Abstract
Accumulation of indigestible lipofuscin and decreased mitochondrial energy production are characteristic age-related changes of post-mitotic retinal pigment epithelial (RPE) cells in the human eye. To test whether these two forms of age-related impairment have interdependent effects, we quantified the ATP-dependent phagocytic function of RPE cells loaded or not with the lipofuscin component A2E and inhibiting or not mitochondrial ATP synthesis either pharmacologically or genetically. We found that physiological levels of lysosomal A2E reduced mitochondrial membrane potential and inhibited oxidative phosphorylation (OXPHOS) of RPE cells. Furthermore, in media with physiological concentrations of glucose or pyruvate, A2E significantly inhibited phagocytosis. Antioxidants reversed these effects of A2E, suggesting that A2E damage is mediated by oxidative processes. Because mitochondrial mutations accumulate with aging, we generated novel genetic cellular models of RPE carrying mitochondrial DNA point mutations causing either moderate or severe mitochondrial dysfunction. Exploring these mutant RPE cells we found that, by itself, only the severe but not the moderate OXPHOS defect reduces phagocytosis. However, sub-toxic levels of lysosomal A2E are sufficient to reduce phagocytic activity of RPE with moderate OXPHOS defect and cause cell death of RPE with severe OXPHOS defect. Taken together, RPE cells rely on OXPHOS for phagocytosis when the carbon energy source is limited. Our results demonstrate that A2E accumulation exacerbates the effects of moderate mitochondrial dysfunction. They suggest that synergy of sub-toxic lysosomal and mitochondrial changes in RPE cells with age may cause RPE dysfunction that is known to contribute to human retinal diseases like age-related macular degeneration.
Retinal pigment epithelial (RPE)5 cells form a polarized monolayer epithelium between the photoreceptors of the neurosensory retina and the choroidal capillary bed. Daily phagocytosis of outer segment (OS) tips shed by adjacent photoreceptors is a vital task of the RPE (recently reviewed by Strauss (1)). RPE cells are post-mitotic and face each ∼30 photoreceptor outer segments in the human eye, all of which shed their distal tip containing stacked membrane disks once a day. Diurnal phagocytosis and digestion of thousands of OS disks for life renders RPE cells the most active phagocytes in the body. Photoreceptor function strictly depends on efficient RPE phagocytosis of spent OS. Complete failure of RPE cells to engulf OS causes rapid photoreceptor degeneration in the Royal College of Surgeons rat (2–4). Impaired RPE phagocytosis also contributes to human retinal disease such as retinitis pigmentosa and, likely, age-related macular degeneration (5, 6).
The continuous nature of outer segment renewal implies that any delay in OS removal by aged or damaged RPE will gradually cause OS components to accumulate. RPE cells are at risk for oxidative damage due to their location in the highly oxygenated environment of the outer retina and their exposure to light (7). Indeed, storage bodies containing autofluorescent age lipids termed “lipofuscin” build up in human RPE over time as a direct consequence of incomplete OS digestion (8). Although some other post-mitotic cells build up age lipid granules with age, excess accumulation of RPE lipofuscin containing specific retinoids is associated with several hereditary retinal degenerations (9, 10) and may contribute to age-related macular degeneration (11). Delayed OS opsin degradation in transgenic mice directly causes lipofuscin accumulation and retinal dysfunction with age (12). Loss of synchronicity of OS clearance in β5 integrin null mice is sufficient to promote accumulation of lipofuscin and loss of photoreceptor function with age (13).
Lipofuscin of the RPE consists of an incompletely characterized and complex mixture of lipids, particularly lipid peroxides, proteins, and different autofluorescent compounds derived mainly from vitamin A as byproducts of the visual cycle. The best characterized fluorophore of lipofuscin is the pyridinium bis-retinoid A2E, a lipophilic quaternary amine that forms from two molecules of all-trans-retinal and one of phosphatidylethanolamine, both components of photoreceptor outer segment membranes (14). Numerous earlier studies have demonstrated that A2E accumulation in RPE cells in culture at levels found in the normal aging human eye are not directly cytotoxic but decrease resistance to light and oxidative stress (15, 16). We found previously that A2E has no effect on OS binding and internalization by RPE cells in culture but slows phagolysosomal digestion specifically of OS phospholipids (17).
Mitochondria play a central role in aging and in the pathogenesis of age-related neurodegenerative diseases, mainly for two reasons: they are the main source of cellular ATP and the major cellular source of reactive oxygen species (18). Oxidative damage to mitochondria can lead to a spiral of noxious effects, whereby damaged mitochondria in turn release more reactive oxygen species, increasing oxidative damage and leading eventually to dysfunctional or defective mitochondria (19, 20). Post-mitotic tissues like the RPE are most susceptible to this damage, particularly because they are more likely to accumulate somatic mutations in their mitochondrial DNA (mtDNA). Mutations of mtDNA and decreases in RPE cell number during aging and age-related macular degeneration have been described (21–23).
We hypothesized that lipofuscin/A2E accumulation and mitochondrial damage could be interrelated problems that cooperate in progressively impairing vital RPE energy-dependent RPE functions, including phagocytosis. To test this hypothesis, we determined the phagocytic activity of RPE cells in culture in the presence and absence of A2E while manipulating mitochondrial function either pharmacologically or genetically. A2E accumulation in RPE lysosomal compartments at levels comparable to those occurring during normal aging in the human eye was sufficient to significantly decrease mitochondrial oxidative phosphorylation (OXPHOS) and membrane potential (ΔΨm). In the presence of physiological concentrations of either glucose or pyruvate as carbon sources, this effect of A2E significantly decreased cellular ATP content and the phagocytic activity of RPE cells. Interestingly, antioxidant supplementation was sufficient to promptly rescue the effect of A2E on ATP synthesis, ΔΨm, and phagocytosis. To complement these studies, we generated and explored novel genetic models of RPE harboring moderate or severe mitochondrial ATP synthesis defects using the cybrid technology (24). As predicted, we found that RPE cybrids phagocytose less if handicapped by severe mitochondrial ATP synthesis defects. Moreover, they become increasingly sensitive to A2E load: A2E load without effect on wild-type cybrids reduced the phagocytic activity of cybrids with moderate OXPHOS capacity and was lethal for cybrids with diminished OXPHOS. Taken together, these results suggest that the lipofuscin component A2E via oxidative mechanisms may enhance effects of moderate mitochondrial defects to impair energy-dependent functions of aging RPE cells.
EXPERIMENTAL PROCEDURES
Reagents—All reagents were from Sigma-Aldrich unless otherwise stated.
A2E Synthesis, Verification, and Quantification—A2E was synthesized according to published procedures (25). Briefly, ethanolamine (19 mg and 352 μmol), all-trans-retinal (200 mg and 704 μmol), and acetic acid (19 μl and 155 μmol) were incubated in ethanol (3 ml) at ambient temperature for 2 days in the dark. The mixture was concentrated in vacuo and purified (3×) by silica gel column chromatography using a gradient elution from 5:95 methanol:CH2Cl2 to 20:80 methanol:CH2Cl2 to give A2E, which was verified by NMR spectroscopy. 50 mm A2E in ethanol was stored in aliquots in the dark at -80 °C. A2E efficacy and integrity were routinely compared among used and new aliquots by quantifying effects on pyruvate-dependent phagocytosis and by assessing separation and fluorescence properties using TLC as described previously (17). Fluorescence scanning using a Typhoon Trio+ scanner (GE Healthcare, Piscataway, NJ) was used to quantify A2E fluorescence signals of cells on coverslips. Evaluation of fluorescence intensities in the red channel (excitation 532 nm and emission 580 nm) using ImageQuaNT v1.2 (Molecular Dynamics, Sunnyvale, CA) yielded relative fluorescence values allowing to compare A2E load between samples.
RPE Cell Lines and Primary RPE Cell Culture—For all experiments in this study, live cells were kept at growth temperatures (32 °C for RPE-J cells, 37 °C for d407 cybrids and primary RPE), under dim white light during manipulation (unless otherwise indicated) and in the dark in 5% CO2 atmosphere during incubations. RPE cell lines rat RPE-J (ATCC) and human d407 (a gift from Dr. R. C. Hunt, University of South Carolina, Columbia, SC), and d407 RPE-derived cybrid clones were routinely grown to confluence in Dulbecco's modified Eagle's medium containing 4.5 g/liter glucose supplemented with 4 and 3% fetal bovine serum, respectively, as described previously (26). The day prior to an experiment, cells on coverslips or on chamber slides received 50 μm (RPE-J) or 10 μm (cybrids) A2E for 6 h followed by incubation with regular growth medium overnight. Rat RPE was isolated from 10-day-old Long Evans rat pups (Charles River, Wilmington, MA) according to established procedures (27). Briefly, cornea, lens, iris, and vitreous body were removed from eyes enucleated from CO2-asphyxiated rats. Eyecups were incubated in 1 mg/ml hyaluronidase in Ca2+, Mg2+-free Hanks' balanced saline solution for 50 min at 37 °C. The neural retina was removed, and eyecups were incubated in 2 mg/ml trypsin in Hanks' balanced saline solution for 45 min at 37 °C. RPE sheets were collected manually from the underlying choroid and plated in 96-well plates or 4-well chamber slides (Nalgene Nunc International, Rochester, NY) at a density of ∼5–10,000 cells/well. Primary RPE was grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum for 4 days before feeding with 50 μm A2E for 3 h followed by overnight incubation in fresh growth medium. Comparative fluorescence scanning (see above) and fluorescence microscopy confirmed that this protocol resulted in A2E accumulation in lysosomes of primary rat RPE at a level similar to the load of RPE-J cell lysosomes following feeding with A2E for 6 h (data not shown). All procedures involving animals were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee.
Generation of RPE Cybrid Cell Lines—143B-derived cybrids containing homoplasmic (i.e. 100%) levels of mutant mtDNA (T8993G, NARP and A8344G, MERRF) or wild-type mtDNA were enucleated by treating with 0.5 μg/ml actinomycin D for 15 h (28). d407 RPE cells devoid of mtDNA (d407-ρ0 cells) were obtained by treating with 1 mm rhodamine 6G for 4 days (29). d407-derived cybrids were obtained by fusion of enucleated 143B-derived cybrids with d407-ρ0 cells, in the presence of 45% polyethylene glycol (24). Cybrids repopulated with 143B mtDNA were selected in medium lacking uridine. Resistant clones were isolated after 15 days of selection, using cloning cylinders. The correct mtDNA genotypes in each clone were confirmed by restriction fragment length polymorphism analysis of PCR fragments of mtDNA encompassing the T8993G (30) and the A8344G (31) mutations as described. MERRF cybrids were grown in the presence of 50 μg/ml uridine.
Biochemistry—ATP synthesis was measured using a luciferin luciferase method with malate plus pyruvate or with succinate as substrates in the presence of 1 μm rotenone, exactly as described (32).
Oxygen consumption in intact cells was measured with a Clark-type electrode in an oxygraph chamber (Hansatech Inc., UK) exactly as described (33). 1 μm carbonyl cyanide p-trifluoromethoxyphenylhydrazone was added to uncouple mitochondrial respiration. 0.53 mm KCN was added to inhibit mitochondrial respiration.
ATP steady-state levels were measured in trichloroacetic acid extracts of cells using ENLITEN-ATP Assay System Bioluminescence Detection Kit for ATP Measurement (Promega, Madison, WI) according to the manufacturer's instructions. A concentration gradient of a commercial ATP standard was also measured for each experiment to calculate absolute ATP concentrations from relative luminescence values.
OS Preparation and Phagocytosis Assays—Bovine OS particles were isolated from fresh cow eyes and covalently labeled with FITC according to standard procedures (34). Cells were preincubated in medium without carbon sources for 1 h prior to feeding with OS. As described in detail previously, cells were fed FITC-conjugated OS at a ratio of 10 OS/cell in media with 5% dialyzed fetal bovine serum and defined carbon sources before fixation in methanol (35). RPE-J cells and d407 cybrids received OS for 5 h. Because primary RPE cells phagocytose at a faster rate than RPE cell lines (26), we fed them with FITC-labeled OS for 1.5 h. To test the effects of antioxidant, 20 μm Trolox was included in the OS suspension for the duration of the uptake assay. Trolox was chosen for pilot experiments because it did not alter OS binding or internalization by control cells in medium with 4.5 g/liter glucose, whereas NAC moderately reduced OS internalization irrespective of energy supplies, which could have confounded results. To quench FITC fluorescence of surface-bound but not engulfed OS, cells were treated with 0.2% trypan blue for 10 min prior to fixation (35). Samples with and without trypan blue treatment were mounted on glass slides, scanned using a Typhoon Trio+ scanner, and quantified using ImageQuaNT v1.2 yielding relative fluorescence values representing total (bound plus internal) and internal OS, respectively. Bound OS were calculated by subtracting internal from total OS (26).
Immunoblotting—Confluent cells without preincubation were solubilized in 50 mm HEPES, pH 7.4, 150 mm NaCl, 10% glycerol, 1.5 mm MgCl2, 1% Triton X-100, and 1% protease inhibitor mixture. Whole cell lysates representing equal numbers of cells were separated on 10% SDS-polyacrylamide gels and electroblotted. Immunoblots were probed with primary antibodies to tubulin (a gift from Dr. G. Kreitzer, Weill Cornell Medical College, New York, NY), αv integrin, focal adhesion kinase (both BD Bioscience, San Jose, CA), β5 integrin (Santa Cruz Biotechnology, Santa Cruz, CA), MerTK (R&D Systems, Minneapolis, MN), porin, and COX-1 (both Invitrogen, Carlsbad, CA). Incubation with horseradish peroxidase-conjugated secondary antibodies was followed by ECL detection (PerkinElmer Life Sciences).
Live-cell Organelle Marker and Immunofluorescence Microscopy—To study subcellular distribution of A2E, RPE-J cells grown on glass coverslips and loaded or not with A2E were incubated for 5 min each with 1 μm LysoSensor blue and/or 1 μm MitoTracker green FM added from stock solutions in DMSO directly into the growth medium (both Invitrogen). Single x-y scans of live cells were acquired in sequential scanning mode on a TSP2 confocal system (Leica, Germany). All signals were acquired in the same order with all laser lines at 10% intensity and with the following parameters: excitation 488 nm, emission read 513–527 nm for MitoTracker green; excitation 543 nm, emission read 603–750 for A2E; and excitation 405 nm, emission read 406–480 nm for LysoSensor blue. Samples with one of the fluorophores omitted were scanned first to establish gain and offset acquisition settings that ensured that each acquired signal was due to emission by individual fluorophores only. All scans were acquired using identical settings and were compiled into panels and processed identically by adjusting image brightness and contrast in Adobe Photoshop 7.0.
To study mitochondrial marker proteins porin and COX-1, cells without preincubation were fixed in ice-cold methanol and incubated sequentially with primary and AlexaFluor488-conjugated donkey anti-mouse secondary antibodies (Invitrogen). Confocal imaging of single x-y scans was performed using a Leica TSP2 system with excitation 488 nm and reading emission 515–540 nm. Images for panels showing the same marker were acquired from one experiment with identical settings and recompiled in Adobe Photoshop 7.0. Identical channel level and contrast adjustments were performed for all panels.
Live Cell ΔΨm Microscopy—Cells grown on four-well chamber slides received medium containing 1 mm pyruvate as sole carbon energy source for 1 h before incubation in 10 nm tetramethyl rhodamine methyl ester (TMRM, Invitrogen) in the same medium for 20 min. Images were acquired on a Zeiss LSM510 Laser Scanning Confocal Microscope (Carl Zeiss Microimaging, Thornwood, NY), equipped with live imaging station (CTI-Controller 3700 and incubator S, Leica) with temperature controller (heating insert P, PeCon, Germany). Acquisition parameters were chosen to allow using minimal laser intensity to prevent photodamage. TMRM signal was acquired at 37 °C using excitation and emission wavelengths of 543 and 575 nm, respectively. Series of optical z-sections were taken in 0.5-μm intervals capturing the entire signal of each cell. Imaging upon mitochondrial depolarization with the uncoupling agent carbonyl cyanide p-trifluoromethoxyphenylhydrazone (3 mm) was used to confirm minimal background fluorescence of both control and A2E-loaded cells. All imaging settings were kept constant for all experiments to allow direct comparison between control and treated cells.
TMRM Fluorescence Quantification—Single cell shapes were outlined and selected for each field. The integrated morphometric analysis feature in Metamorph® (Molecular Devices, Sunnyvale, CA), was used to quantified the fluorescence intensity of each individual z-plane yielding a numerical value representing signal intensity. The sum of all z-plane signals yielded the TMRM intensity value for individual cells. Averages of these total TMRM values are provided in Fig. 4.
FIGURE 4.
Antioxidants restore both lowered RPE mitochondrial membrane potential (ΔΨm) and decreased OS uptake by primary rat RPE caused by A2E load. A, RPE-J cells loaded with A2E or not and incubated with 1 mm NAC overnight or not as indicated in the panels were loaded with the membrane potentiometric dye TMRM and imaged live. Representative maximal projections are shown. Scale bars, 20 μm. B, each bar represents mean ± S.E. of total TMRM signal intensities quantified from individual cells in arbitrary units (A.U.) by Metamorph®, n = 35, cells imaged during three independent experiments. Gray bars, control cells; black bars, cells loaded with A2E. C and D, TMRM live labeling, imaging, and evaluation was performed as described for A and B using unpassaged rat RPE cells in primary culture with or without A2E load as indicated. C, representative maximal projections are shown. Scale bars, 20 μm. D, each bar represents mean ± S.E. of total cellular TMRM signal intensities, n = 30, cells imaged during three independent experiments. Gray bars, control cells; black bars, cells loaded with A2E. Asterisks in B and D indicate significant differences between A2E-loaded cells with and without antioxidant (p < 0.001). NAC did not significantly alter TMRM intensity of control cells (p > 0.05). Imaging and quantification details are provided in the experimental procedures section. E and F, primary, unpassaged rat RPE loaded (black bars) or not (gray bars) with A2E were challenged with FITC-labeled OS in medium containing 1 mm pyruvate with or without 20 μm Trolox as indicated for 1.5 h before quantification of bound (E) and internalized OS (F). All bars represent mean OS per cell ± S.D., of three independent experiments with duplicate samples each. Asterisks indicate significantly different OS uptake by A2E-loaded cells with and without antioxidant (p < 0.01).
Statistical Analysis—Paired or unpaired (as appropriate), two-tailed Student's t test was used to establish whether two values differed from each other. p < 0.05 was considered to indicate significant difference.
RESULTS
A2E Inhibits Mitochondrial ATP Synthesis—To determine whether A2E accumulation affects mitochondrial function in RPE cells, we set out to study cultured rat RPE-J cells in the presence or absence of physiological levels of synthetic A2E. All our experiments exploring RPE-J cells involved exposure to 50 μm A2E in 0.1% ethanol or ethanol-only control in the medium for 6 h followed by overnight chase in fresh high glucose growth medium. We previously established that these conditions do not cause overt cytotoxicity but result in A2E load of RPE lysosomes at levels similar to those of human normal subjects of old age (17, 36). Before any experimental treatment throughout this study, we preincubated cells for 1 h in Dulbecco's modified Eagle's medium without glucose and pyruvate to empty them from internally stored carbon sources. Such brief preincubation without carbon energy sources resulted in basal ATP levels of 12.4 ± 0.2 μmol/106 in control cells and 11.5 ± 0.5 μmol/106 in A2E-loaded cells that were not statistically different from each other (n = 4, p > 0.05). Cells with or without carbon energy source-free preincubation had equal total and mitochondrial protein content and phagocytic function when fed with OS in high glucose medium (data not shown). Thus, the 1-h preincubation did not irreversibly alter cell functionality. As a first assessment whether A2E load altered mitochondrial function, we measured the ATP content of RPE-J cells with or without A2E fed with media containing either glucose or pyruvate as carbon source at physiological concentration for 5 h. ATP content of A2E-loaded RPE-J cells was 25 and 21% less than ATP content of control cells following feeding with glucose and pyruvate, respectively (Fig. 1A, difference of A2E compared with control cells significant with p < 0.001 for both conditions). To test if these reduced steady-state levels of ATP resulted from impaired mitochondrial ATP synthesis, we explored if A2E load inhibits the mitochondrial OXPHOS system. We compared the rate of ATP synthesis by RPE-J cells with and without A2E by providing as sole energy sources either malate plus pyruvate or succinate, which are substrates for complex I or complex II of the mitochondrial respiratory chain, respectively. In the presence of complex II substrate RPE cells with and without A2E only slightly differed in rate of ATP synthesis (Fig. 1B, complex II, n = 4, p = 0.051). In the presence of complex I substrates, ATP synthesis by A2E-loaded cells was more dramatically reduced compared with control cells (Fig. 1B, complex I, n = 4, p < 0.03). Interestingly, the antioxidant N-acetylcysteine (NAC) completely prevented the inhibitory effect of A2E on ATP synthesis (Fig. 1B, A2E + NAC, n = 4, p < 0.001).
FIGURE 1.
Lysosomal A2E inhibits mitochondrial ATP synthesis. RPE-J cells received 50 μm A2E (all black bars) or solvent (all gray bars) for 6 h followed by overnight incubation in growth medium prior to quantification of ATP content (A), ATP synthesis rate (B), or live lysosome and mitochondria organelle labeling (C). A, RPE-J cells received medium supplemented with 5% dialyzed fetal bovine serum and either 1 g/liter glucose or 1 mm pyruvate as indicated for 5 h. ATP content in trichloroacetic acid cell extracts was quantified. B, ATP synthesis rates of RPE-J cells with or without A2E were measured using substrates succinate (in the presence of 1 μm rotenone; complex II) or malate plus pyruvate (complex I) as indicated. Addition of 1 mm NAC antioxidant to growth medium during the overnight incubation following A2E load abolished the effect of A2E on mitochondrial ATP synthesis rate (dark gray bar, complex I). Bars show mean ± S.E. of three independent measurements. Asterisks indicate significant differences (p < 0.05). C, live triple color confocal single x-y scans of A2E, LysoSensor, and MitoTracker as indicated of A2E-loaded RPE-J cells stained with LysoSensor only (upper panels) or with Lyso-Sensor and MitoTracker (lower panels). A2E and LysoTracker signals colocalize like in our previous studies (17, 36). The lower field shows that mitochondria localize distinctly from lysosomes and from A2E. Acquisition parameters for upper and lower fields were identical and are provided under “Experimental Procedures.” Scale bars, 10 μm.
Comparison of subcellular localization of A2E with fluorescent organelle markers, MitoTracker and LysoSensor, in live cells demonstrated that A2E primarily localized to RPE lysosomes (Fig. 1C). Furthermore, LysoSensor signals were of similar intensity and appearance in cells with and without A2E confirming that lysosomal pH and integrity in RPE-J cells loaded with A2E remained normal. These findings are in agreement with the lysosomal distribution of lipofuscin in the human eye, with our previous studies using the same A2E loading protocol (17, 36), as well as with findings by other groups (15, 37, 38). We did not detect A2E in mitochondria of RPE cultures that carried A2E at physiological levels following our established 6-h pulse incubation with 50 μm A2E (Fig. 1C). To assess whether A2E might be present in mitochondria at levels too low to detect by microscopy, we repeated the live cell organelle labeling experiment on RPE-J cells fed for 6 h with 100 μm A2E. We used fluorescence scanning to confirm that this treatment approximately doubled the total cellular load of A2E compared with our standard treatment, as we reported previously (17). Even in these cells with higher than physiological A2E we failed to detect A2E in the MitoTracker-labeled organelles (data not shown). Taken together, these data suggest that A2E in lysosomes inhibits mitochondrial energy production possibly by increasing oxidative stress.
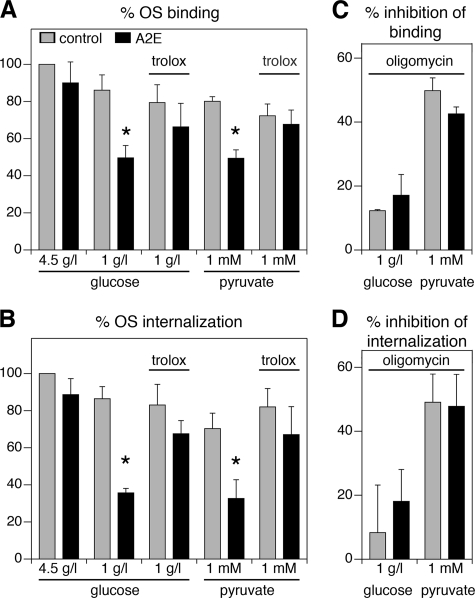
A2E Inhibits Both Binding and Engulfment of OS through Effects on Mitochondrial ATP Generation That Are Reversed by Antioxidants—Like other investigators, we routinely grow RPE cells in media containing 4.5 g/liter glucose, which is about four times higher than physiological (35, 39). In these excess glucose conditions, we previously found that physiological levels of A2E alter neither the binding nor the internalization step of OS phagocytosis (17). Here, we tested whether inhibition of ATP synthesis by A2E with limited type and physiological concentration of carbon energy source (Fig. 1) may affect OS phagocytosis by RPE cells. We thus fed RPE-J cells with or without A2E load with FITC-labeled OS in fresh medium containing either 4.5 g/liter glucose (our previous condition), 1 g/liter glucose, or 1 mm pyruvate. We found that RPE cells without A2E bound and internalized similar numbers of OS in media with 1 g/liter glucose as in 4.5 g/liter glucose (Fig. 2, A and B, compare gray bars). When we provided pyruvate as the sole carbon energy source, control cells bound 19% and internalized 29% fewer OS than in 4.5 g/liter glucose (Fig. 2, A and B, gray bars, pyruvate, difference significant with p < 0.05). Strikingly, cells loaded with A2E bound and internalized significantly fewer OS than control cells in both 1 g/liter glucose and in 1 mm pyruvate (Fig. 2, A and B, compare black to gray bars in each condition, p < 0.01 for all comparisons of cells with and without A2E). This effect of A2E was abolished by adding 20 μm Trolox, a vitamin E-like antioxidant (Fig. 2, A and B, compare black to gray bars marked trolox, p > 0.05 for all comparisons of cells with and without A2E). To test directly whether mitochondrial ATP generation was required for OS uptake, we repeated OS binding and internalization assays in the presence of 0.1 μm oligomycin, a specific inhibitor of the mitochondrial ATPase. Fig. 2 (C and D) shows the relative effect of oligomycin for each condition. Inhibition of mitochondrial ATP synthesis had minimal effect on OS binding or internalization in the presence of physiological levels of glucose but dramatically inhibited both steps of the phagocytic process when pyruvate is the sole substrate (Fig. 2, C and D, compare glucose and pyruvate). These effects of mitochondrial inhibition were independent of the presence of A2E (Fig. 2, C and D, compare gray and black bars in each condition).
FIGURE 2.
A2E reduces binding and internalization of OS by RPE-J cells in the presence of physiological levels of glucose or pyruvate. A and B, RPE-J cells with (black bars) or without (gray bars) A2E received FITC-labeled OS for 5 h in carbon energy source-free medium containing 4.5 g/liter glucose, 1 g/liter glucose, or 1 mm pyruvate with or without 20 μm Trolox as indicated. Bars show relative binding (A) and internalization (B) compared with cells in 4.5 g/liter glucose, which was set at 100%. C and D, experiments as in A and B were repeated with inclusion of 0.1 μm oligomycin in the 5-h uptake assay. Bars show percent inhibition of binding (C) and internalization (D) compared with the same condition without drug. A to D, bars represent mean ± S.D., of three independent assays with triplicate samples each. Asterisks indicate significant differences of cells with A2E compared with cells without A2E in the same condition (p < 0.05).
Exposure of A2E-loaded RPE cells to short wavelength (blue) visible light causes oxidative damage and may result in loss of lysosomal integrity and RPE apoptotic cell death that is regulated by the mitochondrial protein Bcl-2 (40–42). Oxidized forms of A2E promote cell damage even in the dark (43). Auto-oxidative changes of A2E in the dark have also been reported (44). We compared OS uptake by A2E-loaded and control cells in the presence of pyruvate under our routine condition, dim white light illumination during manipulations and incubations in the dark, with dim red light illumination during manipulations and incubations in the dark. We detected the same effect of A2E load on OS uptake regardless of illumination during manipulation (data not shown). Thus, sensitivity of pyruvate-fed RPE cells to A2E load is unlikely due to phototoxic effects of A2E.
Unexpectedly, our data showed that ∼50% of both OS binding and internalization still occurred in pyruvate in the presence of oligomycin (Fig. 2, C and D). We reasoned that this may be due to cellular ATP remaining in the cells despite the 1-h deprivation without carbon energy source. To test this, we first measured cellular ATP content after supplementing cells deprived of carbon sources for 1 h (time 0, our standard condition) for different times with pyruvate and oligomycin. Fig. 3A shows that cellular ATP sharply decreased during the first 30 min after addition of oligomycin and that ATP levels continued to decrease slowly for the next 3 h. This was true for both control and A2E-loaded cells. OS uptake assays with cells that received pyruvate and oligomycin before phagocytic challenge showed that mitochondrial inhibition in advance was sufficient to diminish both OS binding and internalization (Fig. 3, B and C).
FIGURE 3.
Depletion of residual cellular ATP by oligomycin preincubation causes dramatic decline in OS phagocytosis. A, ATP content declines in cells in the presence of oligomycin independently of A2E. RPE-J cells loaded with A2E (black bars) or not (gray bars) received 0.1 μm oligomycin for periods of time as indicated before extraction and ATP quantification. B and C, following our standard 1-h preincubation without carbon sources, RPE-J cells were not further preincubated (shill, clear bars) or preincubated for one additional hour with 0.1 μm oligomycin (oligo, black bars) or solvent (cont, clear bars) in carbon source-free medium before addition of OS in medium containing 1 mm pyruvate and 0.1 μm oligomycin for 5 h. B and C show percent bound and internal OS, respectively, compared with bound and internal OS in RPE-J cells preincubated without carbon sources for 1 h only. Bars represent mean ± S.D. of three independent assays with triplicate samples each.
Antioxidants Reverse the Reduction by A2E of Mitochondrial Membrane Potential ΔΨm and of OS Phagocytosis in Unpassaged Rat RPE in Primary Culture—To further explore the effect of A2E on RPE-J mitochondrial function, we next measured ΔΨm in live cells using the potentiometric dye TMRM. Fig. 4A illustrates that A2E load visibly reduced ΔΨm of RPE-J cells. TMRM quantification confirmed that A2E decreased ΔΨm on average by 46% (Fig. 4B, compare gray and black - NAC bars, p < 0.001). Like the A2E-impaired ATP synthesis, this A2E-impaired ΔΨm was mostly rescued by incubating with the anti-oxidant NAC (Fig. 4B, compare black bars - and + NAC, p < 0.005). The ratiometric dye JC-1 yielded similar results, but its fluorescence signal partially overlapped with the A2E fluorescence, complicating data interpretation (data not shown). These results provided compelling additional evidence for mitochondrial dysfunction caused by oxidative effects due to A2E load in RPE-J cells. Furthermore, they established that we can use TMRM imaging of ΔΨm to assess mitochondrial dysfunction at the single cell level. This was particularly important, because it allowed us to validate our results in unpassaged rat RPE cells in primary culture, which resemble more closely RPE in situ than the RPE cell line but which cannot be obtained in sufficiently large numbers to allow extensive biochemical assays. Fig. 4C shows that the decrease in ΔΨm of primary rat RPE due to A2E load and the striking reversal by NAC appeared obvious. Quantification of results confirmed that A2E reduced ΔΨm of primary RPE cells by 41% on average (Fig. 4D, compare gray and black - NAC bars, p < 0.001), and, similar to RPE-J cells, that the antioxidant NAC abolished the effect of A2E on ΔΨm (Fig. 4D, compare black bars - and + NAC, p < 0.001). Finally, we tested the sensitivity of OS phagocytosis by primary rat RPE to A2E load while limiting their carbon source to pyruvate. We found that A2E load significantly reduced both OS binding and OS internalization by primary rat RPE (Fig. 4, E and F, compare gray and black bars - trolox, p < 0.01). Strikingly, addition of the antioxidant Trolox during particle incubation was sufficient to restore both steps of phagocytosis (Fig. 4, E and F, compare black bars - and + trolox, p < 0.01). Taken together, the data obtained studying primary rat RPE fully support the data obtained studying the RPE-J cell line.
The above data demonstrate that mitochondrial carbon energy production is important for OS phagocytosis. In both RPE-J cells and in primary RPE rat cells without A2E, glycolysis generates enough energy to preserve normal phagocytic function. However, when the carbon energy source is limited to pyruvate, mitochondrial oxidative metabolism becomes crucial for both OS binding and internalization. Earlier results have long suggested that integrin receptor-dependent particle binding and subsequent engulfment during phagocytosis are both active cellular processes, and our results directly confirm that both steps require ATP (45, 46). RPE-J and primary rat RPE cells with A2E bind and engulf fewer OS in the presence of physiological levels of glucose or pyruvate. Complete and acute rescue of this effect by antioxidant suggests that, like mitochondrial ATP synthesis, phagocytosis decreases due to an A2E-dependent oxidative insult. Conversely, inhibiting OXPHOS (but not glycolysis) with oligomycin also abolishes the difference between A2E-loaded and control RPE-J cells. Taken together, these data indicate that A2E load impairs OS binding and internalization by RPE cells by reducing mitochondrial function.
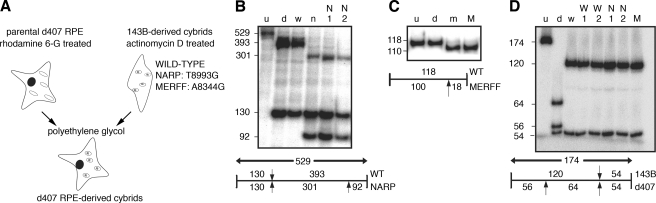
Generation and Characterization of Genetically Modified RPE Cells Deficient in Mitochondrial ATP Synthesis—To support our data demonstrating effects of mitochondrial OXPHOS inhibition with an alternative, genetic, approach, we used cytoplasmic hybrid (cybrid) technology to create RPE cell models of mitochondrial energy dysfunction (24). Because of evolutionary divergence this technology only allows for repopulation of mtDNA in a host cell of the same species, we used the human RPE-derived d407 cell line for all further experiments. We previously showed that d407 cells phagocytose OS and behave like RPE-J cells in response to A2E (17, 36). Here, we treated d407 cells with rhodamine 6G to deplete their mitochondria and then re-populated them with exogenous mitochondria from human patients with different, defined mitochondrial DNA genotypes by fusing them with enucleated 143B-derived osteosarcoma cybrids (Fig. 5A). We generated clonal d407 RPE cell lines carrying either one of two known pathogenic point mutations in the mitochondrial DNA (mtDNA): mutation T8993G in the A6 polypeptide of the ATPase (30), which causes neuropathy, ataxia, and retinitis pigmentosa syndrome (NARP, lines N1 and N2), or mutation A8344G in the tRNALys (47), which causes myoclonic epilepsy with ragged red fibers syndrome (MERRF, line M). Of the two mutant mtDNAs, the MERRF haplotype results in a more severe ATP synthesis defect, because it causes a global loss of mitochondrial respiratory chain protein synthesis. In contrast, the NARP mutation causes a specific reduction in ATP synthase activity. Additionally, we generated control cybrid clones, in which we replaced the parental d407 mtDNA with wild-type 143B-derived osteosarcoma mtDNA (W1 and W2). Cybrids were selected based on their auxotrophy for uridine. Proliferating clonal populations were analyzed by restriction fragment length polymorphism analysis to ensure that they only contained mtDNA molecules originated from the donor cybrids. The NARP mutation creates a restriction site for AvaI, which cuts a 393-bp fragment into two fragments of 301 and 92 bp. The two NARP clones N1 and N2 selected for this study carried 100% mutant mtDNA (Fig. 5B). The MERRF mutation creates a new restriction site for NaeI that generates two fragments of 100 and 18 bp from a 118-bp fragment (Fig. 5C). The d407 MERRF clone M used in this study carried 100% mutant mtDNA. To further ensure that all the mtDNA from d407 parental cells had been eliminated in d407 cybrids, we sequenced the entire hypervariable region of the d-loop of both d407 and 143B cells to find differential polymorphisms susceptible to fragment length polymorphism analysis analysis. Several differential polymorphisms were found that distinguish the two mtDNA genomes (data not shown). The T16519C transition is present in mtDNA of parental d407 but not of 143B cells and creates a new restriction site for the enzyme HaeIII, which cuts a 120-bp fragment into two fragments of 64 and 56 bp. Lack of detectable T16519C mtDNA confirmed that all mtDNA molecules of d407 cybrids selected for further study were derived from donor cybrids (Fig. 5D).
FIGURE 5.
Generation and genetic validation of d407 RPE-derived cybrids by PCR/fragment length polymorphism analysis. A, rhodamine-6G-treated parental d407 RPE cells devoid of mitochondria were fused with actinomycin D-enucleated 143B-derived osteosarcoma cybrids that carried wild-type, NARP, or MERRF mutant mitochondria to generate d407 RPE cybrid clones that carry exclusively donor-derived mitochondria. B, the restriction enzyme AvaI cleaves a 529-bp NARP mutant mtDNA PCR fragment into three fragments of 301, 130, and 92 bp, whereas wild-type mtDNA digest yields two fragment of 392 and 130 bp. u = uncut PCR product; d, d407 parental cell line; w, wild-type 143B-derived cybrid; n, 143B-derived NARP cybrid; N1 and N2, d407-derived NARP mutant clones. C, the restriction enzyme NaeI cleaves a 118-bp PCR fragment of MERRF mtDNA but not parental d407 mtDNA into fragments of 100 bp and 18 bp. u = uncut PCR product; d, d407 parental cell line; m, 143B-derived MERRF cybrid; M, d407-derived MERRF mutant clone. D, PCR/fragment length polymorphism analysis of the neutral polymorphism T16519C. The restriction enzyme HaeIII cleaves a PCR product of 174 bp into three fragments of 64, 56, and 54 bp if the template is d407 parental mtDNA but into only two fragments of 120 and 54 bp if the template is 143B-derived mtDNA. Lane designations are the same as in B and C.
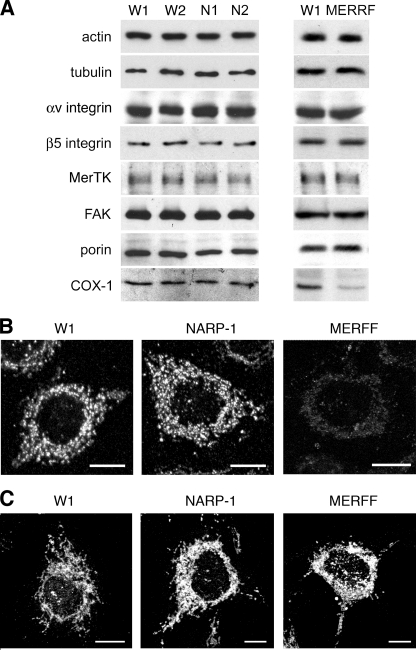
Next, we used immunoblotting to compare expression by our d407-derived cybrid lines of proteins relevant to the phagocytic function of RPE cells. Equal actin and tubulin content indicated that loaded samples represented equal numbers of cells (Fig. 6A). All our wild-type and mutant cybrid clones expressed similar levels of the binding receptor subunits αv and β5 integrin, the essential engulfment receptor MerTK, and focal adhesion kinase whose activation downstream of αvβ5 is required for engulfment (Fig. 6A) (13, 27). In addition, we determined expression levels of mitochondrial proteins porin and cytochrome c-oxidase subunit I (Cox-1, a component of the catalytic subunit of respiratory chain complex IV), which are encoded by nuclear and mtDNA, respectively. MERRF cybrids showed lower steady-state Cox-1 protein levels than NARP and wild-type cybrids by immunoblotting and by immunofluorescence microscopy (Fig. 6, A and B). This result was expected because the NARP mutation affects only complex V, whereas the MERRF mutation affects tRNALys and consequently translation of all 13 polypeptides encoded by the mtDNA, including Cox-1 (31). However, immunofluorescence microscopy of porin showed that all cybrids, regardless of their mtDNA genotype, had a similar mitochondrial content (Fig. 6C). Fig. 6 shows analysis of cells harvested following growth in complete, high glucose growth medium but similar results were obtained by analyzing cells following 1-h preincubation in medium without carbon sources (data not shown). Taken together, these data suggest that wild-type, NARP mutant, and MERRF-mutant d407 RPE cybrids retain similar general cellular morphology and gross molecular characteristics.
FIGURE 6.
NARP and MERRF mutant d407 RPE cybrids retain phagocytic protein expression and form mitochondria of normal appearance. MERRF but not NARP mutant cells lose COX-1 expression. A, comparative immunoblots of lysates prepared from wild-type cybrids W1 and W2, NARP mutant cybrids N1 and N2, and MERRF mutant cybrid clone M show similar protein expression levels of actin, tubulin, αv and β5 integrins, MerTK, focal adhesion kinase, and porin. As expected, COX-1 (encoded by mtDNA) was severely decreased in MERRF cells but remained normal in NARP mutant cells. B, immunofluorescence labeling of COX-1 confirmed reduction of COX-1 exclusively in MERRF mutant cybrids. C, immunofluorescence labeling of porin demonstrated similar abundance of mitochondrial organelles in all cybrids. Representative images acquired and processed identically are shown. Imaging details are provided under “Experimental Procedures.” All scale bars: 10 μm.
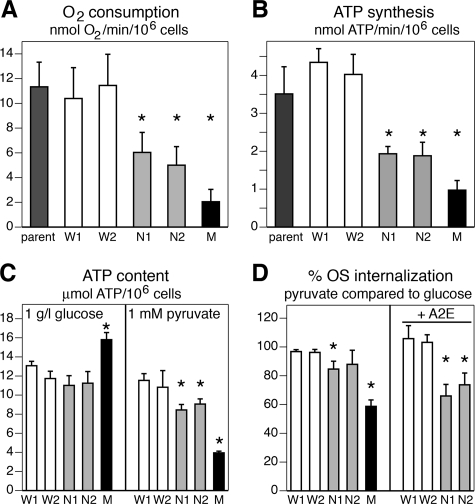
NARP and MERRF Mutant d407 RPE Cybrids Synthesize ATP at Decreased Rates due to Impaired OXPHOS Capacity but NARP Mutant RPE Cells Maintain Normal Steady-state ATP—To characterize OXPHOS function of d407 RPE-derived cybrids we measured oxygen consumption, ATP synthesis rate, and ATP content in the presence of different carbon sources. Like for our analysis of rat RPE, we reduced internal energy stores by preincubating cells without carbon sources for 1 h prior to assays. We assayed KCN-sensitive oxygen consumption (i.e. mitochondrial respiration) by intact cells in medium containing 100 μm pyruvate and 2 mm l-glutamine as sole energy sources. Both wild-type cybrid clones retained the capacity to consume oxygen of parental d407 cells (Fig. 7A, compare W1 and W2 with parent). Compared with wild-type cybrids, oxygen consumption rates of NARP and MERRF mutant cybrids were decreased by ∼50% (p < 0.01) and 81% (p < 0.0001), respectively (Fig. 7A). ATP luminometry in permeabilized cells receiving malate plus pyruvate as substrates showed that the rate of mitochondrial ATP synthesis of NARP mutant clones and of the MERRF mutant clone was reduced by ∼65 and 85%, respectively, compared with wild-type clones (p < 0.001 for all mutant clones) (Fig. 7B). Thus, mitochondrial ATP synthesis directly correlated with oxygen consumption. In media containing a physiological concentration of glucose, NARP mutant and wild-type cybrids had similar ATP steady-state content (Fig. 7C, left panel, white and gray bars). In the presence of glucose, MERRF mutant cells had ∼30% higher levels of ATP than the other cell lines suggesting that glycolytic capacity of these cells was increased to compensate for lack of OXPHOS (Fig. 7C, left panel, black bar). In media containing only pyruvate as carbon source, wild-type and NARP mutant clones maintained ∼80% of ATP content but MERRF mutant cells were severely (by ∼75%) depleted of ATP (Fig. 7C, right panel). Thus, as intended, replacing wild type with NARP and MERRF mutant mtDNA generated d407 RPE cell lines with moderate and severe OXPHOS deficiency, respectively.
FIGURE 7.
The severity of mitochondrial deficiency determines sensitivity to A2E. We compared mitochondrial respiration (A), ATP synthesis rates (B), ATP content (C), and phagocytic activity with and without A2E (D) among parental d407 cells (parent, dark gray bars), wild-type d407 RPE cybrids (W1 and W2, white bars), NARP mutant d407 RPE-derived cybrids (N1 and N2, light gray bars), and MERRF mutant d407 RPE clone M (black bars). A, bars show oxygen consumption by intact cells using pyruvate as substrate (mean ± S.D., n = 5). Asterisks indicate significant differences compared with parental cells (p < 0.05). NARP mutant clones respire significantly less than parental or wild-type cells but significantly more than MERRF cells. B, bars show ATP synthesis rates with malate plus pyruvate as substrates (mean ± S.D., n = 5). Asterisks indicate significant differences compared with wild-type clone W1 (p < 0.05). C, bars show ATP steady-state levels for cybrids following feeding for 3.5 h with physiological concentrations of glucose (left) or pyruvate (right) (mean ± S.E., n = 5). Asterisks indicate significant differences compared with wild-type clone W1 (p < 0.05). D, bars show relative OS internalization for each clone in the presence of pyruvate as compared with in the presence of glucose. For cells without A2E (left panel) asterisks indicate significant differences compared with wild-type clone W1 (p < 0.05). For cells loaded with A2E at about one-fifth of the level of aging human RPE (right panel), asterisks indicate significant differences compared with relative internalization without A2E for the same clone (compare left with right bars). Even low concentrations of A2E were highly toxic to MERRF mutant cells precluding phagocytosis measurements for clone M.
Finally, we tested whether their OXPHOS deficiencies impaired the phagocytic function of our mutant d407 RPE cybrids. To solely focus on differences in activity due to differences in mitochondrial ATP synthesis rather than clonal differences, we compared for each clone OS engulfment in media containing 1 g/liter glucose (in which all clones contain ample ATP) with OS engulfment in media containing 1 mm pyruvate as the sole carbon energy source (in which MERRF cells possess reduced ATP). Clones were preincubated for 1 h in medium without carbon sources before phagocytosis assays. We found that OS engulfment by MERRF cells was 41% less in pyruvate than in glucose (Fig. 7D, p < 0.01). OS engulfment by NARP mutant clone N1 was just 15% less in pyruvate than in glucose (p < 0.05), whereas OS engulfment by NARP mutant clone N2 and both wild-type did not differ in pyruvate and in glucose (all p > 0.05). Strikingly, A2E load at a level of ∼20% of that found on average in aging human RPE in the eye (15) reduced OS engulfment in pyruvate containing media by NARP mutant RPE clones N1 and N2 compared with wild-type RPE by 37 and 30%, respectively (Fig. 7D, right panel, p < 0.05 for either NARP clone compared with W1). We could not assess the effect of A2E on MERRF mutant cells, because A2E load caused massive energy depletion leading to cell death during the phagocytosis assay (data not shown). Thus, if a non-fermentable substrate, such as pyruvate is the sole carbon energy source, A2E accumulation decreases the phagocytic activity of NARP mutant RPE cells that maintain normal activity in oxidative growth conditions. These data imply that even a moderate A2E load of RPE cells exacerbates the effects of mtDNA mutations.
DISCUSSION
Lipofuscin/A2E accumulation and mitochondrial damage are both prominent features of RPE aging. Nonetheless, we and others previously studied molecular mechanisms by which each of them may impact cell viability and function without concomitantly considering the other. Here, we used a combination of biochemical and genetic tools to determine possible synergy between these two age-related cellular changes.
First, we tested whether physiological concentrations of A2E alter mitochondrial ATP synthesis. We found that A2E load reduces ATP synthesis, ATP steady-state levels, and ΔΨm if carbon sources are provided at physiological concentrations. This decline of mitochondrial ATP synthesis reduces OS uptake, used as readout for energy-consuming RPE function. Further, we found that impairment of mitochondrial energy metabolism renders our novel RPE-derived cybrid cells more susceptible to lysosomal A2E load. Taken together, these data provide exciting new insight into the complexity of RPE defects associated with aging: minor lysosomal A2E load and moderate mitochondrial inefficiency each by themselves may cause no detectable harm. However, our results demonstrate that they mutually enhance each other's impact to significantly impair vital RPE activities.
Our findings suggest that RPE cells rely on mitochondria to generate ATP for OS uptake and that A2E interferes with this process. Glucose at 4.5 g/liter protects RPE cells from the effect of A2E on ATP content, because glycolysis fully suffices for ATP generation under these conditions. Our previous work thus found no effect of physiological levels of A2E on binding or internalization of OS by RPE cells (17). However, such excess glucose supplies are likely irrelevant in vivo. It is possible that delayed OS phospholipid digestion in RPE loaded with A2E will further destabilize mitochondria and/or lysosomes over time. Our new RPE-derived cybrid lines with permanently reduced mitochondrial function provide the unique opportunity to test this experimentally in the future.
Earlier in vitro work suggests that A2E may affect mitochondria directly. Adding synthetic A2E to isolated mitochondria reduces cytochrome c oxidase activity (48, 49). Yet, it is not clear that inhibition of purified cytochrome c oxidase in solution with A2E in the micromolar range reflects the activities of A2E in living RPE cells. We and others have previously used microscopy to demonstrate that A2E primarily accumulates in lysosomes of RPE cells in culture (15, 17, 36, 37). Schutt and colleagues (38) recently used biochemical analysis to confirm that A2E efficiently distributes to RPE lysosomes during initial experimental load and that A2E remains in these organelles for extended periods of time unless cells receive more and more A2E in sequential additions. We found no evidence for mitochondrial localization of A2E after our standard one time 6-h load with A2E that results in cellular concentrations within the normal range of aged human RPE (17, 36). Even using our experimental protocol to achieve excess A2E load twice as high as commonly observed in the aging human eye did not reveal mitochondrial A2E. Taken together, we cannot exclude that undetectable levels of A2E localize to mitochondria in our experimental models but we consider it very unlikely that the effects of A2E we report here result from direct mitochondrial localization.
Cells require efficient lysosomal activity for routine autophagic turnover of spent organelles, including mitochondria (50–52). That lipofuscin/A2E burden impairs mitochondrial renewal by RPE lysosomes accelerating mitochondrial aging is an intriguing possibility. However, we consider it unlikely that the effects we observe are related to lack of mitochondrial renewal because of the short duration of our experiments. Furthermore, complete rescue of phagocytic function by acute incubation with antioxidant subsequent to A2E load argues both against irreversible or large scale damage of mitochondria and against interference of antioxidant with A2E uptake or its intracellular mobility. Rather, our results suggest that A2E in our system may favor oxidative processes. In general, mitochondria are particularly vulnerable to oxidative stress (regardless of origin) for instance because they additionally self-produce reactive oxygen species due to constant electron leak and because they lack efficient DNA protection by histones (53). The effects we observed are unlikely due to the well known activity of A2E as photosensitizer, because A2E reduced ATP synthesis in our assays even in complete darkness. However, Wang and colleagues (44) have demonstrated earlier that A2E undergoes auto-oxidative changes in the dark promoting oxidative stress, which may be relevant for the cellular effects of A2E we observed. Although an intriguing challenge, exploring the precise reactive intermediates and molecular mechanisms that link lysosomal A2E to inhibition of mitochondrial ATP generation needs further investigation that is beyond the scope of this study.
Because post-mitotic RPE cells must support overlying photoreceptor neurons for life, decrease in any of their support functions will eventually impair photoreceptor function and vision. Daily phagocytosis of spent outer segments is but one of several critical maintenance functions performed by the RPE to preserve phototransduction activity and viability of photoreceptors.
We chose to measure OS binding and engulfment as an example for the support functions of RPE, because it is an enormously energy-consuming process. Moreover, we could take advantage of reliable, highly quantitative assays for this function that we established previously. Testing how lipofuscin/A2E and mitochondrial defects may synergize to also impair other important RPE support functions and determining how such synergy relates to alterations in RPE functionality in the aging human eye will be important areas for future research.
Acknowledgments
We thank Dr. Geri Kreitzer for reagents and Dr. Richard Hunt for providing the d407 cell line. H. R. V.-S. thanks Kam Lau for excellent assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-EY13295 (to S. C. F.) and K02-NS47306 (to G. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: RPE, retinal pigment epithelium; mtDNA, mitochondrial DNA; MERRF, myoclonic epilepsy with ragged red fibers; NAC, N-acetylcysteine; NARP, neuropathy, ataxia, and retinitis pigmentosa; OS, outer segment fragments; OXPHOS, oxidative phosphorylation; FITC, fluorescein isothiocyanate; TMRM, tetramethyl rhodamine methyl ester.
References
- 1.Strauss, O. (2005) Physiol. Rev. 85 845-881 [DOI] [PubMed] [Google Scholar]
- 2.Dowling, J. E., and Sidman, R. L. (1962) J. Cell Biol. 14 73-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bok, D., and Hall, M. O. (1971) J. Cell Biol. 49 664-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullen, R. J., and LaVail, M. M. (1976) Science 192 799-801 [DOI] [PubMed] [Google Scholar]
- 5.Gal, A., Li, Y., Thompson, D. A., Weir, J., Orth, U., Jacobson, S. G., Apfelstedt-Sylla, E., and Vollrath, D. (2000) Nat. Genet. 26 270-271 [DOI] [PubMed] [Google Scholar]
- 6.Zareparsi, S., Buraczynska, M., Branham, K. E., Shah, S., Eng, D., Li, M., Pawar, H., Yashar, B. M., Moroi, S. E., Lichter, P. R., Petty, H. R., Richards, J. E., Abecasis, G. R., Elner, V. M., and Swaroop, A. (2005) Hum. Mol. Genet. 14 1449-1455 [DOI] [PubMed] [Google Scholar]
- 7.Zarbin, M. A. (2004) Arch. Ophthalmol. 122 598-614 [DOI] [PubMed] [Google Scholar]
- 8.Feeney, L. (1978) Invest. Ophthalmol. Vis. Sci. 17 583-600 [PubMed] [Google Scholar]
- 9.Mata, N. L., Weng, J., and Travis, G. H. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 7154-7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrukhin, K., Koisti, M. J., Bakall, B., Li, W., Xie, G., Marknell, T., Sandgren, O., Forsman, K., Holmgren, G., Andreasson, S., Vujic, M., Bergen, A. A., McGarty-Dugan, V., Figueroa, D., Austin, C. P., Metzker, M. L., Caskey, C. T., and Wadelius, C. (1998) Nat. Genet. 19 241-247 [DOI] [PubMed] [Google Scholar]
- 11.Holz, F. G., Bindewald-Wittich, A., Fleckenstein, M., Dreyhaupt, J., Scholl, H. P., and Schmitz-Valckenberg, S. (2007) Am. J. Ophthalmol. 143 463-472 [DOI] [PubMed] [Google Scholar]
- 12.Rakoczy, P. E., Zhang, D., Robertson, T., Barnett, N. L., Papadimitriou, J., Constable, I. J., and Lai, C. M. (2002) Am. J. Pathol. 161 1515-1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nandrot, E. F., Kim, Y., Brodie, S. E., Huang, X., Sheppard, D., and Finnemann, S. C. (2004) J. Exp. Med. 200 1539-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, J., Itagaki, Y., Ben-Shabat, S., Nakanishi, K., and Sparrow, J. R. (2000) J. Biol. Chem. 275 29354-29360 [DOI] [PubMed] [Google Scholar]
- 15.Sparrow, J. R., Parish, C. A., Hashimoto, M., and Nakanishi, K. (1999) Invest. Ophthalmol. Vis. Sci. 40 2988-2995 [PubMed] [Google Scholar]
- 16.Sparrow, J. R., and Boulton, M. (2005) Exp. Eye Res. 80 595-606 [DOI] [PubMed] [Google Scholar]
- 17.Finnemann, S. C., Leung, L. W., and Rodriguez-Boulan, E. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 3842-3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwong, J. Q., Beal, M. F., and Manfredi, G. (2006) J. Neurochem. 97 1659-1675 [DOI] [PubMed] [Google Scholar]
- 19.Harman, D. (2003) Antioxid. Redox Signal. 5 557-561 [DOI] [PubMed] [Google Scholar]
- 20.Harman, D. (2001) Ann. N. Y. Acad. Sci. 928 1-21 [DOI] [PubMed] [Google Scholar]
- 21.Del Priore, L. V., Kuo, Y. H., and Tezel, T. H. (2002) Invest. Ophthalmol. Vis. Sci. 43 3312-3318 [PubMed] [Google Scholar]
- 22.Barron, M. J., Johnson, M. A., Andrews, R. M., Clarke, M. P., Griffiths, P. G., Bristow, E., He, L. P., Durham, S., and Turnbull, D. M. (2001) Invest. Ophthalmol. Vis. Sci. 42 3016-3022 [PubMed] [Google Scholar]
- 23.Feher, J., Kovacs, I., Artico, M., Cavallotti, C., Papale, A., and Balacco Gabrieli, C. (2006) Neurobiol. Aging 27 983-993 [DOI] [PubMed] [Google Scholar]
- 24.King, M. P., and Attardi, G. (1989) Science 246 500-503 [DOI] [PubMed] [Google Scholar]
- 25.Parish, C. A., Hashimoto, M., Nakanishi, K., Dillon, J., and Sparrow, J. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 14609-14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang, Y., and Finnemann, S. C. (2007) J. Cell Sci. 120 3053-3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finnemann, S. C. (2003) EMBO J. 22 4143-4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayona-Bafaluy, M. P., Manfredi, G., and Moraes, C. T. (2003) Nucleic Acids Res. 31 e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams, A. J., Murrell, M., Brammah, S., Minchenko, J., and Christodoulou, J. (1999) Hum. Mol. Genet. 8 1691-1697 [DOI] [PubMed] [Google Scholar]
- 30.Holt, I. J., Harding, A. E., Petty, R. K., and Morgan-Hughes, J. A. (1990) Am. J. Hum. Genet. 46 428-433 [PMC free article] [PubMed] [Google Scholar]
- 31.Masucci, J. P., Davidson, M., Koga, Y., Schon, E. A., and King, M. P. (1995) Mol. Cell Biol. 15 2872-2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vives-Bauza, C., Yang, L., and Manfredi, G. (2007) Methods Cell Biol. 80 155-171 [DOI] [PubMed] [Google Scholar]
- 33.D'Aurelio, M., Pallotti, F., Barrientos, A., Gajewski, C. D., Kwong, J. Q., Bruno, C., Beal, M. F., and Manfredi, G. (2001) J. Biol. Chem. 276 46925-46932 [DOI] [PubMed] [Google Scholar]
- 34.Molday, R. S., Hicks, D., and Molday, L. (1987) Invest. Ophthalmol. Vis. Sci. 28 50-61 [PubMed] [Google Scholar]
- 35.Finnemann, S. C., Bonilha, V. L., Marmorstein, A. D., and Rodriguez-Boulan, E. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 12932-12937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakkaraju, A., Finnemann, S. C., and Rodriguez-Boulan, E. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 11026-11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holz, F. G., Schutt, F., Kopitz, J., Eldred, G. E., Kruse, F. E., Volcker, H. E., and Cantz, M. (1999) Invest. Ophthalmol. Vis. Sci. 40 737-743 [PubMed] [Google Scholar]
- 38.Schutt, F., Bergmann, M., Holz, F. G., Dithmar, S., Volcker, H. E., and Kopitz, J. (2007) Graefes Arch. Clin. Exp. Ophthalmol. 245 391-398 [DOI] [PubMed] [Google Scholar]
- 39.Robertson, C., Drexler, A. J., and Vernillo, A. T. (2003) J. Am. Dent. Assoc. 134 16S-23S [DOI] [PubMed] [Google Scholar]
- 40.Schutt, F., Davies, S., Kopitz, J., Holz, F. G., and Boulton, M. E. (2000) Invest. Ophthalmol. Vis. Sci. 41 2303-2308 [PubMed] [Google Scholar]
- 41.Sparrow, J. R., Nakanishi, K., and Parish, C. A. (2000) Invest. Ophthalmol. Vis. Sci. 41 1981-1989 [PubMed] [Google Scholar]
- 42.Sparrow, J. R., Zhou, J., and Cai, B. (2003) Invest. Ophthalmol. Vis. Sci. 44 2245-2251 [DOI] [PubMed] [Google Scholar]
- 43.Sparrow, J. R., Vollmer-Snarr, H. R., Zhou, J., Jang, Y. P., Jockusch, S., Itagaki, Y., and Nakanishi, K. (2003) J. Biol. Chem. 278 18207-18213 [DOI] [PubMed] [Google Scholar]
- 44.Wang, Z., Keller, L. M., Dillon, J., and Gaillard, E. R. (2006) Photochem. Photobiol. 82 1251-1257 [DOI] [PubMed] [Google Scholar]
- 45.Bullock, W. E., and Wright, S. D. (1987) J. Exp. Med. 165 195-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finnemann, S. C., and Rodriguez-Boulan, E. (1999) J. Exp. Med. 190 861-874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoffner, J. M., Lott, M. T., Lezza, A. M., Seibel, P., Ballinger, S. W., and Wallace, D. C. (1990) Cell 61 931-937 [DOI] [PubMed] [Google Scholar]
- 48.Shaban, H., Gazzotti, P., and Richter, C. (2001) Arch. Biochem. Biophys. 394 111-116 [DOI] [PubMed] [Google Scholar]
- 49.Suter, M., Reme, C., Grimm, C., Wenzel, A., Jaattela, M., Esser, P., Kociok, N., Leist, M., and Richter, C. (2000) J. Biol. Chem. 275 39625-39630 [DOI] [PubMed] [Google Scholar]
- 50.Brunk, U. T., and Terman, A. (2002) Eur. J. Biochem. 269 1996-2002 [DOI] [PubMed] [Google Scholar]
- 51.Terman, A., Dalen, H., Eaton, J., Neuzil, J., and Brunk, U. T. (2003) Exp. Gerontol. 38 863-876 [DOI] [PubMed] [Google Scholar]
- 52.Terman, A., Gustafsson, B., and Brunk, U. T. (2007) J. Pathol. 211 134-143 [DOI] [PubMed] [Google Scholar]
- 53.Cadenas, E., and Davies, K. J. (2000) Free Radic. Biol. Med. 29 222-230 [DOI] [PubMed] [Google Scholar]