Abstract
We have studied telomere length in Schizosaccharomyces pombe strains carrying mutations affecting cell cycle checkpoints, DNA repair, and regulation of the Cdc2 protein kinase. Telomere shortening was found in rad1, rad3, rad17, and rad26 mutants. Telomere lengths in previously characterized rad1 mutants paralleled the replication checkpoint proficiency of those mutants. In contrast, rad9, chk1, hus1, and cds1 mutants had intact telomeres. No difference in telomere length was seen in mutants affected in the regulation of Cdc2, whereas some of the DNA repair mutants examined had slightly longer telomeres than did the wild type. Overexpression of the rad1+ gene caused telomeres to elongate slightly. The kinetics of telomere shortening was monitored by following telomere length after disruption of the rad1+ gene; the rate was ∼1 nucleotide per generation. Wild-type telomere length could be restored by reintroduction of the wild-type rad1+ gene. Expression of the Saccharomyces cerevisiae RCK1 protein kinase gene, which suppresses the radiation and hydroxyurea sensitivity of Sz. pombe checkpoint mutants, was able to attenuate telomere shortening in rad1 mutant cells and to increase telomere length in a wild-type background. The functional effects of telomere shortening in rad1 mutants were assayed by measuring loss of a linear and a circular minichromosome. A minor increase in loss rate was seen with the linear minichromosome, and an even smaller difference compared with wild-type was detected with the circular plasmid.
INTRODUCTION
Checkpoint genes are involved in monitoring the orderly progress of events in the cell cycle and in arresting or delaying cell cycle progress when there is an indication that an earlier process in the cell cycle has not been completed properly. Mutation in checkpoint genes disrupts these dependencies, and in humans it leads to increased sensitivity to DNA-damaging agents and increased susceptibility to cancer, such as in patients with ataxia telangiectasia. Cells from these patients, with mutation of the ATM gene, beside a defective checkpoint response to γ-ray–induced DNA damage, also have accelerated shortening of telomeres (Metcalfe et al., 1996).
In Schizosaccharomyces pombe, a group of genes has been defined, where a null mutation in any one of the member genes confers simultaneous loss of several checkpoint-related functions. Thus, null alleles of rad1+, rad3+, rad9+, rad17+, rad26+, or hus1+ lead to cells that no longer have the ability to inhibit mitosis in response to DNA damage in G2 or to incomplete DNA replication; they also die rapidly in the presence of the DNA synthesis inhibitor hydroxyurea; finally, these mutations interact with cdc17 or wee1 mutations (Al-Khodairy and Carr, 1992; Enoch et al., 1992; Al-Khodairy et al., 1994; Carr and Hoekstra, 1995; Kanter-Smoler et al., 1995). It has been possible to partially separate these functions in some mutants. The rad26-T12 mutant arrests normally in G2 in response to irradiation but is still moderately radiation sensitive (Al-Khodairy et al., 1994); a similar pattern is seen for, e.g. rad17-E198A (Griffiths et al., 1995). Cells with the rad1-S3 mutant allele have lost the replication checkpoint while largely retaining G2 DNA damage checkpoint function; cells with the rad1-S1 and rad1-S2 alleles are fully checkpoint proficient, but these alleles confer lethality in a cdc17 or wee1 background (Kanter-Smoler et al., 1995).
Telomeres are specialized structures at the ends of eukaryotic chromosomes, essential for preventing loss of genetic material on replication of chromosome ends, for preventing chromosome degradation, and for keeping chromosomes from fusing. In vertebrates, as well as in Sz. pombe and Saccharomyces cerevisiae, the ends of telomeres contain direct repeats of short DNA sequences encoded by the RNA component of the specialized telomere-replicating enzyme, telomerase (Sugawara, 1989; Zakian, 1995). In S. cerevisiae, several mutations affecting telomere length are known, e.g. in TEL1 encoding a putative regulatory protein (a structural homologue of human ATM, of human DNA-dependent protein kinase, and of the S. cerevisiae checkpoint protein Mec1), and in the orphan gene TEL2 (Greenwell et al., 1995; Morrow et al., 1995; Zakian, 1995; Runge and Zakian, 1996). Hdf1 and Hdf2, the S. cerevisiae homologues of the 70- and 80-kDa Ku antigens associated with DNA-dependent protein kinase, respectively, are also required for maintenance of normal telomere length (Boulton and Jackson, 1996; Porter et al., 1996). Interestingly, Hdf1 and Hdf2 have been shown to interact with Sir4 (Tsukamoto et al., 1997), raising the possibility that Sir4 recruits Hdf1 and Hdf2 to telomeres. Mutation in any of the above genes causes telomeres to be established at a reduced steady-state length. By contrast, est2 or tlc1 mutations, resulting in loss of the protein or the RNA component of telomerase, respectively, cause telomeres to shorten progressively. The Cdc13 protein has recently been shown to bind to single-strand DNA at telomeres, and the cdc13-est mutation causes progressive telomere shortening similar to the telomerase mutations (Nugent et al., 1996). Mutations in the gene encoding the general DNA-binding protein Rap1 cause telomeres to elongate or to shorten, depending on the nature of the mutant allele (reviewed in Zakian, 1995).
In Sz. pombe, considerably less is known about the structure of telomeres and of the genetics of telomere regulation. Five of the six telomeric regions in this organism have been cloned and sequenced (Sugawara, 1989). At the terminus, they consist of 200–300 bp with the repeat unit consensus 5′-TTACAG1–8-3′ on the G/T-rich (3′ end) strand (Sugawara, 1989; Duffy and Chambers, 1996). It has been demonstrated that these cloned sequences establish a new telomere onto linear DNA molecules (Nimmo et al., 1994). The taz1+ gene was found to cause telomere shortening when overexpressed and elongation when disrupted (Cooper et al., 1997). Recently, the gene encoding the telomerase-catalytic subunit from fission yeast, trt1+, was identified (Nakamura et al., 1997); trt1 mutants exhibit progressive telomere shortening similar to that in S. cerevisiae est2 mutants. In view of the evidence gathered from humans and S. cerevisiae suggesting an involvement of certain checkpoint-related genes in regulation of telomere length, we have investigated whether telomere length in Sz. pombe is controlled by checkpoint genes.
MATERIALS AND METHODS
Culture Conditions
Vegetative growth under nonselective conditions was in YPD (2% peptone, 1% yeast extract, 2% glucose). For scoring red ade6 cells, YES (0.5% yeast extract, 3% glucose, 10 μg/ml adenine, and 100 μg/ml each of uracil, leucine, and histidine) was used. YNB (0.17% yeast nitrogen base, 0.5% (NH4)2SO4, 2% glucose, and 100 μg/ml each of supplements adenine, uracil, leucine, and histidine as appropriate) was the selective medium except in experiments involving expression from the nmt1 promoter, where instead Edinburgh Minimal Medium (Moreno et al., 1991) was used. Cells were grown at 30°C unless noted otherwise. Genetic crosses were done as described (Gutz et al., 1974). Sz. pombe was transformed by a modified lithium acetate protocol (Kanter-Smoler et al., 1994).
Construction of Sz. pombe Strains
PS46 was made by crossing h+ ade6-704 leu1-32 ura4-D18 with FY759. The genomic rad1+ gene was disrupted in different strains by transformation with BamHI-cut pr1u4 (Sunnerhagen et al., 1990); ura+ transformants were selected on basis on their radiation sensitivity, and the correct disruption event was verified by polymerase chain reaction (PCR) performed directly on cells from the colony (Sunnerhagen, 1993). In this manner, the following strains were created: GK20, SPT1, SPT2, SPT3, SPT4, and SPT5 from strain h+ ura4-D18 leu1-32 his3; PS45 from FY110; and PS47 from PS46. A plasmid containing the rad1-S56 allele, where the in-frame deletions of rad1-S5 and rad1-S6 are combined in one molecule, was constructed as follows. A silent mutation introducing a BstBI site was made at nucleotide 1555 using the mutagenic oligonucleotide 5′-CGGACAATAACGTTCTTCGAAACG-3′ and plasmids pR1S5 and pR1S6 carrying the rad1-S5 and rad1-S6 alleles on a 3.4-kb BamHI fragment as templates (Kanter-Smoler et al., 1995). The resulting plasmids, pR1S5B2 and pR1S6B2, were both cut with BstBI, and the 1.2-kb BstBI fragment of pR1S5B2 was swapped for the corresponding fragment of pR1S6B2 to create pR1S56B2. Strains GK21 through GK24 carrying mutations rad1-S3 through rad1-S6, and PS56 carrying rad1-S56 were created by transformation of strain PS36 and selection for 5-fluoroorotic acid resistance as described (Grimm et al., 1988; Kanter-Smoler et al., 1995). The origins of these and of all other strains are listed in Table 1.
Table 1.
Sz. pombe strain list
| Strain | Genotype | Source or reference |
|---|---|---|
| 972h− | h− (wild-type) | Anwar Nasim |
| h− ura4-D18 leu1-32 his3 | Paul Russell | |
| h+ ura4-D18 leu1-32 his3 | Paul Russell | |
| h− ura4-D18 leu1-32 ade6-704 | Jürg Kohli | |
| h− rad1-1 ura4 | Anwar Nasim | |
| KLP20 | h− rad1-S1 ura4-D18 his3 leu1-32 | (Kanter-Smoler et al., 1995) |
| KLP23 | h− rad1-S2 ura4-D18 his3 leu1-32 | (Kanter-Smoler et al., 1995) |
| GK21 | h− rad1-S3 ura4-D18 his3 leu1-32 | This work |
| GK22 | h− rad1-S4 ura4-D18 his3 leu1-32 | This work |
| GK23 | h− rad1-S5 ura4-D18 his3 leu1-32 | This work |
| GK24 | h− rad1-S6 ura4-D18 his3 leu1-32 | This work |
| PS56 | h− rad1-S56 ura4-D18 his3 leu1-32 | This work |
| PS36 | h− rad1::ura4+ ura4-D18 his3 leu1-32 | (Dahlkvist et al., 1995) |
| PS32r | h− rad1::ura4+ ura4-D18 ade6 leu1 | (Sunnerhagen et al., 1990) |
| GK20 | h+ rad1::ura4+ ura4-D18 his3 leu1-32 | This work |
| rad3-136 ura4 leu1 | Anwar Nasim | |
| NRC2341 | h− rad3-136 ade7 | Anwar Nasim |
| NRC3239 | rad5 ura4 | Anwar Nasim |
| NRC3242 | h− rad8-190 ura4 | Anwar Nasim |
| rad9-192 ade6 ura4 | Anwar Nasim | |
| NRC3241 | h− rad13 ade6 ura4 | Anwar Nasim |
| NRC3240 | h− rad16 ade6 ura4 | Anwar Nasim |
| rad17-W | Anwar Nasim | |
| GK3 | rad17-W his3 ura4 leu1 | (Dahlkvist et al., 1995) |
| rad21-45 ura4 leu1 | Anwar Nasim | |
| h− rad26::ura4+ leu1-32 ade6-704 | Anthony Carr | |
| h− chk1::ura4+ leu1-32 ade6-704 | Anthony Carr | |
| hus1::LEU2 | Anthony Carr | |
| cds1::ura4+ | Hiroto Okayama | |
| cdc2-3w ura4 leu1 his3 | Paul Russell | |
| PR 87.97 | h− cdc2-1w leu1 ura4-D18 | Paul Russell |
| cdc25OP | adh:cdc25+ leu1-32 | Paul Russell |
| PGYQ686 | wee1::ura4+ | Paul Young |
| AD1 | h− ura4::nmt1:RCK1 | (Dahlkvist et al., 1995) |
| AD2 | h− rad1::ura4::nmt1:RCK1 ura4-D18 his3 leu1-32 | (Dahlkvist et al., 1995) |
| FY110 | h+ ura4-D18 leu1-32 ade6-M210 [Ch16 ade6-M216] | (Niwa et al., 1989) |
| FY759 | h− ade6-704 [CM3112 sup3-5] | (Allshire et al., 1995) |
| PS45 | h+ rad1::ura4+ ura4-D18 leu1-32 ade6-M210 [Ch16 ade6-M216] | This work |
| PS46 | ura4-D18 leu1-32 ade6-704 [CM3112 sup3-5] | This work |
| PS47 | rad1::ura4+ ura4-D18 leu1-32 ade6-704 [CM3112 sup3-5] | This work |
| SPT1 | h+ ura4-D18 leu1-32 his3 rad1::ura4+[pIRT2R1 radl+ LEU2] | This work |
| SPT2 | h+ ura4-D18 leu1-32 his3 [pGSR3 nmt1:radl+ LEU2] | This work |
| SPT3 | h+ ura4-D18 leu1-32 his3 rad1::ura4+[pIRT2 LEU2] | This work |
| SPT4 | h+ ura4-D18 leu1-32 his3 rad1::ura4+[pIRT2RCK1 RCK1 LEU2] | This work |
| SPT5 | h+ ura4-D18 leu1-32 his3 rad1::ura4+[pIRT2RCK2 RCK2 LEU2] | This work |
Detection of Telomeres with Southern Blotting
Chromosomal DNA was isolated with a glass bead-phenol extraction protocol (Hoffman and Winston, 1987). To ensure that even amounts of DNA were loaded, agarose gels were photographed after staining with ethidium bromide. As an additional loading control, we used the intensity of a 2.4-kb ApaI band of unique Sz. pombe genomic DNA (GenBank accession no. U33008) detected with a PCR product generated with the primers 5′-GGATAGTCCGTCACGAATAC-3′ and 5′-GGGATTTCGGGGTCCAGG-3′. Transfer to GeneScreen filters was in 25 mM Na2HPO4, pH 6.5. The 1.9-kb ApaI fragment of pEN42 (Nimmo et al., 1994) was used as a probe for telomeric repeat sequences, and the 0.84-kb ApaI fragment of the same plasmid was used as a probe for subtelomeric repeats. Both fragments were labeled with the random hexanucleotide priming method (Feinberg and Vogelstein, 1983). Alternatively, the 1.9-kb fragment was labeled by extension of the telomere-specific primer 5′-CCCTGTAA-3′. Conditions for this labeling method were as for random priming, except 100 ng of primer were used. Hybridization was at 58°C in 1% bovine serum albumin; 1 mM EDTA; 0.5 M NaHPO4, pH 7.2; 7% SDS. Filters were washed at 58°C with 0.2× SSC (1× SSC is 150 mM NaCl plus 15 mM sodium citrate); 0.5% SDS.
Minichromosome Mitotic Stability Test
Strain FY110 and its rad1 derivative PS45, carrying the linear minichromosome Ch16 (Niwa et al., 1989) was used to assess fidelity of mitotic chromosomal transmission. Ch16 carries the ade6-M216 allele, and so in a ade6-M210 chromosomal background cells having lost it are detected by their red color on plates with low adenine concentration. The circular minichromosome CM3112 in PS46 and its rad1 derivative PS47, contains sup3-5, which suppresses the chromosomal ade6-704 allele and gives an ade+ phenotype. Loss of CM3112 likewise yields an ade6 phenotype, scored by red color on low adenine plates. The frequency of minichromosome loss per generation was calculated directly from the fractions of half-sectored and quarter-sectored colonies (Allshire et al., 1995).
RESULTS
Telomeres Are Shorter Only in Mutants Affected in the Replication Checkpoint
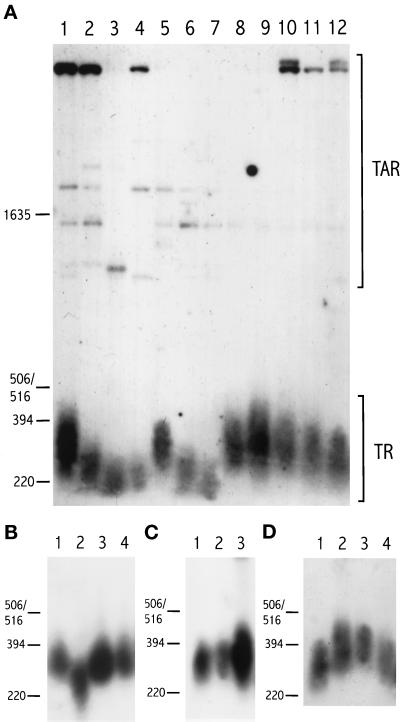
A number of Sz. pombe wild-type and mutant strains were subcultured in liquid medium for 100 generations. Then, chromosomal DNA was prepared and analyzed for the length of telomeres by Southern blot. The rationale for first subculturing for this number of generations was to ensure that each strain had experienced at least 100 divisions since the time the relevant mutation was introduced. This is based on previous experience from Saccharomyces cerevisiae, where 100–150 generations of growth after introduction of the tel1 and tel2 mutations were required for telomeres to reach their shortest length, a phenomenon called phenotypic lag (Lustig and Petes, 1986). The outcome is shown in Figure 1. In the lower part of the blot, the characteristic smear of telomeric DNA is seen; in addition, several higher molecular weight bands are seen corresponding to telomere-associated repeats (TAR), internal to the telomere. This is as expected as the 1.9-kb ApaI fragment of pEN42 used as a probe contains both telomeric repeats and telomere-associated sequences and since telomeric repeat sequences are found also at internal locations near the telomere (Sugawara, 1989; Nimmo et al., 1994). There are considerable strain-to-strain variations in number, intensity, and size of the TAR bands (Figure 1A). This is seen even in strains with a lineage that had split very recently, such as in Figure 2, lanes 2 through 9. Because we have not been able to correlate these variations with either type of mutation or state of the telomeres proper, we conclude that TARs are subject to rapid and apparently stochastic rearrangements. For the remainder of this work, we will deal only with the telomeric repeats.
Figure 1.
Southern blot analysis of telomere length in different mutant and wild-type Sz. pombe strains. Cells were grown for ∼100 generations before harvest. Genomic DNA was restricted with ApaI and separated on 1.2% agarose gels. DNA was blotted to filters and probed with the 1.9-kb ApaI fragment of pEN42 (Nimmo et al., 1994). Positions of DNA size markers and their sizes in base pairs are given on the left. (A) Lane 1, strain 972h− (wild type); lane 2, h− rad1-1 ura4; lane 3, rad3-136 ura4 leu1; lane 4, NRC2341 (h−rad3-136); lane 5, rad9-192 ade6 ura4; lane 6, GK3 (rad17); lane 7, h− rad26::ura4+ leu1-32 ade6-704; lane 8, h− chk1::ura4+ leu1-32 ade6-704; lane 9, PR 87.97 (cdc2-1w); lane 10, cdc2-3w ura4 leu1 his3; lane 11, PGYQ686 (wee1::ura4+); lane 12, cdc25OP (adh:cdc25+). Brackets indicate the position of internal TAR and of the heterogeneous telomeric restriction fragment (TR). (B) Lane 1, 972h−; lane 2, h− rad26::ura4+; lane 3, hus1::LEU2; lane 4, cds1::ura4+. (C) Lane 1, 972h−; lane 2, NRC3242 (rad8-190); lane 3, NRC3239 (rad5). (The amount of DNA loaded in lane 3 is greater than in the other two lanes.) (D) Lane 1, 972h−; lane 2, NRC3241 (rad13); lane 3, NRC3240 (rad16); lane 4, rad21-45 ura4 leu1.
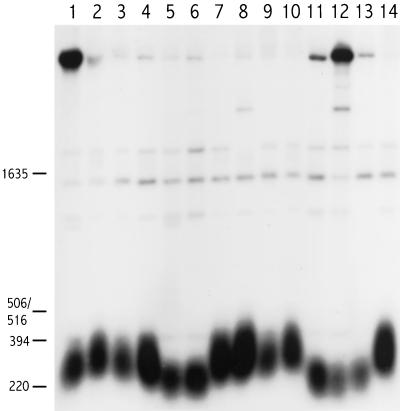
Figure 2.
Telomere lengths in different rad1 mutants. Cells were grown for ∼100 generations, and DNA was analyzed as described in the legend to Figure 1. Lane 1, 972h−; lane 2, h− ura4-D18 leu1-32 his3; lane 3, KLP20 (rad1-S1); lane 4, KLP23 (rad1-S2); lane 5, GK21 (rad1-S3); lane 6, GK22 (rad1-S4); lane 7, GK23 (rad1-S5); lane 8, GK24 (rad1-S6); lane 9, PS56 (rad1-S56); lane 10, h+ ura4-D18 leu1-32 his3; lane 11, PS36 (rad1::ura4+); lane 12, PS32r (rad1::ura4+); lane 13, GK20 (rad1::ura4+); lane 14, h+ ura4-D18 leu1-32 his3.
As seen in Figure 1A, there is a clear reduction in telomere length in rad1, rad3, rad17, and rad26 strains (lanes 2, 3, 4, 6, and 7), most markedly in rad3 and rad26 mutants. To eliminate the possibility that the short telomeres found in these strains were merely the fortuitous result of some cryptic mutation, we examined several strains with mutations in the above genes. Two different rad3 strains (Figure 1A, lanes 3 and 4) had shorter telomeres; further, a rad1-1 strain as well as several Δrad1 strains resulting from independent disruption events all had telomeres shortened to approximately the same length (Figure 1A, lane 2; Figure 2, lanes 11–13).
All of these mutants belong to the “checkpoint-rad” group, the members of which are deficient in both the DNA replication and the G2 DNA damage checkpoints (Al-Khodairy et al., 1994; Carr and Hoekstra, 1995). However, this group also includes the rad9 and hus1 mutants, which have intact telomeres (Figure 1, A, lane 5, and B, lane 3). The cds1 mutation has the distinction of eliminating only the DNA replication checkpoint, while leaving the G2 DNA damage checkpoint response intact (Murakami and Okayama, 1995). The chk1 mutation causes deficiency in the G2 DNA damage checkpoint but does not affect the replication checkpoint (Walworth et al., 1993); thus, it has the complementary effect of cds1. These two latter checkpoint mutants also have telomeres of wild-type length (Figure 1, A, lane 8, and B, lane 4).
Mutants affected in other functions were also examined for telomere length, e.g. strains with alterations in genes the products of which affect the activity of the Cdc2/Cdc13 mitosis-promoting factor kinase. Strain PGYQ686 lacks the Wee1 protein kinase, altering the balance toward the Tyr15 unphosphorylated, active form of Cdc2 (Russell and Nurse, 1987). Strain cdc25OP overproduces the Cdc25 protein phosphatase, giving the same effect (Russell and Nurse, 1986). Cells carrying cdc2-1w or cdc2-3w are smaller at division because of altered sensitivity of the mutant Cdc2 to inhibitory Tyr15 phosphorylation (Russell and Nurse, 1987). None of these strains have significant alterations of telomere length (Figure 1A, lanes 9 through 12). We further examined rad5, rad8, rad13, rad16, and rad21, which are all deficient in various aspects of DNA repair (Birkenbihl and Subramani, 1992; Doe et al., 1993; Carr et al., 1994; Lehmann, 1996). None of these strains have shorter telomeres (Figure 1, C and D). On the contrary, some mutants with DNA repair defects have somewhat longer telomeres. This was most clearly seen for rad13 and rad16 cells. A marginally increased length was found in rad5 cells, whereas rad8 and rad21 strains had telomeres of wild-type length.
Telomere Length in Wild-Type and Checkpoint Mutant Cells
An estimate of the distance from the terminal ApaI site to the start of telomeric repeat sequences on the Sz. pombe chromosome ends (Sugawara, 1989) indicates an average of 50 bp. Given this value, we calculate that in 972h− wild-type cells, the mean length of the telomeric repeat region is about 250 bp (e.g. lane 1 of all panels of Figure 1). This figure is in agreement with several earlier estimates of telomere length in wild-type Sz. pombe (as discussed in Funabiki et al., 1993). In the four mutant strains with shortened telomeres, the corresponding value is 190 bp for rad1 and rad17 mutants and 170 bp for rad3 and rad26 mutants (Figure 1A, lanes 2–4, 6, and 7; Figure 1B, lane 2). In two other wild-type strains, h− ura4-D18 leu1-32 his3 and h+ ura4-D18 leu1-32 his3, telomeres were slightly longer than in 972h− or ∼275 bp (Figure 2, lanes 2, 10, and 14). Nevertheless, rad1 strains directly derived from these strains (PS36, GK20) by genomic disruption have telomeres as short as those of rad1 strains of other origins (rad1-1, PS32r) (Figure 1A, lane 2; Figure 2, lanes 11–13). This size reduction is roughly comparable to that calculated for S. cerevisiae tel2 mutants that carry terminal poly(TG)1–3 tracts of ∼190 bp and the isogenic wild-type strain 360-bp tracts (Lustig and Petes, 1986).
Telomere Length among rad1 Mutants Correlates with Their Replication Checkpoint Proficiency
Having shown that mutation in each of the genes examined with a role in the replication checkpoint cause shorter telomeres, we next examined the influence of different site-specific mutations in the rad1+ gene on telomere length. Previously, we have described site-specific rad1 mutations that affect the various aspects of the rad1 null phenotypes differently (Kanter-Smoler et al., 1995). Strains carrying mutations rad1-S1 through -S6, and rad1-S56 (Table 1) were grown as above and analyzed for telomere length (Figure 2). Five of the mutants, rad1-S1, -S2, -S5, -S6, and -S56, had wild-type telomeres, whereas the remaining two, rad1-S3 and S4, had telomeres as short as strains carrying the rad1::ura4+ null allele. This was as expected for four of the alleles, since rad1-S4 is equivalent to the null allele in all aspects examined thus far; rad1-S5 and -S6 (Kanter-Smoler et al., 1995) and rad1-S56 (data not shown) perform at least as well as the wild-type allele in all other functions studied. The outcome for the other three alleles makes it possible to correlate telomere shortening specifically with one of the phenotypes of the rad1 null allele. Mutants carrying rad1-S1 or -S2 are proficient with respect to both the DNA replication and the G2 DNA damage checkpoint (Kanter-Smoler et al., 1995); these mutants have wild-type telomeres. By contrast, the rad1-S3 mutation completely abolishes the replication checkpoint, while leaving the G2 DNA damage checkpoint largely intact (Kanter-Smoler et al., 1995); such mutants have telomeres as short as those of rad1 null mutants. Thus, in this set of mutants, the presence of the replication checkpoint yields telomeres of wild-type length, whereas its absence causes telomeres to shorten.
Overexpression of Rad1 Lengthens Telomeres
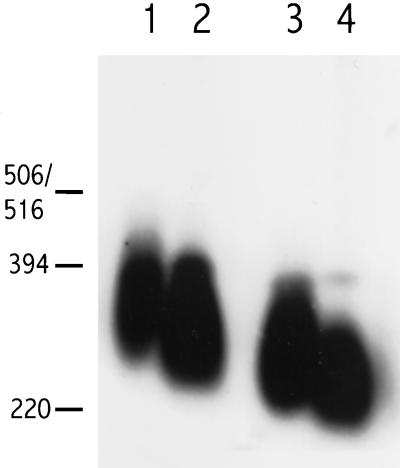
Plasmid pGSR3 (Long et al., 1994), containing full-length rad1+ cDNA under control of the nmt1 promoter, was used to transform h+ ura4-D18 leu1--32 his3 to leu+. Transformants were cultured for ∼100 generations before harvest, allowing full expression from the nmt1 promoter. As seen in Figure 3, cells overexpressing Rad1 have slightly longer telomeres than their wild-type progenitors.
Figure 3.
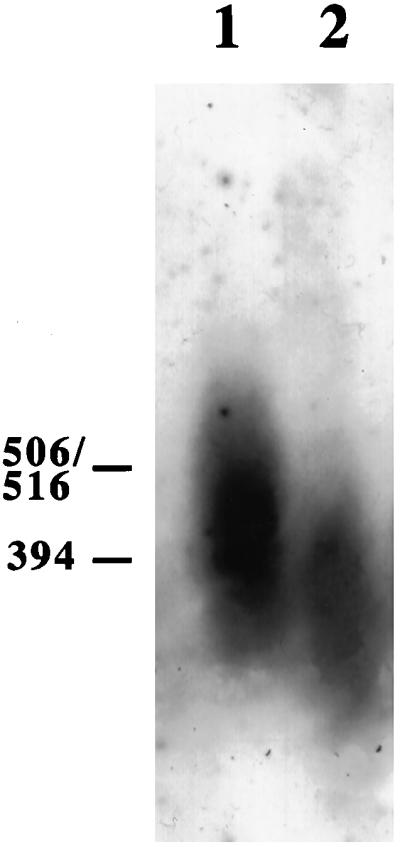
Telomere length after overexpression of rad1+. Cells were grown for 100 generations and Southern blot analysis was as detailed in the legend to Figure 1. Lane 1, SPT2 (wild-type strain h+ ura4-D18 leu1-32 his3 transformed with pGSR3 carrying rad1+ cDNA under control of the nmt1 promoter); lane 2, h+ ura4-D18 leu1-32 his3.
Expression of S. cerevisiae Rck1 Gives Longer Telomeres
The RCK1 and RCK2 genes from S. cerevisiae were isolated as suppressors of radiation and hydroxyurea sensitivity of Sz. pombe checkpoint mutants (Dahlkvist et al., 1995). We wanted to see whether they would also suppress this novel checkpoint mutant phenotype. First, a wild-type strain was transformed with either RCK1 or RCK2 cloned in the multicopy vector pIRT2, or with vector only. Then, the rad1+ gene was disrupted by transformation with pr1 u4 (Sunnerhagen et al., 1990). DNA was harvested after ∼100 generations of growth postdisruption. Under this regimen, telomere shortening was only marginally counteracted by expression of Rck1 or Rck2 (data not shown). Second, strains AD1, containing a chromosomally integrated copy of the S. cerevisiae RCK1 gene under control of the nmt1 promoter, and AD2, containing the same construct in a Δrad1 background, were cultured for 100 generations under conditions allowing maximal expression from the nmt1 promoter (liquid medium lacking thiamine). Figure 4 shows that cells expressing high levels of RCK1 have longer telomeres than their counterparts not expressing this gene. AD1 (Figure 4, lane 1) was constructed from the wild-type strain 972h− (lane 2), and has telomeres clearly longer than those of 972h−. AD2 expressing RCK1 (lane 3) was constructed from the rad1::ura4+strain PS36 (lane 4) and has longer telomeres than PS36, although not as long as in the wild-type case.
Figure 4.
Expression of the S. cerevisiae protein kinase-encoding gene RCK1 from the regulatable nmt1 promoter in a chromosomally integrated construct causes telomere elongation in Sz. pombe. Southern blot analysis as detailed in the legend to Figure 1. Lane 1, AD1 (972h−-derived strain expressing RCK1); lane 2, 972h−; lane 3, AD2 (strain expressing RCK1; derived from PS36); lane 4, PS36 (rad1::ura4+).
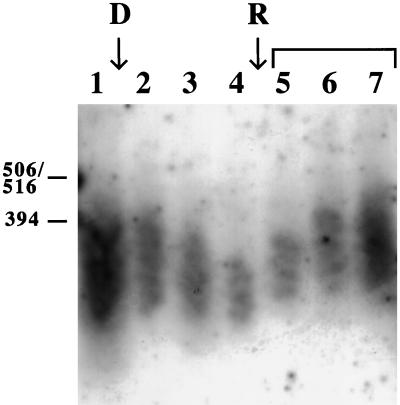
Kinetics of Telomere Shortening on Depletion of Rad1
In an experiment to measure the time after inactivation of the rad1+ gene required for telomeres to shorten below their normal length, the chromosomal rad1+ gene was disrupted as above in a wild-type background (h+ ura4-D18 leu1-32 his3). In Figure 5, the telomere lengths of these freshly generated disruptants at various time intervals are seen and compared with the wild-type progenitor strain. For the first time point, a colony of disruptants was expanded in liquid medium until the total number of progeny cells was 5 × 108; this is equivalent to ∼30 cell divisions since the original gene disruption event, assuming cell death to be insignificant. There is a gradual decrease of average telomere size up to the last time point taken (lanes 1–4).
Figure 5.
Time course of telomere shortening after disruption of the rad1+ gene and of restoration of wild-type telomeres after reintroduction of wild-type rad1+. Wild-type strain h+ ura4-D18 leu1-32 his3 was transformed with pr1u4 and DNA was isolated at various times thereafter. D, point where the rad1+ gene was disrupted; R, point where the rad1+ gene was reintroduced. Brackets above lanes indicate where the rad1+ gene was expressed from a multicopy plasmid (pIRT2R1). Southern blot as described in the legend to Figure 1. Lane 1, DNA was isolated before disruption (0 h); lane 2, 30 generations postdisruption (transformant colony expanded to 5 × 108 cells); lane 3, 60 generations; lane 4, 100 generations postdisruption. The disruptant strain was then transformed with plasmid pIRT2R1 containing the wild-type rad1+ gene. Lane 5, 30 generations after reintroduction of rad1+; lane 6, 50 generations; lane 7, 100 generations.
Shortening of Telomeres after Rad1 Depletion Is Reversible
Having followed the approximate time course of telomere shortening, we then wanted to investigate whether telomere length could be restored by reintroducing the rad1+ gene. The Δrad1 disruptant strain was transformed with pIRT2R1, which carries the wild-type rad1+ gene on a multicopy vector.
Again a transformant colony was expanded, and DNA was prepared 30 generations posttransformation. As seen in Figure 5, lanes 5 through 7, after reexpression of rad1+ telomeres gradually grow longer, and after 100 generations they have regained their original size.
Chromosome Stability in rad1 Mutants
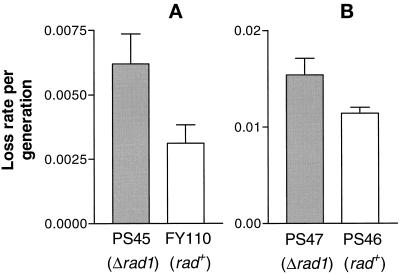
The rad1+ gene was disrupted in strain FY110, which carries the linear 0.5-Mb minichromosome Ch16, to yield strain PS45. This isogenic pair of strains was grown for 100 generations in liquid medium lacking adenine, thus selecting for the minichromosome. As seen in Figure 6A, there is an ∼2-fold increase in loss rate of the linear minichromosome in a rad1 background. In an analogous experiment, the loss rate of the 30-kb circular minichromosome CM3112 was estimated in an isogenic rad+-rad1 pair (PS46 and PS47) after 100 generations of growth following the gene disruption event. In this case the increase in loss rate in the rad1 strain was less, or about 1.5-fold (Figure 6B).
Figure 6.
Frequency of minichromosome loss in wild-type and Δrad1 strains. The genomic rad1+ gene was disrupted in a strain carrying the minichromosome, and the resultant strain and its wild-type parent were grown for ∼100 generations. The frequency of chromosome loss per generation was estimated directly from the fraction of half-sectored colonies plus one-half the fraction of colonies with one-quarter section red and three-quarters white. Each value given is the average of at least three readings and represents counting of at least 5000 colonies. Error bars, ± 1 SE of the mean. (A) Linear minichromosome Ch16; (B) circular minichromosome CM3112.
Temperature-sensitive Slow Growth
The S. cerevisiae tel1 and tel2 mutants exhibit slow growth at 37°C, but only after the phenotypic lag of ∼100 generations (Zakian, 1995; Runge and Zakian, 1996). We investigated the growth rate at 37°C of selected mutants after 100 generations of subculturing in liquid medium. As seen in Table 2, such slow growth was found in rad3, rad21, rad26, hus1, and wee1 strains. All other strains examined, including rad1 and rad17 strains, grew as fast as wild-type strains at this temperature.
Table 2.
Semiquantitative test of growth at elevated temperature (37°C) of selected mutants, after subculturing for 100 generations
| Strain | Growth at 37°C |
|---|---|
| h− ura4-D18 leu1-32 his3 (wild-type) | +++ |
| PS32r (Δrad1) | +++ |
| KLP20 (rad1-S1) | +++ |
| GK21 (rad1-S3) | +++ |
| GK22 (rad1-S4) | +++ |
| PS56 (rad1-S56) | +++ |
| rad3-136 ura4 leu1 | + |
| NRC234 (rad3-136) | + |
| NRC3239 (rad5) | +++ |
| NRC3242 (h− rad8-190) | +++ |
| rad17-W | +++ |
| rad21-45 ura4 leu1 | + |
| h− rad26::ura4+ leu1-32 ade6-704 | ++ |
| h− chk1::ura4+ leu1-32 ade6-704 | +++ |
| hus1::LEU2 | ++ |
| cds1::ura4+ | +++ |
| PGYQ686 (Δwee1) | + |
Cells were deposited as liquid droplets on solid YPD medium and grown at 30°C or 37°C for 2 days. +++, growth at 37°C unaffected relative to 30°C; ++, slightly retarded growth at 37°C; +, markedly retarded growth.
DISCUSSION
We have established short telomeres as a novel phenotype of mutants of four of the Sz. pombe checkpoint genes. Mutations in the human ATM gene cause cell cycle checkpoint defects as well as telomere shortening (Savitsky et al., 1995; Metcalfe et al., 1996). In S. cerevisiae, there is a separation of these phenotypes such that mec1 mutants have checkpoint deficiencies but normal telomeres, whereas tel1 mutants have shortened telomeres but no checkpoint defect (Weinert et al., 1994; Greenwell et al., 1995; Morrow et al., 1995; Paulovich and Hartwell, 1995). Mutation in the structurally related TOR1 and TOR2 genes does not affect telomere length (Greenwell et al., 1995). The situation in Sz. pombe in this respect is similar to the human one in that mutation in the homologous gene rad3+ causes both checkpoint and telomere defects.
Among the mutants studied in this paper, we found shortening of telomeres exclusively in cell cycle checkpoint mutants. We studied rad1 mutants in more detail and found telomere shortening among these to correlate with loss of the replication checkpoint. However, there must be additional criteria since rad9, hus1, and cds1 mutants, all of which lack the replication checkpoint, have normal telomeres. For hus1 and cds1, null mutants were used. For rad9, the rad9-192 point mutation was studied; however, this allele is indistinguishable from the null allele with respect to UV and γ resistance (Murray et al., 1991). Assuming that the six “checkpoint-rad” gene products act as a multiprotein complex (Al-Khodairy et al., 1994; Carr and Hoekstra, 1995) when instigating the cell cycle responses to DNA damage or unreplicated DNA, then a related complex may exist with only four members (Rad1, Rad3, Rad17, and Rad26) with a role in control of telomere length.
We found no reduction of telomere length in mutants affected directly in various aspects of regulation of Tyr15 of the Cdc2 protein. This is noteworthy given that chk1+-dependent signalling from checkpoint proteins has been shown to go through the protein phosphatase Cdc25, responsible for dephosphorylating this residue (Furnari et al., 1997). All the mutants in this group tested here (cdc2-1w, cdc2-3w, adh:cdc25+, Δwee1) have alterations that up-regulate Cdc2 activity and would be predicted to have shorter telomeres if defective Cdc2 Tyr15 signalling were the cause of the short telomeres in checkpoint mutants. Because this is clearly not the case, the signalling from checkpoint proteins which is relevant for telomere length control may be separate from the one involving phosphorylation of Cdc2 Tyr15 and cell cycle arrest.
Among DNA repair mutants, three were found to have slightly longer telomeres than the wild type. These, rad5, rad13, and rad16 are all defective in nucleotide excision repair (NER). We can only speculate that several components of the NER machinery are required for normal function of telomere processing and maintenance. rad8 and rad21 mutants have normal telomeres; rad8+ is implicated in an uncharacterized pathway separate from NER, whereas the rad21 mutation is thought to confer a defect in double-strand break repair (Lehmann, 1996).
More than one mechanism for telomere shortening has been demonstrated in S. cerevisiae. Loss of DNA corresponding to the length of an RNA primer during replication of the 5′ end of one of the strands predicts a rate of ∼10 bp per generation, uniform for all individual chromosome ends. Telomeric rapid deletion (Li and Lustig, 1996) can shorten an individual telomere by >1 kb in a single cell division; however, this process affects only a small subset of the population each generation. After eliminating rad1+ expression, Sz. pombe telomeres gradually shorten with a rate of ∼1 nucleotide per generation. This does not contradict a model in which telomeres are shortened for every round of replication. The length of telomeres in replication checkpoint mutants with an undetermined number of generations of growth prior to subculturing for 100 generations in this work (rad1-1, rad17-W) was the same as in those disrupted in rad1 during the course of this work and then subcultured for 100 generations. This indicates that in these mutants, a new equilibrium is reached, different from that in wild-type cells. Thus, this type of telomere shortening is analogous to what is found in, e.g., S. cerevisiae tel1 and tel2 mutants, but distinct from the ever-shorter phenotype of S. cerevisiae est2 or tlc1 mutants. The short telomeres of S. cerevisiae tel2 mutants revert to wild-type length ∼50 generations after introduction of the TEL2 gene (Runge and Zakian, 1996). The rate of the corresponding reversion in Sz. pombe rad1 mutants on reexpression of the rad1+ gene (wild-type length after 100 generations) indicates similar kinetics.
We investigated the high temperature slow growth found for S. cerevisiae tel1 and tel2 mutants after 100–150 generations of subculturing in several of the Sz. pombe mutant strains in this study. Indeed we did find such a phenotype for some of the mutants, but it did not correlate with telomere length. Thus, rad3 and rad26 mutants (which have short telomeres) display slow growth at 37°C, whereas rad1 and rad17 strains (likewise with short telomeres) do not. There were also examples of mutants with normal telomeres; hus1, rad21 and wee1, that did have the ts phenotype. In Sz. pombe, then, this weak ts phenotype must be the consequence of some other property of checkpoint mutants (and other mutants) than their shortened telomeres. This also raises the possibility that the weak ts phenotype seen for the two S. cerevisiae mutants may not be a consequence of, but only coincide in time with, telomere shortening. Clearly, this temperature sensitivity is not a general property of Sz. pombe mutants with short telomeres.
RCK1 and RCK2 of S. cerevisiae encode protein kinases of similar sequence (Dahlkvist and Sunnerhagen, 1994) and with similar effects in Sz. pombe checkpoint mutants, namely, to increase radiation and hydroxyurea resistance (Dahlkvist et al., 1995). We originally interpreted this effect to be rather indirect, and to result solely from an imposed lengthening of the G2 phase of the cell cycle in these mutants, thus compensating for the lack of G2 delay upon DNA damage or perturbation of DNA replication. However, our present finding that expression of at least RCK1 at high levels in rad1 mutant cells will attenuate telomere shortening indicates that the functional relationship between these two protein kinases and checkpoint proteins may be closer than previously suspected. None of the mutations interfering with the G2-to-M transition studied in this work (cdc2-1w, cdc2-3w, Δwee1, adh:cdc25) affected telomere length, arguing against the possibility that simply spending a longer time in G2 would make telomeres grow longer. High levels of RCK1 expression were able to increase telomere length even in a wild-type background. This can be interpreted as RCK1 acting downstream in the signalling pathway from the checkpoint proteins, analogous to our previous finding that RCK1 expression will cause cell elongation even in a wild-type background (Dahlkvist et al., 1995). The authentic roles of RCK1 and RCK2 in S. cerevisiae remain obscure, and in this context it will be relevant to investigate whether overexpression or disruption of these genes will affect telomere size in S. cerevisiae.
Although a distinct shortening of telomeres was caused by disruption of the rad1+ gene, only a marginal effect on loss rate of linear or circular minichromosomes was seen. The finding that the increase in loss rate was higher with the linear minichromosome (2-fold) than with the circular (1.5-fold) might be taken as evidence that it is the result of a telomere defect given that circular chromosomes lack telomeres. Indeed, for the S. cerevisiae Δtel1 mutation, a similar modest increase in linear chromosome loss rate (∼3-fold) was found (Greenwell et al., 1995). However, we think such a conclusion is not warranted, given the marginal difference between the two situations and the small overall increase. Increased chromosome loss rates above the level of rad1 mutants have previously been observed in mutant Sz. pombe strains shown here not to have shortened telomeres, e.g. chk1 (Griffiths et al., 1995), rad8, or rad13 (Murray et al., 1994). Clearly factors other than length of telomeres determine telomere function as assayed by chromosome stability. Apparently, it is possible for a cell to have drastically shortened telomeres without a profound effect on telomere function as seen in this type of assay.
Defects in DNA metabolism near telomeres in S. cerevisiae, such as in cdc13 mutants, activate the RAD9-dependent checkpoint (Weinert and Hartwell, 1993; Weinert et al., 1994; Garvik et al., 1995). Elimination of an entire telomere activates the RAD9-dependent checkpoint (Sandell and Zakian, 1993); however, inactivation of telomerase does not (Garvik et al., 1995; Lin and Zakian, 1995). A straightforward model of the situation in Sz. pombe would be that lack of activation of the replication checkpoint on shortening of telomeres allows them to decrease from wild-type length to the equilibrium length seen in checkpoint mutants after >100 generations of subculturing. Then, presumably another mechanism for maintenance of telomere size sets in, inasmuch as telomeric repeats do not disappear completely. For the rad1+ gene, a case could be made for a more direct involvement in DNA metabolism at telomeres. The Rad1 product has sequence similarity to the Ustilago maydis Rec1 protein (Long et al., 1994), for which a 3′→5′ exonuclease activity has been found in vitro (Thelen et al., 1994). The S. cerevisiae Rad17 protein is also similar to these two proteins (Lydall and Weinert, 1995; Siede et al., 1996). Garvik et al. (1995) found that cdc13 mutants accumulate single-stranded DNA near telomeres, corresponding to the T/G-rich strand. Based on these observations a model has been proposed (Lydall and Weinert, 1995) where Rad17 degrades one strand of DNA near sites of DNA damage, such as in the vicinity of telomeres in cdc13 mutants. By analogy, one could envisage Sz. pombe Rad1 performing some step essential for, e.g., processing of telomeres before elongation by, e.g., telomerase. These two models are of course in principle compatible with each other. DNA polymerases have been demonstrated to be part of the replication checkpoint (Durso et al., 1995; Francesconi et al., 1995; Navas et al., 1995), and it is reasonable to expect a DNA repair/DNA-processing enzyme to be part of a DNA damage checkpoint. However, the situation in Sz. pombe is not quite the same as the one in S. cerevisiae, since rad1+ is required for both the replication and the G2 DNA damage checkpoints, whereas RAD17 is not required for the replication checkpoint (Weinert et al., 1994). Also, the state discussed by Lydall and Weinert (1995) and Garvik et al. (1995) is not analogous to that studied here, because we demonstrate telomere shortening directly in checkpoint mutants, whereas their work deals with checkpoint activation after mutation in the telomere-binding protein, Cdc13. Further, a more general model must accommodate the fact that not only rad1 mutants but four of the replication checkpoint-deficient mutants have shortened telomeres. We therefore think it is possible that, apart from their general roles in maintaining genomic integrity, the Rad1, Rad3, Rad17, and Rad26 proteins participate together in signalling different aberrations from the normal state of telomeres, be it short telomeric repeats or, e.g., single-stranded regions. To find out about these mechanisms, it will be elucidating to see whether these proteins can be found physically associated with telomeres in vivo. Equally relevant is the issue of whether the clustering of telomeres in G2 seen in wild-type Sz. pombe (Funabiki et al., 1993) is affected in rad1, rad3, rad17, and rad26 mutants, which have shortened telomeres.
ACKNOWLEDGMENTS
This work was supported by the Swedish Cancer Fund, the Swedish Radiation Protection Institute, and Assar Gabrielsson’s Fund. T.O. and A.R. acknowledge graduate fellowships from the Swedish Cancer Fund. Thanks are due to Neal Sugawara for valuable discussions and for making available his thesis. We thank Hiroto Okayama and Anthony Carr for gifts of cds1 and hus1 deletion strains, respectively; Jürg Kohli for a strain carrying ade6-704; and Robin Allshire and Elaine Nimmo for pEN42, strain FY759, and helpful discussions regarding Southern blot techniques for telomeric sequences.
REFERENCES
- Al-Khodairy F, Carr AM. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khodairy F, Fotou E, Sheldrick KS, Griffiths DJF, Lehmann AR, Carr AM. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell. 1994;5:147–160. doi: 10.1091/mbc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire R, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 1995;9:218–233. doi: 10.1101/gad.9.2.218. [DOI] [PubMed] [Google Scholar]
- Birkenbihl RP, Subramani S. Cloning and characterization of rad21, an essential gene of Schizosaccharomyces pombe involved in DNA double-strand-break repair. Nucleic Acids Res. 1992;20:6605–6611. doi: 10.1093/nar/20.24.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton SJ, Jackson SP. Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double strand break rejoining and in telomeric maintenance. Nucleic Acids Res. 1996;24:4639–4648. doi: 10.1093/nar/24.23.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AM, Hoekstra MF. The cellular responses to DNA damage. Trends Cell Biol. 1995;5:32–40. doi: 10.1016/s0962-8924(00)88934-5. [DOI] [PubMed] [Google Scholar]
- Carr AM, Schmidt H, Kirchhoff S, Muriel WJ, Sheldrick KS, Griffiths DJ, Basmacioglu CN, Subramani S, Clegg M, Nasim A, Lehmann AR. The rad16 gene of Schizosaccharomyces pombe: a homolog of the RAD1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:2029–2040. doi: 10.1128/mcb.14.3.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JP, Nimmo ER, Allshire RC, Cech TR. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature. 1997;385:744–747. doi: 10.1038/385744a0. [DOI] [PubMed] [Google Scholar]
- Dahlkvist A, Kanter-Smoler G, Sunnerhagen P. The RCK1 and RCK2 protein kinase genes from Saccharomyces cerevisiae suppress cell cycle checkpoint mutations in Schizosaccharomyces pombe. Mol Gen Genet. 1995;246:316–326. doi: 10.1007/BF00288604. [DOI] [PubMed] [Google Scholar]
- Dahlkvist A, Sunnerhagen P. Two novel deduced serine/threonine protein kinases from Saccharomyces cerevisiae. Gene. 1994;139:27–33. doi: 10.1016/0378-1119(94)90519-3. [DOI] [PubMed] [Google Scholar]
- Doe CL, Murray JM, Shayeghi M, Hoskins M, Lehmann AR, Carr AM, Watts FZ. Cloning and characterisation of the Schizosaccharomyces pombe rad8 gene, a member of the Snf2 helicase family. Nucleic Acids Res. 1993;21:5964–5971. doi: 10.1093/nar/21.25.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy M, Chambers A. DNA-protein interactions at the telomeric repeats of Schizosaccharomyces pombe. Nucleic Acids Res. 1996;24:1412–1419. doi: 10.1093/nar/24.8.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durso G, Grallert B, Nurse P. DNA polymerase α, a component of the replication initiation complex, is essential for the checkpoint coupling S phase to mitosis in fission yeast. J Cell Sci. 1995;108:3109–3118. doi: 10.1242/jcs.108.9.3109. [DOI] [PubMed] [Google Scholar]
- Enoch T, Carr AM, Nurse P. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes Dev. 1992;6:2035–2046. doi: 10.1101/gad.6.11.2035. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for labeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Francesconi S, De Recondo AM, Baldacci G. DNA polymerase Δ is required for the replication feedback control of cell cycle progression in Schizosaccharomyces pombe. Mol Gen Genet. 1995;246:561–569. doi: 10.1007/BF00298962. [DOI] [PubMed] [Google Scholar]
- Funabiki H, Hagan I, Uzawa S, Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J Cell Biol. 1993;121:961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Garvik B, Carson M, Hartwell L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol Cell Biol. 1995;15:6128–6138. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell PW, Kronmal SL, Porter SE, Gassenhuber J, Obermaier B, Petes TD. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell. 1995;82:823–829. doi: 10.1016/0092-8674(95)90479-4. [DOI] [PubMed] [Google Scholar]
- Griffiths DJF, Barbet NC, McCready S, Lehmann AR, Carr AM. Fission yeast rad17: a homologue of budding yeast RAD24 that shares regions of sequence similarity with DNA polymerase accessory proteins. EMBO J. 1995;14:5812–5823. doi: 10.1002/j.1460-2075.1995.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Kohli J, Murray J, Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988;215:81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Gutz H, Heslot H, Leupold U, Loprieno N. Schizosaccharomyces pombe. In: King RC, editor. Bacteria, Bacteriophages, and Fungi. New York: Plenum; 1974. pp. 395–446. [Google Scholar]
- Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Kanter-Smoler G, Dahlkvist A, Sunnerhagen P. Improved method for rapid transformation of intact Schizosaccharomyces pombe cells. Biotechniques. 1994;16:798–800. [PubMed] [Google Scholar]
- Kanter-Smoler G, Knudsen KE, Jimenez G, Sunnerhagen P, Subramani S. Separation of phenotypes in mutant alleles of the Schizosaccharomyces pombe cell cycle checkpoint gene rad1+ Mol Biol Cell. 1995;6:1793–1805. doi: 10.1091/mbc.6.12.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann AR. Molecular biology of DNA repair in the fission yeast Schizosaccharomyces pombe. Mutat Res. 1996;363:147–161. doi: 10.1016/0921-8777(96)00017-1. [DOI] [PubMed] [Google Scholar]
- Li B, Lustig AJ. A novel mechanism for telomere size control in Saccharomyces cerevisiae. Genes Dev. 1996;10:1310–1326. doi: 10.1101/gad.10.11.1310. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Zakian VA. An in vitro assay for Saccharomyces telomerase requires EST1. Cell. 1995;81:1127–1135. doi: 10.1016/s0092-8674(05)80017-0. [DOI] [PubMed] [Google Scholar]
- Long KE, Sunnerhagen P, Subramani S. The Schizosaccharomyces pombe rad1 gene consists of three exons and the cDNA sequence is partially homologous to the Ustilago maydis REC1 cDNA. Gene. 1994;148:155–159. doi: 10.1016/0378-1119(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Lustig AJ, Petes TD. Identification of yeast mutants with altered telomere structure. Proc Natl Acad Sci USA. 1986;83:1398–1402. doi: 10.1073/pnas.83.5.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydall D, Weinert T. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science. 1995;270:1488–1491. doi: 10.1126/science.270.5241.1488. [DOI] [PubMed] [Google Scholar]
- Metcalfe JA, Parkhill J, Campbell L, Stacey M, Biggs P, Byrd PJ, Taylor MR. Accelerated telomere shortening in ataxia telangiectasia. Nat Genet. 1996;13:350–353. doi: 10.1038/ng0796-350. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Morrow DM, Morrow M, Tagle DA, Shiloh Y, Collins FS, Hieter P. TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell. 1995;82:831–840. doi: 10.1016/0092-8674(95)90480-8. [DOI] [PubMed] [Google Scholar]
- Murakami H, Okayama H. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature. 1995;374:817–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- Murray JM, Carr AM, Lehmann AR, Watts FZ. Cloning and characterization of the rad9 DNA repair gene from Schizosaccharomyces pombe. Nucleic Acids Res. 1991;19:3525–3531. doi: 10.1093/nar/19.13.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JM, Tavassoli M, Al-Harithy R, Sheldrick KS, Lehmann AR, Carr AM, Watts FZ. Structural and functional conservation of the human homolog of the Schizosaccharomyces pombe rad2 gene, which is required for chromosome segregation and recovery from DNA damage. Mol Cell Biol. 1994;14:4878–4888. doi: 10.1128/mcb.14.7.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- Navas TA, Zhou Z, Elledge SJ. DNA polymerase, links the DNA replication machinery to the S phase checkpoint. Cell. 1995;80:29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- Nimmo ER, Cranston G, Allshire RC. Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J. 1994;13:3801–3811. doi: 10.1002/j.1460-2075.1994.tb06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O, Matsumoto Y, Chikasage Y, Yanagida M. Characterization of Schizosaccharomyces pombe minichromosome deletion derivatives and a functional allocation of their centromere. EMBO J. 1989;8:3045–3052. doi: 10.1002/j.1460-2075.1989.tb08455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent CI, Hughes TR, Lue NF, Lundblad V. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science. 1996;274:249–252. doi: 10.1126/science.274.5285.249. [DOI] [PubMed] [Google Scholar]
- Paulovich AG, Hartwell LH. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell. 1995;82:841–847. doi: 10.1016/0092-8674(95)90481-6. [DOI] [PubMed] [Google Scholar]
- Porter SE, Greenwell PW, Ritchie KB, Petes TD. The DNA-binding protein Hdf1p (a putative Ku homologue) is required for maintaining normal telomere length in Saccharomyces cerevisiae. Nucleic Acids Res. 1996;24:582–585. doi: 10.1093/nar/24.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge KW, Zakian VA. TEL2, an essential gene required for telomere length regulation and telomere position effect in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3094–3105. doi: 10.1128/mcb.16.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Sandell LL, Zakian VA. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993;75:729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- Savitsky K, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Siede W, Nusspaumer G, Portillo V, Rodriguez R, Friedberg EC. Cloning and characterization of RAD17, a gene controlling cell cycle responses to DNA damage in Saccharomyces cerevisiae. Nucleic Acids Res. 1996;24:1669–1675. doi: 10.1093/nar/24.9.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N. DNA sequences at the telomeres of the fission yeast S. pombe. Ph.D. Thesis. Cambridge, MA: Harvard University; 1989. [Google Scholar]
- Sunnerhagen P. PCR used to determine mating type in Schizosaccharomyces pombe. Biotechniques. 1993;14:18. [PubMed] [Google Scholar]
- Sunnerhagen P, Seaton BL, Nasim A, Subramani S. Cloning and analysis of a gene involved in DNA repair and recombination, the rad1 gene of Schizosaccharomyces pombe. Mol Cell Biol. 1990;10:3750–3760. doi: 10.1128/mcb.10.7.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen MP, Onel K, Holloman WK. The REC1 gene of Ustilago maydis involved in the cellular response to DNA damage encodes an exonuclease. J Biol Chem. 1994;269:747–754. [PubMed] [Google Scholar]
- Tsukamoto Y, Kato J, Ikeda H. Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature. 1997;388:900–903. doi: 10.1038/42288. [DOI] [PubMed] [Google Scholar]
- Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- Weinert TA, Hartwell LH. Cell cycle arrest of cdc mutants and specificity of the RAD9 checkpoint. Genetics. 1993;134:63–80. doi: 10.1093/genetics/134.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert TA, Kiser GL, Hartwell LH. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- Zakian VA. Telomeres. E.H. Blackburn and C.W. Greider. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1995. Saccharomyces telomeres: function, structure and replication; pp. 107–137. [Google Scholar]