Abstract
Vasopressin controls water excretion through regulation of aquaporin-2 (AQP2) trafficking in renal collecting duct cells. Using mass spectrometry, we previously demonstrated four phosphorylated serines (Ser256, Ser261, Ser264, and Ser269) in the carboxyl-terminal tail of rat AQP2. Here, we used phospho-specific antibodies and protein mass spectrometry to investigate the roles of vasopressin and cyclic AMP in the regulation of phosphorylation at Ser269 and addressed the role of this site in AQP2 trafficking. The V2 receptor-specific vasopressin analog dDAVP increased Ser(P)269-AQP2 abundance more than 10-fold, but at a rate much slower than the corresponding increase in Ser256 phosphorylation. Vasopressin-mediated changes in phosphorylation at both sites were mimicked by cAMP addition and inhibited by protein kinase A (PKA) antagonists. In vitro kinase assays, however, demonstrated that PKA phosphorylates Ser256, but not Ser269. Phosphorylation of AQP2 at Ser269 did not occur when Ser256 was replaced by an unphosphorylatable amino acid, as seen in both S256L-AQP2 mutant mice and in Madin-Darby canine kidney cells expressing an S256A mutant, suggesting that Ser269 phosphorylation depends upon prior phosphorylation at Ser256. Immunogold electron microscopy localized Ser(P)269-AQP2 solely in the apical plasma membrane of rat collecting duct cells, in contrast to the other three phospho-forms (found in both apical plasma membrane and intracellular vesicles). Madin-Darby canine kidney cells expressing an S269D “phosphomimic” AQP2 mutant showed constitutive localization at the plasma membrane. The data support a model in which vasopressin-mediated phosphorylation of AQP2 at Ser269:(a) depends on prior PKA-mediated phosphorylation of Ser256 and (b) enhances apical plasma membrane retention of AQP2.
Aquaporins are molecular water channels that mediate rapid water transport across lipid membranes in a variety of cell types (1). Aquaporin-2 (AQP2),3 the vasopressin-regulated water channel, is responsible for control of water excretion by the kidney (2). AQP2-mediated osmotic water transport from the lumens of the renal collecting ducts returns filtered water to the bloodstream under the control of the peptide hormone arginine vasopressin (AVP). This control is exerted through binding of AVP to the V2 receptor (a G protein-coupled receptor) in the collecting duct cells. The V2 receptor, working via Gs-mediated elevation of intracellular cAMP, increases epithelial osmotic water permeability via regulated trafficking of AQP2-containing intracellular vesicles to and from the apical plasma membrane (3). This process is impaired in several common disorders of water balance (2), e.g. in congestive heart failure, in lithium-induced nephrogenic diabetes insipidus associated with treatment of bipolar disorder, and in the syndrome of inappropriate antidiuresis seen in many cancer patients.
Exo- and endocytosis of AQP2 are believed to be independently regulated, and the amount of AQP2 in the plasma membrane is dependent on a balance between the two processes (4–6). Membrane trafficking processes that control the quantity of AQP2 in the apical plasma membrane have been proposed to depend on changes in phosphorylation of AQP2 at Ser256 (7–9). Recently, we have demonstrated by phosphoproteomic analysis of native rat renal inner medullary collecting duct (IMCD) cells that Ser256 is part of a polyphosphorylated region containing four phosphorylated serines (Ser256, Ser261, Ser264, and Ser269) within the last 16 amino acids of the AQP2 COOH-terminal tail (10). Prior studies have established that the abundance of the Ser256-phosphorylated form of AQP2 is increased in response to AVP (11). In addition, we have recently shown that the Ser261 phosphorylation of AQP2 is decreased (12), whereas Ser264 phosphorylation is increased by AVP (13).
The role of the Ser269 phosphorylation site in AQP2 trafficking has not been investigated. In this study, we use a novel phospho-specific antibody to Ser(P)269-AQP2, liquid chromatography-tandem mass spectrometry (LC-MS/MS), site-directed mutagenesis in MDCK cells, as well as mice expressing mutant forms of AQP2 to investigate the role of AQP2 phosphorylation at Ser269 in regulated trafficking of AQP2. The findings show that vasopressin markedly increases Ser269-AQP2 phosphorylation and that this phosphorylated form is localized exclusively in the apical plasma membrane of collecting duct cells. Studies using site-directed mutagenesis in MDCK cells support the hypothesis that Ser269 phosphorylation serves as a plasma membrane retention signal. The results also indicate that dependence of Ser269-AQP2 phosphorylation on protein kinase A (PKA) is indirect and is due to requirement for a PKA-dependent “priming” phosphorylation at Ser256 prior to Ser269 phosphorylation.
EXPERIMENTAL PROCEDURES
Antibodies
Affinity-purified rabbit polyclonal antibodies recognizing Ser(P)256-AQP2 (11), Ser(P)261-AQP2 (12), and Ser(P)264-AQP2 (13) were previously described. Here, an affinity-purified rabbit polyclonal antibody to Ser(P)269-AQP2 was generated against a synthetic peptide corresponding to the COOH terminus of rat AQP2 that included Ser(P)269 (PhosphoSolutions, Aurora, CA) as described (12). Specificity was documented by dot blotting against synthetic phosphopeptides and non-phosphopeptides (supplemental Fig. S1). A goat polyclonal antibody directed against the amino terminus of AQP2 (N-20; Santa Cruz) recognizes all known modified forms of AQP2. Another antibody recognizing total AQP2 (L127) has been described previously (14). A new antibody recognizing all forms of AQP2 investigated in this paper was created using a synthetic peptide corresponding to amino acids in the COOH terminus upstream from the polyphosphorylated region of rat AQP2 (CLKGLEPDTDWEEREVRRRQ) as described (15). The antiserum (K5007) was affinity purified using the synthetic peptide linked to agarose beads (Sulfo-Link; Pierce). A 1:5000 dilution was used for the immunoblotting.
Protein Mass Spectrometry
Data-dependent Mode—The samples were trypsinized, and the phosphopeptides were enriched by immobilized metal affinity chromatography exactly as described (10). They were analyzed on an Agilent 1100 nanoflow LC system (Palo Alto, CA) connected to an LTQ-FT hybrid mass spectrometer (Thermo, San Jose, CA) run in “neutral loss scanning” mode as described (10). Label-free phosphopeptide quantification was performed as described (16).
Multiple Reaction Monitoring (MRM) Mode—Freshly isolated rat IMCD suspensions were prepared as above and incubated in the presence or absence of 1 nm of dDAVP (synthetic analog of vasopressin) for 0, 5, and 30 min. Upon reaction, the cell suspensions were flash lysed in 6 m guanidine HCl, and a standard reduction/alkylation and enzymatic digestion (trypsin or chymotrypsin) protocols were carried out as previously described (12) except that immobilized metal affinity chromatography was not used. The samples were subjected to nanoLC using the TEMPO™ nanoMDLC System and PepMap100 C18, 75 μm × 5 μm × 15 cm nanoLC column (Dionex LC Packings). The chromatographic method employed a 10-min on-line trapping and desalting step followed by a 1-h 5–40% mobile B gradient (mobile B = 98% ACN, 0.1% formic acid). The samples were analyzed on the 4000 QTrap™ system by a MRM-triggered MS/MS information-dependent acquisition. Several MRM transitions for each phosphorylated form of the AQP2 COOH-terminal peptide were designed and optimized using MRM Pilot™ 0.9 beta software (Applied Biosystems, Foster City, CA). Q1 to Q3 transitions were generated to encompass both the characteristic H3PO4 neutral loss fragments and distinguishable peptide backbone fragments for Ser(P)256, Ser(P)261, Ser(P)264, Ser(P)269, and nonphosphorylated forms of the AQP2 COOH-terminal tail peptide. Triplicate data sets of both untreated and 5–30-min vasopressin-treated IMCD lysates were submitted to MultiQuant™ 1.0 software (Applied Biosystems) to quantitate the MRM profiles of all COOH-terminal phosphopeptides of AQP2. MRM traces were matched for retention time, and areas were integrated for relative quantitation between controls and vasopressin-treated samples.
IMCD Tubule Suspensions
IMCD tubule suspensions were prepared from kidneys of rats (Sprague-Dawley strain, NHLBI animal protocol H-0110) as described (17). Suspensions were incubated for the designated time periods in the absence or presence of 1 nm [deamino-Cys,1D-Arg8]vasopressin (dDAVP) or 0.1 mm 8-(4-chlorophenylthio)-cAMP (cpt-cAMP). In some experiments, H-89 (10 μm) was included for a 20-min preincubation prior to addition of cpt-cAMP to assess the effect of PKA inhibition. In osmotic stress experiments, IMCD suspensions were incubated in either isotonic (290 mOsm) or hypertonic (490 mOsm, addition of NaCl) medium. After incubation, the cells were pelleted by centrifugation at 10,000 × g for 30 s and resuspended in 1× sample buffer (1.5% SDS, Tris-HCl, pH 6.8), followed by DNA shearing via QIAShredder column (Qiagen) and immunoblotted as described (14), with reagent and protocol modifications based on the Odyssey Infrared Imaging System (LiCor, Lincoln, NE).
In Vitro Phosphorylation of Synthetic Peptides
Three microgram aliquots of a synthetic, unmodified AQP2 carboxyl-terminal peptide (Anaspec, EPDTDWEEREVRRRQSVELHSPQSLPRGSKA) or the same peptide prephosphorylated at Ser256 were incubated at 30 °C for 1 h with 0.1 mm ATP, 50 mm Tris, pH 7.5, 10 mm MgCl2, and each of the following kinases: 60 ng of PKA, 200 ng of ERK1, 400 ng of p38α, 300 ng of JNK1, 300 ng of Cdk1, 200 ng of Cdk5 plus 60 units of Cdk5 activator, 100 ng of protein kinase Cα. All of the kinases were from Cell Signaling Technology, Inc. (Danvers, MA). Phosphorylation of the peptide was assessed by both LC-MS/MS (data-dependent mode, described above) and immunoblotting. To stop the reaction, Laemmli solubilization buffer (18) was added to the peptide/kinase incubation product. The mixture was heated to 60 °C for 10 min and was run on 4–15% gradient gels for immunoblotting as described (14). Total AQP2 peptide was detected using the AQP2 COOH-terminal antibody (K5007), whereas the various phosphorylated forms of the AQP2 peptide were detected with appropriate phospho-specific AQP2 antibodies.
Cph Mice
Ten-day-old homozygotic mice with an S256L mutation (19) and WT control mice from the same litters were injected intraperitoneally with dDAVP (4 μl/g of body weight of a 2 μm solution in water). After 45 min, the right kidney was removed and immersion fixed in 10% formalin. Kidneys were processed for immunohistochemistry as described (20).
Short Term dDAVP Infusion of Brattleboro Rats
Homozygous Brattleboro rats had free access to standard rat chow and water before the experiment. Four rats were treated with intramuscular injection of 5 ng of dDAVP in 50 μl of saline/animal, and four saline-injected rats served as controls. After 30 min, the rats were anesthetized, and the kidneys were perfusion fixed through the abdominal aorta with 3% paraformaldehyde in 0.1 m cacodylate buffer, pH 7.4.
Immunogold Electron Microscopy of Kidney Tissue
The kidneys were perfusion fixed, cryoprotected in 2.3 m sucrose, frozen in liquid nitrogen, embedded in Lowicryl, and immunogold-labeled as previously described (20).
Immunogold Electron Microscopy of MDCK Cells
The cells were grown to confluency on semi-permeable supports, fixed with 4% paraformaldehyde in 0.01 m PBS for 20 min at room temperature, layered with gelatin, cryo-protected with 2.3 m sucrose, mounted on holders, and frozen in liquid nitrogen. Immunogold electron microscopy was performed on thin (80-nm) cryo-sections (Reichert Ultracut S cryo-ultramicrotome, Leica) with an antibody (N-20) that recognizes total AQP2.
Constructs
A cDNA for aquaporin-2 was created by reverse transcription-PCR from mouse inner medulla cDNA, cloned into the vector pcDNA5/FRT (Invitrogen). Site-directed mutagenesis (Stratagene) was used to introduce mutations into AQP2 at positions Ser256 and Ser269 by standard methods. All of the constructs were verified by sequencing.
Stable Transfection and Cell Culture
Stable cell lines were generated using the Flp-In™ system (Invitrogen). The MDCK host cell line containing one FRT site (21) was transfected with aquaporin-2 constructs using Lipofectamine™ 2000 reagent (Invitrogen). Transfected cells were selected using 500 μg/ml hygromycin. Clonal cell lines were subjected to reverse transcription-PCR followed by sequencing to confirm their identity. A minimum of two cell lines expressing the same protein were selected for further studies. With this system, all cell lines contain a single copy of AQP2 inserted into an identical position in the genome. Stably transfected cells were grown at 37 °C and 5% CO2 in Dulbecco's modified Eagle's medium/GlutaMAX (Invitrogen) supplemented with 10% donor bovine serum, 100 IU/ml each of penicillin, streptomycin, and hygromycin (250 μg/ml). Cells seeded on semi-permeable supports (0.02 μm; Nunc) were grown to confluence in hygromycin-free medium. From 24 h before experiments, all of the media contained 10 μm indomethacin. The cells were incubated in serum- and antibiotic-free media for 1 h followed by 25 μm forskolin for 30 min at 37 °C. For immunocytochemistry, the cells were fixed in 4% paraformaldehyde at room temperature for 20 min, washed in PBS, permeabilized for 30 min at 4 °C in 0.3% Triton X-100, 0.1% bovine serum albumin, and blocked in 1% bovine serum albumin for 30 min. The filters were incubated with 1:1000 goat anti-AQP2 (Santa Cruz) at 4 °C overnight, washed in PBS, and incubated for 1 h with Alexa Fluor 488-conjugated donkey anti-goat antibody (1:1000; Molecular Probes). The filters were washed in PBS and mounted on glass slides using Glycergel mounting medium (Dako). A Leica TCS SL confocal microscope was used for imaging of the cells. Image stacks were taken using a 63× oil objective lens and a z distance of 0.3 μm between images.
RESULTS
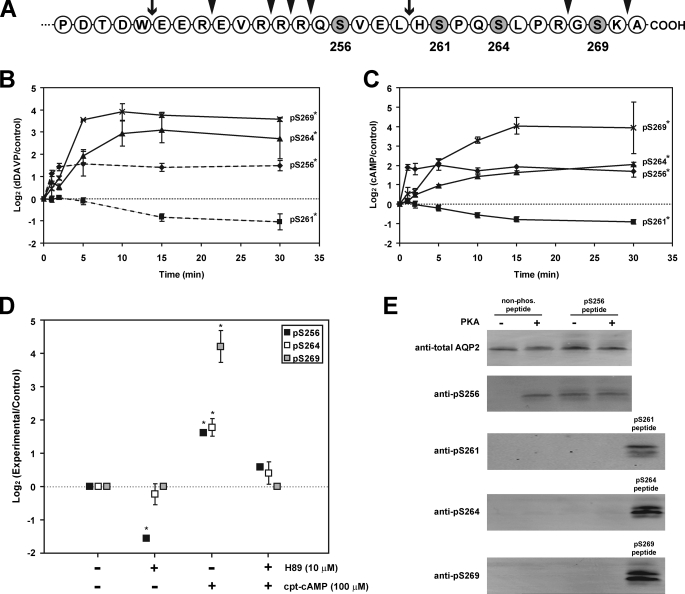
Time Course of Vasopressin-induced AQP2 Polyphosphorylation—The terminal 30 amino acids of the AQP2 COOH-terminal tail (amino acids 242–271) are shown in Fig. 1A, indicating the four phosphorylated amino acids (10). A phospho-specific antibody was produced against Ser(P)269-AQP2, complementing our existing antibodies against total AQP2, Ser(P)256-AQP2 (11), Ser(P)261-AQP2 (12), and Ser(P)264-AQP2 (13). Immunoblotting of rat IMCD tubule suspensions in the absence and presence of V2 vasopressin receptor agonist dDAVP demonstrated a >10-fold increase in phosphorylation of AQP2 at Ser269 (Fig. 1B). The time course of this increase is compared with vasopressin-induced changes at the other three phosphorylation sites in Fig. 1B (see supplemental Fig. S2 for examples of the original immunoblots). As we previously reported (12), dDAVP rapidly increases AQP2 phosphorylation at Ser256 (t½ = 41 s), whereas phosphorylation at Ser261 falls following AVP exposure (t½ = 10.6 min). dDAVP-induced increases in phosphorylation at Ser264 occurred with a t½ of 4.2 min, and the increase in phosphorylation at Ser269 occurred with a t½ of 3.2 min. Thus, the vasopressin-mediated increase in AQP2 phosphorylation at both Ser264 and Ser269 occurred significantly more slowly than the increase in Ser256 phosphorylation.
FIGURE 1.
AQP2 polyphosphorylation is altered by vasopressin acting through cyclic AMP and protein kinase A. A, terminal 30 amino acids of COOH-terminal tail of rat AQP2, showing the four serines (shaded) that are phosphorylated. The arrowheads indicate sites of trypsin cleavage; the arrows indicate sites of chymotrypsin cleavage. B, time course of changes in phosphorylation in response to 1 nm dDAVP in rat IMCD tubule suspensions. The results are based on immunoblotting using phospho-specific antibodies recognizing Ser(P)256-, Ser(P)261-, Ser(P)264-, and Ser(P)269-AQP2. Control suspensions for each time point were exposed to vehicle for the same time period. n = 3 for each time point (mean ± S.E.). The data for Ser(P)256- and Ser(P)261-AQP2 were previously reported by Hoffert et al. (12) but are included here for comparison (dashed lines). * indicates area under curve significantly different from zero (p < 0.01) (see supplemental Fig. 2 (top) for examples of the original immunoblots). C, same as A except that 0.1 mm cpt-cAMP was added instead of dDAVP. n = 3 for each time point (mean ± S.E.). * indicates area under curve significantly different from zero (p < 0.01) (see supplemental Fig. 2 (bottom) for examples of the original immunoblots). D, effect of H-89 (10 μm) on COOH-terminal phosphorylation in absence and presence of 0.1 mm cpt-cAMP. n = 3 for each point (mean ± S.E.). * indicates significantly different from zero (p < 0.01). E, in vitro phosphorylation of COOH-terminal AQP2 synthetic peptides by PKA.
Role of Cyclic AMP and PKA in Vasopressin-induced AQP2 Polyphosphorylation—Binding of AVP to V2 receptors triggers an increase in intracellular cyclic AMP (2). When rat IMCD cell suspensions were exposed to the cyclic AMP analog cpt-cAMP, the entire sequence of phosphorylation events observed with dDAVP was recapitulated (Fig. 1C), indicating that all phosphorylation changes in response to vasopressin are downstream from cyclic AMP-dependent steps (see supplemental Fig. S2 for examples of the original immunoblots). We conclude that vasopressin, acting through an increase in intracellular cAMP concentration, regulates phosphorylation at all four serines in a distinct time-dependent manner.
cpt-cAMP-induced phosphorylation at Ser256, Ser264, and Ser269 was markedly reduced by the PKA inhibitor H-89 (Fig. 1D). When a nonphosphorylated synthetic peptide corresponding to the polyphosphorylated region of the AQP2 COOH-terminal tail was incubated with PKA in the presence of ATP in vitro, phosphorylation at Ser256 occurred, as demonstrated both by immunoblotting with the phosphospecific antibody (Fig. 1E) and by LC-MS/MS analysis (not shown). However, PKA-induced phosphorylation at the other three sites was not detectable in vitro. Similarly, when a synthetic peptide prephosphorylated at Ser256 was incubated with PKA, no downstream phosphorylation was detectable. Thus, PKA is likely to be responsible for the phosphorylation at Ser256 but does not appear to phosphorylate the other sites directly. Control incubations of synthetic AQP2 COOH-terminal peptides with several other kinases (ERK1, p38α, JNK1, Cdk1, and Cdk5) did not result in phosphorylation, whereas protein kinase Cα produced only a faint signal at Ser256 (supplemental Fig. S3).
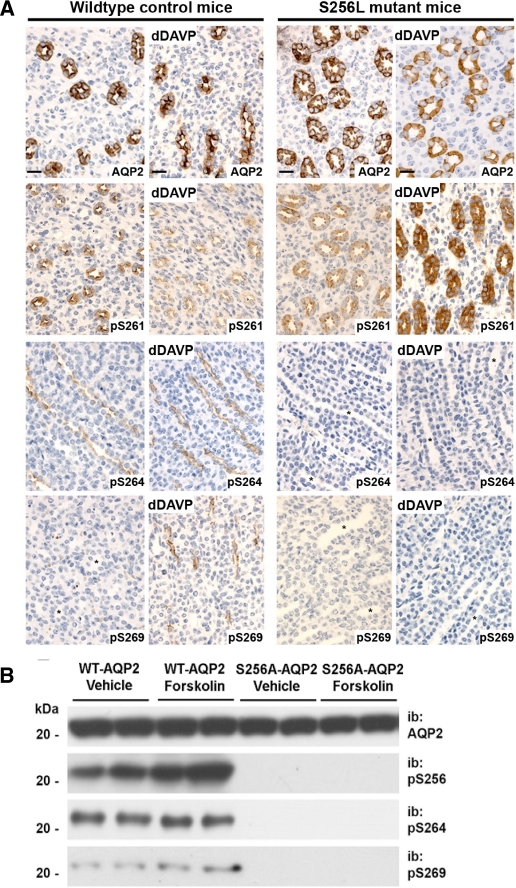
Does Phosphorylation of AQP2 at Ser264 and Ser269 Depend on Prior Phosphorylation at Ser256?—The inhibition of AQP2 phosphorylation at Ser264 and Ser269 by the PKA inhibitor H-89 could be explained if the downstream phosphorylation events were mediated by other kinases but had a requirement for prior PKA-mediated phosphorylation of AQP2 at Ser256. To address this possibility, we performed immunolabeling, using the phospho-specific antibodies, on kidney sections from mice with a spontaneous S256L-AQP2 mutation (19) (Fig. 2A). Consistent with the hypothesis, there was an absence of labeling for Ser(P)264-AQP2 or Ser(P)269-AQP2 in S256L-AQP2 mice, either with or without dDAVP administration. Furthermore, dDAVP administration resulted in increased Ser(P)261 labeling in contrast to the decrease seen in WT mice (Fig. 2A) and in rat IMCD suspensions (Fig. 1B). To test the hypothesis further, MDCK cells were stably transfected with either WT-AQP2 or AQP2 with an S256A mutation. The AQP2-S256A mutation resulted in an elimination of detectable Ser264 or Ser269 phosphorylation (Fig. 2B). In contrast, WT-AQP2 MDCK cells manifested abundant phosphorylation at both Ser264 and Ser269. Overall, the data from the S256L substitution in mice and the S256A substitution in MDCK cells are compatible with the view that Ser256 phosphorylation is required for downstream phosphorylation at both Ser264 and Ser269.
FIGURE 2.
Phosphorylation of AQP2 at Ser264 and Ser269 is dependent on phosphorylation at Ser256. A, immunoperoxidase labeling of inner medullas from WT control and S256L-AQP2 cph mice with total aquaporin-2 antibody (top row), anti-Ser(P)261-AQP2 antibody (second row), anti-Ser(P)264-AQP2 antibody (third row), and anti-Ser(P)269-AQP2 antibody (bottom row). Brown pigment indicates sites of labeling. A complete absence of p264 and p269 labeling is apparent, even after dDAVP administration in kidney sections from mice with an AQP2-S256L mutation. * indicates collecting duct lumen in nonlabeled tubules. Scale bars, 20 μm. B, immunoblotting of MDCK cells expressing WT-AQP2 or S256A-AQP2 mutation. The cells were either treated with forskolin (25 μm) or vehicle and immunoblotted with total AQP2 antibody, anti-Ser(P)264-AQP2 antibody, or anti-Ser(P)269-AQP2 antibody. Mutation at Ser256 blocked phosphorylation at downstream sites.
Quantification of Multiply Phosphorylated Forms of AQP2 Using Mass Spectrometry—Phospho-specific antibodies have the limitation that they cannot give information about what phosphorylated amino acids are found in combination. To address what multiply phosphorylated forms of AQP2 are present and how they change in response to the vasopressin analog dDAVP, we used protein mass spectrometry (Fig. 3).
FIGURE 3.
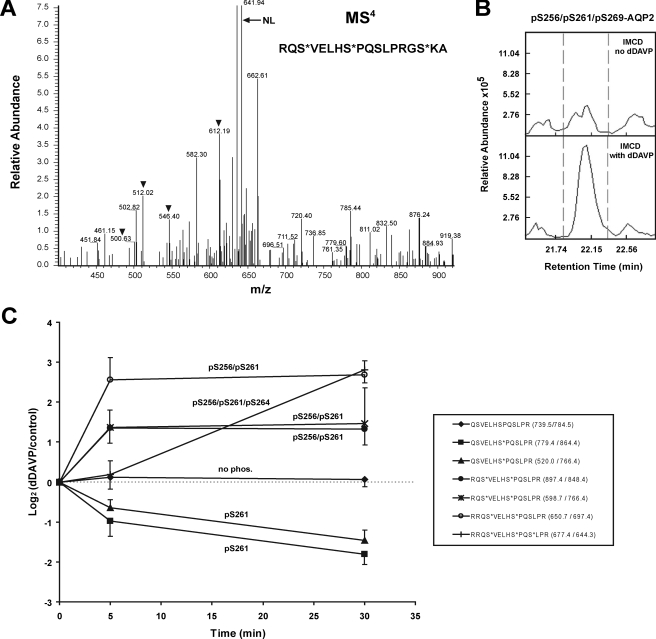
Detection and quantification of AQP2 phosphopeptides by LC-MS/MS. A, detection of triply phosphorylated Ser(P)256/Ser(P)261/Ser(P)269 AQP2 tryptic peptide by LC-MS/MS in data-dependent mode. MS4 spectrum showing neutral loss peak (NL) and site determining fragmentation peaks (arrowheads). Peptides injected were from rat IMCD suspension exposed to dDAVP. B, reconstructed ion chromatograms for peptide shown in A. dDAVP increased the abundance of this peptide 2.6-fold. C, quantification of tryptic phosphopeptides by LC-MS/MS using multiple reaction monitoring. The values are the means ± S.E. for n = 3 experiments.
Previous studies have demonstrated that dDAVP-treated IMCD cells contain readily detectable quantities of AQP2 singly phosphorylated at Ser256 or Ser261 (10), doubly phosphorylated at Ser256 and Ser261 (10), and triply phosphorylated at Ser256, Ser261, and Ser264 (16). To detect and quantify Ser269-phosphorylated AQP2 peptides in the rat IMCD using LC-MS/MS in a data-dependent mode, we took advantage of the fact that a missed trypsin cleavage at Arg267 (Fig. 1A) produces peptides that contain all four phosphorylation sites. Using an LTQ-FTICR LC-MS/MS system (Thermo, San Jose), two multiply phosphorylated forms that included Ser(P)269 were detected at the MS4 level, namely Ser(P)256/Ser(P)261/Ser(P)269-AQP2 (Fig. 3) and Ser(P)256/Ser(P)269-AQP2 (supplemental Fig. S4). Based on integration under the curves representing reconstructed ion chromatograms (see Fig. 3B for an example), the abundance of both phosphopeptides appeared to be substantially increased in response to dDAVP. Despite repeated analyses of rat IMCD homogenates, we could not detect AQP2 peptides that were phosphorylated at Ser269 but not at Ser256.
To quantify AQP2 tryptic phosphopeptides, we used targeted LC-MS/MS (multiple reaction monitoring or MRM). In contrast to the data-dependent approach (previous paragraph), MRM ignores all peptides other than those with preset MS1 and MS2 mass-to-charge (m/z) ratios, thereby assuring that a given peptide can be successfully quantified in each experiment (22), permitting statistical analysis of the results. We carried out MRM in a triple quadrupole-based LC-MS/MS system (QTrap 4000, Applied Biosystems, Foster City, CA) to sequence and quantify several AQP2 tryptic phosphopeptides from rat IMCD suspensions incubated for 0, 5, or 30 min with dDAVP after an initial equilibration period (Fig. 3C). The MRM results confirmed our prior observations that dDAVP decreases the abundance of Ser261 monophosphorylated AQP2 and increases the abundance of Ser(P)256/Ser(P)261 doubly phosphorylated AQP2 (10). The abundance of Ser(P)256/Ser(P)261-AQP2 was maximal within 5 min of dDAVP exposure. MRM also showed that the abundance of the Ser(P)256/Ser(P)261/Ser(P)264 triply phosphorylated form of AQP2 was increased by dDAVP but that most of the increase in this form occurred beyond the 5-min time point.
In general, AQP2 tryptic phosphopeptides containing Ser(P)269 were not quantifiable in our hands via MRM, presumably because of the low frequency of missed cleavages combined with the negative charges associated with multiply phosphorylated forms of AQP2. Chymotrypsin digestion afforded a partial solution to this problem, yielding peptides containing the last three phosphorylation sites (Ser261, Ser264, and Ser269) (Fig. 1A). MRM experiments to quantify the triply phosphorylated AQP2 chymotryptic peptide Ser(P)261/Ser(P)264/Ser(P)269-AQP2 in IMCD suspensions demonstrated that the abundance of this peptide was markedly increased in response to dDAVP but that most of the increase was delayed beyond the 5-min time point (supplemental Fig. S5).
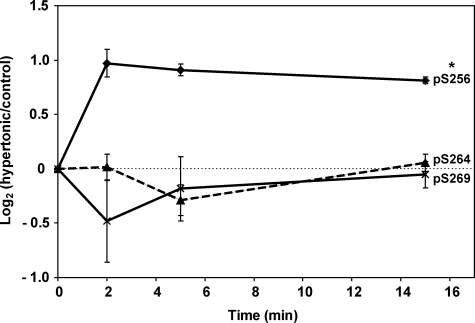
Is Phosphorylation of AQP2 at Ser256 Sufficient to Cause Downstream Phosphorylation of AQP2?—The question of whether phosphorylation of AQP2 at Ser256 is sufficient to cause downstream phosphorylation was answered in experiments in IMCD tubule suspensions exposed to high osmolality in the absence of AVP (Fig. 4). Previous studies had demonstrated that PKA can be activated by high osmolality via a cAMP-independent mechanism (23). IMCD cells pre-equilibrated at an osmolality of 290 mOsm were switched to a solution of osmolality 490 mOsm at time 0, and aliquots were immunoblotted for total and phospho-AQP2 as a function of time. An increase in osmolality elicited a large increase in Ser256-AQP2 phosphorylation, consistent with a role for PKA, but did not alter phosphorylation at Ser264 or Ser269. Thus, Ser256 phosphorylation can increase without augmenting phosphorylation at the downstream sites.
FIGURE 4.
Effect of hypertonicity on aquaporin-2 phosphorylation. Time course of changes in phosphorylation in response to change of osmolality from 290 to 490 mOsm in rat IMCD tubule suspensions. The results are based on immunoblotting using phospho-specific antibodies recognizing Ser(P)256-, Ser(P)264-, and Ser(P)269-AQP2. Control suspensions for each time point were subjected to sham exchange and collected after the same time periods as corresponding hypertonic samples. Vasopressin was not present in these experiments. n = 3 for each time point (mean ± S.E.).
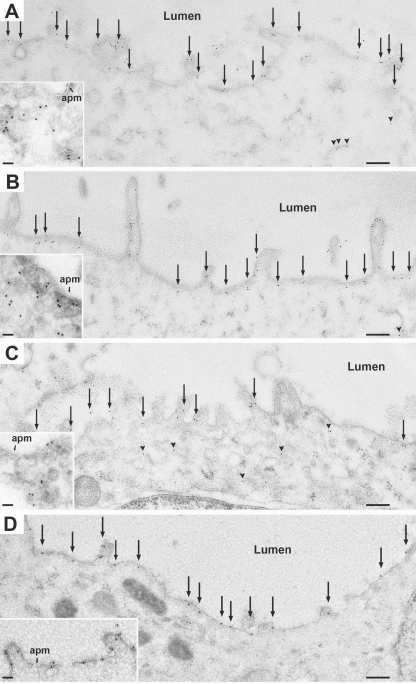
AQP2 Phosphorylated at Ser269 Is Restricted to the Plasma Membrane in Collecting Duct Cells—Immunogold electron microscopy of IMCD cells was carried out on Lowrycyl-embedded renal inner medullary sections isolated from dDAVP-treated Brattleboro rats (Fig. 5). Total AQP2 (Fig. 5A), Ser(P)256-AQP2 (Fig. 5B) and Ser(P)264-AQP2 (Fig. 5C) were detectable both inside the cell in “vesicle-like” structures and at the apical plasma membrane. In contrast, Ser(P)269-AQP2 was present exclusively at the apical plasma membrane of collecting duct cells in dDAVP-treated Brattleboro rats (Fig. 5D). Immunogold labeling for Ser(P)269-AQP2 was undetectable in Brattleboro rats in the absence of vasopressin (not shown).
FIGURE 5.
Immunogold labeling in IMCD cells from dDAVP-treated Brattleboro rats using antibody to total AQP2 (A), anti-Ser(P)256-AQP2 (B), anti-Ser(P)264-AQP2 (C), or anti-Ser(P)269-AQP2 (D). Ser(P)269-AQP2 labeling was detected exclusively in the apical plasma membrane (apm, arrows), whereas labeling with the other antibodies as seen in both the apical plasma membrane (arrows) and intracellularly (arrowheads). For low magnification overview, scale bar, 200 nm. For the high magnification inset, scale bar, 50 nm.
Does Phosphorylation of AQP2 at Ser269 Alter Its Cellular Distribution?—The immunogold labeling described above (Fig. 5) raises the possibility that Ser269 phosphorylation plays a special role in the mechanism of AVP-induced retention of AQP2 in the apical plasma membrane. To address this hypothesis, MDCK cells were stably transfected with AQP2 in which Ser269 was mutated either to aspartate (S269D mimicking the charge state of Ser(P)269) or to alanine (S269A) and were compared with WT-AQP2 MDCK cells (Fig. 6A). Fixed cells were observed by immunofluorescence microscopy using a “total
FIGURE 6.
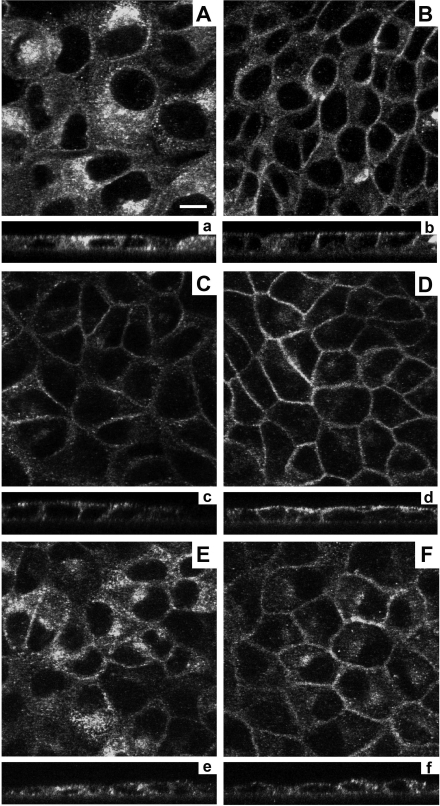
Effect of Ser269 mutations on membrane accumulation of AQP2. In stably transfected MDCK cells, WT-AQP2 is predominantly intracellular (A) and moves to the membrane upon forskolin (25 μm) stimulation (B). S269D-AQP2 is seen predominantly in plasma membrane a in either the nonstimulated (C) or forskolin-stimulated state (D). AQP2-S269A is predominantly intracellular (E) but moves to the plasma membrane upon stimulation (F). Panels a–f indicate equivalent images in the x-z plane. Scale bar, 10 μm.
AQP2” antibody that recognizes all forms of AQP2. With WT-AQP2, there was diffuse particulate labeling throughout the cell (Fig. 6A), and forskolin caused redistribution to both the apical and lateral plasma membranes (Fig. 6B), mimicking the response seen in native collecting ducts. With S269D-AQP2 cells, mimicking the charge state of phosphorylated Ser269, labeling was already present at the plasma membrane without forskolin (Fig. 6C), and a similar distribution was seen after the addition of forskolin (Fig. 6D). Immunogold labeling at the electron microscopic level confirmed the presence of the S269D form of AQP2 in the plasma membrane (Fig. 7). With S269A-AQP2 cells, AQP2 was predominantly intracellular in the absence of forskolin (Fig. 6E) but with a somewhat different distribution compared with wild type untreated cells, viz. a focal perinuclear distribution consistent with the Golgi apparatus. With forskolin S269A-AQP2 was partially redistributed to the plasma membrane, indicating that the separately regulated AQP2 exocytic process is not dependent on Ser269 phosphorylation (Fig. 6F). However, intracellular S269A-AQP2 continued to have the same perinuclear distribution in response to forskolin. The overall results are consistent with the postulated role of Ser269 phosphorylation as part of the mechanism responsible for regulated trafficking of AQP2 via vasopressin-induced AQP2 retention in the plasma membrane (presumably via inhibition of endocytosis) but with no direct role in vasopressin-regulated exocytosis.
FIGURE 7.
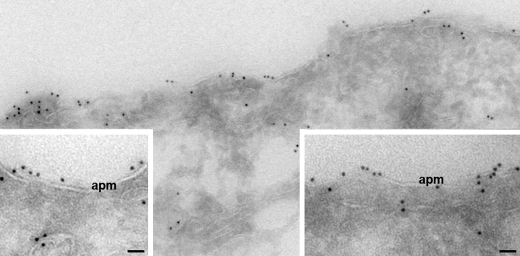
In unstimulated MDCK cells, S269D-AQP2 is located in the plasma membrane. Immunogold electron microscopy on ultrathin cryosections using total AQP2 antibody detected abundant labeling of apical plasma membrane domains in absence of forskolin stimulation. At high magnification (insets) it is clear that a substantial proportion of S269D-AQP2 is present within the plasma membrane (apm). Gold particles, 10 nm. Scale bars, 50 nm.
DISCUSSION
Our previous studies using protein mass spectrometry-based phosphoproteomic analysis of rat renal collecting duct cells revealed that the vasopressin-regulated water channel AQP2 is phosphorylated at four sites, all serines, within the terminal 16 amino acids of the COOH-terminal tail (10). Here, we have demonstrated a special role for the last of these four phosphorylation sites, Ser269, which is located within two amino acids of the COOH-terminal end of the protein (269SKA-COOH). We conclude that vasopressin, acting through cyclic AMP and PKA, markedly enhances AQP2 phosphorylation at Ser269 and that this phosphorylation event plays a critical role in vasopressin-mediated aquaporin-2 trafficking. This action of PKA appears indirect because purified PKA failed to phosphorylate Ser269 in vitro, and the residues surrounding Ser269 do not suggest a strong consensus for recognition by PKA. Additional studies revealed that the PKA dependence of Ser269 phosphorylation is due to the ability of PKA to phosphorylate Ser256 (8, 24), which appears to be a priming phosphorylation event necessary for subsequent phosphorylation at Ser269. In this Discussion, we expand upon these conclusions and place them in context of current knowledge regarding the physiology and biochemistry of AQP2 regulation as well as clinical disorders of water balance.
Regulation of AQP2 Phosphorylation by Cyclic AMP-dependent Processes—Of the four phosphorylation sites in AQP2 detected in our prior phosphoproteomic study (10), only the Ser256 site was previously identified directly (11). Ser256 is part of a consensus motif (RRQS) for phosphorylation by the cAMP-dependent kinase PKA (25). The present study using purified PKA in in vitro kinase assays with synthetic peptide substrates provides direct evidence that PKA can phosphorylate this site as demonstrated by immunoblotting and mass spectrometry. PKA-induced phosphorylation of AQP2 does not substantially change the water conductance of AQP2 (24, 26) but has been demonstrated to play an essential role in cyclic AMP-mediated regulation of AQP2 trafficking to the plasma membrane (7, 8, 27).
Kinetic studies in rat collecting duct suspensions revealed that cyclic AMP addition mimics the complex time course elicited by addition of vasopressin (Fig. 1). With both agonists, Ser256 showed a rapid increase in phosphorylation in less than 1 min in freshly isolated IMCD cells. Prior experiments demonstrated similarly rapid increases in Ser256 phosphorylation in response to vasopressin (11, 28). The increase in Ser256 phosphorylation was followed by slower increases in phosphorylation at Ser264 and Ser269 (Fig. 1) in response to cyclic AMP (Fig. 1C) or vasopressin (Fig. 1B). Cyclic AMP addition also reproduced the previously demonstrated effect of vasopressin to decrease Ser261 phosphorylation (12). Overall, the concordance between the changes elicited by vasopressin and those elicited by exogenously added cyclic AMP implicates the latter as a mediator of the action of vasopressin to alter AQP2 phosphorylation at all four sites. These results fit well with the general view from a large number of studies that the downstream effects of vasopressin to regulate water permeability in the renal collecting duct are mediated by cyclic AMP (2).
Role of Ser269-AQP2 Phosphorylation in Regulation of AQP2 Trafficking—Immunogold localization using the phospho-specific antibodies to each site singled out Ser(P)269-AQP2 as the only phosphorylated form that was detectable solely in the apical plasma membrane of renal collecting duct cells. All of the other phosphorylated forms were seen both in intracellular vesicles and on the plasma membrane. Ser(P)264-AQP2 has also been detected in the basolateral plasma membranes of collecting duct cells (13). The fact that Ser(P)269-AQP2 is present only in the apical plasma membrane points to the likelihood that this phosphorylation event plays an important role in the mechanism that mediates retention of AQP2 in the apical plasma membrane in the presence of vasopressin. This result therefore provided the rationale for further experiments focusing on the role of Ser269 in AQP2 trafficking.
As noted in the Introduction, regulation of AQP2 trafficking by vasopressin is believed to involve two independent regulatory processes: one that regulates AQP2 exocytosis and another that regulates AQP2 endocytosis (4, 5). The steady state abundance of AQP2 in the apical plasma membrane represents a balance between the rates of exo- and endocytosis, which are continuously occurring even in the absence of vasopressin (6, 29–31) and even when Ser256 of AQP2 is replaced by the nonphosphorylatable amino acid, alanine (31). Phosphorylation of AQP2 at Ser269 could theoretically alter either exo- or endocytosis, although the localization of Ser(P)269-AQP2 (apical plasma membrane only) can be more readily fit into a model of regulated endocytosis, where the phosphorylation event occurs at the apical plasma membrane and retards AQP2 internalization (see below for possible mechanisms). Consistent with this possibility, when we mutated AQP2 to replace Ser269 with an aspartate creating a fixed negative charge mimicking a phosphoserine and expressed the mutant AQP2 in MDCK cells, the AQP2 was localized predominantly in the plasma membrane, even without measures to increase intracellular cyclic AMP (Fig. 6). The plasma membrane localization of S269D-AQP2 was confirmed by immunoelectron microscopy (Fig. 7). Interestingly, the S269A mutant AQP2 retained the ability to move to the cell periphery in response to forskolin, suggesting that Ser269 phosphorylation is not necessary for regulation of AQP2 exocytosis, pointing therefore to the possibility of a selective role in AQP2 endocytosis.
How could cyclic AMP-dependent phosphorylation of Ser269 slow the endocytosis of AQP2, thereby increasing the water permeability of the apical plasma membrane? The dependence of apical retention of AQP2 on Ser269 phosphorylation is consistent with three extant hypotheses regarding AQP2 trafficking. The first hypothesis, proposed by Sasaki and co-workers (32), posits that trafficking of AQP2 depends on recognition of a COOH-terminal PDZ ligand (269SKA271) by PDZ domain proteins. Candidate interactors include SPA-1 (Rap1GAP) identified as an AQP2-interacting protein by Noda et al. (32) or other PDZ domain proteins such as Semacap3 (Pdzrn3), SPA1-like protein (Sipa1l1), and nitric oxide synthase 1 (Nos1), which have been identified in association with the apical plasma membrane of the renal inner medullary collecting duct in proteomic studies (33). Such a PDZ binding interaction could theoretically be modified by phosphorylation of Ser269, a component of the PDZ ligand motif. Phosphorylation of a PDZ ligand motif as a mechanism for controlling protein-protein interactions and membrane trafficking has been previously identified for the tetraspan protein stargazin. Stargazin interacts with AMPA-type glutamate receptors in the postsynaptic region of neurons to regulate their trafficking to the plasma membrane. Phosphorylation of stargazin at Thr321, an element of the COOH-terminal PDZ-I ligand (321TPV-COOH) that normally binds to the PDZ domain scaffold protein PSD-95, blocked stargazin binding to and clustering with PSD-95 (34, 35).
The second hypothesis, proposed by Deen and co-workers (36), is based on the observation that Lys270 (the penultimate amino acid) of AQP2 is ubiquitylated and that the ubiquitylation triggers endocytic removal of AQP2 from the apical plasma membrane. The ability of ubiquitin ligases to ubiquitylate Lys270 could theoretically be altered by phosphorylation of the adjacent serine Ser269, thereby slowing or eliminating AQP2 endocytosis. Recognizing that AQP2 endocytosis occurs by a clathrin-dependent process (30), a possible mechanism involves interaction between ubiquitinated AQP2 with proteins of the epsin family, which are clathrin adaptors that recognize ubiquitinated protein via their so-called ubiquitin interaction motifs (37). Such a mechanism has recently been demonstrated for clathrin-mediated endocytosis of another transport protein expressed in renal collecting duct cells, viz. the epithelial sodium channel ENaC (38).
A third hypothesis derives from the recent observation that the 70-kDa heat shock cognate protein (hsc70) binds selectively to AQP2 molecules that are not phosphorylated at Ser256 (39). These authors proposed that, when bound to AQP2, hsc70 potentiates AQP2 endocytosis via clathrin-interactions and that Ser256 phosphorylation (by removing the associated hsc70) inhibits AQP2 endocytosis. Whether hsc70-AQP2 interactions or other protein interactions are affected by phosphorylation at Ser269 are yet to be investigated.
Several mutations of AQP2 have been reported in humans with nephrogenic diabetes insipidus (40), a clinical disorder characterized by high rates of urinary water excretion that are unresponsive or poorly responsive to administered AVP. In contrast to mutations in other regions of the AQP2 coding sequence, mutations in the COOH-terminal tail (including the polyphosphorylated region from Ser256 to Ala271) are inherited in an autosomal dominant fashion and result in cellular missorting, often to the basolateral, rather than apical, plasma membrane (40). It is plausible that misfolding of the COOH-terminal tail of AQP2 in this class of mutations limits phosphorylation and thus inhibits correct targeting of AQP2. As a result, the osmotic water permeability of the collecting duct cells would not increase in response to vasopressin resulting in the inexorable water loss seen clinically. The COOH-terminal mutations include R254L (which prevents Ser256 phosphorylation presumably by ablating recognition by PKA), E258K, P262L (which putatively interferes with a proline-directed phosphorylation at Ser261), and several frameshift mutations that replace the polyphosphorylated region of the COOH-terminal tail of AQP2.
Ser256 Phosphorylation as a Priming Event for Downstream Phosphorylation—The kinases responsible for phosphorylation at three of the four sites remain unidentified. Although our in vitro phosphorylation studies confirmed that Ser256 is phosphorylated by PKA, none of the downstream sites (Ser261, Ser264, and Ser269) have motifs consistent with PKA sites or were phosphorylated by PKA in vitro. This contrasts with the effect of the PKA inhibitor H-89 in IMCD cells, which reduced phosphorylation at both Ser264 and Ser269. This apparent inconsistency is explained by our finding that Ser256 phosphorylation is required for Ser264 and Ser269 phosphorylation (Fig. 2). Thus, PKA acting either directly or indirectly regulates phosphorylation at all of the known COOH-terminal sites.
Ser261, in contrast to Ser264 and Ser269, shows a decrease in phosphorylation in response to vasopressin (12) or cyclic AMP (Fig. 1C). This response is abrogated in S256L-AQP2 mutant mice (Fig. 2), suggesting that it may be dependent on phosphorylation at Ser256 or that the S256L mutation effects a conformational change that blocks access of Ser261 by a putative phosphatase.
Based on the results described in this paper, PKA-mediated phosphorylation of Ser256 can be viewed as a priming phosphorylation event that creates a single control point for regulation. The concept of a priming phosphorylation event is not new. For example, sequential serine/threonine phosphorylation of β-catenin by GSK-3β at a series of three closely spaced sites (Ser33, Ser37, and Ser41) requires a priming phosphorylation at Ser45 by casein kinase Iα (41). A similar priming event is required for GSK-3β-mediated phosphorylation of other substrates (42). The mechanism by which Ser256 phosphorylation alters downstream phosphorylation remains to be investigated. For the priming phosphorylation of β-catenin-mediated by casein kinase Iα, the phosphoserine at Ser45 becomes part of the recognition motif for GSK-3β-mediated phosphorylation of Ser41, resulting in a cascade of phosphorylation events in which GSK-3β sequentially phosphorylates Ser41, Ser37, and Ser33. A similar mechanism is possible for AQP2 but unlikely because of the longer distance needed to bridge from Ser256 to Ser264. Other hypothetical mechanisms for the requirement for Ser256 phosphorylation for downstream phosphorylation include 1) a change in the conformation of the COOH-terminal tail after Ser256 phosphorylation to alter substrate susceptibility to a kinase or phosphatase; 2) binding to Ser256 of as-yet-unidentified accessory proteins involved in regulation of kinases or phosphatases; or 3) induction of AQP2 translocation to distinct membrane domains (e.g. plasma membrane lipid rafts or endosomes) containing distinct compartmentalized kinases or phosphatases (43).
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants RO1DK067386 and P30DK079333 (to F. C.) and by NHLBI, National Institutes of Health Intramural Budget Project Z01-HL001285 (to J. D. H., M. Y., T. P., and M. A. K.). This work was also supported by a Marie Curie Intra-European Fellowship and funds from the Danish National Research Foundation (Danmarks Grundforskningsfond) (to R. A. F.) and by funds from the Department of Internal Medicine/Renal Division at Washington University School of Medicine and March of Dimes Award FY06-343 (to F. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
Footnotes
The abbreviations used are: AQP2, aquaporin-2; PKA, protein kinase A; AVP, arginine vasopressin; IMCD, inner medullary collecting duct; LC-MS/MS, liquid chromatography-tandem mass spectrometry; dDAVP, deamino-[Cys,1D-Arg8]vasopressin; MRM, multiple reaction monitoring; cpt-cAMP, 8-(4-chlorophenylthio)-cyclic adenosine monophosphate; MDCK, Madin-Darby canine kidney; WT, wild type; PBS, phosphate-buffered saline; ERK, extracellular signal-regulated kinase; JNK, c-Jun NH2-terminal kinase.
References
- 1.Kozono, D., Yasui, M., King, L. S., and Agre, P. (2002) J. Clin. Investig. 109 1395-1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen, S., Frokiaer, J., Marples, D., Kwon, T. H., Agre, P., and Knepper, M. A. (2002) Physiol. Rev. 82 205-244 [DOI] [PubMed] [Google Scholar]
- 3.Nielsen, S., Chou, C. L., Marples, D., Christensen, E. I., Kishore, B. K., and Knepper, M. A. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 1013-1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knepper, M. A., and Nielsen, S. (1993) Am. J. Physiol. 265 F214-F224 [DOI] [PubMed] [Google Scholar]
- 5.Nielsen, S., and Knepper, M. A. (1993) Am. J. Physiol. 265 F204-F213 [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. (2003) Am. J. Physiol. 284 F893-F901 [DOI] [PubMed] [Google Scholar]
- 7.Katsura, T., Gustafson, C. E., Ausiello, D. A., and Brown, D. (1997) Am. J. Physiol. 272 F817-F822 [PubMed] [Google Scholar]
- 8.Fushimi, K., Sasaki, S., and Marumo, F. (1997) J. Biol. Chem. 272 14800-14804 [DOI] [PubMed] [Google Scholar]
- 9.van Balkom, B. W., Graat, M. P., van Raak, M., Hofman, E., van der, S. P., and Deen, P. M. (2004) Am. J. Physiol. 286 C372-C379 [DOI] [PubMed] [Google Scholar]
- 10.Hoffert, J. D., Pisitkun, T., Wang, G., Shen, R. F., and Knepper, M. A. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 7159-7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimoto, G., Zelenina, M., Li, D., Yasui, M., Aperia, A., Nielsen, S., and Nairn, A. C. (1999) Am. J. Physiol. 276 F254-F259 [DOI] [PubMed] [Google Scholar]
- 12.Hoffert, J. D., Nielsen, J., Yu, M. J., Pisitkun, T., Schleicher, S. M., Nielsen, S., and Knepper, M. A. (2007) Am. J. Physiol. 292 F691-F700 [DOI] [PubMed] [Google Scholar]
- 13.Fenton, R. A., Moeller, H. B., Hoffert, J. D., Yu, M.-J., Nielsen, S., and Knepper, M. A. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 3134-3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiGiovanni, S. R., Nielsen, S., Christensen, E. I., and Knepper, M. A. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 8984-8988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knepper, M. A., and Masilamani, S. (2001) Acta Physiol. Scand. 173 11-21 [DOI] [PubMed] [Google Scholar]
- 16.Hoffert, J. D., Wang, G., Pisitkun, T., Shen, R. F., and Knepper, M. A. (2007) J. Proteome Res. 6 3501-3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou, C. L., DiGiovanni, S. R., Luther, A., Lolait, S. J., and Knepper, M. A. (1995) Am. J. Physiol. 268 F78-F85 [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. (1970) Nature 227 680-685 [DOI] [PubMed] [Google Scholar]
- 19.McDill, B. W., Li, S. Z., Kovach, P. A., Ding, L., and Chen, F. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 6952-6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frokiaer, J., Marples, D., Valtin, H., Morris, J. F., Knepper, M. A., and Nielsen, S. (1999) Am. J. Physiol. 276 F179-F190 [DOI] [PubMed] [Google Scholar]
- 21.Frohlich, O., Klein, J. D., Smith, P. M., Sands, J. M., and Gunn, R. B. (2004) Am. J. Physiol. 286 C1264-C1270 [DOI] [PubMed] [Google Scholar]
- 22.Pisitkun, T., Hoffert, J. D., Yu, M. J., and Knepper, M. A. (2007) Physiol. (Bethesda) 22 390-400 [DOI] [PubMed] [Google Scholar]
- 23.Ferraris, J. D., Persaud, P., Williams, C. K., Chen, Y., and Burg, M. B. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 16800-16805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwahara, M., Fushimi, K., Terada, Y., Bai, L., Marumo, F., and Sasaki, S. (1995) J. Biol. Chem. 270 10384-10387 [DOI] [PubMed] [Google Scholar]
- 25.Fushimi, K., Uchida, S., Hara, Y., Hirata, Y., Marumo, F., and Sasaki, S. (1993) Nature 361 549-552 [DOI] [PubMed] [Google Scholar]
- 26.Lande, M. B., Jo, I., Zeidel, M. L., Somers, M., and Harris, Jr., H. W. (1996) J. Biol. Chem. 271 5552-5557 [DOI] [PubMed] [Google Scholar]
- 27.Kamsteeg, E. J., Heijnen, I., van Os, C. H., and Deen, P. M. (2000) J. Cell Biol. 151 919-930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou, C. L., Christensen, B. M., Frische, S., Vorum, H., Desai, R. A., Hoffert, J. D., de Lanerolle, P., Nielsen, S., and Knepper, M. A. (2004) J. Biol. Chem. 279 49026-49035 [DOI] [PubMed] [Google Scholar]
- 29.Gustafson, C. E., Katsura, T., McKee, M., Bouley, R., Casanova, J. E., and Brown, D. (2000) Am. J. Physiol. 278 F317-F326 [DOI] [PubMed] [Google Scholar]
- 30.Sun, T. X., Van, H. A., Huang, Y., Bouley, R., McLaughlin, M., and Brown, D. (2002) Am. J. Physiol. 282 F998-F1011 [DOI] [PubMed] [Google Scholar]
- 31.Lu, H., Sun, T. X., Bouley, R., Blackburn, K., McLaughlin, M., and Brown, D. (2004) Am. J. Physiol. 286 F233-F243 [DOI] [PubMed] [Google Scholar]
- 32.Noda, Y., Horikawa, S., Furukawa, T., Hirai, K., Katayama, Y., Asai, T., Kuwahara, M., Katagiri, K., Kinashi, T., Hattori, M., Minato, N., and Sasaki, S. (2004) FEBS Lett. 568 139-145 [DOI] [PubMed] [Google Scholar]
- 33.Yu, M. J., Pisitkun, T., Wang, G., Shen, R. F., and Knepper, M. A. (2006) Mol. Cell Proteomics 5 2131-2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chetkovich, D. M., Chen, L., Stocker, T. J., Nicoll, R. A., and Bredt, D. S. (2002) J. Neurosci. 22 5791-5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi, J., Ko, J., Park, E., Lee, J. R., Yoon, J., Lim, S., and Kim, E. (2002) J. Biol. Chem. 277 12359-12363 [DOI] [PubMed] [Google Scholar]
- 36.Kamsteeg, E. J., Hendriks, G., Boone, M., Konings, I. B., Oorschot, V., van der, S. P., Klumperman, J., and Deen, P. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 18344-18349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonifacino, J. S., and Traub, L. M. (2003) Annu. Rev. Biochem. 72 395-447 [DOI] [PubMed] [Google Scholar]
- 38.Wang, H., Traub, L. M., Weixel, K. M., Hawryluk, M. J., Shah, N., Edinger, R. S., Perry, C. J., Kester, L., Butterworth, M. B., Peters, K. W., Kleyman, T. R., Frizzell, R. A., and Johnson, J. P. (2006) J. Biol. Chem. 281 14129-14135 [DOI] [PubMed] [Google Scholar]
- 39.Lu, H. A., Sun, T. X., Matsuzaki, T., Yi, X. H., Eswara, J., Bouley, R., McKee, M., and Brown, D. (2007) J. Biol. Chem. 282 28721-28732 [DOI] [PubMed] [Google Scholar]
- 40.Robben, J. H., Knoers, N. V., and Deen, P. M. (2006) Am. J. Physiol. 291 F257-F270 [DOI] [PubMed] [Google Scholar]
- 41.Liu, C., Li, Y., Semenov, M., Han, C., Baeg, G. H., Tan, Y., Zhang, Z., Lin, X., and He, X. (2002) Cell 108 837-847 [DOI] [PubMed] [Google Scholar]
- 42.Cohen, P., and Frame, S. (2001) Nat. Rev. Mol. Cell. Biol. 2 769-776 [DOI] [PubMed] [Google Scholar]
- 43.Stefan, E., Wiesner, B., Baillie, G. S., Mollajew, R., Henn, V., Lorenz, D., Furkert, J., Santamaria, K., Nedvetsky, P., Hundsrucker, C., Beyermann, M., Krause, E., Pohl, P., Gall, I., MacIntyre, A. N., Bachmann, S., Houslay, M. D., Rosenthal, W., and Klussmann, E. (2007) J. Am. Soc. Nephrol. 18 199-212 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.