Abstract
The sphingolipid ceramide has been implicated in mediating cell death that is accompanied by mitochondrial functional alterations. Moreover, ceramide has been shown to accumulate in mitochondria upon induction of apoptotic processes. In this study, we sought to evaluate the effects of natural, highly hydrophobic long-chain ceramides on mitochondrial function in vitro. Ceramide in a dodecane/ethanol delivery system inhibited the opening of the mitochondrial permeability transition pore (PTP) induced by either oxidative stress, SH group cross-linking, or high Ca2+ load, suggesting that the inhibitory point is at a level at which major PTP regulatory pathways converge. Moreover, ceramide had no effect on well known mitochondrial components that modulate PTP activity, such as cyclophilin D, voltage-dependent anion channel, adenine nucleotide transporter, and ATP synthase. The inhibitory effect of ceramide on PTP was not stereospecific, nor was there a preference for ceramide over dihydroceramide. However, the effect of ceramide on PTP was significantly influenced by the fatty acid moiety chain length. These studies are the first to show that long-chain ceramide can influence PTP at physiologically relevant concentrations, suggesting that it is the only known potent natural inhibitor of PTP. These results suggest a novel mechanism of ceramide regulation of mitochondrial function.
In recent years, ceramide has become increasingly appreciated as a bioactive lipid that modulates apoptotic/necrotic cellular processes that are integrated at the mitochondrial level, as a result of studies demonstrating correlation between the intensity of cell death stimuli and the level of ceramide production (1–4). It has been suggested that ceramide conveys death signals to mitochondria by two mechanisms. One mechanism assumes selective permeabilization of the outer mitochondrial membrane for proapoptotic proteins as a process independent of inner membrane functions. The second mechanism presumes that loss of the permeability barrier of the inner mitochondrial membrane is a prerequisite for the subsequent outer membrane permeabilization.
The simplest model of selective outer membrane permeabilization arises from experiments with artificial membranes (liposomes and black lipid membranes) (5–7). Within this model, proapoptotic protein release due to large pore formation in the outer mitochondrial membrane is attributed to ceramide itself, whereas the inner membrane is viewed as being ceramide-insensitive. Although supported by extensive experimental material with isolated mitochondria (8–11), there are a number of studies in which ceramide alone did not affect cytochrome c release (12–16) or cytochrome c release was related to an inner membrane perturbation (15–19). This could be due to differences in cell types and experimental differences. Moreover, in Bax- and Bak-deficient cells, ceramide-induced cell death was completely suppressed (20), indicating a likely role of Bcl-2 family proteins in ceramide-induced apoptosis. In fact it was recently shown that Bcl-2 can disassemble ceramide channels in the outer mitochondrial membrane (21).
Another model of selective outer membrane permeabilization considers the Bcl-2 protein family localized in the cytoplasm and on the mitochondrial outer membrane to be major participants in the manifestation of the proapoptotic potential for ceramide. Activation of proapoptotic proteins, including Bid, Bax, and Bad, is mediated by interaction of ceramides with protein phosphatases PP1 and PP2A or with cathepsin D (22–28). However, the direct effect of ceramides on Bax binding/insertion has also been reported (14, 29, 30). An increase in proapoptotic proteins bound to mitochondria is thought to trigger specific permeabilization of the outer mitochondrial membrane for cytochrome c and other mitochondrial intermembrane-resident apoptosis-inducing proteins (31, 32).
A mechanism that accounts for participation of the inner mitochondrial membrane in the release of proapoptotic proteins involves the obligatory opening of the permeability transition pore in the inner membrane (PTP)2 (31–34). The resulting osmotic swelling of the mitochondrial inner membrane compartment leads to rupture of the outer mitochondrial membrane and release of proapoptotic proteins. It is thought that PTP opening occurs as a result of direct interaction of ceramide with the pore in the presence of Ca2+ (15, 35) or that PTP opening follows mitochondrial Ca2+ overload. In the latter scenario, the source of Ca2+ is the Ca2+ stores of the endoplasmic reticulum (ER), which are considered primary ceramide targets (20, 36). Support for this mechanism arises from experiments with many cell types in which ceramide-induced cell death is suppressed by PTP inhibitors, such as cyclosporine A (CSA) and bongkrekic acid (15, 19, 35, 37–39).
Models of mitochondrial Ca2+ overload as a consequence of ceramide-induced Ca2+ release from the ER and the direct activation of PTP by ceramides were largely based on data obtained using artificial C2-ceramide. Whether these models are applicable in vivo with endogenously generated natural ceramide is unclear. Thus, traditional extrinsic and intrinsic pathways that involve endogenous ceramide generation (tumor necrosis factor, CD95/Fas/APO-1 (40–43); etoposide, staurosporine (44–47)) are either Ca2+-insensitive (CD95/Fas/APO-1 (36, 48), tumor necrosis factor (48, 49)) or have limited sensitivity to Ca2+ (etoposide, staurosporine (20, 50)), which might be related to interaction of Ca2+ with targets other than mitochondria (51, 52). Thus, this model apparently more closely reflects “accidental” apoptosis during ischemia/reperfusion, which is accompanied by Ca2+ overload and oxidative stress and in which participation of PTP is expected (33, 34).
The critical role of PTP as a major apoptotic checkpoint has also been questioned. Recent studies have indicated that cells with inactivated PTP due to cyclophilin D (a main regulatory component of PTP and the CSA target) deficiency undergo normal apoptosis when challenged by variety of stimuli, including etoposide, staurosporine, tunicamycin, thapsigargin, brefeldin A, tumor necrosis factor, TRAIL, moderate Ca2+ overload, and Bax overexpression (53–55). Moreover, overexpression of cyclophilin D delays apoptosis induced by mild oxidative stress and staurosporine (56, 57). At the same time, cyclophilin D deficiency was protective under high intensity oxidative stress and high Ca2+ overload, conditions that precipitate necrosis. These data seem to rule out a critical role for PTP in both extrinsic and intrinsic apoptotic pathways but suggest that PTP opening is instrumental in necrosis. These studies raised questions about the extrapolation of results obtained with artificial short-chain ceramide analogs to in vivo studies and whether long- and short-chain ceramides affect the same binding sites on mitochondria with similar consequences. To address this question, we reinvestigated the effect of ceramides on the permeability of the inner mitochondrial membrane using natural long-chain ceramides delivered in dodecane/ethanol. This system had been successfully utilized for ceramide delivery to subcellular organelles in situ (41, 58) and to enzymes in vitro (59). Our results show that long-chain but not short-chain ceramides suppress PTP activity at physiologically relevant concentrations. These studies raise the interesting possibility that long-chain ceramides promote apoptosis at the level of the mitochondria by causing PTP closure.
EXPERIMENTAL PROCEDURES
Materials—Ceramides were from the Lipidomics Core of the Medical University of South Carolina; α-chymotrypsin (bovine pancreas) and cyclosporin A were from Calbiochem. All other reagents were from Sigma.
Preparation of Mitochondria from Rat Liver—Liver mitochondria were isolated by differential centrifugation as described (60, 61) with some modifications. Briefly, mitochondria were prepared from livers of male Sprague-Dawley rats (220–250 g) fasted overnight. Livers from two rats were homogenized in 100 ml of isolation medium containing 230 mm mannitol, 70 mm sucrose, 2 mm EDTA, and 10 mm HEPES (pH 7.4 adjusted by KOH). Homogenate was centrifuged at 579 × gmax for 10 min to pellet the nucleus and unbroken cells. Supernatant from the previous step was centrifuged at 8,000 × gmax for 10 min to pellet mitochondria. The mitochondrial pellet was resuspended in isolation medium and centrifuged at 8,000 × gmax for 10 min. This step was repeated again, except that isolation medium contained 10 μm of EGTA instead of EDTA. The final mitochondrial pellet was resuspended in the above medium (final protein concentration 60 mg/ml). Where indicated, further mitochondrial purification was performed by self-generated Percoll gradient centrifugation (62). Mitochondrial protein concentration was determined with a bicinchoninic acid assay using bovine serum albumin as a standard (63).
Mitochondrial Incubation Medium—Unless otherwise specified, incubations of isolated mitochondria were conducted at 25 °C, using 1 mg/ml of protein in a medium containing 150 mm KCl, 10 mm HEPES (pH 7.4 adjusted by KOH), 5 mm succinate, 3 mm KH2PO4, and rotenone (2 μm). Deviations from this medium and other reagents employed are described in the figure legends. Mitochondrial membrane potential and swelling were followed simultaneously in a water-jacketed cell equipped with magnetic stirring.
Ceramide Delivery—Ceramides were delivered in ethanol or in 2% dodecane solution in ethanol. In all experiments, ceramides were delivered as a 5-μl aliquot per 1 ml of incubation medium to maintain dodecane 0.01% (0.6 mm) and ethanol at 0.5% (0.1 m). The ceramide solution was warmed to 37 °C and introduced into the medium immediately after adding mitochondria.
Mitochondrial Respiration—Oxygen consumption by mitochondria was measured in a chamber equipped with a Clark type oxygen electrode (Instech Laboratories) at the conditions described under “Mitochondrial Incubation Medium.”
Measurement of Mitochondrial Permeabilization—Inner membrane permeabilization was assayed by measurements of ΔΨ and mitochondrial swelling. ΔΨ as estimated from the accumulation of TPP+ in the mitochondrial matrix as described by Kamo et al. (64). TPP+ (2 μm) was added to the incubation medium as indicated in the figure legends. Mitochondrial swelling was measured by changes in absorbance at 520 nm using a Brinkmann PC 900 probe colorimeter and a fiberoptic probe.
Calcium Retention Capacity (CRC) of Mitochondria—CRC was evaluated according to previously published methods (65) in medium containing 250 mm sucrose, 10 mm Hepes-Tris, 5 mm succinate-Tris, 1 mm Pi-Tris, 10 μm EGTA-Tris, and 2 μm rotenone (pH 7.4) by gradual addition to mitochondria (1 mg/ml at 25 °C) of known amounts of Ca2+. The concentration of Ca2+ in the incubation medium was followed with a Ca2+-selective electrode (Orion).
Cytochrome c Release from Mitochondria—Aliquots of mitochondrial suspension were taken as indicated in the figure legends and centrifuged at 15,000 × g for 3 min. The supernatants and mitochondria were frozen and stored at -80 °C. Cytochrome c in supernatants and mitochondria was quantified using the Quantikine cytochrome c ELISA kit (R & D Systems, Minneapolis, MN). Quantification was performed in duplicates according to the manufacturer's recommendations.
Measurement of Mitochondrial Peptidyl-prolyl cis-trans Isomerase (Cyclophilin D) Activity—Mitochondria (200 μg/ml), untreated or treated with 5 μm C18-ceramide or 5 μm cyclosporin A at 25 °C for 15 min, were permeabilized for 5 min with alamethicin (7 μg/ml) and kept at 10 °C before the analysis. Peptidyl-prolyl cis-trans isomerase activity was assayed at 10 °C as described by Halestrap and Davidson (66). Equal volumes of mitochondrial suspension and medium containing 0.52 mg/ml chymotrypsin were mixed, and the reading at A390 was allowed to stabilize for 2 min. The reaction was initiated by the addition of N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (final concentration, 25 μm) from 25 mm stock dissolved in trifluoroethanol/LiCl (470 mm) (67).
Mitochondrial Ceramide Measurements—Mitochondrial ceramide species were determined by tandem mass spectrometry (MS) (68). Mitochondria were incubated at 25 °C, using protein (1 mg/ml) in a medium containing 150 mm KCl, 10 mm HEPES (pH 7.4 adjusted by KOH), 5 mm succinate, 3 mm KH2PO4, and rotenone (2 μm). The incubations were performed in the absence or in the presence of C18-ceramide (1 nmol/mg protein). After 3 min, aliquots of mitochondrial suspension were taken and centrifuged at 15,000 × g for 3 min. Mitochondrial pellets were lysed in a buffer containing 10 mm Tris and 1% Triton X-100, pH 7.4, for electrospray ionization/MS/MS analysis of ceramides, which was performed on a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer, operating in a multiple reaction-monitoring, positive ionization mode. Mitochondrial lysates, fortified with internal standards, were extracted with ethyl acetate/isopropyl alcohol/water (60:30:10, v/v/v), evaporated to dryness, and reconstituted in 100 μl of methanol. The samples were injected into the HP1100/TSQ 7000 liquid chromatography/MS system and gradient-eluted from the BDS Hypersil C8, 150 × 3.2-mm, 3-μm particle size column, with a 1.0 mm methanolic ammonium formate, 2 mm aqueous ammonium formate mobile phase system. The peaks for the target analytes and internal standards were collected and processed with the Xcalibur software system. Calibration curves were constructed by plotting peak area ratios of synthetic standards, representing each target analyte, to the corresponding internal standard. The target analyte peak area ratios from the samples were similarly normalized to their respective internal standard and compared with the calibration curves using a linear regression model.
Statistical Analysis—The standard curve and the data for cytochrome c release were computed by generation of a four-parameter logistic curve fit. Where indicated, values were expressed as the mean value of the treatment groups ± S.D. Data were analyzed for statistically significant differences between groups using the two-tailed Student's t test. Statistical significance was ascribed to the data when p was <0.05.
RESULTS
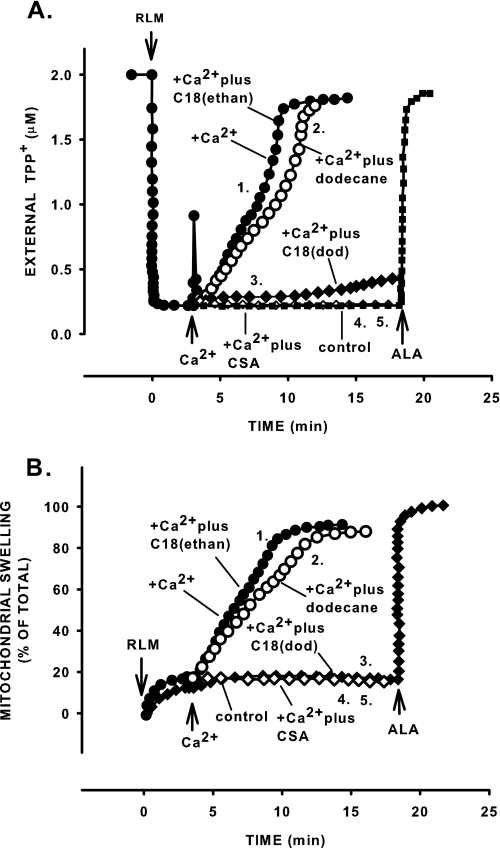
C18-ceramide Suppresses Ca2+-induced Permeability Transition and Increases Mitochondrial CRC—To determine whether natural long chain ceramide modulates the PTP, we used a dodecane/ethanol delivery system, which promotes the incorporation of long-chain ceramide into subcellular compartments (41, 58). Induction of permeability transition under conditions relevant to ischemia/reperfusion (presence of a large amount of Ca2+ and inorganic phosphate) was nearly completely suppressed by 1 nmol/mg protein C18-ceramide (Fig. 1). A 3-min preincubation with C18-ceramide resulted in 74% (736.4 ± 12.4 pmol/mg protein from 1 nmol added) incorporation into mitochondria as measured by MS. C18-ceramide prevented both the Ca2+-induced decline in ΔΨ (Fig. 1A, trace 2 versus trace 3) and large amplitude swelling (Fig. 1B, trace 2 versus trace 3). The suppression was similar to that seen with the potent PTP inhibitor CSA (Fig. 1, A and B, traces 5). Dodecane alone had no effect on these parameters (Fig. 1, A and B, trace 1 versus trace 2). When C18-ceramide was delivered as an ethanol solution, the Ca2+-induced decline in ΔΨ and large amplitude swelling were indistinguishable from those observed in the presence of Ca2+ alone. The lack of effect of C18-ceramide delivered in ethanol is in line with previous reports that suggest the ability of dodecane to improve the delivery of long-chain hydrophobic ceramides into subcellular compartments (41, 58, 59). Cellular contaminants (ER, Golgi, etc.) do not mediate effects of ceramide on mitochondrial PTP opening; similar results were obtained with mitochondria purified by Percoll self-generated gradient centrifugation (results not shown).
FIGURE 1.
C18-ceramide suppresses Ca2+-induced decline in ΔΨ (A) and mitochondrial large amplitude swelling (B). Mitochondria were incubated as described under “Experimental Procedures” except that 2 μm of TPP+ was present from the beginning of the experiment. C18-ceramides were added immediately after the mitochondria. Alamethicin (ALA), a pore-forming peptide (7 μg/mg protein), was added as indicated to induce permeabilization and determine the full extent of potential changes in the parameters of interest. Where indicated, Ca2+ at 200 μm was added to the incubation medium. A and B show time courses of C18-ceramide effect on ΔΨ and mitochondrial swelling, respectively. Traces 1, no additions or 1 μm C18-ceramide in ethanol; traces 2, 5 μl of 2% dodecane; traces 3, 1 μm C18-ceramide in dodecane; traces 4, control without Ca2+ and dodecane; traces 5, Ca2+ plus CSA. The results are typical representative of at three independent experiments.
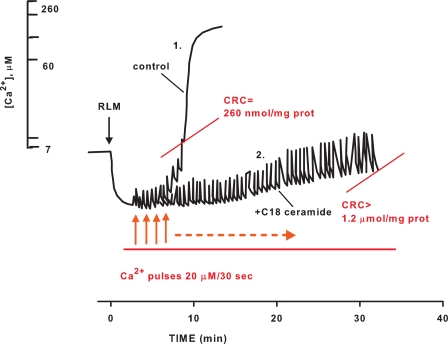
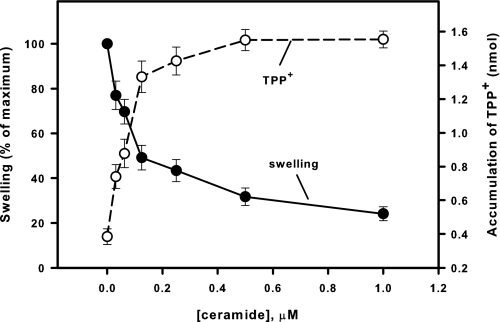
To quantitatively assess the desensitization of PTP to Ca2+ in the presence of C18-ceramide, we studied the effect of C18-ceramide on CRC, which is a quantitative and sensitive measure of the ability of mitochondria to open the PTP in response to accumulated Ca2+. In this assay, mitochondria were loaded with a train of 20 μm Ca2+ pulses until the load reached the threshold of about 260 nmol/mg protein, after which Ca2+ was spontaneously released (Fig. 2, trace 1), and mitochondria underwent CSA-sensitive large amplitude swelling and the discharge of the inner membrane potential (not shown). The presence of C18-ceramide (1 nmol/mg protein) increased CRC greater than 4-fold (Fig. 2, trace 2), indicating considerable desensitization of PTP to Ca2+. The ability of mitochondria to buffer the Ca2+ concentration in the incubation medium after Ca2+ pulses also indicates that suppression of PTP by C18-ceramide is not related to inactivation of electrogenic Ca2+ uptake by the Ca2+ uniporter. Fig. 3 shows the concentration dependence of PTP inhibition by C18-ceramide at conditions similar to those in Fig. 1. It can be appreciated that ceramide suppresses Ca2+-induced PTP opening in the pmol range (IC50 ∼ 130 pmol/mg protein). The data strongly suggest that C18-ceramide-induced inhibition of PTP opening cannot be attributed to the inhibition of the energy-dependent Ca2+ uptake pathway.
FIGURE 2.
Effect of C18-ceramide on calcium retention capacity of mitochondria. Mitochondria were incubated as described under “Experimental Procedures.” Mitochondria were incubated in the presence of dodecane (trace 1) or 1 μm C18-ceramide (trace 2) for 3 min and challenged with the trains of 20 μm Ca2+ pulses. The results are typical representative of three independent experiments.
FIGURE 3.
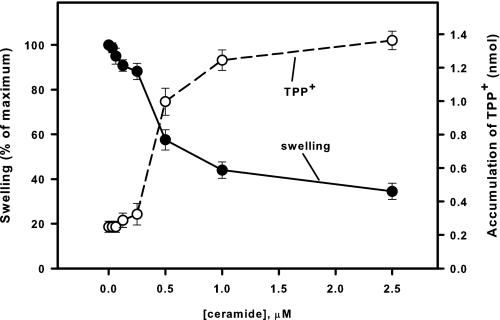
Dose-response curves of the suppression by C18-ceramide of mitochondrial large-amplitude swelling (closed circles) and discharge of ΔΨ (open circle) induced by Ca2+. Mitochondria were incubated as described under “Experimental Procedures” except that 2 μm TPP+ was present from the beginning of the experiment. C18-ceramides were added immediately after the mitochondria. Then 200 μm Ca2+ was added at 3 min to initiate the swelling. The degree of swelling and the amount of accumulated TPP+ were evaluated at the time point where swelling in control attained 90% of maximum of the Ca2+-induced swelling. The values for the swelling in the presence of C18-ceramide were normalized to the swelling observed at this point in the presence of dodecane alone. The results represent the average± S.D. of three independent experiments.
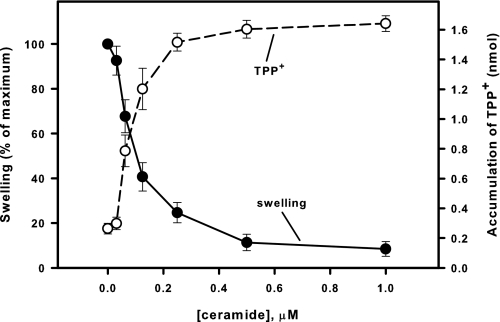
C18-ceramide Suppresses Permeability Transition Induced by Oxidation of Mitochondrial Redox Components—Next, we tested whether C18-ceramide specifically suppresses the Ca2+-related PTP induction pathway or whether this inhibition involves inactivation of a common component on which other pathways of PTP induction converge. In the experiment depicted in Fig. 4, PTP was induced by t-BuOOH, and dependence of PTP induction on concentration of C18-ceramide in the incubation medium was determined. t-BuOOH induces PTP via oxidation of NADH, NADPH, and GSH through a cascade of coupled enzymatic reactions involving glutathione peroxidase and transhydrogenase. C18-ceramide inhibited t-BuOOH-induced PTP opening to an extent similar to inhibition of Ca2+-induced PTP (IC50 ∼ 108 pmol/mg protein). In addition, PTP opening was induced by cross-linking of the vicinal SH-groups of one of the PTP components (presumably ANT (69)) using PhAsO (70–72). C18-ceramide suppressed PhAsO-induced PTP opening but at higher concentrations (IC50 ∼ 790 pmol/mg protein; Fig. 5). The data indicate that natural ceramide inhibits a common component of PTP induction pathways, presumably a level at which PTP is modulated by oxidation of SH groups.
FIGURE 4.
Dose-response curves of the suppression by C18-ceramide of mitochondrial large amplitude swelling (closed circles) and discharge of ΔΨ (open circle) induced by t-BuOOH. Mitochondria were incubated as described under “Experimental Procedures” except that 20 μm Ca2+ and 2 μm of TPP+ were present from the beginning of the experiment. C18-ceramides were added immediately after the mitochondria. Then 200 μm t-BuOOH was added at 3 min to initiate the swelling. The degree of swelling and the amount of accumulated TPP+ were evaluated at the time point where swelling in control attained 90% of the maximum of the Ca2+-induced swelling. The values for the swelling in the presence of C18-ceramide were normalized to the swelling observed at this point in the presence of dodecane alone. The results represent the average ± S.D. of three independent experiments.
FIGURE 5.
Dose-response curves of the suppression by C18-ceramide of mitochondrial large amplitude swelling (closed circles) and discharge of ΔΨ (open circle) induced by PhAsO. Mitochondria were incubated as described under “Experimental Procedures” except that 10 μm Ca2+ and 2 μm of TPP+ were present from the beginning of the experiment. C18-ceramides were added immediately after the mitochondria. Next, 10 μm PhAsO was added at 3 min to initiate the swelling. The degree of swelling and the amount of accumulated TPP+ were evaluated at the time point where swelling in control attained 90% of maximum of the Ca2+-induced swelling. The values for the swelling in the presence of C18-ceramide were normalized to the swelling observed at this point in the presence of dodecane alone. The results represent the average ± S.D. of three independent experiments.
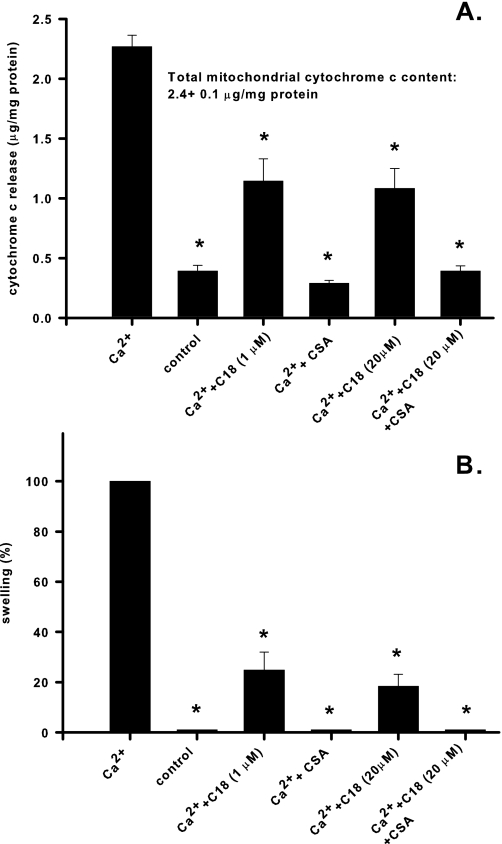
C18-ceramide Suppresses Ca2+-induced Cytochrome c Release—Mitochondrial permeability transition is accompanied by cytochrome c release due to rupture of the outer membrane. Although inhibition of PTP opening by long chain ceramide is expected to prevent cytochrome c release, available data also suggest that natural ceramide could form lipid channels in the outer mitochondrial membrane that are readily permeable to cytochrome c (8). To evaluate the relative contribution of these two ceramide-dependent processes to the net efflux of cytochrome c from the mitochondrial intermembrane space, we measured Ca2+-induced cytochrome c release into the medium in the presence of C18-ceramide. As shown in Fig. 6A, C18-ceramide (1 nmol/mg protein) substantially suppressed Ca2+-induced cytochrome c release. In the presence of CSA, Ca2+-induced cytochrome c release was completely prevented. The degree of suppression of cytochrome c release by C18-ceramide and CSA correlates well with the degree of suppression of large amplitude swelling (Fig. 6B). Further increases in ceramide (up to 20 nmol/mg protein) provided little additional suppression of cytochrome c release and swelling. Interestingly, the addition of C18-ceramide at 20 nmol/mg protein to mitochondria with PTP inhibited by CSA released only a small (∼4.2% of total) fraction of cytochrome c within 18 min.
FIGURE 6.
Effect of C18-ceramide on Ca2+-induced cytochrome c release. Mitochondria were incubated as described under “Experimental Procedures” except that 10 μm EGTA was present from the beginning of the experiment. C18-ceramides (at the indicated concentrations) in dodecane, or dodecane alone, as well as 1 μm CSA (where indicated) were added immediately after the mitochondria. Then, 300 μm Ca2+ was added at 3 min to initiate cytochrome c release. At 15 min after the addition of Ca2+, 1 mm EGTA and 1 μm CSA were added to prevent further permeabilization, and after 2 min samples were taken, treated, and analyzed as described under “Experimental Procedures.” The degree of swelling at 15 min after the addition of Ca2+ was evaluated and expressed as a percentage of the maximum of the Ca2+-induced swelling. The results represent the average ± S.D. of three independent experiments. A two-tailed Student's t test was performed, and the asterisks denote values significantly different from the values obtained in the presence of Ca2+ alone (p < 0.05).
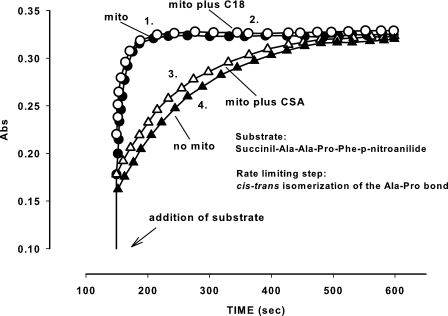
Suppression by C18-ceramide of Permeability Transition Cannot Be Explained by Inhibition of Cyclophilin D Activity— Cyclophilin D is a major component of PTP activity modulation (32, 73). Thus, we tested whether C18-ceramide affects cyclophilin D activity in mitochondria. Because cyclophilin D localizes in the matrix, its catalytic site is inaccessible to the commonly used hydrophilic substrate succinil-Ala-Ala-Pro-Phe-p-nitroanilide. To monitor cyclophilin D activity in mitochondria, the inner mitochondrial membrane was permeabilized using the pore-forming peptide, alamethicin. Cyclophilin D isomerizes the substrate along the Ala-Pro bond, readying it for subsequent cleavage by chymotrypsin with the formation of a colored product (Fig. 7, trace 1). As expected, CSA completely suppressed cyclophilin D activity (trace 3), but C18-ceramide did not have any effect (trace 2). These experiments demonstrate that C18-ceramide does not inhibit cyclophilin D isomerase activity in a manner similar to CSA. The data, however, do not rule out the possibility that C18-ceramide might provoke the dissociation of cyclophilin D from its binding site on PTP.
FIGURE 7.
Effect of C18-ceramide on mitochondrial peptidyl-prolyl cis-trans isomerase activity. Mitochondria were incubated as described under “Experimental Procedures.” Trace 1, mitochondria plus dodecane; trace 2, mitochondria plus C18-ceramide; trace 3, mitochondria plus CSA; trace 4, no mitochondria added. The results are typical, representative of three independent experiments.
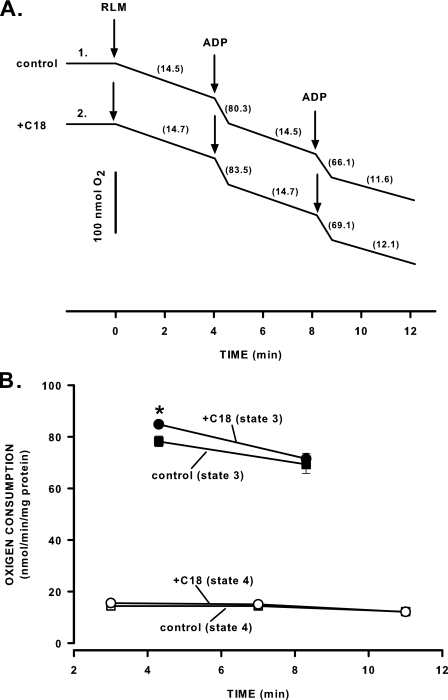
Suppression by C18-ceramide of Permeability Transition Cannot Be Explained by Inhibition of Oxidative Phosphorylation Complex—The activation of PTP could be prevented by a change in the conformation state of VDAC, stabilization of ANT in an m-state, or inhibition of the F0 component of ATP synthase. To verify the potential contribution of these components to the manifestation of C18-ceramide action, we evaluated mitochondrial oxidative phosphorylation in the presence of C18-ceramide. The rate of oxygen consumption in state 3 is determined by passage of substrates through VDAC of the outer membrane, passage of substrates across the inner membrane mediated by the specific transporters, including ANT, and passage of H+ across the F0 portion of ATP synthase. Inhibition of these transport systems results in an interruption of substrate fluxes and a subsequent decrease in the rate of oxygen consumption. Fig. 8 shows that incubation of mitochondria with C18-ceramide (2 nmol/mg protein) for 4 min did not inhibit state 3 respiration. The data indicate that C18-ceramide-induced inhibition of PTP opening does not involve the blockage of the major mitochondrial transport systems. Also, we confirm that higher concentrations of ceramide (5–10 nmol/mg protein) inhibit state 3 respiration in a time-dependent manner (results not shown). It can be related to the known ability of ceramides to inhibit an electron transfer in complex I and complex III of the mitochondrial respiratory chain (74–76).
FIGURE 8.
Effect of C18-ceramide on mitochondrial respiration. Mitochondria were incubated as described under “Experimental Procedures” except that 2 mm Mg2+ was present from the beginning of the experiment. A, mitochondria were incubated with dodecane (trace 1) or 2 μm C18-ceramide (trace 2) for 4 min followed by the addition of 200 μm ADP at 4 and 8 min. The numbers on the plot show respiratory rate expressed in nmol of O2/min/mg of protein. The results are typical representative of three independent experiments. B, the plot of the respiratory rate data obtained in experiments similar to one shown in A. The results represent the average ± S.D. of three independent experiments. A two-taled Student's t test was performed, and the asterisk denotes value significantly different from the values obtained with untreated mitochondria (p < 0.05).
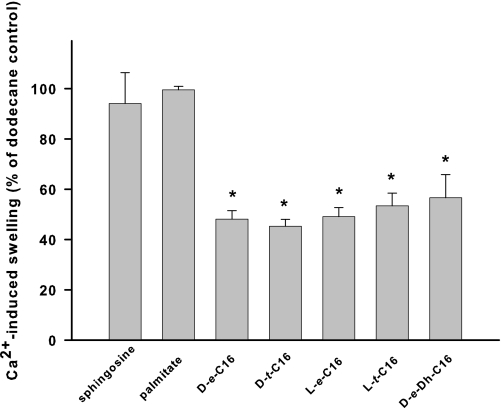
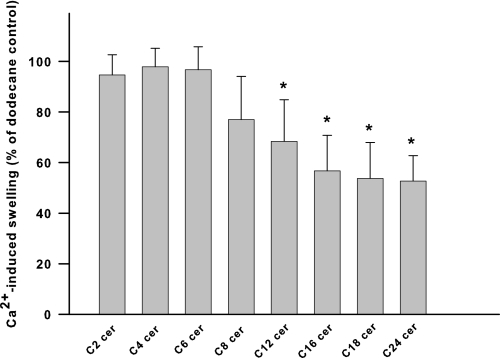
Structural Specificity of Ceramide Suppression of the PTP— To better characterize a potential ceramide-binding site responsible for inhibition of PTP opening, we evaluated the effects of ceramide stereoisomers and the degree of saturation of the 4,5-trans-double bond on PTP inhibition. Stereochemical variations at the C2 and C3 positions of the sphingoid backbone (Fig. 9; d-e-versus l-e-, d-t-, and l-t-C16-ceramide stereoisomers) or the level of saturation at the C4–C5 positions (Fig. 9; d-e-versus d-e-dihydro-C16-ceramide) had similar inhibitory effects on the PTP. Next, modulation of the length of the fatty acyl moiety of ceramide side chain was evaluated for the effect of different chain lengths on the inhibition of PTP opening. Increasing length of the N-acyl chain of ceramides augmented the potency of ceramide as the inhibitor of the permeability transition pore (Fig. 10).
FIGURE 9.
Effect of ceramide stereochemistry on the inhibition of Ca2+-induced large amplitude swelling. Mitochondria were incubated as described under “Experimental Procedures.” Ceramides (250 pmol/mg protein) in dodecane or dodecane alone was added immediately after the mitochondria. Then 100–200 μm Ca2+ was added at 3 min to initiate the swelling. The degree of swelling in the presence of ceramides was evaluated at the moment when the swelling in the control reached 90% of the maximum Ca2+-induced swelling observed in the presence of dodecane. The values for the swelling in the presence of C18-ceramide were normalized to the swelling observed at this point in the presence of dodecane alone. The results represent the average ± S.D. of three independent experiments. A two-tailed Student's t test was performed, and the asterisk denotes a value significantly different from the values obtained with sphingosine (p < 0.05).
FIGURE 10.
Effect of ceramide fatty acid chain length on inhibition of Ca2+-induced large amplitude swelling. Mitochondria were incubated as described under “Experimental Procedures.” Ceramides (250 pmol/mg protein) in dodecane or dodecane alone were added immediately after the mitochondria. Then 100–300 μm Ca2+ was added at 3 min to initiate the swelling. The degree of swelling in the presence of ceramides was evaluated at the moment when the swelling in the control reached 90% of the maximum Ca2+-induced swelling observed in the presence of dodecane. The values for the swelling in the presence of C18-ceramide were normalized to the swelling observed at this point in the presence of dodecane alone. The results represent the average ± S.D. of three independent experiments. A two-tailed Student's t test was performed, and the asterisk denotes a value significantly different from the values obtained with C2-ceramide (p < 0.05).
DISCUSSION
Prior to this study, long-chain ceramides had not been shown to affect PTP activity per se. The difficulty in utilization of long-chain ceramides in vivo and in vitro arises from solubility problems, because long-chain neutral lipids are poorly soluble in aqueous environments.
Establishing the conditions necessary for delivery of long-chain ceramides to mitochondria allowed us to demonstrate that long-chain but not short-chain ceramides almost completely inhibit permeability transition at a concentration as low as 1 nmol/mg protein. Ardail et al. (77) reported the value of ceramide content in rat liver mitochondria equal to 1.66 ± 0.78 nmol/mg protein. Later estimates (78) provide values between 0.14 ± 0.01 and 0.25 ± 0.14 nmol/mg protein, depending on the animal age. Mitochondrial ceramide content can be increased ∼5 times in hepatocytes after tumor necrosis factor stimulation (from 1.28 ± 0.32 to 6.24 ± 0.8 nmol/mg protein) (74). Thus, variations in ceramide content in the range of 0.1–6 nmol/mg protein can be considered to be physiological. In our experiments, 74% (736.4 ± 12.4 pmol, n = 3, from added 1 nmol/mg protein) of ceramide is bound to mitochondria within 3 min. However, it is important to note that in our experiments, unbound ceramide introduced into the medium as dodecane/ethanol solution had a propensity to co-sediment with mitochondria (data not shown). It thus makes it difficult to determine the exact amount of ceramide needed to suppress the PTP. Therefore, 736.4 ± 12.4 pmol/mg protein represents the upper limit needed to suppress PTP. Assuming this upper limit of ceramide binds to mitochondria, it increases total mitochondrial ceramide from 361.8 ± 33.5 to 1066.6 ± 1.9 pmol/mg protein (n = 3) (i.e. within the physiological range). Moreover, it has been shown that ceramide added in alcohols to aqueous solutions may also in part co-sediment with mitochondria (9). Despite these observations, in our studies ceramide dissolved in alcohol failed to suppress the PTP, indicating that it is unlikely to be effectively delivered to mitochondria. Furthermore, it is possible that the dodecane/ceramide combination has effects other than delivery and/or proper insertion of ceramide into mitochondrial membranes. It may, for example, also facilitate diffusion of ceramide to the site of its action, as it had been demonstrated in experiments by Ji et al. (79) and Ardail et al. (58). As for the more potent nature of long chain v/s short chain ceramides as inhibitors of PTP opening, the difference in the degree of ceramide incorporation into mitochondrial membranes may also potentially contribute to this observation.
Nevertheless, inhibition of the PTP by long-chain ceramides in dodecane could represent a physiological phenomenon that might be of importance under pathological conditions that involve accumulation of ceramides in mitochondria (30, 74, 80, 81). Suppression by ceramides of PTP opening induced by either oxidative stress, SH group cross-linking, or high Ca2+ load suggests an inhibitory point at a level at which major PTP regulatory pathways converge, thus underscoring the importance of this regulatory step. It is well known that mitochondrial proteins, such as ANT, ATP synthase, VDAC, and cyclophilin D, modulate PTP activity (32–34, 73, 82–86). The PTP channel was previously thought to be formed by association of ANT, localized in the inner mitochondrial membrane, with VDAC of the outer mitochondrial membrane, with the opening of this channel complex to be regulated by association of cyclophilin D with ANT from the matrix space. Recent experiments with mitochondria from ANT- or VDAC-deficient mice clearly demonstrated that they do not constitute the PTP channel by itself but may be considered regulatory components (87–89).
The nature of proteins that constitute the PTP channel remains unknown. Whether this channel is a separate entity, as had been originally postulated by Hunter and Haworth (90, 91), or whether any random misfolded protein in the inner membrane can form such a channel, as suggested recently (92), remains to be determined.
Our results demonstrate that ceramide does not inhibit activity of ANT, ATP synthase, VDAC, and cyclophilin D when PTP opening is already suppressed. One option is that ceramides can dissociate regulatory enzyme complexes from the PTP channel without interfering with their activity, as documented for ceramide-induced dissociation of cytochrome c from the inner mitochondrial membrane (11, 13). The second possibility is the inhibition by ceramides of some unidentified PTP component.
Another interesting aspect of these studies is the structural specificity of the ceramide binding site involved in PTP inhibition. Often dihydroceramide and ceramide stereoisomers, except d-erythro-ceramide, behave as neutral molecules or exert effects on biological systems that are opposite to those attributed to ceramide (93–96). In the context of isolated mitochondria, hydrophobic binding sites are diverse with respect to saturation of the ceramide 4–5-trans-double bond. Generation of reactive oxygen species by mitochondria in response to ceramide treatment appears to lack specificity with respect to ceramide versus dihydroceramide (74). Also, a report from Richter and co-workers (11) shows a greater than 3-fold increase in cytochrome c release from isolated mitochondria under the effect of C2 dihydroceramide compared with control. Positively charged mimetics of conventional ceramides that readily accumulate in the mitochondrial matrix have only partial selectivity between saturated and unsaturated compounds in the induction of PTP opening (17). At the same time, activation of the PTP in Ca2+-loaded mitochondria in digitonin-permeabilized HepG2 cells (15) and potentiation of Bax-induced PTP opening (16) show considerable specificity for ceramide compared with dihydroceramide. In our studies, variations in dihydroceramide during the time course of apoptosis do not exceed 10% of the total ceramide change.3,4 This suggests that it is the increase in ceramide species that might provide regulatory effects on PTP activity during apoptosis.
To explain these seemingly contradictory data regarding activation/inhibition of PTP by ceramides, one can speculate that PTP possesses at least two regulatory sites. Occupation of the site that is accessible for short chain ceramides may promote pore opening, whereas long-chain ceramide binding to another site may result in pore closure.
The existence of such hydrophobic sites with opposing functions in PTP regulation is well documented, and they are relatively nonspecific. A biphasic dose response was observed with the 1.3-nanosiemens megachannel (counterpart of PTP in electrophysiological studies) and the hydrophobic compounds antimycin A and protoporphyrin IX in studies of the inner mitochondrial membrane of mitoplasts using patch clamp techniques (97, 98). Low concentrations of these compounds decrease the open probability of the 1.3-nanosiemens megachannel, whereas higher concentrations facilitate channel opening. These studies suggest that two separate high and low affinity binding sites for antimycin A and protoporphyrin IX produce these opposing effects. These studies are supportive of subsequent investigations into the effects of a variety of chemically unrelated hydrophobic drugs (butylhydroxytoluene, trifluoperazine, dicyclohexylcarbodiimide, and pentamethylchromanol) on PTP activity in isolated mitochondria (99). Researchers have concluded that, depending on concentration, occupation of relatively nonspecific hydrophobic binding sites in the inner mitochondrial membrane results in PTP opening or closure, but physiological effectors of these sites were undetermined. In a series of recent studies, Fontaine and co-workers (100–102) have provided evidence that these sites might represent the point of PTP regulation by mitochondrial quinones. Using a variety of quinone derivatives, the authors showed that minor structural changes in these compounds drastically modify their effect on the PTP, although quinones of diverse structures may display qualitatively similar properties and may appear to occupy the same binding site that affects PTP activity. Our data suggest that these sites can be candidates for PTP regulation by lipid second messengers.
How ceramide inhibition of the PTP may have a physiological role requires further investigation. One possibility is the preservation of barrier function of the inner mitochondrial membrane during apoptosis.
Mitochondrial dysfunction accompanying apoptosis was attributed to two mechanisms (31, 32, 103, 104). One mechanism is the increase in the outer mitochondrial membrane permeability as a result of translocation/insertion of Bax and Bad, followed by counterdiffusion of cytochrome c (out of the intermembrane space) and caspases (into the intermembrane space). This results in activation of “executioner” caspases by the Apaf-1-cytochrome c-caspase 9 complex formed in the cytoplasm and a decrease in ΔΨ and inactivation of respiration as a consequence of cytochrome c release (105) and proteolysis of complex I and II of the respiratory chain by caspases at the outer surface of the inner membrane (106).
The second mechanism considers alterations in the inner mitochondrial membrane as a primary event of mitochondrial dysfunction in apoptosis, during which PTP opening is thought to be a major event. PTP opening, in turn, completely dissipates ΔΨ and provokes large amplitude swelling with the subsequent rapture of the outer membrane and release of the intermembrane proteins, including cytochrome c.
Both of these mechanisms were considered to be possible candidates for execution of mitochondrial steps in apoptosis. However, recent data support the hypothesis that only the first mechanism operates in apoptosis, because PTP is closed during execution of the apoptotic program (53–55). One could speculate that ceramide elevation in mitochondria at the initial stage of apoptosis (30, 74, 80, 81) keeps the PTP closed, despite the increase in reactive oxygen species (107, 108), which are well known PTP inducers (32–34), in the cytoplasm. This PTP inactivation would allow mitochondria to support adequate ATP production for formation of the apoptosome and might be responsible for the initial rise in ATP production (and hence ΔΨ) during apoptosis (109, 110), an observation that corresponds well with the reported transient mitochondrial hyperpolarization in the apoptosis induced by interleukin-3 withdrawal (111, 112). Subsequent inactivation of the respiratory chain by proteolysis or by ceramides itself (74–76, 113) may account for the frequently observed decrease in ΔΨ at later stages. However, PTP opening by an uncontrolled increase in reactive oxygen species, Ca2+ levels, and ceramide can also contribute to this phenomenon.
In this scenario, ceramide may play a dual role. It promotes translocation/activation of Bax/Bad to mitochondria, allowing formation of cytochrome c-permeable pores, and keeps PTP closed, because Bax by itself has the propensity to activate PTP (16, 114, 115).
This does not mean that ceramide cannot directly open the PTP when its level exceeds some threshold or when an additional combination of factors occurs. However, an indication for such a mechanism at the level of isolated mitochondria is limited and at times contradictory (no effect in the range 2–60 μm (12, 16, 17)) and restricted to short-chain ceramides only. At the cellular level, indications that ceramides open the pore are also limited to short-chain ceramides. Thus, the data presented here suggest a novel mechanism for the regulation of cellular function at the mitochondrial level by lipid second messengers.
Acknowledgments
We thank Kathy Wiita-Fisk for administrative assistance and Dr. Jennifer Schnellmann for help with preparation of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant AG16583 (to L. M. O.), National Institutes of Health Grant P20 RR 17677-04 (to T. I. G.), and National Institutes of Health Grant CO6 RR018823. This work was also supported by Veterans Affairs Merit Awards (to T. I. G. and L. M. O.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PTP, permeability transition pore; ΔΨ, mitochondrial inner membrane potential; CSA, cyclosporine A; CRC, mitochondrial calcium retention capacity; ER, endoplasmic reticulum; MS, mass spectrometry; PhAsO, phenylarsine oxide; t-BuOOH, t-butyl-hydroperoxide; TPP+, tetraphenylphosphonium.
L. M. Obeid and T. D. Mullen, unpublished results.
T. I. Gudz and D. Chudakova, unpublished results.
References
- 1.Taha, T. A., Mullen, T. D., and Obeid, L. M. (2006) Biochim. Biophys. Acta 17582027 -2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pettus, B. J., Chalfant, C. E., and Hannun, Y. A. (2002) Biochim. Biophys. Acta 1585114 -125 [DOI] [PubMed] [Google Scholar]
- 3.Mimeault, M. (2002) FEBS Lett. 530 9-16 [DOI] [PubMed] [Google Scholar]
- 4.Kolesnick, R. N., and Kronke, M. (1998) Annu. Rev. Physiol. 60643 -665 [DOI] [PubMed] [Google Scholar]
- 5.Montes, L. R., Ruiz-Arguello, M. B., Goni, F. M., and Alonso, A. (2002) J. Biol. Chem. 27711788 -11794 [DOI] [PubMed] [Google Scholar]
- 6.Siskind, L. J., and Colombini, M. (2000) J. Biol. Chem. 27538640 -38644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon, C. G., Jr., and Gear, A. R. (1998) Biochemistry 372059 -2069 [DOI] [PubMed] [Google Scholar]
- 8.Siskind, L. J., Kolesnick, R. N., and Colombini, M. (2002) J. Biol. Chem. 27726796 -26803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siskind, L. J., Kolesnick, R. N., and Colombini, M. (2006) Mitochondrion 6 118-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Paola, M., Zaccagnino, P., Montedoro, G., Cocco, T., and Lorusso, M. (2004) J. Bioenerg. Biomembr. 36 165-170 [DOI] [PubMed] [Google Scholar]
- 11.Ghafourifar, P., Klein, S. D., Schucht, O., Schenk, U., Pruschy, M., Rocha, S., and Richter, C. (1999) J. Biol. Chem. 2746080 -6084 [DOI] [PubMed] [Google Scholar]
- 12.Kristal, B. S., and Brown, A. M. (1999) J. Biol. Chem. 27423169 -23175 [DOI] [PubMed] [Google Scholar]
- 13.Yuan, H., Williams, C. D., Adachi, S., Oltersdorf, T., and Gottlieb, R. A. (2003) Mitochondrion 2 237-244 [DOI] [PubMed] [Google Scholar]
- 14.Kashkar, H., Wiegmann, K., Yazdanpanah, B., Haubert, D., and Kronke, M. (2005) J. Biol. Chem. 28020804 -20813 [DOI] [PubMed] [Google Scholar]
- 15.Szalai, G., Krishnamurthy, R., and Hajnoczky, G. (1999) EMBO J. 186349 -6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastorino, J. G., Tafani, M., Rothman, R. J., Marcinkeviciute, A., Hoek, J. B., Farber, J. L., and Marcineviciute, A. (1999) J. Biol. Chem. 27431734 -31739 [DOI] [PubMed] [Google Scholar]
- 17.Novgorodov, S. A., Szulc, Z. M., Luberto, C., Jones, J. A., Bielawski, J., Bielawska, A., Hannun, Y. A., and Obeid, L. M. (2005) J. Biol. Chem. 28016096 -16105 [DOI] [PubMed] [Google Scholar]
- 18.Arora, A. S., Jones, B. J., Patel, T. C., Bronk, S. F., and Gores, G. J. (1997) Hepatology 25 958-963 [DOI] [PubMed] [Google Scholar]
- 19.Pastorino, J. G., Simbula, G., Yamamoto, K., Glascott, P. A., Jr., Rothman, R. J., and Farber, J. L. (1996) J. Biol. Chem. 27129792 -29798 [DOI] [PubMed] [Google Scholar]
- 20.Scorrano, L., Oakes, S. A., Opferman, J. T., Cheng, E. H., Sorcinelli, M. D., Pozzan, T., and Korsmeyer, S. J. (2003) Science 300135 -139 [DOI] [PubMed] [Google Scholar]
- 21.Siskind, L. J., Feinstein, L., Yu, T., Davis, J. S., Jones, D., Choi, J., Zuckerman, J. E., Tan, W., Hill, R. B., Hardwick, J. M., and Colombini, M. (2008) J. Biol. Chem. 2836622 -6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang, C. W., Kanies, C., Kim, K. W., Fang, W. B., Parkhurst, C., Xie, M., Henry, T., and Yang, E. (2003) Mol. Cell. Biol. 236350 -6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruvolo, P. P., Deng, X., Ito, T., Carr, B. K., and May, W. S. (1999) J. Biol. Chem. 27420296 -20300 [DOI] [PubMed] [Google Scholar]
- 24.Datta, S. R., Dudek, H., Tao, X., Masters, S., Fu, H., Gotoh, Y., and Greenberg, M. E. (1997) Cell 91 231-241 [DOI] [PubMed] [Google Scholar]
- 25.Bidere, N., Lorenzo, H. K., Carmona, S., Laforge, M., Harper, F., Dumont, C., and Senik, A. (2003) J. Biol. Chem. 27831401 -31411 [DOI] [PubMed] [Google Scholar]
- 26.Xin, M., and Deng, X. (2006) J. Biol. Chem. 28118859 -18867 [DOI] [PubMed] [Google Scholar]
- 27.Chiang, C. W., Harris, G., Ellig, C., Masters, S. C., Subramanian, R., Shenolikar, S., Wadzinski, B. E., and Yang, E. (2001) Blood 971289 -1297 [DOI] [PubMed] [Google Scholar]
- 28.Heinrich, M., Neumeyer, J., Jakob, M., Hallas, C., Tchikov, V., Winoto-Morbach, S., Wickel, M., Schneider-Brachert, W., Trauzold, A., Hethke, A., and Schutze, S. (2004) Cell Death Differ. 11550 -563 [DOI] [PubMed] [Google Scholar]
- 29.Birbes, H., El Bawab, S., Hannun, Y. A., and Obeid, L. M. (2001) FASEB J. 152669 -2679 [DOI] [PubMed] [Google Scholar]
- 30.Birbes, H., Luberto, C., Hsu, Y. T., El Bawab, S., Hannun, Y. A., and Obeid, L. M. (2005) Biochem. J. 386445 -451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong, J. S. (2006) BioEssays 28253 -260 [DOI] [PubMed] [Google Scholar]
- 32.Kroemer, G., Galluzzi, L., and Brenner, C. (2007) Physiol. Rev. 8799 -163 [DOI] [PubMed] [Google Scholar]
- 33.Crompton, M. (1999) Biochem. J. 341233 -249 [PMC free article] [PubMed] [Google Scholar]
- 34.Crompton, M. (2000) J. Physiol. 52911 -21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacher, P., and Hajnoczky, G. (2001) EMBO J. 204107 -4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinton, P., Ferrari, D., Rapizzi, E., Di Virgilio, F., Pozzan, T., and Rizzuto, R. (2001) EMBO J. 202690 -2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoica, B. A., Movsesyan, V. A., Lea, P. M. T., and Faden, A. I. (2003) Mol. Cell Neurosci. 22 365-382 [DOI] [PubMed] [Google Scholar]
- 38.Lin, C. F., Chen, C. L., Chang, W. T., Jan, M. S., Hsu, L. J., Wu, R. H., Tang, M. J., Chang, W. C., and Lin, Y. S. (2004) J. Biol. Chem. 27940755 -40761 [DOI] [PubMed] [Google Scholar]
- 39.Gendron, M. C., Schrantz, N., Metivier, D., Kroemer, G., Maciorowska, Z., Sureau, F., Koester, S., and Petit, P. X. (2001) Biochem. J. 353357 -367 [PMC free article] [PubMed] [Google Scholar]
- 40.Osawa, Y., Uchinami, H., Bielawski, J., Schwabe, R. F., Hannun, Y. A., and Brenner, D. A. (2005) J. Biol. Chem. 28027879 -27887 [DOI] [PubMed] [Google Scholar]
- 41.Paris, F., Grassme, H., Cremesti, A., Zager, J., Fong, Y., Haimovitz-Friedman, A., Fuks, Z., Gulbins, E., and Kolesnick, R. (2001) J. Biol. Chem. 2768297 -8305 [DOI] [PubMed] [Google Scholar]
- 42.Dbaibo, G. S., El-Assaad, W., Krikorian, A., Liu, B., Diab, K., Idriss, N. Z., El-Sabban, M., Driscoll, T. A., Perry, D. K., and Hannun, Y. A. (2001) FEBS Lett. 503 7-12 [DOI] [PubMed] [Google Scholar]
- 43.Gamard, C. J., Dbaibo, G. S., Liu, B., Obeid, L. M., and Hannun, Y. A. (1997) J. Biol. Chem. 27216474 -16481 [DOI] [PubMed] [Google Scholar]
- 44.Sawada, M., Nakashima, S., Banno, Y., Yamakawa, H., Hayashi, K., Takenaka, K., Nishimura, Y., Sakai, N., and Nozawa, Y. (2000) Cell Death Differ. 7761 -772 [DOI] [PubMed] [Google Scholar]
- 45.Ferraro, C., Quemeneur, L., Fournel, S., Prigent, A. F., Revillard, J. P., and Bonnefoy-Berard, N. (2000) Cell Death Differ. 7197 -206 [DOI] [PubMed] [Google Scholar]
- 46.Tepper, A. D., de Vries, E., van Blitterswijk, W. J., and Borst, J. (1999) J. Clin. Invest. 103971 -978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiesner, D. A., and Dawson, G. (1996) J. Neurochem. 661418 -1425 [DOI] [PubMed] [Google Scholar]
- 48.Hampton, M. B., Vanags, D. M., Porn-Ares, M. I., and Orrenius, S. (1996) FEBS Lett. 399277 -282 [DOI] [PubMed] [Google Scholar]
- 49.McFarlane, S. M., Anderson, H. M., Tucker, S. J., Jupp, O. J., and MacEwan, D. J. (2000) Mol. Cell Biochem. 211 19-26 [DOI] [PubMed] [Google Scholar]
- 50.Rabkin, S. W., and Kong, J. Y. (2002) Cell Biol. Int. 26433 -440 [DOI] [PubMed] [Google Scholar]
- 51.Aoyama, M., Grabowski, D. R., Dubyak, G. R., Constantinou, A. I., Rybicki, L. A., Bukowski, R. M., Ganapathi, M. K., Hickson, I. D., and Ganapathi, R. (1998) Biochem. J. 336727 -733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grabowski, D. R., Dubyak, G. R., Rybicki, L., Hidaka, H., and Ganapathi, R. (1998) Biochem. Pharmacol. 56 345-349 [DOI] [PubMed] [Google Scholar]
- 53.Schinzel, A. C., Takeuchi, O., Huang, Z., Fisher, J. K., Zhou, Z., Rubens, J., Hetz, C., Danial, N. N., Moskowitz, M. A., and Korsmeyer, S. J. (2005) Proc. Natl. Acad. Sci. U. S. A. 10212005 -12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakagawa, T., Shimizu, S., Watanabe, T., Yamaguchi, O., Otsu, K., Yamagata, H., Inohara, H., Kubo, T., and Tsujimoto, Y. (2005) Nature 434652 -658 [DOI] [PubMed] [Google Scholar]
- 55.Baines, C. P., Kaiser, R. A., Purcell, N. H., Blair, N. S., Osinska, H., Hambleton, M. A., Brunskill, E. W., Sayen, M. R., Gottlieb, R. A., Dorn, G. W., Robbins, J., and Molkentin, J. D. (2005) Nature 434658 -662 [DOI] [PubMed] [Google Scholar]
- 56.Lin, D. T., and Lechleiter, J. D. (2002) J. Biol. Chem. 27731134 -31141 [DOI] [PubMed] [Google Scholar]
- 57.Li, Y., Johnson, N., Capano, M., Edwards, M., and Crompton, M. (2004) Biochem. J. 383101 -109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ardail, D., Popa, I., Bodennec, J., Famy, C., Louisot, P., and Portoukalian, J. (2002) Biochim. Biophys. Acta 1583305 -310 [DOI] [PubMed] [Google Scholar]
- 59.Chalfant, C. E., Kishikawa, K., Mumby, M. C., Kamibayashi, C., Bielawska, A., and Hannun, Y. A. (1999) J. Biol. Chem. 27420313 -20317 [DOI] [PubMed] [Google Scholar]
- 60.Graham, J. M. (1999) in Current Protocols in Cell Biology (Bonifacino, J. S., Dasso, M., Harford, J. B., Lippincott-Schwartz, J., and Yamada, K. M., eds) pp.3.3.1 -3.3.3, John Wiley & Sons, Inc., New York
- 61.Frezza, C., Cipolat, S., and Scorrano, L. (2007) Nat. Protocols 2287 -295 [DOI] [PubMed] [Google Scholar]
- 62.Hoppel, C. L., Kerner, J., Turkaly, P., Turkaly, J., and Tandler, B. (1998) J. Biol. Chem. 27323495 -23503 [DOI] [PubMed] [Google Scholar]
- 63.Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J., and Klenk, D. C. (1985) Anal. Biochem. 15076 -85 [DOI] [PubMed] [Google Scholar]
- 64.Kamo, N., Muratsuga, M., Hongoh, R., and Kobatake, Y. (1979) J. Membr. Biol. 49 105-121 [DOI] [PubMed] [Google Scholar]
- 65.Soriano, M. E., Nicolosi, L., and Bernardi, P. (2004) J. Biol. Chem. 27936803 -36808 [DOI] [PubMed] [Google Scholar]
- 66.Halestrap, A. P., and Davidson, A. M. (1990) Biochem. J. 268153 -160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Echeverria, C., Kofron, J. L., Kuzmic, P., and Rich, D. H. (1993) Biochem. Biophys. Res. Commun. 191 70-75 [DOI] [PubMed] [Google Scholar]
- 68.Bielawski, J., Szulc, Z. M., Hannun, Y. A., and Bielawska, A. (2006) Methods 39 82-91 [DOI] [PubMed] [Google Scholar]
- 69.McStay, G. P., Clarke, S. J., and Halestrap, A. P. (2002) Biochem. J. 367541 -548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chernyak, B. V., and Bernardi, P. (1996) Eur. J. Biochem. 238623 -630 [DOI] [PubMed] [Google Scholar]
- 71.Costantini, P., Chernyak, B. V., Petronilli, V., and Bernardi, P. (1996) J. Biol. Chem. 2716746 -6751 [DOI] [PubMed] [Google Scholar]
- 72.Lenartowicz, E., Bernardi, P., and Azzone, G. F. (1991) J. Bioenerg. Biomembr. 23 679-688 [DOI] [PubMed] [Google Scholar]
- 73.Rasola, A., and Bernardi, P. (2007) Apoptosis 12815 -833 [DOI] [PubMed] [Google Scholar]
- 74.Garcia-Ruiz, C., Colell, A., Mari, M., Morales, A., and Fernandez-Checa, J. C. (1997) J. Biol. Chem. 27211369 -11377 [DOI] [PubMed] [Google Scholar]
- 75.Gudz, T. I., Tserng, K. Y., and Hoppel, C. L. (1997) J. Biol. Chem. 27224154 -24158 [DOI] [PubMed] [Google Scholar]
- 76.Di Paola, M., Cocco, T., and Lorusso, M. (2000) Biochemistry 396660 -6668 [DOI] [PubMed] [Google Scholar]
- 77.Ardail, D., Popa, I., Alcantara, K., Pons, A., Zanetta, J. P., Louisot, P., Thomas, L., and Portoukalian, J. (2001) FEBS Lett. 488160 -164 [DOI] [PubMed] [Google Scholar]
- 78.Tserng, K. Y., and Griffin, R. (2003) Anal. Biochem. 32384 -93 [DOI] [PubMed] [Google Scholar]
- 79.Ji, L., Zhang, G., Uematsu, S., Akahori, Y., and Hirabayashi, Y. (1995) FEBS Lett. 358211 -214 [DOI] [PubMed] [Google Scholar]
- 80.Dai, Q., Liu, J., Chen, J., Durrant, D., McIntyre, T. M., and Lee, R. M. (2004) Oncogene 233650 -3658 [DOI] [PubMed] [Google Scholar]
- 81.Matsko, C. M., Hunter, O. C., Rabinowich, H., Lotze, M. T., and Amoscato, A. A. (2001) Biochem. Biophys. Res. Commun. 2871112 -1120 [DOI] [PubMed] [Google Scholar]
- 82.Zamzami, N., Larochette, N., and Kroemer, G. (2005) Cell Death Differ. 12 Suppl. 2, 1478-1480 [DOI] [PubMed] [Google Scholar]
- 83.Novgorodov, S. A., Gudz, T. I., Mohr Yu, E., Goncharenko, E. N., and Yaguzhinsky, L. S. (1989) FEBS Lett. 247255 -258 [DOI] [PubMed] [Google Scholar]
- 84.Novgorodov, S. A., Gudz, T. I., Brierley, G. P., and Pfeiffer, D. R. (1994) Arch. Biochem. Biophys. 311219 -228 [DOI] [PubMed] [Google Scholar]
- 85.Shimizu, S., Matsuoka, Y., Shinohara, Y., Yoneda, Y., and Tsujimoto, Y. (2001) J. Cell Biol. 152237 -250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Azoulay-Zohar, H., Israelson, A., Abu-Hamad, S., and Shoshan-Barmatz, V. (2004) Biochem. J. 377347 -355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kokoszka, J. E., Waymire, K. G., Levy, S. E., Sligh, J. E., Cai, J., Jones, D. P., MacGregor, G. R., and Wallace, D. C. (2004) Nature 427461 -465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krauskopf, A., Eriksson, O., Craigen, W. J., Forte, M. A., and Bernardi, P. (2006) Biochim. Biophys. Acta 1757590 -595 [DOI] [PubMed] [Google Scholar]
- 89.Baines, C. P., Kaiser, R. A., Sheiko, T., Craigen, W. J., and Molkentin, J. D. (2007) Nat. Cell Biol. 9 550-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haworth, R. A., and Hunter, D. R. (1979) Arch. Biochem. Biophys. 195460 -467 [DOI] [PubMed] [Google Scholar]
- 91.Haworth, R. A., and Hunter, D. R. (1980) J. Membr. Biol. 54231 -236 [DOI] [PubMed] [Google Scholar]
- 92.He, L., and Lemasters, J. J. (2002) FEBS Lett. 5121 -7 [DOI] [PubMed] [Google Scholar]
- 93.Dobrowsky, R. T., Kamibayashi, C., Mumby, M. C., and Hannun, Y. A. (1993) J. Biol. Chem. 26815523 -15530 [PubMed] [Google Scholar]
- 94.Chalfant, C. E., Szulc, Z., Roddy, P., Bielawska, A., and Hannun, Y. A. (2004) J. Lipid Res. 45 496-506 [DOI] [PubMed] [Google Scholar]
- 95.Wolff, R. A., Dobrowsky, R. T., Bielawska, A., Obeid, L. M., and Hannun, Y. A. (1994) J. Biol. Chem. 26919605 -19609 [PubMed] [Google Scholar]
- 96.Bielawska, A., Crane, H. M., Liotta, D., Obeid, L. M., and Hannun, Y. A. (1993) J. Biol. Chem. 26826226 -26232 [PubMed] [Google Scholar]
- 97.Campo, M. L., Kinnally, K. W., and Tedeschi, H. (1992) J. Biol. Chem. 2678123 -8127 [PubMed] [Google Scholar]
- 98.Kinnally, K. W., Zorov, D. B., Antonenko, Y. N., Snyder, S. H., McEnery, M. W., and Tedeschi, H. (1993) Proc. Natl. Acad. Sci. U. S. A. 901374 -1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gudz, T., Eriksson, O., Kushnareva, Y., Saris, N. E., and Novgorodov, S. (1997) Arch. Biochem. Biophys. 342143 -156 [DOI] [PubMed] [Google Scholar]
- 100.Fontaine, E., Ichas, F., and Bernardi, P. (1998) J. Biol. Chem. 27325734 -25740 [DOI] [PubMed] [Google Scholar]
- 101.Walter, L., Miyoshi, H., Leverve, X., Bernard, P., and Fontaine, E. (2002) Free Radic. Res. 36 405-412 [DOI] [PubMed] [Google Scholar]
- 102.Walter, L., Nogueira, V., Leverve, X., Heitz, M. P., Bernardi, P., and Fontaine, E. (2000) J. Biol. Chem. 27529521 -29527 [DOI] [PubMed] [Google Scholar]
- 103.Martinou, J. C., and Green, D. R. (2001) Nat. Rev. Mol. Cell. Biol. 2 63-67 [DOI] [PubMed] [Google Scholar]
- 104.Scorrano, L., and Korsmeyer, S. J. (2003) Biochem. Biophys. Res. Commun. 304437 -444 [DOI] [PubMed] [Google Scholar]
- 105.Mootha, V. K., Wei, M. C., Buttle, K. F., Scorrano, L., Panoutsakopoulou, V., Mannella, C. A., and Korsmeyer, S. J. (2001) EMBO J. 20 661-671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ricci, J. E., Gottlieb, R. A., and Green, D. R. (2003) J. Cell Biol. 16065 -75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Curtin, J. F., Donovan, M., and Cotter, T. G. (2002) J. Immunol. Methods 26549 -72 [DOI] [PubMed] [Google Scholar]
- 108.Chen, Q., Crosby, M., and Almasan, A. (2003) Korean J. Biol. Sci. 71 -9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Atlante, A., Giannattasio, S., Bobba, A., Gagliardi, S., Petragallo, V., Calissano, P., Marra, E., and Passarella, S. (2005) Biochim. Biophys. Acta 1708 50-62 [DOI] [PubMed] [Google Scholar]
- 110.Sanchez-Alcazar, J. A., Ruiz-Cabello, J., Hernandez-Munoz, I., Pobre, P. S., de la Torre, P., Siles-Rivas, E., Garcia, I., Kaplan, O., Munoz-Yague, M. T., and Solis-Herruzo, J. A. (1997) J. Biol. Chem. 27230167 -30177 [DOI] [PubMed] [Google Scholar]
- 111.Vander Heiden, M. G., Chandel, N. S., Williamson, E. K., Schumacker, P. T., and Thompson, C. B. (1997) Cell 91627 -637 [DOI] [PubMed] [Google Scholar]
- 112.Khaled, A. R., Reynolds, D. A., Young, H. A., Thompson, C. B., Muegge, K., and Durum, S. K. (2001) J. Biol. Chem. 2766453 -6462 [DOI] [PubMed] [Google Scholar]
- 113.Corda, S., Laplace, C., Vicaut, E., and Duranteau, J. (2001) Am. J. Respir. Cell Mol. Biol. 24 762-768 [DOI] [PubMed] [Google Scholar]
- 114.Narita, M., Shimizu, S., Ito, T., Chittenden, T., Lutz, R. J., Matsuda, H., and Tsujimoto, Y. (1998) Proc. Natl. Acad. Sci. U. S. A. 9514681 -14686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gogvadze, V., Robertson, J. D., Zhivotovsky, B., and Orrenius, S. (2001) J. Biol. Chem. 27619066 -19071 [DOI] [PubMed] [Google Scholar]