Abstract
Mutations in the late endosomal/lysosomal membrane protein Niemann-Pick C1 (NPC1) are known to cause a generalized block in retrograde vesicle-mediated transport, resulting in the hyper-accumulation of multiple lysosomal cargos. An important, yet often overlooked, category of lysosomal cargo includes the vast array of small molecular weight amine-containing molecules that are substrates for ion trapping in the highly acidic organelle lumen. We show here that the introduction of amine-containing molecules in lysosomes can significantly stimulate NPC1-mediated late endosome/lysosome fusion, and subsequently the secretion of lysosomal cargo. To illustrate the physiological importance of this NPC1-mediated transport pathway, we show that NPC1-deficient cells are more susceptible to the toxic effects of a lysosomotropic polyamine metabolite 3-aminopropanal. Moreover, NPC fibroblasts are shown to have higher levels of polyamine oxidase, an enzyme involved in the formation of 3-aminopropanal. Collectively, these findings provide strong support for a novel functional role for NPC1 and may also provide clues toward understanding NPC disease progression.
Mutations in Niemann-Pick C1 (NPC1),2 a membrane protein associated with late endosomes/lysosomes, result in a fatal neurodegenerative lysosomal storage disorder referred to as Niemann-Pick type C (NPC) disease (1, 2). The early work of Pentchev and others showed that NPC-diseased cells are laden with LDL-derived free unesterified cholesterol and other lipids in their lysosomes, which was thought to be the direct cause of NPC disease pathology (2–4). Therapeutic interventions aimed at limiting the biosynthesis and/or uptake of cholesterol and other lipids have been quite effective in reducing the hyper-accumulation phenotype; however, such approaches have not been successful in significantly decreasing NPC disease progression in various human and animal disease models (5–8). Accordingly, the true functional role of NPC1 has been controversial and is, perhaps, unknown (9).
It is clear that NPC1 is required for the trafficking of LDL-derived cholesterol from lysosomes; however, NPC1 is also known to be required for the trafficking of multiple cargos from lysosomes (10). Is there a “primary cargo” that regulates this NPC1-mediated lysosomal egress pathway? To address this, we considered other molecules that accumulate in lysosomes but do not have an established pathway for their removal. Cells are known to be continuously exposed to very high concentrations of endogenous biogenic amines, polyamines, and their metabolites (11, 12). Despite the fact that these molecules are not fluorescent, making it difficult to analyze their intracellular localization, a number of reports have suggested that these molecules are extensively compartmentalized in lysosomes (13–15). The propensity for weakly basic amine-containing molecules to be sequestered into acidic organelles of cells, principally lysosomes, has long been observed and theoretically understood (16–18). The basic substrates are referred to as lysosomotropics. The driving force for accumulation relies on the magnitude of the pH gradient separating the lysosomal lumen (∼pH 4.5) and the cell cytosol (∼pH 7.0). Amine-containing molecules, depending on their pKa, can be significantly unionized and membrane-permeable in the cytosol and readily cross lipid bilayers of organelles. When such compounds enter the acidic environment of lysosomes, they experience a dramatic change in their ionization state and can now exist virtually 100% in their ionized and membrane-impermeable form, which severely impairs their rate of diffusion back out. The lysosomal proton concentration is kept high through the action of the vacuolar H+ ATPase, thus allowing excessive accumulation of the amines (19). Some lysosomotropic agents when incubated with cells have been shown to induce massive vacuolization of lysosomes (20–22). Their subsequent clearance and the loss of the vacuolar appearance have been observed, but the mechanistic basis for this pathway has not been previously understood (18).
There are three separate lines of evidence that support a potential functional role for NPC1 in the regulation of endogenous amines in lysosomes: 1) We and others have shown that NPC1 is required for efficient clearance of amines from lysosomes (23, 24); 2) endogenous hydrophobic amines have been shown to be over 20-fold elevated in livers of mice with NPC disease compared with control mice and were shown to be concentrated in lysosomes (25); and 3) alterations in endogenous polyamine levels and/or their metabolizing enzymes have been associated with neurodegenerative disorders similar to Alzheimer and NPC disease (26–28).
The rational development of successful therapeutic strategies for treating NPC disease have, for the most part, failed due to the lack of understanding of the function of NPC1. We show here that the formation of late endosome/lysosome hybrid organelles and the subsequent egress of lysosomal cargo are significantly enhanced when cells are exposed to certain lysosomotropic amines. Moreover, cells deficient in NPC1 are shown to be highly sensitive to the toxic effects of an endogenous polyamine metabolite, which illustrates the significance of the function. Collectively, our findings support a role for NPC1 in regulating lysosomal amine concentrations and should help to understand NPC disease pathology.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents—Anti-EEA1 (early endosomes), anti-golgin-84, and anti-GM130 (Golgi apparatus) mouse monoclonal antibodies were from BD Biosciences (San Jose, CA). Mouse monoclonal anti-mannose 6-phosphate receptor (MPR, late endosomes) was obtained from Abcam (Cambridge, MA). Mouse monoclonal anti-LAMP-1 (lysosomes) was from Developmental Studies Hybridoma (Iowa City, IA). Rabbit polyclonal anti-NPC1 was obtained from Novus Biologicals (Littleton, CO). Goat polyclonal anti-polyamine oxidase was purchased through Santa Cruz Biotechnology (Santa Cruz, CA). LysoTracker Red DND-99 (LTR), Alexa Fluor 647 goat anti-mouse and anti-rabbit secondary antibodies, biotinylated dextran amine, and Alexa Fluor 555 and 647 succinimidyl esters were obtained from Invitrogen. Carboxylate-modified polystyrene beads were purchased from Polysciences (Warrington, PA). 3-Aminopropanal diethyl acetal was obtained from TCI America (Portland, OR). All other materials, unless otherwise stated, were acquired from Sigma-Aldrich.
Cell Culture and Conditions—Normal human fibroblasts (CRL-2076, designated NPC1+/+) were cultured according to suggestions from Coriell Cell Repository (Camden, NJ). NPC disease fibroblasts with mutated and dysfunctional NPC1 protein (GM-03123, designated NPC1-/-), mucolipidosis type IV (GM-02408, designated MLIV), and Sandhoff disease (GM-11707) fibroblasts were all cultured according to ATCC (Manassas, VA) instructions. All media was supplemented with fetal bovine serum and penicillin/streptomycin. Cultures were maintained at 37 °C in a humidified 5% carbon dioxide atmosphere. For all experiments, cells were plated 24 h prior to evaluations to allow for adherence.
Amine-induced Vacuole Size Determinations—A confocal microscopy method was developed to obtain average diameters of NR-containing compartments. Briefly, cells were treated with 50 μm nocodazole (NOC) for 1 h, washed with PBS (3×), and then exposed to 70 μm neutral red (NR) in culture medium for 6 h. Cells without NOC pretreatment were included as positive controls for vacuolization. Cells were viewed with a Zeiss Meta 510 confocal microscope equipped with an argon laser set at 458 nm. Images were captured with a Hamamatsu Orca ER camera. For size determination, NR-containing compartments were measured using MetaMorph software version 7.0 from Universal Imaging Corp. (Downington, PA). The diameters of 150 NR-containing vesicles from three separate experiments were averaged.
Silencing of NPC1 Expression Using siRNA—An siRNA construct was utilized to silence NPC1 expression as previously reported (29). Nonspecific scrambled siRNA (Ambion, Austin, T×) was used as a negative control under identical transfection conditions per the manufacturer's suggestions. All siRNA transfections were performed using siPORT-Amine (Ambion) transfection reagent for 72 h. To confirm NPC1 knockdown, cells were harvested post-transfection, and lysates were subjected to Western blot analysis with subsequent densitometry performed using ImageJ software. For siRNA experiments requiring subsequent amine exposure, transfections were performed as described, at which point cells were exposed to 70 μm NR or 100 μm chloroquine for 6 h.
Immunofluorescence—Fibroblasts were fixed using freshly prepared 4% paraformaldehyde in PBS for 10 min and permeabilized by incubating cells with PBS containing 0.1% saponin and 10% fetal bovine serum for 30 min at room temperature. Primary and secondary antibody solutions were made in 0.05% saponin and 10% fetal bovine serum in PBS. Cells were incubated with primary antibodies for 2 h using the following dilutions: EEA1 (1:50), MPR (1:50), LAMP-1 (1:100), NPC1 (1:100), and GM130 (1:100). Cells were subsequently washed with PBS (3×), and secondary antibodies (Alexa Fluor 647-conjugated anti-mouse or rabbit IgG, 1:1000) were applied for 2 h. Cells were washed again with PBS (4×) prior to microscopy with standard epifluorescence optics (Nikon).
Isolation and Characterization of NR-containing Lysosomes— Lysosomes were isolated using a previously described magnetic chromatography approach. The isolation procedure, as well as control experiments, was performed as previously described with minor modifications to allow for localization of the super-paramagnetic iron dextran in lysosomes of amine-treated cells (30). To confirm that the iron dextran was localizing in amine-containing compartments, cells were incubated with 2 mg/ml Alexa Fluor 647 dextran in PBS for 2 h, washed with PBS (4×), and subsequently given chase in complete dextran-free medium for 20 h at 37 °C. Cells were then exposed to 100 μm CQ for 6 h, and phase-contrast and fluorescence images were taken.
DNA Transfections—NPC1-GFP and Rab9-YFP plasmid DNA were kindly provided by Dr. Suzanne Pfeffer and Dr. Matthew Scott, respectively (Stanford University School of Medicine, Stanford, CA). Transfections were performed using FuGene6 (Roche Applied Science) per the manufacturer's suggestions. Transfections were carried out for 48 h to allow adequate protein expression. To assess the effects of amine exposure on single and dual transfected cells, 100 μm chloroquine was added post-transfection to the culture medium for 6 h. Microscopy was then performed using standard epifluorescence optics (Nikon).
Sucrosome Formation—Sucrosomes were formed by culturing cells in complete medium containing 0.1% sucrose for 48 h.
Evaluating Amine Secretion from Cells—Quantitative and qualitative methods were developed to determine secretion of amines from cells. First, fibroblasts were exposed to either 70 μm NR for 6 h or 2 μm LTR for 1 h. After incubation, cells were washed with warm PBS (3×), and complete medium was replaced. For LTR, cells were imaged as a function of time using fluorescence microscopy under identical microscope settings for each time point. For NR, monolayers were washed with PBS (3×), and NR was extracted using a solution containing 49% methanol and 1% acetic acid. Samples were analyzed by spectrophotometry measuring absorbance at 540 nm using a 96-well plate reader.
Dextran Secretion Assay—Cellular release of [3H]dextran (70,000 molecular weight, 125 μCi/mg, American Radiolabeled Chemicals, St. Louis, MO) into culture medium, as well as controls, was performed as described by Gong et al. (23). For assays that included amine treatments, cells were incubated with amines for 3 h in complete medium at the end of the [3H]dextran chase, washed with warm PBS (2×), and medium replaced. Detection of [3H]dextran began immediately after replacement of the medium as described. The cumulative amount of dextran released into the medium is reported as a percentage of the total amount of dextran endocytosed.
Late Endosome/Lysosome Fusion Assay—The fusion between late endosomes and lysosomes was evaluated in fibroblasts using a method described by Jahrus et al. (31) with modifications to allow quantitative assessments based on fluorescence resonance energy transfer (FRET) of late endosome/lysosome contents upon fusion. To accomplish this, biotinylated dextran, fluorescently labeled with Alexa Fluor 647 (FRET acceptor), was localized to lysosomes using the above optimized pulse/chase scheme for dextran. Next, streptavidin-conjugated latex beads (0.792 ± 0.037 μm), fluorescently labeled with Alexa Fluor 555 (FRET donor), were localized to late endosomes per Jahrus et al. (31). Amine treatments were performed after localization of the latex bead to the late endosome. Intracellular localizations of dextran and latex beads were confirmed by immunofluorescence (data not shown). To detect fusion, FRET measurements were made using a Photon Technologies International (Birmingham, NJ) Ratiomaster microscope-mounted spectrofluorometer with PMT detection. Complete details on tracer construction, measurements, and analysis can be found in the supplemental “Experimental Procedures.”
3-Aminopropanal Synthesis and Toxicity Studies—3-Aminopropanal (3-AP) was prepared, as previously described, from the hydrolysis of 3-aminopropanal diethyl acetal (32). The reactant mixture was applied to a strong cation exchange resin (Dowex 50 × 2, H+ form) and eluted using a 0–3 m HCl step gradient. Fractions were collected, and aldehyde content was assayed by the method of Bachrach and Reches (33) using a standard curve constructed of propionaldehyde. For cytotoxicity studies, fractions of 3-AP were neutralized to physiological pH before use. Cell viability was assessed after 12 h of 3-AP exposure using the Quick Cell Proliferation WST-1 assay from BioVision (Mountain View, CA). To determine the effects of 3-AP exposure on lysosomal membrane integrity, cellular cytosol was purified as described previously by our laboratory (34). Fractions of purified cytosol were assayed for β-hexosaminidase activity, a lysosomal enzyme, as described by Duvvuri et al. (19).
RESULTS
Amine-induced Vacuolization of Lysosomes Requires Functional NPC1—When cells grown in culture are exposed to high concentrations of lysosomotropic amines, their lysosomes typically become vacuolated (20, 22). The vacuolar appearance rapidly disappears when the amines are removed from the cell culture medium, which suggests that the amines are efficiently cleared (18). The mechanism for release is not clearly understood, but it has been shown to require intact microtubules and its efficiency differed between cell types (22, 23).
If lysosomes were to rupture, the release of enzymes (i.e. cathepsins) into the cell cytosol would potentially initiate apoptosis (35). When amines accumulate in the lysosome, a significant osmotic imbalance can result causing water to enter the organelle and exert pressure on the organelle lipid bilayer. Accordingly, the vacuolization would serve to increase the organelle volume, decrease amine concentration, and help relieve pressure to avoid organelle rupture.
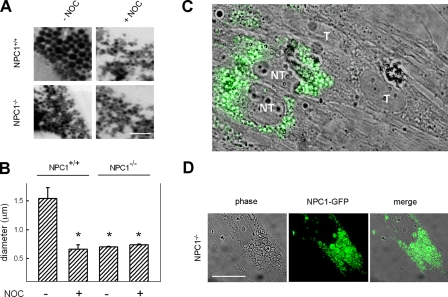
We examined if NPC1 was involved in the amine-induced vacuolization of lysosomes. Human foreskin fibroblasts (normal or with a mutated and dysfunctional NPC1, represented by NPC1+/+ and NPC1-/-, respectively) were incubated with the lysosomotropic amine NR, and high resolution images of cells were taken with a confocal microscope to examine the average diameter of NR-containing compartments. Normal cells exposed to NR had an approximate vacuole size of 1.5 μm (Fig. 1, A and B). NPC1-/- cells exposed to the amine had NR-containing compartments approximately one-half the size of those from NPC1+/+ cells. NPC1+/+ cells treated with nocodazole also had small NR-containing vacuoles. This illustrates the involvement of microtubules in vacuolization, suggesting that the membrane is not obtained, at least significantly, from internal sources such as multivesicular bodies. To more specifically illustrate the involvement of NPC1 in amine-induced vacuolization, we utilized a small interfering RNA (siRNA) designed to transiently suppress the expression of NPC1. When performing the siRNA transfections, we were sometimes able to view, on a single image, cells that were not transfected (i.e. had normal NPC1 expression) alongside those that were efficiently transfected and had depleted NPC1. Immunofluorescence staining of NPC1 allowed us to demonstrate that only cells expressing NPC1 formed amine-induced vacuoles (Fig. 1C). Conversely, cells that did not have visible amounts of NPC1 did not form the amine-induced vacuoles. We were also able to rescue the amine-induced vacuolization phenotype in NPC1-/- fibroblasts by transfecting them with a functional NPC1-GFP construct (Fig. 1D). Collectively, these results illustrate the requirement for NPC1 in the amine-induced vacuolization of lysosomes.
FIGURE 1.
Amine-induced vacuolization requires functional NPC1 and an intact microtubule network. A, NPC1+/+ and NPC1-/- fibroblasts incubated with NR and imaged using confocal microscopy. NR causes vacuolization in NPC1+/+ fibroblasts but not in NPC1-/- cells. Nocodazole (NOC) pretreatment disrupts the NPC1-dependent vacuolization process. Scale bars represent 5 μm. B, quantitative analysis of average amine-induced vacuole diameters. NR-containing compartments in normal cells are approximate twice the size of NR compartments in NPC1-/- fibroblasts or from fibroblasts pretreated with NOC (*, p < 0.01 by unpaired t test). C, combined phase contrast and NPC1 immunofluorescence (green) images of NPC1+/+ fibroblasts pretreated with NPC1 siRNA and subsequently incubated with 100 μm chloroquine (CQ) to induce vacuolization. Cells that did not contain NPC1 (i.e. efficiently transfected, T) did not form visible vacuoles. Conversely, cells inefficiently transfected (NT) contained NPC1 and were able to form amine-induced vacuoles. Scale bars represent 10 μm. D, the amine-induced vacuolization phenotype is rescued in NPC1-/- cells by transfection with a functional NPC1-GFP construct. Cells were transfected with a NPC1-GFP plasmid and subsequently exposed to 100 μm CQ to form vacuoles. Scale bars represent 10 μm.
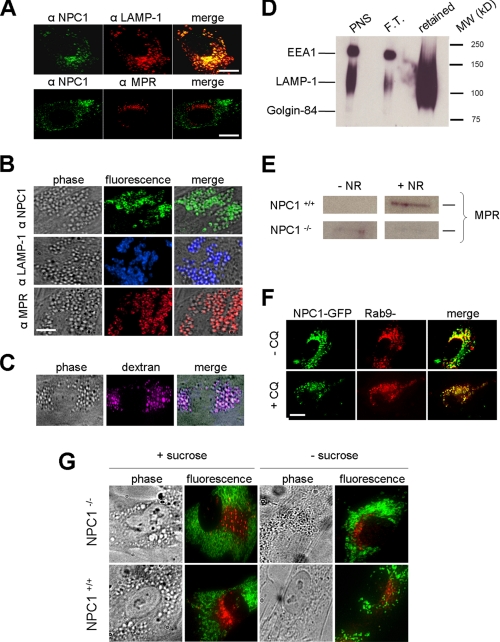
Amine-induced Vacuoles Are Formed through a Heterotypic Fusion of Lysosomes with Late Endosomes—When considering vacuolization, it is important to realize that amine-induced vacuolization requires the recruitment of membranes to allow lipid bilayers to expand (36). This is consistent with our previous results that illustrated the requirement of intact microtubules in this process (Fig. 1, A and B). Accordingly, we examined if amines facilitated the fusion of lysosomes with other organelles or vesicles. Immunofluorescence analysis of NPC1+/+ fibroblasts demonstrated that without amine treatment NPC1 is shown to reside predominantly on a subset of a lysosome-specific membrane protein (LAMP-1)-positive lysosomes and not to a significant extent on late endosomes containing the cation-independent mannose 6-phosphate receptor (MPR) (Fig. 2A). However, when NPC1+/+ cells were treated with NR the resultant vacuoles contained LAMP-1, MPR, and the NPC1 protein (Fig. 2B). The vacuoles did not colocalize with a Golgi-specific protein GM130 or with an early endosome specific protein EEA1 (supplemental Fig. S1). Terminal endocytic compartments (vacuoles) were purified from fibroblasts using a previously described magnetic chromatographic approach that utilizes endocytosed iron-coated dextran (10,000 molecular weight) (19). A fluorescence-labeled dextran of the same size was incubated with cells to establish optimal pulse-chase parameters to ensure localization with amine-induced vacuoles (Fig. 2C). The isolated compartment was highly enriched in the lysosomal protein LAMP-1 and was not significantly contaminated with other compartments such as the Golgi or early endosomes (Fig. 2D). The isolated compartment was analyzed for the presence of MPR, which was shown to become enriched only in NPC1+/+ and not in NPC1-/- fibroblasts that were treated with amines prior to the isolation (Fig. 2E). To confirm the involvement of late endosomes in the vacuolization, NPC1+/+ fibroblasts were co-transfected with Rab9-YFP, a late endosome-specific protein, and with NPC1-GFP. Consistent with prior results, colocalization of these proteins occurred extensively on amine-induced vacuoles, yet a much smaller degree of colocalization was observed in cells not incubated with the amine (Fig. 2F).
FIGURE 2.
Amine-induced vacuoles are created through a heterotypic fusion of lysosomes with late endosomes. A, immunofluorescence images of NPC1+/+ cells showing the localization of NPC1 (without amine treatment). NPC1 (green) does not significantly colocalize with the late endosome-specific mannose 6-phosphate receptor (MPR, red) and does colocalize with the lysosome-associated membrane protein-1 (LAMP-1, red). B, immunofluorescence and phase contrast images of NPC1+/+ cells incubated with NR to induce vacuolization. NPC1, LAMP-1, and MPR are all localized to the membrane of the newly formed vacuoles. C, fluorescence-labeled dextran macromolecules specifically colocalize with amine-induced vacuoles in normal fibroblasts following a pulse-chase protocol (see “Experimental Procedures”). D, Western blot analysis of organelle-specific proteins contained in the purification of terminal endocytic compartments (see “Experimental Procedures”). Aliquots of the post nuclear supernatant (PNS), the material flowing through the magnetic column (flow through, F.T.), and the fraction retained on the column (retained) were all analyzed for the Golgi-specific protein Golgin-84, the lysosome-specific protein LAMP-1, and the early endosome antigen 1 (EEA1). E, Western blot analysis of MPR in purified terminal endocytic compartments before and after amine (NR) treatments. NPC1+/+ fibroblasts become enriched in MPR after amine treatment, which does not occur with NPC1-/- fibroblasts. F, fluorescence images of NPC1+/+ fibroblasts transfected with NPC1-GFP (green) and with the late endosome-specific Rab9-YFP (red). An increase NPC1/Rab9 colocalization is observed following treatment with the amine CQ. G, vacuolization of lysosomes with sucrose occurs regardless of NPC1 functional status and does not involve fusion with late endosomes. Phase contrast images show that the addition of 0.1% sucrose induced vacuolization (sucrosomes formation) in both NPC1+/+ and NPC1-/- fibroblasts. Immunofluorescence analysis of LAMP-1 (green) and MPR (red) reveals that they do not colocalize with or without sucrose. Scale bars represent 10 μm.
We next evaluated whether NPC1 was required for any vacuolization event involving lysosomes or if it was specific to vacuolization induced by amines. It is well known that lysosomes can be vacuolated by incubating cells in high concentrations of sucrose (37). The resultant vacuoles are referred to as sucrosomes. Interestingly, the formation of sucrosomes occurred regardless of NPC1 functional status (Fig. 2G). This result suggests that this vacuolization occurs by a different mechanism, independent of NPC1. Moreover, formation of sucrosomes did not appear to involve fusion of late endosomes with lysosomes since MPR and LAMP-1 did not significantly colocalize (Fig. 2G).
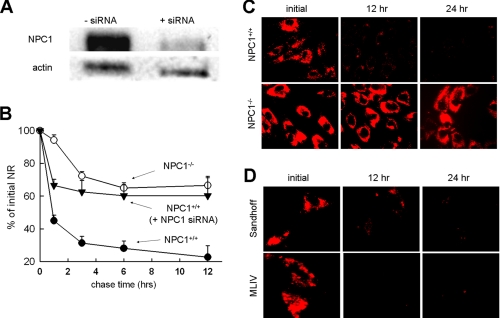
NPC1 Is Required for the Efficient Clearance of Amines Trapped in Lysosomes—We and others have previously shown that the release of protonated amines or other membrane-impermeable small molecules such as sucrose from lysosomes is significantly impeded in NPC-diseased fibroblasts relative to normal cells (10, 23, 24). The use of fibroblasts from NPC-diseased patients to study the function of NPC1 is routinely done but could yield misleading results considering the multiple compensatory pathways that could be established. To more specifically investigate the involvement of NPC1, we evaluated the trafficking of amines in lysosomes from cells treated with siRNA to transiently deplete it. NPC1 siRNA treatment conditions were optimized to enable efficient suppression of NPC1 expression (Fig. 3A). The release of two well known lysosomotropic amines, NR and LTR, was evaluated. Using a quantitative release assay, we showed that the release of NR was efficient in normal fibroblasts compared with NPC1 siRNA-treated normal fibroblasts (Fig. 3B). The NR release profile from NPC1-/- fibroblasts was similar to the profile obtained from NPC1 siRNA-treated cells. The release of the lysosomal vital stain LTR from NPC1+/+ and NPC1-/- cells, evaluated using fluorescence microscopy, provides visual evidence for the differences in release (Fig. 3C).
FIGURE 3.
NPC1 is required for the efficient clearance of amines that accumulate in lysosomes. A, Western blot analysis of NPC1 expression in normal fibroblasts before and after transfection with NPC1 siRNA. Actin expression was used as a sample loading control. B, quantitative evaluation of NR release shows NPC1+/+ fibroblasts (○) clear NR more efficiently than NPC1-/- fibroblasts (•) or NPC1+/+ fibroblasts pretreated with NPC1 siRNA (▾). Each time point represents the mean ± S.D. of 3–10 individual measurements. C, fluorescence micrographs of the lysosomal vital stain LTR showing the enhanced clearance in NPC1+/+ fibroblasts relative to the NPC1-/- fibroblasts. D, lysosomal clearance of LTR is not impaired in lysosomal storage disorder fibroblasts mucolipidosis type IV (MLIV) and Sandhoff disease. All LTR images are representative of data collected from at least three independent experiments.
It has been previously shown that the hyper-accumulation of lipids in late endosomes/lysosomes can impede their ability to fuse (38). Considering this, it is possible that the decreased cellular clearance of amines observed with the NPC1-deficient cell lines could be secondary to the lipid accumulation and not directly related to the inactivity of NPC1. To examine this possibility, we visually examined the clearance of LTR in two additional fibroblasts obtained from patients with other lysosomal lipid storage disorders, including mucolipdosis IV (MLIV) and Sandhoff disease. MLIV fibroblasts have defective mucolipin, a protein involved in the subcellular transport of sphingolipids, phospholipids, and acid mucopolysaccharides (39). Sandhoff disease is characterized by hyper-accumulation of gangliosides (40). LTR was efficiently cleared from both of these cell lines using the same conditions in which LTR was not cleared in NPC1-/- fibroblasts (Fig. 3D). Because both of these cell lines have functional NPC1, these results further support the specific involvement of NPC1 in the efficient efflux of amines from lysosomes.
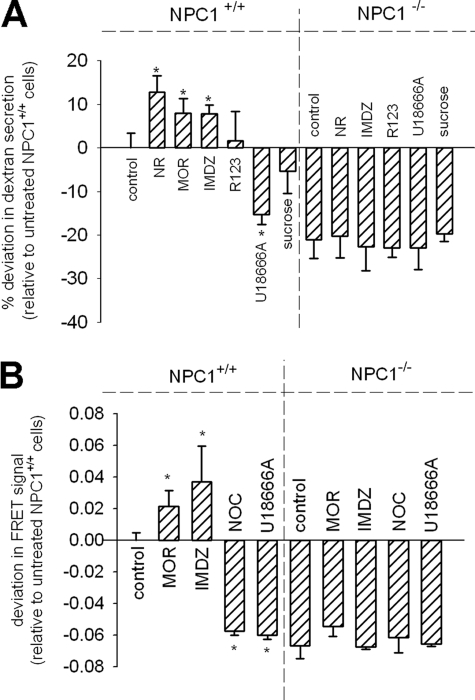
Lysosomotropic Amines Stimulate NPC1-mediated Secretion of Lysosomal Cargo—The evaluations presented in the previous section support the notion NPC1 plays a role in the egress of protonated amines from lysosomes (Fig. 3B). In this work we propose that NPC1 plays an important functional role in regulating the concentrations of amines that are sequestered in lysosomes. If this is the case, NPC1 should “sense” the accumulation of amines and subsequently facilitate their clearance. To evaluate this we examined the influence of exogenously supplied amines on the release of a different, membrane-impermeable, cargo from lysosomes. Here we evaluated the influence of amines on the NPC1-mediated lysosomal egress of 10,000 molecular weight dextran polymers. We specifically localized [3H]dextran to terminal endocytic compartments using the previously described pulse-chase protocol used for fluorescence-labeled dextran (Fig. 2C) and evaluated its release into the cell culture medium as a function of time in both normal and NPC1-/- fibroblasts (see supplemental Fig. S2 for representative plots of the release profiles). The amount of dextran released at the 24-h time point was found to be suitable for representing differences between normal and NPC cells; therefore, this single time point measurement was obtained for all subsequent experiments evaluating the influence of amines and other molecules on dextran release. For comparison purposes, the amount of dextran released at the 24-h time point for the untreated NPC1+/+ fibroblasts was set to zero (Fig. 4A). Accordingly, molecules having a stimulatory effect on dextran release will have positive deviations from control. Alternatively, those treatments that inhibit dextran secretion relative to control will have negative values. Three lysosomotropic amines, NR, imidazole (IMDZ), and morpholine (MOR), had the unique ability to significantly stimulate the release of dextran from lysosomes of normal fibroblasts (Fig. 4A). It is important to note that not every amine will induce secretion. For example, rhodamine 123, a basic amine that is known to accumulate into mitochondria instead of lysosomes (41), did not induce nor inhibit NPC1-mediated secretion (Fig. 4A). Somewhat paradoxically, it is well known that certain amphiphilic hydrophobic amines, instead of having a stimulatory role, cause lysosomal trafficking defects (42). The best known inducer of the NPC disease phenotype is the basic molecule U18666A. As anticipated, U18666A had a negative influence on NPC1-mediated dextran secretion in normal fibroblasts (Fig. 4A). It is likely that such amines elicit an inhibitory function unrelated to the propensity of the molecules for lysosomal sequestration (see “Discussion”). We also examined the effect of the formation of vacuoles with sucrose on dextran secretion and found no statistically significant change (Fig. 4A). As expected, the release of dextran from NPC1-/- fibroblasts was less than that from normal untreated fibroblasts and similar to that from U18666A-treated normal fibroblasts (Fig. 4A). Importantly, the amines found to have stimulatory effects of NPC1-mediated lysosomal secretion from normal cells had no significant impact on the dextran release from NPC1-/- cells, which illustrates the specificity of the amines for NPC1. Considering the relatively short incubation time with these reagents (6 h), up-regulation of NPC1 should not have had time to occur, which was confirmed by Western blot analysis (data not shown).
FIGURE 4.
Lysosomotropic amines can stimulate the function of NPC1 in the clearance of lysosomal contents and late endosome/lysosome hybrid organelle formation. A, cumulative lysosomal-dextran secreted into the cell culture medium after 24 h in both NPC1+/+ and NPC1-/- fibroblasts pretreated with indicated molecules. Bars represent average ± S.D. for the percent deviation in dextran amount released relative to untreated NPC1+/+ fibroblasts (value set to zero). Positive differences in dextran release were observed for the lysosomotropic amines neutral red (70 μm, NR, n = 12), morpholine (10 mm, MOR, n = 8), and imidazole (10 mm, IMDZ, n = 7) in NPC1+/+ fibroblasts relative to the untreated control (*, p < 0.001 by unpaired t test). Statistical differences were not observed in NPC1-/- cells treated with the same compounds. Amine-containing molecules that are known not to accumulate in the lysosome, such as rhodamine-123 (R123, 70 μm, n = 7), had no effect on dextran release. U18666A (10 μm, n = 7) inhibited NPC1 function in dextran secretion in normal cells and had no influence on NPC cells. The formation of sucrosomes with sucrose (0.1% w/v, n = 4) had no statistically significant influence of dextran secretion. B, the degree of late endosome/lysosome hybrid organelle formation in NPC1+/+ and NPC1-/- fibroblasts pretreated with indicated molecules. Bars represent average ± S.D. for the percent deviation in FRET signal relative to untreated NPC1+/+ fibroblasts (value set to zero). Positive deviations in hybrid organelle formation (relative to untreated normal fibroblasts) were observed for MOR (5 mm, n = 3) and IMDZ (10 mm, n = 3) with NPC1+/+ fibroblasts. Treatments with U18666A (10 μm, n = 3) and nocodazole (50 μm, n = 3) decreased late endosome/lysosome fusion relative to control using NPC1+/+ fibroblasts (*, p < 0.01 by unpaired t test). All treatments using NPC1-/- fibroblasts (n = 3 for each) showed no differences in FRET signal in comparison to untreated NPC1-/- fibroblasts.
These results demonstrate that select lysosomotropic amines can stimulate the NPC1-mediated release of lysosomal cargo to the plasma membrane. There are at least two different pathways that lysosomal contents (i.e. dextran) could be trafficked to the cell surface, direct fusion with the plasma membrane and retrograde transport through the endocytic pathway. Previous studies by Reddy and colleagues had shown that stimulation of lysosome secretion in membrane resealing was mediated by cytosolic elevations of Ca2+ that interact with lysosomal synaptotagmin VII (43). To determine if amines could facilitate a direct fusion of lysosomes with the plasma membrane through a Ca2+-mediated pathway, we examined if the addition of lysosomotropic amines caused elevations in cytosolic Ca2+ levels; however, none of the stimulatory amines were able to significantly change cytosolic Ca2+ levels (data not shown). Some models of lysosome membrane dynamics suggest that lysosomes continually fuse with late endosomes to form a hybrid organelle (44). Ko et al. (45) have suggested that NPC1 might facilitate the release of lysosomal cargo from a late endocytic hybrid organelle. Therefore, we investigated if the stimulatory amines had an impact on the creation of a lysosome/late endosome hybrid organelle. To evaluate this fusion event, we utilized a concept for studying late endosome/lysosome fusion originally described by Jahrus et al. (31). The approach involved the localization of specific tracers to each of these compartments and observed evidence of their meeting. First, terminal lysosomes were loaded with sucrose to form sucrosomes. Next, late endosomes were specifically populated with endocytosed latex beads (∼800 nm diameter) that were covalently coupled with invertase. The beads have been shown not to reach terminal lysosomes due to an apparent size restriction in this transport step (46). The fusion of late endosomes with lysosomes was observed by the disappearance of sucrosomes as sucrose was enzymatically converted into fructose and glucose. In our modified method we utilized FRET to quantitatively evaluate the fusion event in real time (see supplemental Fig. S3). This was accomplished by endocytosing a biotinylated dextran (10,000 molecular weight), covalently labeled with the FRET acceptor Alexa Fluor 647, into lysosomes using the same pulse-chase scheme as described previously (Fig. 2C). Subsequently, a streptavidin-conjugated latex bead (∼800 nm diameter) tagged with the FRET donor Alexa Fluor 555 was added to cells to label late endosomes. We evaluated the FRET signal in untreated normal fibroblasts at 2 h and set this value to zero for comparison purposes, as was done in Fig. 4A. The lysosomotropic amines IMDZ and MOR stimulated retrograde fusion in normal cells and had no influence on NPC1-/- fibroblasts (Fig. 4B). U18666A-treated normal fibroblasts had hybrid organelle formation rates that were similar to those of NPC1-/- fibroblasts, which showed minimal hybrid organelle formation regardless of treatment (Fig. 5B). The requirement of microtubules in this fusion step was shown by the decrease in FRET signal with nocodazole-treated NPC1+/+ fibroblasts (Fig. 4B). It was not possible to utilize rhodamine 123 or NR, as was done in dextran release studies (Fig. 4A), because their intrinsic fluorescence interfered with the spectral properties of Alexa Fluor 555.
FIGURE 5.
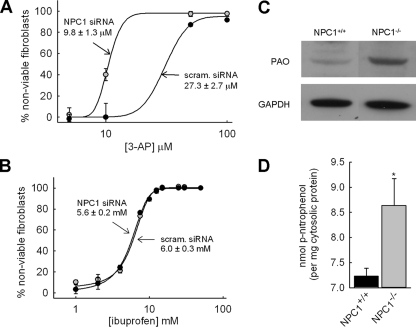
NPC1-deficient cells are more susceptible to the toxic effects of the lysosomotropic aldehyde and polyamine metabolite 3-AP. A, NPC1+/+ fibroblasts exposed to 3-AP were used in comparative cytotoxicity evaluations upon knockdown of NPC1 expression using siRNA. NPC1 depleted cells (•) exposed to 3-AP were found to have an IC50 of 9.8 ± 1.3 μm, whereas cells transfected with a nonspecific scrambled control siRNA (•) had an IC50 value of 27.3 ± 2.7 μm. Each point represents average ± S.D. from three independent experiments. B, ibuprofen (a non-lysosomotropic drug) had no preferential toxicity toward NPC1-deficient fibroblasts relative to normal fibroblasts. Data points represent averages ± S.D. from three independent experiments. C, polyamine oxidase, the enzyme responsible for the metabolism of spermine to spermidine and 3-AP, and spermidine to putrescine and 3-AP, is differentially expressed in fibroblasts with dysfunctional NPC1 as shown by Western blot analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was included for protein loading comparisons. D, β-hexosaminidase activity, a lysosomal enzyme, is greater in isolated cytosol from 3-AP-treated NPC cells (gray bar) relative to that from 3-AP-treated normal fibroblasts (black bar). Cells were treated with 100 μm 3-AP for 12 h prior to analysis. Bars represent average ± S.D. from five independent experiments (*, p < 0.01 by unpaired t test).
Collectively, these studies illustrate that select lysosomotropic amines can stimulate the release of lysosomal cargo through a NPC1-dependent vesicle-mediated pathway. Our results are consistent with this occurring through the formation of a hybrid lysosome/late endosome organelle. It is possible that vesicles containing lysosomal cargo bud off from this hybrid organelle and fuse with the plasma membrane for release (see “Discussion”).
NPC Cells Are More Susceptible to 3-AP-induced Toxicity Than Are Normal Cells—Thus far we have presented findings that support a role for NPC1 in sensing and regulating lysosomal amine content. Why would uncontrolled compartmentalization of amines in lysosomes be a concern? Theoretically, there are many possible reasons. First, a vast array of endogenous small molecular weight compounds exist in cells, the most ubiquitous are polyamines (i.e. spermine, spermidine, putrescine, and cadaverine), sphingoid bases, ammonia, and other biogenic amines. Many of these amines are found in high concentrations in cells (47) and have physicochemical properties making them ideal substrates for lysosomal sequestration (12). Sequestration of amines without an efficient clearance pathway could have several negative consequences on lysosomes, including osmotic rupture, pH imbalances, and destabilization of the membrane.
In work presented here, we have focused on polyamines. The precise biological function of polyamines is controversial; however, it is known that changes in their levels and/or their biosynthetic enzymes have been associated with neurodegenerative-type diseases (26). Specifically, polyamine levels have been shown to be significantly altered in Alzheimer disease (28). Polyamines have been speculated to play a role in neurodegeneration through several possible pathways. Here, we specifically examined the comparative toxicity of 3-AP in normal and NPC1-depleted fibroblasts. 3-AP is formed endogenously from the metabolism of spermidine to putrescine by the enzyme polyamine oxidase. This compound was selected because it has been shown to be specifically toxic to lysosomes through its ability to facilitate membrane rupture, and there have been numerous reports on its neurotoxic effects (48, 49). Consistent with the NPC disease pathology, 3-AP has been shown to be particularly toxic to neuronal cells relative to other cells for unknown reasons (50). In cells with depleted NPC1 protein we reasoned that the 3-AP should have more contact time with lysosomes and therefore an increased propensity to exert toxic effects relative to normal cells. To test this hypothesis 3-AP was synthesized and exposed to fibroblasts transiently transfected with NPC1 siRNA and compared with those cells treated with a nonspecific scrambled siRNA (Fig. 5A). As expected, NPC1-depleted cells were more susceptible to the toxic effects of 3-AP than were cells expressing NPC1. As a control, NPC1 depletion had no influence on the IC50 of a non-lysosomotropic drug ibuprofen (Fig. 5B).
Because polyamine oxidase is the enzyme responsible for the generation of 3-AP in cells, we decided to comparatively examine its expression in NPC1-/- versus NPC1+/+ cells and found it to be significantly elevated in the diseased cell line (Fig. 5C). The enhanced levels of lysosomal enzymes in the cell cytosol are often indicative of damage to lysosomal membranes. Using a previously established assay for isolating cell cytosol (34), we evaluated the levels of the lysosomal enzyme β-hexosaminidase in both cells and found significantly higher levels in NPC1-/- fibroblasts following a 12-h incubation with 100 μm 3-AP (Fig. 5D).
Collectively, these results suggest that NPC1-deficient cells are more susceptible to the toxic effects of a well known reactive polyamine metabolite 3-AP. The finding that polyclonal anti-polyamine oxidase levels are increased in NPC1-/- cells does not directly establish that levels of 3-AP are enhanced, but it does support the notion that the homeostasis of endogenous polyamines is disturbed in NPC cells.
DISCUSSION
Contained in this report are experimental findings that, collectively, provide evidence to support a novel functional role for NPC1 in regulating amine accumulation in lysosomes, thereby avoiding several possible deleterious consequences that may otherwise occur. The following lines of experimental evidence support this hypothesis: 1) amine-induced vacuolization of lysosomes requires functional NPC1; 2) the egress of lysosomal cargo is stimulated by certain amines; 3) amines can facilitate the formation of late endosomal/lysosomal hybrid organelles; and 4) cells with reduced NPC1 expression were shown to more susceptible to toxic effects of an endogenous amine.
Lysosomes are now understood to be dynamic organelles that are involved in several membrane-trafficking events (44). They are capable of fusing with late endosomes to create hybrid organelles (51). It has also been suggested that lysosomes from most cells are capable of regulated fusion with the plasma membrane (52–54). Despite evidence supporting the importance of lysosomal secretion, the physiological stimuli that regulate it are incompletely understood. Andrews and colleagues have made significant contributions toward understanding how increases in free intracellular Ca2+ levels stimulate lysosomal exocytosis through interactions with the lysosomal protein synaptotagmin VII and how cells utilize this lysosomal secretion pathway to reseal plasma membranes following damage (53, 55).
The amine-stimulated release of lysosomal cargo described in this report does not appear to involve direct fusion with the plasma membrane, because we do not observe elevations in free cytosolic Ca2+; however, more work will be needed to conclusively evaluate this. The data presented here are consistent with a mechanism whereby amines accumulate into lysosomes and stimulate the NPC1-mediated formation of a hybrid organelle that results from fusion between late endosomes and lysosomes. This statement is supported by several different experimental approaches that all show enhanced colocalization of lysosomal and late endosomal markers in the presence of amines, which was only observed in NPC1-competent cells (Figs. 2 and 5). The formation of hybrid organelles and their subsequent fission to reform lysosomes and late endosomes is a dynamic process (44). Our data suggest that, at steady state, a relatively small fraction of lysosomes are fused with late endosomes, at any given time, under normal cell culture conditions. This may or may not be relevant with in vivo situations. It is possible that rate of hybrid organelle formation (fusion) is small relative to the rate of reformation of lysosomes (fission) under normal conditions. When cells in culture are exposed to select lysosomotropic amines in high concentration, we observe nearly 100% colocalization of lysosomes with late endosomes in normal cells (Fig. 2A). One possible explanation is that the amines stimulate NPC1-mediated fusion events, leading to the creation of hybrid organelles, yet have no influence on the subsequent fission events that are responsible for the disappearance of the hybrid organelle.
Considering the fact that lysosomes are much denser than the hybrid organelles they originate from, there must be steps involved in hybrid organelle fission that function to reduce membrane content. In support of this, studies in live cells have shown small tubular structures budding off of late endosome/lysosome hybrid organelles (56). Similarly, Ko et al. (45) have visualized VAMP-7-positive vesicles budding from NPC1-positive hybrid organelles. It is likely that some of these vesicles would transport late endosomal membrane proteins back to their origin. However, it is possible that some of the vesicles may fuse with the plasma membrane. We propose that it is in these types of vesicles that the trafficking of lysosomal cargo, such as undigested macromolecules (i.e. dextran), LDL-derived cholesterol, and protonated amines, to the plasma membrane may occur.
The similarity of the NPC1 sequence and topology with the resistance-nodulation-division family of prokaryotic permeases suggests NPC1 may function as a molecular transport pump (57). Accordingly, Davies et al. (24) have previously established a role for NPC1 in the intracellular trafficking of the amine-containing compound acriflavine from late endocytic compartments. The molecular mechanism for NPC1-mediated trafficking of amines published in their work substantially differs from ours in that they proposed that NPC1 functioned as a transmembrane pump. Passegio and Liscum (58) have since shown that NPC1 does not appear to act as a molecular pump for the amines, including acriflavine. Moreover, we have previously established that the NPC1-mediated clearance of amine-containing molecules from lysosomes requires an intact microtubule network, which is consistent with a vesicle-mediated transport pathway rather than a transmembrane pump (23).
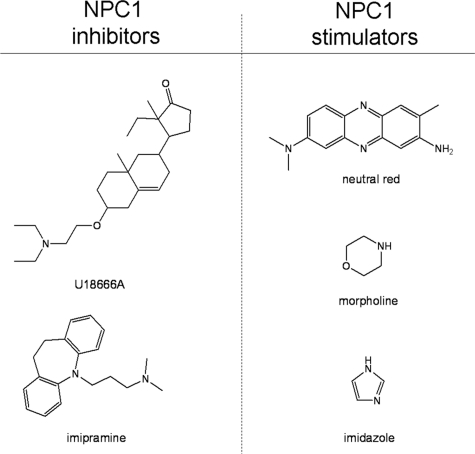
The results showing that some amines had a stimulatory influence on NPC1-mediated lysosome-trafficking events would initially appear counterintuitive. Nearly two decades ago, Liscum and Faust (59) had shown that a weakly basic compound named U18666A was able to impair the intracellular transport of LDL-derived cholesterol from lysosomes. This group has since identified a number of other, so-called, class II amphiphilic agents with related activity (60). The two most studied amines are U18666A and imipramine, and both are used to chemically induce a NPC disease phenotype. The exact mechanism of action for these agents remains poorly understood, but it is thought that they interfere with some aspect of lysosome to endoplasmic reticulum cholesterol transport. It is important to consider what might differentiate weakly basic molecules that stimulate NPC1 from those that inhibit it. We have previously evaluated how physicochemical properties of weakly basic molecules influence sequestration in lysosomes. Specifically, we have found that pKa value, membrane permeability and charge delocalization are all important factors (41, 61). According to these parameters, we would predict that NPC1 inhibitors (imipramine and U18666A) as well as the NPC1 stimulators (neutral red, imidazole, and morpholine) all have lysosomotropic properties. However, there are differences in the apparent amphiphilic nature of the molecules (see Fig. 6). Imipramine and U1866A have clear separation between the basic ionizable tertiary amine and the hydrophobic moiety, which is not the case for the NPC1 stimulatory molecules evaluated in this work. It could be that the amphiphilic nature allows the molecule to accumulate in membranes to a greater extent and consequently disrupt the activity of membrane proteins such as NPC1. In support of this, the degree of amphiphilicity of a group of molecules has been shown to correlate well with their propensity to inhibit the activity of a membrane-bound transporter protein P-glycoprotein (62).
FIGURE 6.
Structures of weakly basic amine-containing compounds shown to either stimulate or inhibit the NPC1-dependent trafficking of lysosomal cargo. All molecules shown are lysosomotropic with respect to their physicochemical properties. U18666A and imipramine are amphiphilic and have been shown to inhibit NPC1 function. NR, MOR, and IMDZ are not considered to be as amphiphilic and were all found to stimulate NPC1 function.
Progressive neurodegeneration is the ultimate cause of death in NPC patients, yet it remains unknown why alterations in lipid homeostasis would lead to such profound neurological deterioration (63). Alleviation of cholesterol accumulation in late endocytic compartments using pharmacological agents as wells as genetic approaches does little to slow the onset of neurodegeneration in mice and feline models of the disease (5, 7). Likewise, alleviation of neuronal ganglioside storage had no significant impact on disease progression (64).
NPC research has traditionally revolved around cholesterol, which has limited the number of exploratory studies that are designed to reveal other potential causes of NPC disease pathology. It is clear that a more generalized view of NPC1 as a protein required for normal transport along the late endocytic pathway is warranted, rather than viewing it narrowly as a protein specifically involved in cholesterol/lipid trafficking. An opinion, published by Ioannou (9), suggests that NPC1 may play an essential role as a lipid flippase, thereby allowing late endosomal/lysosomal compartments to maintain the correct degree of lipid asymmetry, which in turn facilitates the correct membrane curvature required to drive vesicle fusion. Our work described here demonstrates that certain lysosomotropic amines are able to facilitate NPC1-mediated fusion of late endosomes with lysosomes. We believe that this work is consistent with the previously described hypothetical role for NPC1 in vesicle fusion. The accumulation of amines in late endocytic compartments will undoubtedly cause increases in both ionic strength and osmotic pressure in the organelle lumen, which, in turn, could favorably influence membrane curvature to facilitate NPC1-mediated membrane fusion. More work will be needed to address these mechanistic details, however, it is clear the work presented here represents a significant step in bringing us closer to understanding an alternative and important function of NPC1.
Supplementary Material
Acknowledgments
We thank D. Moore and the Kansas University Microscopy and Analytical Imaging facility for help with imaging analysis, J. Easterlund for technical assistance, and N. Harmony for the reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 CA106655. This work was also supported by the Ara Parseghian Medical Research Foundation (to J. P. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” Figs. S1–S3, and references.
Footnotes
The abbreviations used are: NPC1, Niemann-Pick C1 protein; NPC, Niemann-Pick type C disease; LDL, low density lipoprotein; EEA1, early endosome antigen-1; LAMP-1, lysosome-associated membrane protein-1; MPR, mannose 6-phosphate receptor; MLIV, mucolipidosis type IV; NR, neutral red; LTR, LysoTracker red DND-99; IMDZ, imidazole; MOR, morpholine; CQ, chloroquine; NOC, nocodazole; 3-AP, 3-aminopropanal; FRET, fluorescence resonance energy transfer; BDA, biotinylated dextran amine; GFP, green fluorescent protein; PBS, phosphate-buffered saline; siRNA, small interference RNA.
References
- 1.Sturley, S. L., Patterson, M. C., Balch, W., and Liscum, L. (2004) Biochim. Biophys. Acta 1685 83-87 [DOI] [PubMed] [Google Scholar]
- 2.Pentchev, P. G. (2004) Biochim. Biophys. Acta 1685 3-7 [DOI] [PubMed] [Google Scholar]
- 3.Garver, W. S., and Heidenreich, R. A. (2002) Curr. Mol. Med. 2 485-505 [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee, S., and Maxfield, F. R. (2004) Biochim. Biophys. Acta 1685 28-37 [DOI] [PubMed] [Google Scholar]
- 5.Erickson, R. P., Garver, W. S., Camargo, F., Hossain, G. S., and Heidenreich, R. A. (2000) J. Inherit. Metab. Dis. 23 54-62 [DOI] [PubMed] [Google Scholar]
- 6.Patterson, M. C., Di Bisceglie, A. M., Higgins, J. J., Abel, R. B., Schiffmann, R., Parker, C. C., Argoff, C. E., Grewal, R. P., Yu, K., Pentchev, P. G., Brady, R. O., and Barton, N. W. (1993) Neurology 43 61-64 [DOI] [PubMed] [Google Scholar]
- 7.Somers, K. L., Brown, D. E., Fulton, R., Schultheiss, P. C., Hamar, D., Smith, M. O., Allison, R., Connally, H. E., Just, C., Mitchell, T. W., Wenger, D. A., and Thrall, M. A. (2001) J. Inherit. Metab. Dis. 24 427-436 [DOI] [PubMed] [Google Scholar]
- 8.Patterson, M. C., and Platt, F. (2004) Biochim. Biophys. Acta 1685 77-82 [DOI] [PubMed] [Google Scholar]
- 9.Ioannou, Y. A. (2005) Trends Biochem. Sci. 30 498-505 [DOI] [PubMed] [Google Scholar]
- 10.Neufeld, E. B., Wastney, M., Patel, S., Suresh, S., Cooney, A. M., Dwyer, N. K., Roff, C. F., Ohno, K., Morris, J. A., Carstea, E. D., Incardona, J. P., Strauss, J. F., 3rd, Vanier, M. T., Patterson, M. C., Brady, R. O., Pentchev, P. G., and Blanchette-Mackie, E. J. (1999) J. Biol. Chem. 274 9627-9635 [DOI] [PubMed] [Google Scholar]
- 11.Davis, B. A. (1989) J. Chromatogr. 466 89-218 [DOI] [PubMed] [Google Scholar]
- 12.Masuko, T., Kusama-Eguchi, K., Sakata, K., Kusama, T., Chaki, S., Okuyama, S., Williams, K., Kashiwagi, K., and Igarashi, K. (2003) J. Neurochem. 84 610-617 [DOI] [PubMed] [Google Scholar]
- 13.Dai, H., Kramer, D. L., Yang, C., Murti, K. G., Porter, C. W., and Cleveland, J. L. (1999) Cancer Res. 59 4944-4954 [PubMed] [Google Scholar]
- 14.Soulet, D., Gagnon, B., Rivest, S., Audette, M., and Poulin, R. (2004) J. Biol. Chem. 279 49355-49366 [DOI] [PubMed] [Google Scholar]
- 15.Cullis, P. M., Green, R. E., Merson-Davies, L., and Travis, N. (1999) Chem. Biol. 6 717-729 [DOI] [PubMed] [Google Scholar]
- 16.de Duve, C., de Barsy, T., Poole, B., Trouet, A., Tulkens, P., and Van Hoof, F. (1974) Biochem. Pharmacol. 23 2495-2531 [DOI] [PubMed] [Google Scholar]
- 17.Ohkuma, S., and Poole, B. (1978) Proc. Natl. Acad. Sci. U. S. A. 75 3327-3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulychev, A., Trouet, A., and Tulkens, P. (1978) Exp. Cell Res. 115 343-355 [DOI] [PubMed] [Google Scholar]
- 19.Duvvuri, M., and Krise, J. P. (2005) Mol. Pharm. 2 440-448 [DOI] [PubMed] [Google Scholar]
- 20.Yang, W. C., Strasser, F. F., and Pomerat, C. M. (1965) Exp. Cell Res. 38 495-506 [DOI] [PubMed] [Google Scholar]
- 21.Finnin, B. C., Reed, B. L., and Ruffin, N. E. (1969) J. Pharm. Pharmacol. 21 114-117 [DOI] [PubMed] [Google Scholar]
- 22.Morissette, G., Moreau, E., C-Gaudreault, R., and Marceau, F. (2004) J. Pharmacol. Exp. Ther. 310 395-406 [DOI] [PubMed] [Google Scholar]
- 23.Gong, Y., Duvvuri, M., Duncan, M. B., Liu, J., and Krise, J. P. (2006) J. Pharmacol. Exp. Ther. 316 242-247 [DOI] [PubMed] [Google Scholar]
- 24.Davies, J. P., Chen, F. W., and Ioannou, Y. A. (2000) Science 290 2295-2298 [DOI] [PubMed] [Google Scholar]
- 25.Goldin, E., Roff, C. F., Miller, S. P., Rodriguez-Lafrasse, C., Vanier, M. T., Brady, R. O., and Pentchev, P. G. (1992) Biochim. Biophys. Acta 1127 303-311 [DOI] [PubMed] [Google Scholar]
- 26.Virgili, M., Crochemore, C., Pena-Altamira, E., and Contestabile, A. (2006) Neurochem. Int. 48 201-207 [DOI] [PubMed] [Google Scholar]
- 27.Bernstein, H. G., and Muller, M. (1999) Prog. Neurobiol. 57 485-505 [DOI] [PubMed] [Google Scholar]
- 28.Morrison, L. D., and Kish, S. J. (1995) Neurosci. Lett. 197 5-8 [DOI] [PubMed] [Google Scholar]
- 29.Ganley, I. G., and Pfeffer, S. R. (2006) J. Biol. Chem. 281 17890-17899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diettrich, O., Mills, K., Johnson, A. W., Hasilik, A., and Winchester, B. G. (1998) FEBS Lett. 441 369-372 [DOI] [PubMed] [Google Scholar]
- 31.Jahraus, A., Storrie, B., Griffiths, G., and Desjardins, M. (1994) J. Cell Sci. 107 145-157 [DOI] [PubMed] [Google Scholar]
- 32.Ivanova, S., Botchkina, G. I., Al-Abed, Y., Meistrell, M., 3rd, Batliwalla, F., Dubinsky, J. M., Iadecola, C., Wang, H., Gregersen, P. K., Eaton, J. W., and Tracey, K. J. (1998) J. Exp. Med. 188 327-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachrach, U., and Reches, B. (1966) Analyt. Biochem. 17 38-48 [DOI] [PubMed] [Google Scholar]
- 34.Duvvuri, M., Feng, W., Mathis, A., and Krise, J. P. (2004) Pharm. Res. 21 26-32 [DOI] [PubMed] [Google Scholar]
- 35.Ferri, K. F., and Kroemer, G. (2001) Nat. Cell Biol. 3 E255-E263 [DOI] [PubMed] [Google Scholar]
- 36.Solheim, A. E., and Seglen, P. O. (1983) Biochim. Biophys. Acta 763 284-291 [DOI] [PubMed] [Google Scholar]
- 37.Bright, N. A., Lindsay, M. R., Stewart, A., and Luzio, J. P. (2001) Traffic 2 631-642 [DOI] [PubMed] [Google Scholar]
- 38.Zhang, M., Dwyer, N. K., Love, D. C., Cooney, A., Comly, M., Neufeld, E., Pentchev, P. G., Blanchette-Mackie, E. J., and Hanover, J. A. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 4466-4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen, C. S., Bach, G., and Pagano, R. E. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 6373-6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolhuis, P. A., Oonk, J. G., Kamp, P. E., Ris, A. J., Michalski, J. C., Overdijk, B., and Reuser, A. J. (1987) Neurology 37 75-81 [DOI] [PubMed] [Google Scholar]
- 41.Duvvuri, M., Gong, Y., Chatterji, D., and Krise, J. P. (2004) J. Biol. Chem. 279 32367-32372 [DOI] [PubMed] [Google Scholar]
- 42.Roff, C. F., Goldin, E., Comly, M. E., Cooney, A., Brown, A., Vanier, M. T., Miller, S. P., Brady, R. O., and Pentchev, P. G. (1991) Dev. Neurosci. 13 315-319 [DOI] [PubMed] [Google Scholar]
- 43.Andrews, N. W. (2005) Sci. STKE 2005, pe19. [DOI] [PubMed]
- 44.Luzio, J. P., Pryor, P. R., and Bright, N. A. (2007) Nat. Rev. Mol. Cell Biol. 8 622-632 [DOI] [PubMed] [Google Scholar]
- 45.Ko, D. C., Gordon, M. D., Jin, J. Y., and Scott, M. P. (2001) Mol. Biol. Cell 12 601-614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabinowitz, S., Horstmann, H., Gordon, S., and Griffiths, G. (1992) J. Cell Biol. 116 95-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janne, J., Alhonen, L., Pietila, M., and Keinanen, T. A. (2004) Eur. J. Biochem. 271 877-894 [DOI] [PubMed] [Google Scholar]
- 48.Wood, P. L., Khan, M. A., Kulow, S. R., Mahmood, S. A., and Moskal, J. R. (2006) Brain Res. 1095 190-199 [DOI] [PubMed] [Google Scholar]
- 49.Wood, P. L., Khan, M. A., and Moskal, J. R. (2007) Brain Res. 1145 150-156 [DOI] [PubMed] [Google Scholar]
- 50.Yu, Z., Li, W., Hillman, J., and Brunk, U. T. (2004) Brain Res. 1016 163-169 [DOI] [PubMed] [Google Scholar]
- 51.Luzio, J. P., Poupon, V., Lindsay, M. R., Mullock, B. M., Piper, R. C., and Pryor, P. R. (2003) Mol. Membr. Biol. 20 141-154 [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez, A., Webster, P., Ortego, J., and Andrews, N. W. (1997) J. Cell Biol. 137 93-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrews, N. W. (2000) Trends Cell Biol. 10 316-321 [DOI] [PubMed] [Google Scholar]
- 54.Griffiths, G. (2002) Semin. Cell Dev. Biol. 13 279-284 [DOI] [PubMed] [Google Scholar]
- 55.Reddy, A., Caler, E. V., and Andrews, N. W. (2001) Cell 106 157-169 [DOI] [PubMed] [Google Scholar]
- 56.Bright, N. A., Gratian, M. J., and Luzio, J. P. (2005) Curr. Biol. 15 360-365 [DOI] [PubMed] [Google Scholar]
- 57.Tseng, T. T., Gratwick, K. S., Kollman, J., Park, D., Nies, D. H., Goffeau, A., and Saier, M. H., Jr. (1999) J. Mol. Microbiol. Biotechnol. 1 107-125 [PubMed] [Google Scholar]
- 58.Passeggio, J., and Liscum, L. (2005) J. Biol. Chem. 280 10333-10339 [DOI] [PubMed] [Google Scholar]
- 59.Liscum, L., and Faust, J. R. (1989) J. Biol. Chem. 264 11796-11806 [PubMed] [Google Scholar]
- 60.Liscum, L. (1990) Biochim. Biophys. Acta 1045 40-48 [DOI] [PubMed] [Google Scholar]
- 61.Duvvuri, M., Konkar, S., Funk, R. S., Krise, J. M., and Krise, J. P. (2005) Biochemistry 44 15743-15749 [DOI] [PubMed] [Google Scholar]
- 62.Konig, G., Chiba, P., and Ecker, G. F. (2008) Monatsh. Chem. 139 401-405 [Google Scholar]
- 63.Vincent, I., Bu, B., and Erickson, R. P. (2003) Curr. Opin. Neurol. 16 155-161 [DOI] [PubMed] [Google Scholar]
- 64.Liu, Y., Wu, Y. P., Wada, R., Neufeld, E. B., Mullin, K. A., Howard, A. C., Pentchev, P. G., Vanier, M. T., Suzuki, K., and Proia, R. L. (2000) Hum. Mol. Genet. 9 1087-1092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.