, 3 mm MgCl2, 1 mm EDTA, 3 mm CaCl2, 1 mm phenylmethylsulfonyl fluoride, and 1 mm diisopropyl fluorophosphate. Binding of [3H]CP55940 (specific activity 168 Ci/mmol; PerkinElmer Life Sciences) to plasma membranes (50 μg of membrane protein/150-μl reaction) was performed essentially as described previously (25). Different concentrations of unlabeled CP55940, 2-EGs, EETs, arachidonic acid, 2-AG, AM251, or AM630 were used to conduct competition binding in the presence of 0.5 nm [3H]CP55940. Specific binding was determined by subtracting the nonspecific binding component measured in the presence of excess unlabeled CP55940 (1 μm). Competition binding data were analyzed using Prism 4.01 software (GraphPad) by entering Kd values of 2.3 and 0.608 nm as determined for [3H]CP55940 in cell membranes expressing either CB1 or CB2 receptors, respectively (26).
Immunoblotting—CHO cells transfected with human CB1 or CB2 receptor were treated with the indicated agents, and cell lysates were prepared and subjected to immunoblotting analysis with the indicated antibodies as described previously (6, 8, 9, 24).
In Vivo Studies to Measure Cannabimimetic Effects of 2-EG—Male, 10-week-old C57BL/6J mice (Jackson) were used to examine the biological effects of 2-EG. Each animal was used only once in the sequential experiments. 2-EG, 2-AG, or vehicle alone was administered by tail-vein injection. Core temperature was determined by a thermistor probe (inserted 25 mm) and a telethermometer (Yellow Springs Instrument Co.); the difference in temperature before and 30 min after injection was calculated for each animal. The open field mobility experiments were performed at the Vanderbilt Murine Neurobehavioral Core Laboratory of the Center for Molecular Neuroscience, essentially as described previously (27). Mice were placed individually in the center of each open field activity chamber equipped with three vectors of infrared photo beam emitters and detectors spaced 1.5 cm apart (Med Associates). Spontaneous locomotor activity was recorded as the total distance traveled in cm for each 5-min block of the continuous 30-min session.
Vasodilatory Response of the Renal Glomerular Afferent Arteriole to 2-EG—Experiments were conducted using the in vitro perfused juxtamedullary nephron preparation as described (28). Briefly, the isolated rat kidney was perfused with a reconstituted red blood cell-containing solution, and renal artery perfusion pressure was set to 100 mm Hg. Effects of 2-11,12-EG or 2-14,15-EG on glomerular afferent arterioles preconstricted with 500 nm norepinephrine were determined with or without pretreatment by the CB receptor antagonists, AM251 or AM630 (each at 1 μm for 20 min), respectively. Data are presented as percentage relaxation, with 100% relaxation equal to the control diameter before the addition of norepinephrine (28).
Bradykinin-increased 2-EG Production in Renal Microvessels—Renal microvessels were isolated as we described previously (29) and incubated at 37 °C in a vial containing Dulbecco's modified Eagle's medium solution gassed with 95% O2 and 5% CO2 for 30 min to determine 2-EG levels. In a subset of renal microvessel incubations, 1 μm bradykinin was included. Incubations were terminated by placing the samples on ice and adding 5 mg of triphenylphosphine. Samples were then snap frozen in liquid N2, and lipids were extracted and analyzed for 2-EG levels using LC/MS/MS.
Cell Migration Assay—HL-60 cells were treated with 100 nm 1,25-(OH)2 vitamin D3 for 5 days to induce differentiation into macrophage-like cells. 1 × 106 differentiated cells suspended in 0.1 ml of RPMI 1640 medium containing 0.1% bovine serum albumin were plated into each Transwell™ insert (pore size, 5 μm) with 0.6 ml of the same cell-free medium in the lower compartment of 24-well culture plates (Corning Costar), followed by the addition of increasing concentrations of 2-11,12-EG, 2-14,15-EG, 11,12-EET, 14,15-EET, 2-AG, or vehicle (0.1% ethanol) alone to the lower compartment. After a 4-h treatment at 37 °C in the cell culture incubator (95% air and 5% CO2), cells that migrated from the upper compartment to the lower compartment were counted using a hemocytometer. For the experiments with inhibitors, the cells were pretreated with or without either AM251 or AM630 at 1 μm for 15 min or pertussis toxin (100 ng/ml) for 18 h before exposure to 1 μm 2-11,12-EG or 11,12-EET.
Statistical Analysis—Data are presented as means ± S.E. for at least four separate experiments (each in triplicate). An unpaired Student's t test was used for statistical analysis, and analysis of variance and Bonferroni t tests were used for multiple group comparisons. A value of p < 0.05 in comparison was considered statistically significant.
RESULTS
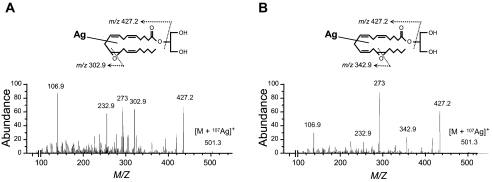
Characterization of the Mass Spectral Properties of Synthetic 2-EG—Our initial experiments characterized the mass spectral properties of synthetic 2-14,15-EG and 2-11,12-EG using Ag+ cationization and positive ion ESI/LC/MS/MS analysis. Since 2-acyl-glycerols isomerize spontaneously to mixtures of the corresponding 1- and 2-acyl-derivatives, the synthetic standards contain variable mixtures of the 1- and 2-positional isomers. As shown in Fig. 1 (A and B), collision-induced dissociation of the 107Ag adduct of synthetic 11,12- and 14,15-EG produced molecular ions at m/z 501.3 ([M + 107Ag]+) and yielded common ion fragments at m/z 427.2 (base peak, loss of glycerol) and 273. Regioisomer-specific EG fragments originating from transannular cleavage of the oxido functions are found at m/z 302.9 and 342.9, which are diagnostic for 11,12-EG (Fig. 1A) and 14,15-EG (Fig. 1B), respectively. A similar analysis using [13C3]glycerol-labeled 11,12- and 14,15-EG yielded the corresponding molecular ions at m/z 504.3 ([M + 107Ag] + 3) and the same common and diagnostic ion fragments observed with both the unlabeled standards (Fig. 1, A and B) and fragments generated after the loss of [13C3]glycerol from the m/z 504.3 molecular ion.
FIGURE 1.
Characterization of the mass spectral properties of synthetic 2-EG. Shown are collision-induced fragmentation patterns for the Ag107 isotope adducts [M + 107Ag]+ of synthetic 11,12-EG (A) and 14,15-EG (B). See “Experimental Procedures” for detailed procedures.
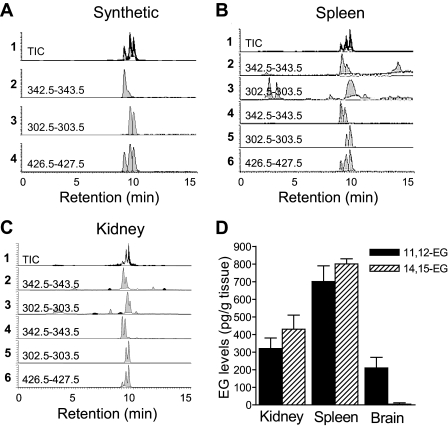
Identification of Endogenous 2-EG in Fresh Tissues by ESI/LC/MS/MS Analysis—We then utilized collision-induced fragmentation of the m/z 501.3 ion ([M + 107Ag]+) and selective reaction monitoring (SRM) at m/z 342.5–343.5 (for 14,15-EG), 302.5–303.5 (for 11,12-EG), and 426.5–427.5 (common for both EG isoforms) to analyze an equimolar mixture of 11,12-EG and 14,15-EG (2 ng each). As shown by the HPLC elution profiles of the total ion current (TIC) and of the common ion at m/z 427.2, the mixture of synthetic 11,12- and 14,15-EG was partially resolved into three fractions eluting from the column between 8.6 and 9.8 min and containing the 1- and 2-glycerol positional isomers of 14,15-EG, followed by those derived from 11,12-EG (Fig. 2A). By monitoring the elution profiles of the respective diagnostic ion fragments, 11,12-EG was able to be selectively detected in the presence of 14,15-EG, and vice versa (Fig. 2A). These results confirmed the validity of the diagnostic ESI/LC/MS/MS SRM method for the analysis of mixtures of these EGs in biological samples. SRM monitoring of EG diagnostic ions derived from the collision-induced fragmentation of the 13C3-labeled (internal standard; m/z 504.3) and unlabeled (biological; m/z 501.3) 11,12- and 14,15-EG and comparisons of HPLC retention times and diagnostic ion intensities at m/z 342.5–343.5 and 302.5–303.5 provided evidence for the presence of mixtures of the glycerol 1- and 2-positional isomers of these EGs in samples extracted from rat spleen (Fig. 2B) and kidney (Fig. 2C). By comparison of diagnostic ion intensities for the labeled and nonlabeled 2-EGs, we detected 750 and 1500 pg of 2-EGs (320 and 700 pg of 11,12-EG and 430 and 800 pg of 14,15-EG)/g of rat kidney and spleen tissues, respectively (Fig. 2D). In contrast, only 11,12-EG was detected in rat brain (210 pg/g of tissue) (Fig. 2D). The presence of endogenous 2-EG in rat kidney, spleen, and brain was confirmed in additional experiments in which the EG pools were extracted and purified by reversed HPLC and then submitted to hydrolysis in methanolic KOH (30). The resulting products were then converted to the corresponding pentafluorobenzyl esters by reaction with pentafluorobenzyl bromide and characterized as EETs using NICI/GC/MS (data not shown) (30).
FIGURE 2.
Identification of endogenous 2-EG in fresh spleen and kidney tissues by ESI/LC/MS/MS analysis. A, HPLC elution profile of product ions derived from the collision induced fragmentation of mixtures of synthetic 11,12- and 14,15-EG. Shown are the ion products derived from the fragmentation of the EG molecular ions at m/z 501.3 ([M + 107Ag]+) and detected by SRM at m/z 426.5–427.5 (for 11,12- and 14,15-EG), 302.5–303.5 (for 11,12-EG), and 342.5–343.5 (for 14,15-EG). TIC, total ion current. B and C, chromatograms of endogenous 11,12- and 14,15-EG detection in fresh tissues of spleen (B) and kidney (C). SRM-HPLC chromatograms of the EG-derived ions fragmented from the 107Ag adduct of the biological, unlabeled EGs at m/z 501.3 (B2, C2 and B3, C3) and the 13C3-labeled EG internal standard at m/z 504.3 (B4, C4 and B5, C5). The HPLC elution profiles of the TIC and of the common ion at m/z 427.2 are also shown (B1, C1 and B6, C6), respectively; shown are representative data from four separate experiments with similar results. D, levels of endogenous 11,12- and 14,15-EG present in rat kidney, spleen, and brain quantified from the ratios of intensities of the diagnostic ion fragments originated for the internal standard and the biological EG isomers (n = 3–6).
To compare the endogenous levels of 2-EGs with that of 2-AG, we measured the levels of 2-AG in the same tissues using the same methodology side by side. By comparison with the internal 2-AG standard, we detected 7.41, 11.42, and 5.21 ng of 2-AG in each gram of fresh kidney, spleen, and brain tissues, respectively (the values are mean of n = 3 animals).
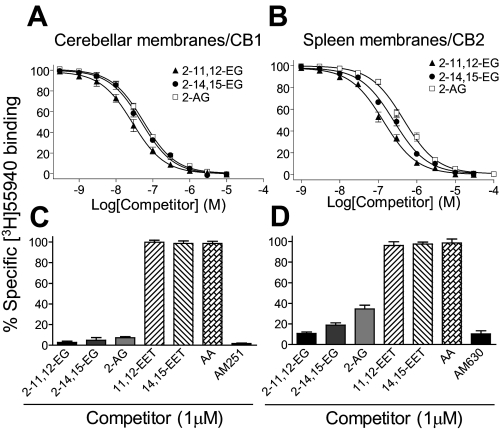
2-EG but Not EET Bound both CB1 and CB2 Cannabinoid Receptors with High Affinity—After confirmation of the existence of endogenous 2-EG in multiple tissues, we attempted to identify potential receptors for these cP450-dependent arachidonic acid metabolites. Radioligand binding experiments revealed that both 2-11,12-EG and 2-14,15-EG dose-dependently competed with [3H]CP55940 for binding to cerebellar plasma membranes (Fig. 3A), which express high levels of CB1 receptors (31), with a Ki value of 23 ± 3 and 40 ± 5 nm, respectively. [3H]CP55940 is a well characterized synthetic ligand specific for both CB1 and CB2 cannabinoid receptor subtypes with equally high affinity (26, 32). Since the spleen is the organ that expresses the highest levels of CB2 receptor (25), we also isolated plasma membranes from rat spleen and performed competition binding assays. As shown in Fig. 3B, both 2-EG isoforms also competed with [3H]CP55940 for binding to spleen plasma membranes, with a Ki of 75 ± 6 nm for 2-11,12-EG and 138 ± 9 nm for 2-14,15-EG. As indicated in Table 1, both 2-11,12-EG and 2-14,15-EG bind CB1 and CB2 receptors with an affinity comparable with 2-AG, a well established endogenous ligand for both CB1 and CB2 receptors (33–37). In contrast, neither arachidonic acid nor EETs competed with [3H]CP55940 for binding to CB1 receptors (Fig. 3C) or CB2 receptors (Fig. 3D). Included as positive controls, the CB1-selective antagonist AM251 and the CB2-selective antagonist AM630, each at 1 μm, almost completely blocked 0.5 nm [3H]CP55940 binding to CB1 receptors (Fig. 3C) and CB2 receptors (Fig. 3D), respectively. Thus, these new cP450-dependent arachidonate metabolites are endogenous ligands for both CB1 and CB2 receptors.
FIGURE 3.
2-EG but not EET bound both CB1 and CB2 receptors with high affinity. A and B, both 2-11,12-EG and 2-14,15-EG competed with [3H]CP55940 (0.5 nm) for binding to rat cerebellar membranes, which express high levels of CB1 (A), and rat spleen membranes, which express high levels of CB2 (B). C and D, the parental compounds, arachidonic acid and EETs, did not inhibit [3H]CP55940 binding to CB1 receptors (C) or CB2 receptors (D).
TABLE 1.
Comparison of the Ki values of endocannabinoids
Shown are binding affinities of 2-EG and 2-AG for CB1 receptors expressed in rat cerebellar membranes and CB2 receptors expressed in rat spleen membranes. Ki values were determined using Prism 4.01 software (GraphPad) by entering the concentration of [3H]CP55940 (0.5 nm) and the characterized Kd of 2.3 nm for CB1 or 0.608 nm for CB2 (26), respectively. Results are mean ± S.E. of 5-7 independent experiments, each performed in triplicate.
|
CB ligands |
Ki |
|
|---|---|---|
| CB1 | CB2 | |
| nm | nm | |
| 2-11,12-EG | 23 ± 3 | 75 ± 6 |
| 2-14,15-EG | 40 ± 5 | 138 ± 9 |
| 2-AG | 45 ± 4 | 251 ± 15 |
2-EG Activated Gi/o-coupled ERK Signaling in Cells Expressing CB1 or CB2 Cannabinoid Receptors—Since 2-11,12-EG possesses a relatively higher affinity than 2-14,15-EG for both CB1 and CB2 receptor subtypes, as indicated in Fig. 3, we used 2-11,12-EG to study the signaling events and biological responses in the subsequent experiments.
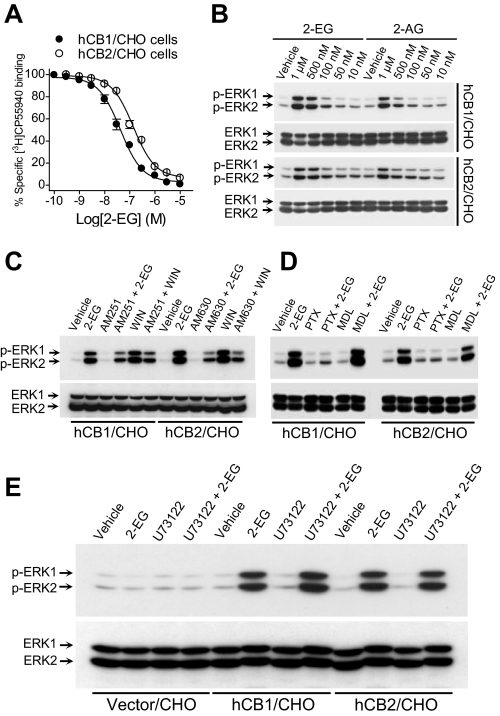
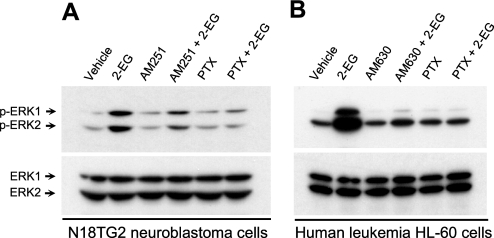
To determine whether binding of 2-EG to cannabinoid receptors could activate the receptors, we transfected the mammalian expression cDNA constructs of hCB1 (31) and hCB2 (25) into CHO cells, respectively. High affinity 2-EG binding to cannabinoid receptors was further confirmed using plasma membranes isolated from CHO cells expressing either human CB1 receptors or human CB2 receptors (Fig. 4A). There was no specific [3H]CP55940 binding to plasma membranes isolated from either nontransfected or control vector-transfected CHO cells (data not shown). Immunoblotting analysis revealed that 2-EG activated the p44/p42 extracellular signal-regulated kinases (ERKs) in CHO cells transfected with either hCB1 or hCB2 receptors, respectively (Fig. 4B), but not in nontransfected (data not shown) or control vector-transfected CHO cells (Fig. 4E). The ERK activation was inhibited by pretreatment of the cells with either the selective CB1 antagonist AM521 or CB2 antagonist AM630, respectively; the synthetic CB1 and CB2 activator, WIN55212-2 (WIN), was included as a positive control (Fig. 4C). EETs did not activate ERKs in hCB1-, hCB2-, or empty vector-transfected control CHO cells. In contrast, the synthetic 2-EG analogs, 2-(11,12-epoxyeicosatrienoyl)glycerol ether and 2-(14,15-epoxyeicosatrienoyl)glycerol ether, which are unable to be cleaved to free EETs because the ester linkage in 2-EGs has been replaced by an ether bond in these 2-EG analogs (see Fig. 10), also stimulated ERK activation in hCB1- or hCB2-transfected CHO cells but not in the empty vector-transfected control CHO cells (data not shown). Of note, the ether analog of 2-AG, 2-arachidonyl glyceryl ether (noladin ether), has recently been demonstrated to be an endogenous agonist of CB1 receptors (Ki = 21.2 nm) (38). 2-EG-induced ERK activation mediated by both CB receptor subtypes was inhibited by pertussis toxin, whereas the cell-permeable and irreversible adenylyl cyclase inhibitor MDL12,330A (Fig. 4D) and the protein kinase A inhibitor H89 (data not shown) had no effect. In addition, the phospholipase C inhibitor U73122 did not affect 2-EG-induced ERK activation in either hCB1- or hCB2-transfected CHO cells; 2-EG did not activate ERK in empty vector-transfected CHO cells (Fig. 4E). These data suggest that 2-EG induced ERK activation in the CHO cells expressing either human CB1 or CB2 cannabinoid receptors by binding to and activating the transfected, human cannabinoid receptors coupled to Gi/o proteins.
FIGURE 4.
Binding of 2-EG to CB1 or CB2 receptors activated Gi/o-coupled ERK signaling. A, 2-11,12-EG concentration-dependently competed with [3H]CP55940 (0.5 nm) for binding to plasma membranes isolated from CHO cells expressing either hCB1 or hCB2 receptors, as indicated. B, 2-11,12-EG concentration-dependently increased tyrosine phosphorylation of p44/p42 ERKs in either hCB1- or hCB2-transfected CHO cells. C, the selective CB1 antagonist AM251 (1 μm) or CB2 antagonist AM630 (1 μm) inhibited 2-11,12-EG-induced ERK activation in hCB1- or hCB2-transfected CHO cells, respectively. WIN55212-2 (WIN), which is known to activate CB1 and CB2 signaling to ERK phosphorylation, was included as a positive control. D, 2-EG activation of ERKs was inhibited by pertussis toxin (PTX; 100 ng/ml for 18 h before exposure to 1 μm 2-11,12-EG for 10 min) but not by the cell-permeable and irreversible adenylyl cyclase inhibitor MDL12,330A (MDL; 100 μm). E, pretreatment with the phospholipase C (PLC) inhibitor U73122 (10 μm) did not affect 2-11,12-EG (1 μm)-induced tyrosine phosphorylation of ERKs in either human CB1- or CB2-transfected CHO cells. The blots are representative of n = 3–4 separate experiments with similar results.
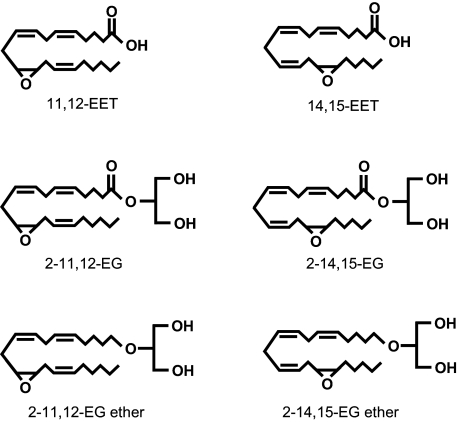
FIGURE 10.
Chemical structures of the synthetic analogs of 2-EGs along with those of EETs and 2-EGs for comparison.
We also examined the effect of 2-EG in N18TG2 neuroblastoma cells, which express endogenous CB1 but not CB2 cannabinoid receptors (39). As indicated in Fig. 5A, administration of 2-EG activated ERK signaling in N18TG2 neuroblastoma cells. This effect was inhibited by pretreatment of the cells with either the selective CB1 antagonist AM251 or pertussis toxin (Fig. 5A), indicating 2-EG activation of the native CB1 receptor-ERK signaling in a Gi/o-dependent manner. Similarly, the selective CB2 antagonist AM630 as well as pertussis toxin blocked 2-EG-induced ERK activation in human promyelocytic leukemia HL-60 cells (Fig. 5B); these cells are known to express functional native CB2 but not CB1 receptors (40).
FIGURE 5.
2-EG activated ERK signaling in cells naturally expressing CB1 or CB2 cannabinoid receptors. A, 2-11,12-EG (1 μm) induced ERK activation in N18TG2 neuroblastoma cells, which express functional native CB1 but not CB2 receptors. This effect of 2-EG was blocked by pretreatment with either the selective CB1 antagonist AM251 (1 μm) or pertussis toxin (PTX; 100 ng/ml for 18 h). B, 2-11,12-EG (1 μm)-stimulated ERK activation in human promyelocytic leukemia HL-60 cells, which are known to endogenously express native CB2 but not CB1 receptors, was prevented by pretreatment with either pertussis toxin (100 ng/ml for 18 h) or the selective CB2 antagonist AM630 (1 μm). The blots are representative of n = 3 separate experiments with similar results.
The above signaling experiments further confirmed that 2-EG binds and activates cannabinoid receptors coupled to Gi/o protein and thus initiates intracellular signaling events, resulting in activation of downstream p44/p42 ERKs in cells expressing either transfected or endogenous CB1 or CB2 receptors. These data collectively suggest that 2-EG is a full agonist for both CB1 and CB2 receptors (Figs. 4 and 5).
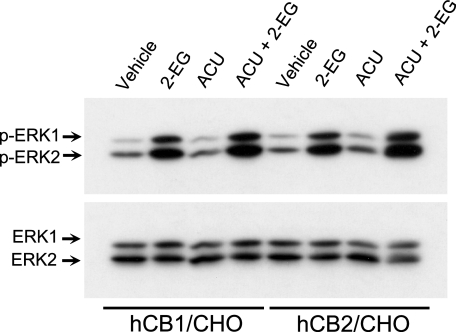
Effect of Soluble Epoxide Hydrolase on the CB Receptor Agonist Activity of 2-EG—Additional experiments revealed that 1-adamantyl-3-cyclohexylurea, a potent soluble epoxide hydrolase inhibitor that has been shown to increase Ca2+ influx in CYP2C9-expressing but not in CYP2C9-deficient endothelial cells in culture (41), potentiated 2-EG-stimulated ERK phosphorylation in CHO cells expressing either CB1 or CB2 receptors (Fig. 6). These data suggest that the presumed hydrolysis products, 2-dihydroxyeicosatrienoyl-glycerols may be biologically less active or inactive. Thus, such hydrolysis is likely to serve as a mechanism of 2-EG inactivation. However, more rigorous analysis in future studies focusing on the elucidation of 2-EG biosynthesis and metabolism/inactivation will be required to definitively address the related questions.
FIGURE 6.
Effect of soluble epoxide hydrolase on the CB receptor agonist activity of 2-EG. hCB1- or hCB2-transfected CHO cells were pretreated with the potent soluble epoxide hydrolase inhibitor, 1-adamantyl-3-cyclohexylurea (ACU;10μm) for 15 min before exposure to 1μm 2-11,12-EG for 10 min. Cell lysates were subjected to immunoblotting analysis with an antibody recognizing only phosphorylated ERK1 (44 kDa) and ERK2 (42 kDa) (top), followed by stripping of the same blot and reprobing with an antibody to total ERKs to ensure equal loading (bottom). Shown is a set of representative blots of four separate experiments with similar results.
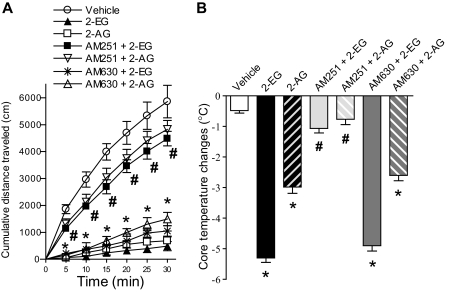
2-EG Elicited Hypomotility and Hypothermia Responses in Intact Mice—To evaluate whether 2-EG could elicit CB1-mediated cannabimimetic responses, such as simultaneous hypomotility and hypothermia (26, 33, 42), we administered 2-EG (76 nmol/g body weight) into C57BL/6J mice through tail vein injection. 2-EG profoundly suppressed spontaneous locomotor activity (Fig. 7A) and significantly decreased the core temperature of the mice (Fig. 7B). At the same concentration, 2-EG was slightly more potent than 2-AG in inhibiting locomotor activity (Fig. 7A) and significantly more potent in lowering the core temperature (Fig. 7B). Pretreatment of C57BL/6J mice with the selective CB1 antagonist AM251 (2 mg/kg) abolished the effects of hypomotility (Fig. 7A) and hypothermia (Fig. 7B) induced by either 2-EG or 2-AG. In contrast, the selective CB2 antagonist AM630 (2 mg/kg) had no effect on the hypomotility and hypothermia responses elicited by 2-EG or 2-AG (Fig. 7, A and B). Similar to the effects of 2-AG, these cannabimimetic effects of 2-EG began to decrease 30 min after intravenous administration and gradually diminished (data not shown), suggesting that 2-EG undergoes rapid biological inactivation, which is another characteristic of cannabinoid substances (26, 33, 42, 43).
FIGURE 7.
2-EG elicited hypomotility and hypothermia responses in intact mice. 2-11,12-EG or 2-AG (both at 76 nmol/g of body weight, intravenously) suppressed spontaneous locomotor activity (A) and simultaneously decreased core temperature in C57BL6/J mice (B). These effects were inhibited by pretreatment with the selective CB1 antagonist AM251 (2 mg/kg) but not by the selective CB2 antagonist AM630 (2 mg/kg). The error bars indicate S.E.; n = 7 animals per group in both A and B;*, p < 0.001 compared with corresponding vehicle controls. #, p < 0.001 compared with AM251 plus 2-EG or AM251 plus 2-AG with 2-EG or 2-AG alone, respectively.
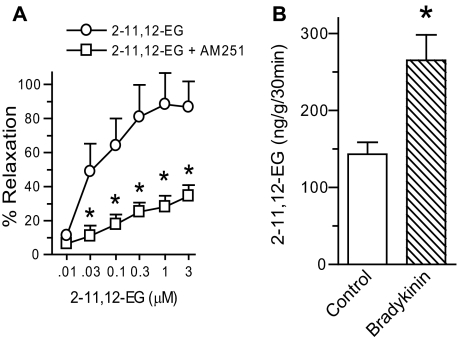
2-EG Induced Vasorelaxation, and Bradykinin Increased 2-EG Production in Renal Microvasculature—Utilizing an in vitro perfused juxtamedullary nephron preparation, we observed that 2-EG induced relaxation of renal glomerular afferent arterioles preconstricted with norepinephrine, an effect blocked by the selective CB1 antagonist AM251 (Fig. 8A) but not by the CB2 antagonist AM630 (data not shown).
FIGURE 8.
2-EG induced vasorelaxation, and bradykinin increased 2-EG production in renal microvasculature. Renal microvessels were isolated as we described (29), and experiments using the in vitro perfused juxtamedullary nephron preparation were conducted as we described previously (28). A, 2-11,12-EG concentration-dependently relaxed renal glomerular afferent arterioles preconstricted with norepinephrine (500 nm), which was inhibited by pretreatment with the CB1 antagonist AM251 (1 μm). Data are presented as percentage relaxation, with 100% relaxation equal to the control diameter before the addition of norepinephrine (28). B, renal microvessels produced 2-11,12-EG, and this production was increased by the vasodilating hormone, bradykinin (1 μm), determined by ESI/LC/MS/MS analysis. The error bars indicate S.E. in both panels; n = 7 vessels/group in A; n = 3 vessel samples/group in B.*, p < 0.05 compared with control 2-EG responses (A) or vehicle controls (B).
ESI/LC/MS/MS analysis revealed that renal microvessels produced 2-11,12-EG, and this production was increased by the vasodilator, bradykinin (Fig. 8B). 2-14,15-EG was also produced by renal microvasculature and induced similar vasorelaxation in renal glomerular afferent arterioles (data not shown).
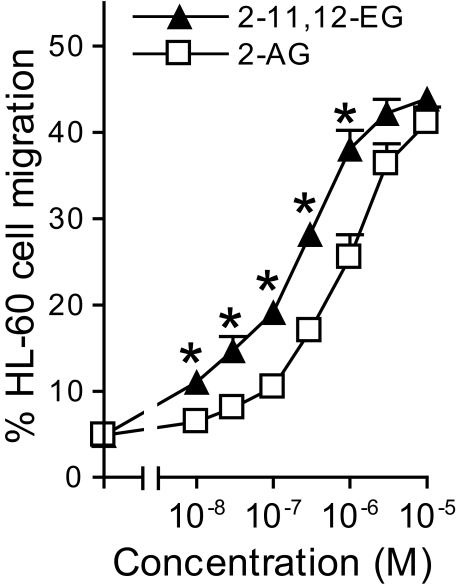
2-EG-induced Migration of HL-60 Cells—To investigate whether 2-EG could also induce CB2 receptor-mediated responses, we examined migration of HL-60 cells, which express CB2 but not CB1 receptors (40). As indicated in Fig. 8, 2-EG stimulated HL-60 cell migration in a concentration-dependent manner with an EC50 of ∼130 nm, whereas 2-AG exhibited a similar effect but had an EC50 of ∼800 nm (Fig. 9). These effects were inhibited by pretreatment with either pertussis toxin or the selective CB2 receptor antagonist, AM630, but not by the selective CB1 receptor antagonist, AM251 (data not shown). The parental compounds, 11,12-EET and 14,15-EET, had no effect on HL-60 cell migration (data not shown).
FIGURE 9.
2-EG concentration-dependently induced migration of HL-60 cells. HL-60 cells were treated with 100 nm 1,25-(OH)2 vitamin D3 for 5 days to induce differentiation into macrophage-like cells, which were then used for cell migration assays as described under “Experimental Procedures.” The error bars indicate S.E.; n = 4–6 separate experiments with each concentration in triplicate; *, p < 0.05 compared with control 2-AG responses at the respective concentrations indicated.
To determine whether the EGs are derived from the cP450-catalyzed epoxidation of 2-AG, we incubated [14C]2-AG (40 Ci/mol; 50–75 μm) with microsomes isolated from the livers and kidneys of untreated, salt-loaded, or phenobarbital-treated rats in the presence of NADPH and an NADPH-generating system (2). Although [14C]arachidonic acid was effectively epoxidized to EETs, no NADPH-dependent epoxidation or hydroxylation of [14C]2-AG was observed, even after extended incubations at 37 °C (n = 4). Furthermore, incubation of [14C]2-AG with the recombinant epoxygenases CYP2C8, 2C11, or 2C23 (44, 45) in the presence of purified cP450 reductase, dilauroylphosphatidylcholine, and NADPH failed to yield cP450-dependent epoxidation of [14C]2-AG (n = 4). These results are consistent with the fact that methyl-arachidonate is not a substrate for cP450, suggesting that the carboxylic acid functionality is required for fatty acid binding and metabolism (46). Since EETs are found primarily esterified to the sn-2 carbon of phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol in vivo (47), we hypothesize that endogenous 2-EG is produced by phospholipase C-catalyzed hydrolysis of the EET-containing glycerophospholipids coupled to diacylglycerol lipase-mediated cleavage of the fatty acid moiety at the sn-1-position and/or by phospholipase A1-catalyzed hydrolysis of the EET-phospholipids coupled to subsequent lysophospholipase C-mediated hydrolysis.
DISCUSSION
cP450 enzymes were first recognized to metabolize arachidonic acid in 1981 (2–4), and the demonstration of such cP450 arachidonate metabolites in various tissues (such as the liver, kidney, brain, heart, lung, and vasculature), plasma, and urine unequivocally established the cP450-dependent metabolism of arachidonic acid as a third metabolic pathway of arachidonic acid in vivo (14, 16, 46). In the past 2 decades, considerable advances have been made in understanding the potent biological effects of the cP450 arachidonate metabolites (5, 6, 10–16). The present study identified a novel group of cP450-dependent arachidonate metabolites that we termed 2-EGs that can be identified in kidney, spleen, and brain. We determined that these metabolites can act as high affinity ligands for both CB1 and CB2 cannabinoid receptors. 2-EG activation of cannabinoid receptors was prevented by pertussis toxin but not by inhibition of either phospholipase C, adenylyl cyclase, or protein kinase A, indicating coupling to the Gi/o subtypes of G proteins. When administered into mice, 2-EG induced profound hypomotility and hypothermia, both of which were inhibited by the selective CB1 receptor antagonist AM251. In addition, 2-EG induced migration in HL60 cells through activation of CB2 receptors. These data suggest that we have identified a novel group of cP450-dependent arachidonate metabolites that are endogenously produced and biologically active lipid mediators with the characteristics of cannabinoid substances.
Cannabinoids, such as δ-9-tetrahydrocannabinol, are the active constituent compounds in marijuana. Cannabinoids were initially recognized to be clinically relevant due to their pharmacological effects, such as analgesia, anti-inflammation, antianxiety, and other central nervous system effects, including alterations in mood and cognition experienced by users of marijuana (48–50). However, the widespread acceptance and clinical application of cannabinoid compounds as therapeutic agents has been limited by their psychoactive effects. This stimulated investigation of the biochemical basis of their actions. The development of the synthetic cannabinoid agonist [3H]CP55940 allowed the demonstration of high affinity, saturable, and stereospecific binding sites in mouse brain plasma membranes; this study provided definitive evidence for the existence of specific cannabinoid receptors that mediate the cellular effects of cannabinoids (51). The cloning of cannabinoid receptors (25, 31) led to the search for their endogenous ligands and resulted in the discovery of N-arachidonoyl ethanolamine (anandamide) (52) and 2-AG (33, 34). Additional endogenous ligands for cannabinoid receptors have been reported, such as N-arachidonoyldopamine (53), 2-arachidonylglyceryl ether (noladin) (38), and O-arachidonoylethanolamine (virhodamine) (54), but anandamide and 2-AG remain to be the best characterized endocannabinoids to date. Of interest, all of the endocannabinoids identified thus far are endogenous arachidonic acid derivatives, including the 2-EG described in the present study.
Although accumulating pharmacological evidence suggests the existence of one or more additional cannabinoid receptors (55–57), only two cannabinoid receptors, CB1 and CB2, have been cloned to date (25, 31). CB1 and CB2 receptors both belong to the superfamily of G protein-coupled receptors; they share 68% homology within the transmembrane domains and 44% identity throughout the whole molecules (25, 31). Although the CB1 receptor is highly expressed in the central nervous system and is thought to be the most abundant receptor in the mammalian brain (31, 56, 58), it is also expressed in vasculature and a variety of peripheral tissues and cells (56, 59, 60). Similarly, the CB2 receptor primarily expressed in cells of the immune and hematopoietic systems (25) is also expressed in brainstem neurons (61) and in bone (62). More recently, the CB2 receptor has been shown to be present in the pancreatic β-cells and to regulate Ca2+ signaling and insulin secretion (63). The involvement of cannabinoid receptors in various physiologic and pathophysiologic processes (such as food intake, pain, mood, and cell growth) has stimulated interest in this pathway as a potential therapeutic target (50, 56, 58, 64, 65).
Although CB1 receptors have been detected in vascular smooth muscle as well as endothelium (59, 60), and EETs elicit a vasodilatory response in renal arterioles (29), our data indicated that the effects of EETs are not mediated by cannabinoid receptors, because EETs do not bind or activate cannabinoid receptors. In addition, our experiments have confirmed that synthetic analogs of 2-EG that are unable to be cleaved to free EETs (Fig. 10) also activate cannabinoid receptor-mediated effects. Of interest, the production of 2-EG in renal microvessels is increased in response to bradykinin, suggesting that 2-EG may serve as an effector for vasoactive hormones in renal microvasculature. The potential roles of 2-EG and the underlying mechanisms in regulation of local tissue blood supply and systemic blood pressure as well as their implications in the pathogenesis of hypertension certainly deserve further investigation, which may lead to new therapeutic strategies for hypertension.
We also observed a potent stimulatory effect of 2-EG on the migration of human promyelocytic leukemia HL-60 cells that had been differentiated into macrophage-like cells, which are known to express functional native CB2 but not CB1 receptors (40). Although EETs have no effect on migration of these macrophage-like cells, the effect of 2-EG is mediated by activation of CB2 receptors coupled to Gi/o protein. Since the spleen is the organ that expresses the highest levels of CB2 receptors (26), it is possible that 2-EG may serve as a modulator of immune responses through activation of CB2 receptors.
Reported Ki values of (endo)cannabinoids for CB1 and CB2 receptors have shown variation, depending on the binding assay system used (33, 34, 51, 66). The reported Ki values of 2-AG ranged from 58.3 to 472 nm for CB1 receptors and from 145 to 1400 nm for CB2 receptors, as summarized in the tables of related reviews (49, 67). Our preliminary experiments initially optimized the conditions for our binding assay system by including different protease inhibitors in the homogenizing buffer and different concentrations of diisopropyl fluorophosphate and/or phenylmethylsulfonyl fluoride in the binding buffer. Under the optimized binding conditions described under “Experimental Procedures,” the Ki values of 2-EGs for both CB1 and CB2 are comparable with those of 2-AG (which had a Ki of 45 nm for CB1 and 251 nm for CB2).
In the present study, using ESI/LC/MS/MS analysis, we detected 750, 1500, and 210 pg of 2-EGs in each gram of rat kidney, spleen, and brain tissues, respectively. However, 143 ng of 2-11,12-EG was detected in each gram of renal microvessels, and this level was increased to 265 ng within 30 min in response to treatment with bradykinin (Fig. 8B). In contrast, using the same methodology, we detected 7.41, 11.42, and 5.21 ng of 2-AG in each gram of rat kidney, spleen, and brain tissues, respectively. These data suggest that both 2-EGs and 2-AG are produced at significantly different levels in different tissues and even in different structures of the same tissue, with the basal level of 2-EG production significantly higher in the microvessels of the kidney and regulated by vasodilatory hormones, such as bradykinin (Fig. 8B).
To our knowledge, there are a total of three published research articles that have tested the effects of synthetic EGs (also called GEETs) to date (20, 68, 69). Gauthier et al. (68) showed that the synthetic EG, 14,15-GEET, activated smooth muscle large conductance K+ channels and relaxed the bovine coronary arteries, an effect not dependent on hydrolysis to 14,15-EET, whereas Fang et al. (69) showed that exogenously added 14,15-GEET was first hydrolyzed to release 14,15-EET, which was then converted to 14,15-DHET, which can directly bind and activate PPARα in COS-7 cells transfected with β-galactosidase, PPARα, and luciferase reporter genes. We have recently reported that synthetic 2-14,15-EG induced mitogenic effect by transactivation of the EGF receptor in LLCPKcl4 cells, a renal proximal tubule epithelial cell line that does not express either CB1 or CB2 receptors (20). However, all of the studies published to date have only reported noncannabinoid receptor-mediated effects of synthetic 2-EGs. Obviously, chemically synthesized compounds may not necessarily be produced in biological tissues, and no previous study examined the endogenous production of 2-EG or reported 2-EG as a ligand for CB receptors.
In summary, the present study describes novel cP450-dependent metabolites of arachidonic acid, 2-EG, which are endogenously produced and biologically active. We have also identified that CB1 and CB2 represent functional receptors of 2-EG, which represents the first documentation that cP450 arachidonate metabolites can exert their biological effects by binding to and activating G-protein-coupled cell membrane receptors and suggests that 2-EG are new members of the endocannabinoid family. These findings suggest that cP450 epoxygenase may play a previously unrecognized role in cannabinoid receptor-mediated effects, since endocannabinoid ligand availability plays an important role in mediating biological responses in both central and peripheral tissues that express cannabinoid receptors (70).
Acknowledgments
We are grateful to Dr. Tom Bonner (NIMH, National Institutes of Health, Bethesda, MD) for providing the human CB1 expression plasmid, to Dr. Ken Mackie (University of Washington, Seattle) for providing the human CB2 cDNA construct, and to Dr. David Merkler (University of South Florida, Tampa, FL) for providing N18TG2 neuroblastoma cells. We thank Drs. Michael P. McDonald and John D. Allison (The Center for Molecular Neuroscience, Vanderbilt University School of Medicine, Nashville, TN) for technical assistance during the behavioral experiments and Dr. Richard M. Breyer (Departments of Medicine and Pharmacology, Vanderbilt University School of Medicine, Nashville, TN) for assistance in analysis of radioligand-receptor binding data.
This work was supported, in whole or in part, by National Institutes of Health Grant Program Project Grant DK38226 (to J.-K. C., J. D. I., J. R. F., J. H. C., and R. C. H.). This work was also supported by the Department of Veterans Affairs (to R. C. H.) and American Heart Association Scientist Development Grant 0630274N (to J.-K. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: cP450, cytochrome P450 enzyme system; EET, epoxyeicosatrienoic acid; 2-EG, 2-epoxyeicosatrienoylglycerol; 2-11,12-EG, 2-(11,12-epoxyeicosatrienoyl)glycerol; 2-14,15-EG, 2-(14,15-epoxyeicosatrienoyl)glycerol; 11,12-EG, 11,12-epoxyeicosatrienoylglycerol; 14,15-EG, 14,15-epoxyeicosatrienoylglycerol; 2-AG, 2-arachidonylglycerol; CB, cannabinoid; CB1 and -2, cannabinoid receptor 1 and 2, respectively; hCB1 and -2, human CB1 and CB2, respectively; ERK, extracellular signal-regulated kinase; ESI, electrospray ionization; LC, liquid chromatography; MS, mass spectrometry; HPLC, high pressure liquid chromatography; CHO, Chinese hamster ovary; SRM, selective reaction monitoring; TIC, total ion current.
References
- 1.Needleman, P., Turk, J., Jakschik, B. A., Morrison, A. R., and Lefkowith, J. B. (1986) Annu. Rev. Biochem. 5569 –102 [DOI] [PubMed] [Google Scholar]
- 2.Capdevila, J., Chacos, N., Werringloer, J., Prough, R. A., and Estabrook, R. W. (1981) Proc. Natl. Acad. Sci. U. S. A. 785362 –5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison, A. R., and Pascoe, N. (1981) Proc. Natl. Acad. Sci. U. S. A. 787375 –7378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliw, E. H., Lawson, J. A., Brash, A. R., and Oates, J. A. (1981) J. Biol. Chem. 2569924 –9931 [PubMed] [Google Scholar]
- 5.Graier, W. F., Simecek, S., and Sturek, M. (1995) J. Physiol. (Lond.) 482259 –274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, J. K., Wang, D. W., Falck, J. R., Capdevila, J., and Harris, R. C. (1999) J. Biol. Chem. 2744764 –4769 [DOI] [PubMed] [Google Scholar]
- 7.Rzigalinski, B. A., Willoughby, K. A., Hoffman, S. W., Falck, J. R., and Ellis, E. F. (1999) J. Biol. Chem. 274175 –182 [DOI] [PubMed] [Google Scholar]
- 8.Chen, J. K., Falck, J. R., Reddy, K. M., Capdevila, J., and Harris, R. C. (1998) J. Biol. Chem. 27329254 –29261 [DOI] [PubMed] [Google Scholar]
- 9.Chen, J. K., Capdevila, J., and Harris, R. C. (2002) Proc. Natl. Acad. Sci. U. S. A. 996029 –6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell, W. B., Gebremedhin, D., Pratt, P. F., and Harder, D. R. (1996) Circ. Res. 78415 –423 [DOI] [PubMed] [Google Scholar]
- 11.Fisslthaler, B., Popp, R., Kiss, L., Potente, M., Harder, D. R., Fleming, I., and Busse, R. (1999) Nature 401493 –497 [DOI] [PubMed] [Google Scholar]
- 12.Node, K., Huo, Y., Ruan, X., Yang, B., Spiecker, M., Ley, K., Zeldin, D. C., and Liao, J. K. (1999) Science 2851276 –1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe, H., Vriens, J., Prenen, J., Droogmans, G., Voets, T., and Nilius, B. (2003) Nature 424434 –438 [DOI] [PubMed] [Google Scholar]
- 14.McGiff, J. C., and Quilley, J. (2001) Curr. Opin. Nephrol. Hypertens. 10231 –237 [DOI] [PubMed] [Google Scholar]
- 15.Pratt, P. F., Medhora, M., and Harder, D. R. (2004) Curr. Opin. Investig. Drugs 5952 –956 [PubMed] [Google Scholar]
- 16.Sarkis, A., Lopez, B., and Roman, R. J. (2004) Curr. Opin. Nephrol. Hypertens. 13205 –214 [DOI] [PubMed] [Google Scholar]
- 17.Seubert, J. M., Zeldin, D. C., Nithipatikom, K., and Gross, G. J. (2007) Prostaglandins Other Lipid Mediat. 8250 –59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breyer, R. M., Bagdassarian, C. K., Myers, S. A., and Breyer, M. D. (2001) Annu. Rev. Pharmacol. Toxicol. 41661 –690 [DOI] [PubMed] [Google Scholar]
- 19.Brink, C., Dahlen, S. E., Drazen, J., Evans, J. F., Hay, D. W., Nicosia, S., Serhan, C. N., Shimizu, T., and Yokomizo, T. (2003) Pharmacol. Rev. 55195 –227 [DOI] [PubMed] [Google Scholar]
- 20.Chen, J., Chen, J. K., Falck, J. R., Guthi, J. S., Anjaiah, S., Capdevila, J. H., and Harris, R. C. (2007) Mol. Cell. Biol. 273023 –3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang, S. H., Tsai, H. J., Liu, J. Y., Morisseau, C., and Hammock, B. D. (2007) J. Med. Chem. 503825 –3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morisseau, C., Goodrow, M. H., Newman, J. W., Wheelock, C. E., Dowdy, D. L., and Hammock, B. D. (2002) Biochem. Pharmacol. 631599 –1608 [DOI] [PubMed] [Google Scholar]
- 23.Merkler, D. J., Chew, G. H., Gee, A. J., Merkler, K. A., Sorondo, J. P., and Johnson, M. E. (2004) Biochemistry 4312667 –12674 [DOI] [PubMed] [Google Scholar]
- 24.Chen, J. K., Capdevila, J., and Harris, R. C. (2000) J. Biol. Chem. 27513789 –13792 [DOI] [PubMed] [Google Scholar]
- 25.Munro, S., Thomas, K. L., and Abu-Shaar, M. (1993) Nature 36561 –65 [DOI] [PubMed] [Google Scholar]
- 26.Pertwee, R. G. (1997) Pharmacol. Ther. 74129 –180 [DOI] [PubMed] [Google Scholar]
- 27.Schramm, N. L., McDonald, M. P., and Limbird, L. E. (2001) J. Neurosci. 214875 –4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imig, J. D., Navar, L. G., Roman, R. J., Reddy, K. K., and Falck, J. R. (1996) J. Am. Soc. Nephrol. 72364 –2370 [DOI] [PubMed] [Google Scholar]
- 29.Imig, J. D., Falck, J. R., Wei, S., and Capdevila, J. H. (2001) J. Vasc. Res. 38247 –255 [DOI] [PubMed] [Google Scholar]
- 30.Capdevila, J. H., Dishman, E., Karara, A., and Falck, J. R. (1991) Methods Enzymol. 206441 –453 [DOI] [PubMed] [Google Scholar]
- 31.Matsuda, L. A., Lolait, S. J., Brownstein, M. J., Young, A. C., and Bonner, T. I. (1990) Nature 346561 –564 [DOI] [PubMed] [Google Scholar]
- 32.Thomas, B. F., Gilliam, A. F., Burch, D. F., Roche, M. J., and Seltzman, H. H. (1998) J Pharmacol. Exp. Ther. 285285 –292 [PubMed] [Google Scholar]
- 33.Mechoulam, R., Ben-Shabat, S., Hanus, L., Ligumsky, M., Kaminski, N. E., Schatz, A. R., Gopher, A., Almog, S., Martin, B. R., Compton, D. R., Pertweee, R. G., Griffine, G., Bayewitchf, M., Bargf, J., and Vogelf, Z. (1995) Biochem. Pharmacol. 5083 –90 [DOI] [PubMed] [Google Scholar]
- 34.Sugiura, T., Kondo, S., Sukagawa, A., Nakane, S., Shinoda, A., Itoh, K., Yamashita, A., and Waku, K. (1995) Biochem. Biophys. Res. Commun. 21589 –97 [DOI] [PubMed] [Google Scholar]
- 35.Sugiura, T., Kondo, S., Kishimoto, S., Miyashita, T., Nakane, S., Kodaka, T., Suhara, Y., Takayama, H., and Waku, K. (2000) J. Biol. Chem. 275605 –612 [DOI] [PubMed] [Google Scholar]
- 36.Sugiura, T., Kishimoto, S., Oka, S., and Gokoh, M. (2006) Prog. Lipid Res. 45405 –446 [DOI] [PubMed] [Google Scholar]
- 37.Di Marzo, V., and Petrosino, S. (2007) Curr. Opin. Lipidol. 18129 –140 [DOI] [PubMed] [Google Scholar]
- 38.Hanus, L., Abu-Lafi, S., Fride, E., Breuer, A., Vogel, Z., Shalev, D. E., Kustanovich, I., and Mechoulam, R. (2001) Proc. Natl. Acad. Sci. U. S. A. 983662 –3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abood, M. E., Ditto, K. E., Noel, M. A., Showalter, V. M., and Tao, Q. (1997) Biochem. Pharmacol. 53207 –214 [DOI] [PubMed] [Google Scholar]
- 40.Kishimoto, S., Gokoh, M., Oka, S., Muramatsu, M., Kajiwara, T., Waku, K., and Sugiura, T. (2003) J. Biol. Chem. 27824469 –24475 [DOI] [PubMed] [Google Scholar]
- 41.Fleming, I., Rueben, A., Popp, R., Fisslthaler, B., Schrodt, S., Sander, A., Haendeler, J., Falck, J. R., Morisseau, C., Hammock, B. D., and Busse, R. (2007) Arterioscler. Thromb. Vasc. Biol. 272612 –2618 [DOI] [PubMed] [Google Scholar]
- 42.Zimmer, A., Zimmer, A. M., Hohmann, A. G., Herkenham, M., and Bonner, T. I. (1999) Proc. Natl. Acad. Sci. U. S. A. 965780 –5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Marzo, V., Fontana, A., Cadas, H., Schinelli, S., Cimino, G., Schwartz, J. C., and Piomelli, D. (1994) Nature 372686 –691 [DOI] [PubMed] [Google Scholar]
- 44.Zeldin, D. C., DuBois, R. N., Falck, J. R., and Capdevila, J. H. (1995) Arch. Biochem. Biophys. 32276 –86 [DOI] [PubMed] [Google Scholar]
- 45.Holla, V. R., Makita, K., Zaphiropoulos, P. G., and Capdevila, J. H. (1999) J. Clin. Invest. 104751 –760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capdevila, J. H., Falck, J. R., and Harris, R. C. (2000) J. Lipid Res. 41163 –181 [PubMed] [Google Scholar]
- 47.Karara, A., Dishman, E., Falck, J. R., and Capdevila, J. H. (1991) J. Biol. Chem. 2667561 –7569 [PubMed] [Google Scholar]
- 48.Dewey, W. L. (1986) Pharmacol. Rev. 38151 –178 [PubMed] [Google Scholar]
- 49.Howlett, A. C., Barth, F., Bonner, T. I., Cabral, G., Casellas, P., Devane, W. A., Felder, C. C., Herkenham, M., Mackie, K., Martin, B. R., Mechoulam, R., and Pertwee, R. G. (2002) Pharmacol. Rev. 54161 –202 [DOI] [PubMed] [Google Scholar]
- 50.Mackie, K. (2006) Annu. Rev. Pharmacol. Toxicol. 46101 –122 [DOI] [PubMed] [Google Scholar]
- 51.Devane, W. A., Dysarz, F. A., III, Johnson, M. R., Melvin, L. S., and Howlett, A. C. (1988) Mol. Pharmacol. 34605 –613 [PubMed] [Google Scholar]
- 52.Devane, W. A., Hanus, L., Breuer, A., Pertwee, R. G., Stevenson, L. A., Griffin, G., Gibson, D., Mandelbaum, A., Etinger, A., and Mechoulam, R. (1992) Science 2581946 –1949 [DOI] [PubMed] [Google Scholar]
- 53.Bisogno, T., Melck, D., Bobrov, M., Gretskaya, N. M., Bezuglov, V. V., De Petrocellis, L., and Di Marzo, V. (2000) Biochem. J. 351817 –824 [PMC free article] [PubMed] [Google Scholar]
- 54.Porter, A. C., Sauer, J. M., Knierman, M. D., Becker, G. W., Berna, M. J., Bao, J., Nomikos, G. G., Carter, P., Bymaster, F. P., Leese, A. B., and Felder, C. C. (2002) J. Pharmacol. Exp. Ther. 3011020 –1024 [DOI] [PubMed] [Google Scholar]
- 55.Begg, M., Pacher, P., Batkai, S., Osei-Hyiaman, D., Offertaler, L., Mo, F. M., Liu, J., and Kunos, G. (2005) Pharmacol. Ther. 106133 –145 [DOI] [PubMed] [Google Scholar]
- 56.Pacher, P., Batkai, S., and Kunos, G. (2006) Pharmacol. Rev. 58389 –462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mackie, K., and Stella, N. (2006) Am. Assoc. Pharm. Sci. J. 8E298 –E306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pagotto, U., Marsicano, G., Cota, D., Lutz, B., and Pasquali, R. (2006) Endocr. Rev. 2773 –100 [DOI] [PubMed] [Google Scholar]
- 59.Deutsch, D. G., Goligorsky, M. S., Schmid, P. C., Krebsbach, R. J., Schmid, H. H., Das, S. K., Dey, S. K., Arreaza, G., Thorup, C., Stefano, G., and Moore, L. C. (1997) J. Clin. Invest. 1001538 –1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugiura, T., Kodaka, T., Nakane, S., Kishimoto, S., Kondo, S., and Waku, K. (1998) Biochem. Biophys. Res. Commun. 243838 –843 [DOI] [PubMed] [Google Scholar]
- 61.Van Sickle, M. D., Duncan, M., Kingsley, P. J., Mouihate, A., Urbani, P., Mackie, K., Stella, N., Makriyannis, A., Piomelli, D., Davison, J. S., Marnett, L. J., Di Marzo, V., Pittman, Q. J., Patel, K. D., and Sharkey, K. A. (2005) Science 310329 –332 [DOI] [PubMed] [Google Scholar]
- 62.Idris, A. I., van't Hof, R. J., Greig, I. R., Ridge, S. A., Baker, D., Ross, R. A., and Ralston, S. H. (2005) Nat. Med. 11774 –779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Juan-Pico, P., Fuentes, E., Bermudez-Silva, F. J., Javier Diaz-Molina, F., Ripoll, C., Rodriguez de Fonseca, F., and Nadal, A. (2006) Cell Calcium 39155 –162 [DOI] [PubMed] [Google Scholar]
- 64.Piomelli, D. (2005) Curr. Opin. Investig. Drugs 6672 –679 [PubMed] [Google Scholar]
- 65.Di Marzo, V., and Petrocellis, L. D. (2006) Annu. Rev. Med. 57553 –574 [DOI] [PubMed] [Google Scholar]
- 66.Ben-Shabat, S., Fride, E., Sheskin, T., Tamiri, T., Rhee, M. H., Vogel, Z., Bisogno, T., De Petrocellis, L., Di Marzo, V., and Mechoulam, R. (1998) Eur. J. Pharmacol. 35323 –31 [DOI] [PubMed] [Google Scholar]
- 67.Coutts, A. A., and Izzo, A. A. (2004) Curr. Opin. Pharmacol. 4572 –579 [DOI] [PubMed] [Google Scholar]
- 68.Gauthier, K. M., Baewer, D. V., Hittner, S., Hillard, C. J., Nithipatikom, K., Reddy, D. S., Falck, J. R., and Campbell, W. B. (2005) Am. J. Physiol. 288H1344 –H1351 [DOI] [PubMed] [Google Scholar]
- 69.Fang, X., Hu, S., Xu, B., Snyder, G. D., Harmon, S., Yao, J., Liu, Y., Sangras, B., Falck, J. R., Weintraub, N. L., and Spector, A. A. (2006) Am. J. Physiol. 290H55 –H63 [DOI] [PubMed] [Google Scholar]
- 70.Hohmann, A. G., Suplita, R. L., Bolton, N. M., Neely, M. H., Fegley, D., Mangieri, R., Krey, J. F., Walker, J. M., Holmes, P. V., Crystal, J. D., Duranti, A., Tontini, A., Mor, M., Tarzia, G., and Piomelli, D. (2005) Nature 4351108 –1112 [DOI] [PubMed] [Google Scholar]