Abstract
High density lipoproteins (HDL) are major plasma carriers of sphingosine 1-phosphate (S1P). Here we show that HDL increases endothelial barrier integrity as measured by electric cell substrate impedance sensing. S1P was implicated as the mediator in this process through findings showing that pertussis toxin, an inhibitor of Gi-coupled S1P receptors, as well as antagonists of the S1P receptor, S1P1, inhibited barrier enhancement by HDL. Additional findings show that HDL stimulates endothelial cell activation of Erk1/2 and Akt, signaling pathway intermediates that have been implicated in S1P-dependent endothelial barrier activity. HDL was also found to promote endothelial cell motility, a process that may also relate to endothelial barrier function in the context of a vascular injury response. The effects of HDL on endothelial cell Erk1/2 and Akt activation and motility were suppressed by pertussis toxin and S1P1 antagonists. However, both HDL-induced barrier enhancement and HDL-induced motility showed a greater dependence on Akt activation as compared with Erk1/2 activation. Together, the findings indicate that HDL has endothelial barrier promoting activities, which are attributable to its S1P component and signaling through the S1P1/Akt pathway.
Sphingosine 1-phosphate (S1P)2 is a plasma-borne lysosphingolipid that has been shown to regulate endothelial barrier integrity (1). For example, treatment of cultured endothelial cells with S1P associated with the carrier albumin acts to increase endothelial barrier activity as indicated by increased transendothelial electrical resistance (2, 3). Moreover, S1P administration greatly reduces lung capillary leakage induced in mice by lipopolysaccharide treatment (4). Mechanistically, S1P acts to enhance tight junction formation in neighboring endothelial cells by influencing subcellular distributions of tight junction components including ZO-1 and claudin-5 (5). In addition, S1P induces endothelial cortical actin assembly (1) and relocation of endothelial cell junctional adhesion molecules including platelet endothelial cell adhesion molecule and vascular endothelial-cadherin to cell-cell junctional areas (5).
In plasma, S1P is found associated with multiple lipoproteins including low density lipoproteins, very low density lipoproteins, and high density lipoproteins (HDL). However, the bulk of the lipoprotein particle-associated S1P (54%) is bound to HDL (6). A number of recent studies point to the S1P cargo of HDL as being a mediator of many of the cardiovascular effects of HDL including the ability to promote vasodilation, vasoconstriction, and angiogenesis, protect against ischemia/reperfusion injury, and inhibit/reverse atherosclerosis (7). One important cardiovascular-related effect of S1P that has not yet been attributed to HDL is regulation of endothelial barrier activity, a major physiological function of the endothelium. Here we investigate the role of HDL as a regulator of endothelial barrier integrity processes known to be dependent on S1P signaling.
EXPERIMENTAL PROCEDURES
Reagents—d-Erythro-S1P was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Stock solutions of S1P (100 μm) were prepared by dissolving S1P into Dulbecco's phosphate-buffered saline (DPBS) containing 4 mg/ml fatty acid-free bovine serum albumin (BSA; Sigma). S1P levels in the stock solution were confirmed by the Lipidomics Core Facility at the Medical University of South Carolina, Charleston, SC (Department of Biochemistry) using liquid chromatography/mass spectrometry (LC-MS-MS). Pertussis toxin (PTX) was purchased from Sigma. The S1P1 antagonist 857390 and the S1P1/S1P3 antagonist VPC23019 were purchased from Avanti Polar Lipids and reconstituted at 1 mm in acidified DMSO, 4 mg/ml BSA. The Akt inhibitor, LY294002, and Erk inhibitor, PD98059, were purchased from R&D Systems, Inc. (Minneapolis, MN).
HDL Preparation—Blood for HDL isolation was collected from healthy volunteers in 0.4 mm EDTA after 12 h of fasting. The donors were normolipemic volunteers, not receiving prescription medication for any acute or chronic condition and without family history of coronary artery disease, peripheral vascular disease, or stroke. None of the volunteers was receiving antioxidant therapy. Low density lipoprotein was isolated from pooled plasma (typically from 4 to 5 donors) after density adjustment (1.019 < d < 1.063 g/ml) with potassium bromide (KBr), by preparative ultracentrifugation at 50,000 rpm (about 360,000 × g) for 17 h, 5 °C, on a Beckman Optima™ XL-100K ultracentrifuge, using a type 70 Ti rotor. HDL was then isolated after density adjustment (1.063 < d < 1.21 g/ml) with KBr at 68,000 rpm (500,000 × g) for 22 h using the same rotor. Isolated HDL was washed by ultracentrifugation (SW 41 Ti rotor, 40,000 rpm, 280,000 × g, 36 h), dialyzed against a 0.15 m sodium chloride solution containing 0.3 mm EDTA, pH 8.0. Prior to use in cell culture, the HDL was dialyzed against DPBS containing 0.3 mm EDTA. Protein levels in HDL preparations were measured by the Lowry method. To determine S1P levels in the purified HDL preparations, aliquots were diluted 1:50 in DPBS and subjected to LC-MS-MS described by Bielawski et al. (8). These analyses showed that the average S1P level was 381.87 ± 32.22 pmol/mg of HDL protein (n = 4).
Cells—Human umbilical vein endothelial cells (HUVEC) (Cascade Biologics, Inc., Portland, OR) were maintained on Corning tissue culture dishes (Corning Inc., Corning, NY) in a humidified atmosphere of 5% CO2, 95% air in endothelial growth medium-2 (EGM-2; Lonza, Walkersville, MD), which contains 2% fetal bovine serum. Cells were used between passages 3 and 7.
Electrical Cell Substrate Impedance Sensing Assay—Electric cell substrate impedance sensing (ECIS) is a non-invasive means of measuring electrical resistance across cultured cell monolayers in real time (9). In ECIS, cells are grown on the surface of gold-film electrodes. From readings of the electrical impedance (a measure of opposition to a sinusoidal alternating current) of the cell-covered electrode performed with low amplitude-sensing voltages, alterations in cell-cell contacts can be deduced. Changes of impedance are attributed to changes of the resistance in the paracellular pathway and the resistance between the ventral cell surface and the electrode (α: α = R(ρ/h)0.5, where R is the cell radius, ρ is the resistivity of the culture medium, and h is the gap between the cells and the electrode) (10, 11). Disruption in the barrier integrity of an epithelial monolayer results in increased ion flow across the cell layer leading to decreased electrical resistance. ECIS has been used to monitor transendothelial electrical resistance (TEER) as an index of endothelial cell barrier function (1, 5).
Herein, TEER was measured using an ECIS Model 1600 instrument (Applied Biophysics, Troy, NY). HUVEC in EGM-2 medium were seeded into the wells of ECIS 8W10E+ electrode arrays at a density of 1 × 105 cells/well. The arrays were precoated with human plasma fibronectin (Invitrogen) at 100 μg/ml in 0.15 m NaCl, 0.01 m Tris, pH 8.0. The cells were cultured in EGM-2 medium, and impedance was measured every 5 min at 15 kHz frequency. For every 48 h of culture, 50% volume of medium was replaced with fresh EGM-2. When the electrical resistance reached a maximal plateau (∼3 days), the medium was replaced with serum-free endothelial basal medium (EBM; Lonza) containing 1× penicillin-streptomycin-glutamine (Invitrogen). Electrical resistance was monitored until a minimal plateau was reached (∼24 h). Effectors (i.e. S1P-albumin, HDL, or albumin in DPBS) were introduced into the culture medium by removing a volume corresponding to that of the effector to be added. The maximum volume of each effector added did not exceed 1/25 of the 400-μl volume of conditioned culture medium in each well. For experiments evaluating the effects of PTX on TEER, PTX (2 μg/ml in 50% glycerol with 50 mm Tris, 10 mm glycine, and 0.5 m NaCl, pH 7.5) was added to EBM during the final 12 h of serum starvation. Controls received PTX buffer alone. S1P or HDL was then added to the medium.
For experiments evaluating the effects of S1P1 antagonists on TEER, antagonist stocks (1 mm in 5% acidified DMSO, 4 mg/ml BSA) were diluted 1:100 into the conditioned EBM at the same time that S1P or HDL was added. For experiments evaluating the effects of Akt and Erk inhibition on TEER, inhibitor stocks (25 μm LY294002 in DMSO or 50 μm PD98059 in DMSO) were diluted to 50 nm into the conditioned EBM 1 h prior to the addition of S1P or HDL.
Bio-Plex Phosphoprotein Detection Assay—HUVEC were seeded into 6-well dishes (Corning) at 1 × 106 cells/well and grown for 18 h in EGM-2. The medium was then replaced with EBM containing 0.1% fetal bovine serum, and the cells were grown for 24 h. The medium was then supplemented with a range of S1P concentrations (37 nm-1 μm) or a range of HDL concentrations (37 μg-1 mg of protein/ml, corresponding to 18-493 nm S1P). For experiments evaluating the effects of PTX, PTX (100 ng/ml) or the PTX buffer was added to EBM during the final 12 h of serum starvation. For experiments evaluating the effects of the S1P1 antagonist 857390 and the S1P1/S1P3 antagonist VPC23019, the antagonists (1 mm in 5% acidified DMSO, 4 mg/ml BSA) were diluted 1:100 into the conditioned EBM during the final 15 min of serum starvation. S1P or HDL was then added to the medium and allowed to incubate for 3 min. The cells were then extracted with Bio-Plex cell lysis buffer (Bio-Rad), and protein concentration was determined by the Bio-Rad DC protein assay (Bio-Rad). Levels of phospho-Erk1/2 and phospho-Akt in the extracts were determined by multiplex bead assay using kits (Bio-Rad) and a Bioplex-200 instrument (Bio-Rad).
HUVEC Migration Assay—ECIS was also used to evaluate the effects of S1P and HDL on HUVEC motility. HUVEC were seeded onto 8W1E electrode arrays at 1 × 105 cells/well in EGM-2 medium. The cells were grown until electrical resistance reached a maximal plateau. Each cell-covered electrode was subjected to a pulse of 4 V for 20 s. This brief elevated field effectively killed the cells on the electrode as indicated by a drop in impedance. Electrical resistance was then monitored for the subsequent 10-h period during which, under control conditions, electrical resistance recovers as cells migrate and fill in the void. During this period, the EBM medium was supplemented either with S1P (250 nm) or with HDL (333 μg/ml), each in the presence or absence of PTX (1 μg/ml) or S1P1 receptor antagonists (10 μm). For experiments that evaluated the effects of Akt and Erk inhibitors, the cells were treated with inhibitors or buffer vehicle 1 h prior to wounding and throughout the period in which the cells migrate to fill the void.
RESULTS
HDL Augments TEER—As shown in Fig. 1A, S1P induces a dose-dependent increase in TEER. The TEER response to S1P displays a bimodal distribution over a 5-h period. Each of the two modes spans 2 h. For all of the S1P doses tested, the height of the second peak was always less than that of the first. HDL treatment also produces a dose-dependent increase in TEER (Fig. 1B). Like S1P, HDL also produced a bimodal TEER response. By contrast, the effect of HDL on the second TEER peak was sustained longer at the higher HDL doses than observed with any S1P dose tested.
FIGURE 1.
HDL enhances transendothelial electrical resistance. A minimal TEER plateau was reached within ∼24 h of replacing the culture medium of confluent endothelial cells with serum-free medium. The monolayers were then incubated with varying concentrations S1P (A) or HDL (B). In B, the concentration of S1P in the HDL tested ranged from 5 to 403 nm. The TEER tracings shown in panels A and B each represent mean data from three independent experiments each with two replicates per condition. As a control, monolayers were treated with 40 μg/ml BSA, a concentration corresponding to the amount of BSA carrier used for the highest concentration of S1P tested. Impedance values were normalized by dividing each value by the level of impedance measured just prior to the addition of effectors.
HDL-induced Enhancement of TEER Is Pertussis Toxin-sensitive—Gi protein-coupled S1P receptor signaling in endothelial cells is inhibited by PTX (12). As shown in Fig. 2A, the TEER response of endothelial cell monolayers to S1P treatments was inhibited by PTX. Similarly, the TEER response to HDL was completely abrogated by PTX (Fig. 2B), suggesting the requirement for Gi-coupled S1P receptors.
FIGURE 2.
HDL-enhanced transendothelial electrical resistance is inhibited by pertussis toxin and S1P1 antagonists. Confluent endothelial monolayers were grown under serum-free conditions until a minimal TEER plateau had been reached. The cells were then incubated with S1P or HDL in the presence or absence of PTX (A and B) or S1P1 antagonists (C and D). In A and B, S1P was used at 833 nm, HDL was used at 1000 μg/ml (containing 400 nm S1P), and PTX was used at 1 μg/ml. In C and D, S1P was used at 250 nm, HDL was used at 621 μg/ml (containing 250 nm S1P), the S1P1 antagonist 857390 was used at 10 μm, and the S1P1/S1P3 antagonist VPC23019 was used at 10 μm. As controls, monolayers were treated with BSA-containing serum-free medium (SFM) plus or minus vehicle buffer. Each of the TEER tracings shown is an average of two replicate wells and representative of three independent experiments. Impedance values were normalized by dividing each value by the level of impedance measured just prior to the addition of effectors.
S1P Receptor Antagonists Inhibit HDL-induced Enhancement of TEER—The S1P receptor, S1P1, has been shown to mediate S1P-stimulated augmentation of endothelial barrier activity (1). We therefore tested the effect of S1P1 receptor antagonists on the barrier enhancement response of endothelial cell monolayers to HDL. Both the S1P1 antagonist, 857390, and the S1P1/S1P3 antagonist, VPC23019, inhibited the TEER response to HDL as well as the TEER response to S1P (Fig. 2, C and D).
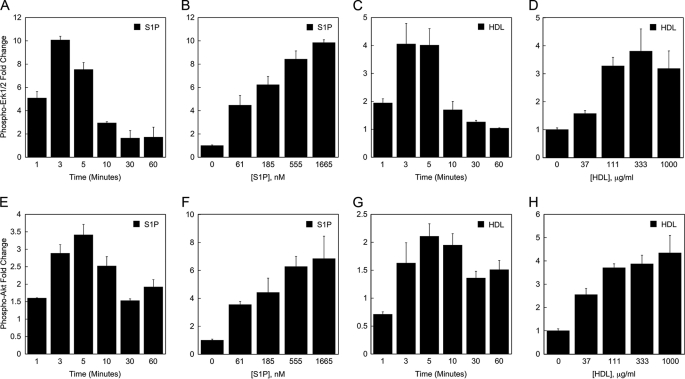
HDL Stimulates Erk1/2 and Akt Activation in Endothelial Cells—S1P signaling in endothelial cells involves Erk1/2 and Akt activation (13). Multiplex microbead suspension array analysis was performed to evaluate the effect of HDL on the activation of Erk1/2 and Akt signaling pathway intermediates, which have been implicated in endothelial barrier function (5, 14, 15). As shown in Fig. 3A, S1P elicits a transient increase in Erk1/2 phosphorylation with peak phosphorylation detectable within 3-5 min of treatment. The response to S1P was dose-dependent, although a plateau was not reached using the highest dosage of S1P tested (Fig. 3B). Similarly, HDL produced a transient increase in Erk1/2 phosphorylation with peak phosphorylation detectable within 3-5 min of treatment (Fig. 3C). The activation response to HDL was also dose-dependent, reaching a maximal plateau at 333 μg/ml (based on LC-MS-MS analysis, this amount of HDL contained 135 nm S1P) (Fig. 3D). Similar effects of HDL and S1P on the activation of Akt were also observed (Fig. 3, E-H). Furthermore, the findings indicate that when equimolar amounts of S1P were carried either on HDL or on albumin, they elicit a similar magnitude of Erk and Akt activation (Fig. 3, compare B with D and compare F with H).
FIGURE 3.
HDL stimulates Erk1/2 and Akt activation in endothelial cells. The effect of S1P and HDL on activation of Erk1/2 (A-D) and Akt (E-H) in HUVEC was determined by multiplex bead array assay. In A-D, the values for the -fold difference in Erk1/2 phosphorylation were derived from the level of phospho-Erk1/2 fluorescence in S1P- or HDL-treated cells divided by the level of phospho-Erk1/2 fluorescence in control cells. In E-G, the values for the -fold increase in Akt phosphorylation were derived from the level of phospho-Akt fluorescence in cells treated with S1P or HDL divided by the level of phospho-Akt fluorescence measured in control cells. The data depicted in panels A, C, E, and G is based on treating HUVEC for the indicated times with 833 nm S1P or 333 μg/ml HDL (containing 133 nm S1P). The data depicted in panels B, D, F, and H are based on treating HUVEC with the indicated concentrations of S1P or HDL for 3 min. The level of S1P in the 3-fold dilutions of HDL tested in panel H ranged from 12 to 337 nm. Data are shown from a representative experiment. Each experiment was performed two times; each data point is an average from two independent wells.
Activation of Erk1/2 and Akt by HDL Is Blocked by Pertussis Toxin and an S1P1 Antagonist—Previous studies have shown that S1P-mediated activation of Erk and Akt is PTX-sensitive (16, 17) and mediated by the S1P receptor, S1P1 (18, 19). We performed multiplex microbead suspension array analysis to evaluate the effect of PTX on HDL-induced activation of Erk1/2 and Akt in endothelial cells. The activation of Akt and Erk1/2 by either HDL or S1P was inhibited by PTX (Fig. 4, A and B), suggesting the requirement for Gi-coupled S1P receptors. The S1P receptor, S1P1, was implicated in this process by the finding that the S1P1 antagonist, 857390, and the S1P1/S1P3 antagonist, VPC23019, inhibited HDL-induced and S1P-induced activation of Erk1/2 and Akt (Fig. 4, C and D).
FIGURE 4.
HDL activation of Erk1/2 and Akt in HUVEC is inhibited by pertussis toxin and S1P1 antagonists. In A and B, PTX (100 ng/ml) or the PTX buffer was added to EBM during the final 12 h of serum starvation. BSA, S1P (200 nm), or HDL (containing 200 nm S1P) were then added to the medium and allowed to incubate with the cells for 3 min. In C and D, the S1P1 antagonist 857390 or the S1P1/S1P3 antagonist or vehicle was added to the medium 15 min prior to addition of S1P or HDL and allowed to incubate with the cells for 3 min. The graphed values were derived from the level of phospho-Erk1/2 or phospho-Akt fluorescence in S1P- or HDL-treated cells divided by the level of phospho-Erk1/2 or phospho-Akt fluorescence in BSA-treated cells. Data are shown from representative experiments, antagonist and inhibitor experiments were performed 2-4 times (e.g. PTX, n = 3; S1P1 antagonist, n = 4; S1P1/S1P3 antagonist, n = 2). Each data point is an average from two independent wells.
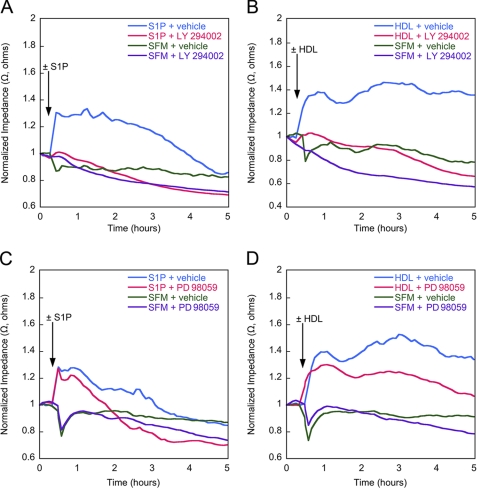
Inhibition of Akt Blocks HDL-induced Enhancement of TEER—In light of the finding that HDL stimulates activation of both Erk and Akt, we next evaluated the effects of antagonists of activation of these signaling pathway intermediates on endothelial barrier function. Inhibition of both S1P-induced and HDL-induced TEER was observed using the Akt inhibitor LY294002 (Fig. 5, A and B). By contrast, partial inhibition of both S1P-induced and HDL-induced TEER was observed using the Erk inhibitor PD98059 (Fig. 5, C and D). Control multiplex microbead suspension array analyses showed that LY294002 blocked 100% of Akt phosphorylation and PD98059 blocked ∼70% of Erk1/2 phosphorylation in response to either S1P or HDL stimulation in HUVEC (data not shown).
FIGURE 5.
Inhibition of Akt and Erk1/2 blocks HDL-induced enhancement of TEER. Confluent endothelial monolayers were grown under serum-free conditions until a minimal TEER plateau had been reached. The cells were then incubated with S1P or HDL in the presence or absence of the Akt inhibitor LY294002 ((LY) A and B) or the Erk inhibitor PD98059 ((PD) C and D). Each of the TEER tracings shown is an average of two replicate wells and representative of three independent experiments. Impedance values were normalized by dividing each value by the level of impedance measured just prior to the addition of effectors. SFM, serum-free medium.
HDL Promotes Endothelial Cell Motility via S1P1/Akt Signaling—Endothelial cell motility has been shown to be regulated by the S1P1/Akt/Rac small GTPase pathway (18, 20). An ECIS-based electrical wound healing assay (5) was used to investigate the ability of HDL to influence endothelial cell motility. Endothelial cells attached to circular micro-electrodes were killed by a burst of electrical current. The migration of viable cells into the wounded area was monitored using ECIS. In the presence of either S1P or HDL, there was an increase in TEER (Fig. 6, A and B), which correlated with increased numbers of cells migrating into the wound area as determined by microscopic examination. The rate of endothelial migration into the wounded area achieved by HDL treatment was reproducibly greater than observed using an amount of S1P equimolar to that carried on HDL.
FIGURE 6.
HDL promotes S1P receptor-dependent endothelial migration in ECIS wounding assay. HUVEC grown on the microelectrodes of the ECIS wells were killed with a burst of high electrical current. Culture medium was supplemented with S1P (833 nm) or HDL (1000 μg/ml containing 400 nm S1P) in the presence or absence of PTX (A and B) or S1P1 antagonists (C and D), and the migration of cells into the wound areas was measured in real time by electrical impedance. Data depicted in each of the TEER tracings are the average of two independent experiments each with two replicates per condition. SFM, serum-free medium.
The effects of S1P and HDL on endothelial cell motility were inhibited by PTX (Fig. 6, A and B), suggesting the requirement for Gi-coupled S1P receptors. Given evidence that the S1P receptor, S1P1, can mediate S1P-induced HUVEC migration (18, 20), we evaluated the effect of S1P1 receptor antagonists on HDL induced endothelial cell motility. The results show that S1P1 antagonists inhibit endothelial cell motility promoted by either HDL or S1P (Fig. 6, C and D). We next evaluated the impact of Akt and Erk inhibitors on the process of HDL-induced endothelial cell motility. As a result, it was found that inhibition of Akt abrogated HDL-induced motility (Fig. 7B). Similarly, inhibition of Akt also abrogated motility induced by S1P carried on albumin (Fig. 7A). By contrast, inhibition of Erk1/2 activation produced partial inhibition of the motility induced by either HDL or S1P (Fig. 7, C and D). Together, these findings indicate that HDL is capable of enhancing endothelial cell motility via S1P signaling primarily through the S1P1/Akt pathway. This signaling may play an important role in the process by which an injured endothelium is repaired.
FIGURE 7.
HDL-stimulated endothelial migration is dependent on Akt activation to greater extent than Erk activation. HUVEC grown on the microelectrodes of the ECIS wells were killed with a burst of high electrical current. One hour before the electrical wounding, the culture medium was supplemented with the Akt inhibitor LY294002 ((LY) A and B) or the Erk inhibitor PD98059 ((PD) C and D). S1P (250 nm) and HDL (234 μg/ml containing 250 nm S1P) were added after electrical wounding. Each of the TEER tracings shown is representative of three independent experiments. SFM, serum-free medium.
DISCUSSION
Here we explored the effects of HDL on endothelial permeability. A number of studies have established that HDL administration can reduce organ injury and mortality in animal models of shock and ischemia/reperfusion (21-23). Mechanistically, such beneficial effects have been attributed to a variety of HDL properties including the ability of HDL to bind and inactivate lipopolysaccharide, to inhibit cytokine-induced expression of endothelial cell adhesion molecules, and to prevent neutrophil adhesion and transmigration through the endothelium (23-26). These latter activities involve effects on the semipermeable barrier function of the endothelium. Alteration in endothelial barrier function is a factor underlying postischemic edema, the recruitment and migration of monocytes, as well as the introduction of triglyceride-rich lipoprotein particles into the intima of the blood vessel (27). The findings presented in the present study establish that HDL can act to promote endothelial barrier integrity as evidenced by its ability to increase TEER in the ECIS assay.
This study also provides insights into the mechanisms underlying HDL-mediated endothelial barrier enhancement. It is shown that the elevated TEER response to HDL is PTX-sensitive, thus implicating Gi-coupled S1P receptor signaling. This inference was substantiated by the finding that HDL enhancement of TEER is blocked by S1P1 antagonists. These results are consistent with findings presented here and elsewhere showing that TEER is augmented by S1P carried on albumin and mediated by S1P1 (1, 5). The physiological relevance of this activity of HDL will likely relate to endothelial barrier function in vivo particularly in light of findings showing that S1P prevents pulmonary edema in rodent and canine models of acute lung injury (28).
The present study also showed that HDL stimulates several known endothelial cell responses to S1P including activation of Erk1/2 and Akt and augmentation of cell motility. These responses to HDL were each found to be mediated by S1P1 signaling. Furthermore, the activation of Akt and Erk1/2 was found to be important in HDL-induced enhancement of endothelial barrier function. However, barrier enhancement showed a greater dependence on Akt activation as compared with Erk1/2 activation. Together, the observed HDL effects on barrier enhancement are consistent with reported evidence that stimulation of endothelial cells by S1P carried on albumin leads to redistribution of tight junction-associated proteins to cell-cell junctions via the S1P1/G(i)/Akt/Rac pathway (5).
It was also observed that both HDL and S1P (carried on albumin) induced bimodal TEER responses over a range of doses. Considering that S1P receptors are G-coupled receptors, these findings may relate to the process by which many G protein-coupled receptors when exposed to agonist become refractory to further stimulation by the same agonist (29). This process, known as agonist-induced desensitization, can be mediated by receptor internalization, down-regulation, and G protein uncoupling. Importantly, S1P has been shown to induce internalization and recycling of the endothelial cell S1P receptor, S1P1 (30). Thus, agonist-induced internalization and recycling of S1P receptors may underlie the observed bimodal TEER response to HDL-S1P.
Acknowledgments
We thank the Lipidomics Core Facility (Drs. J. Bielawski and A. Bielawska) at the Medical University of South Carolina, which is supported by National Institutes of Health Grant C06 RR018823. We thank Charlyne Chassereau for assistance with lipoprotein isolation.
This work was supported, in whole or in part, by National Institutes of Health Grants HL80404 and RR107677 (to K. M. A.), HL52813 (to W. S. A.), and HL079274 (to S. M. H.). This work was also supported by American Heart Association-Jon DeHaan Foundation Scientist Development Grant in Angiogenesis 0230245N (to K. M. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: S1P, sphingosine 1-phosphate; HDL, high density lipoprotein(s); HUVEC, human umbilical vein endothelial cell(s); TEER, transendothelial electrical resistance; PTX, pertussis toxin; ECIS, electric cell substrate impedance sensing; LC-MS-MS, liquid chromatography/mass spectrometry; DPBS, Dulbecco's phosphate-buffered saline; BSA, bovine serum albumin; EGM-2, endothelial growth medium-2; EBM, endothelial basal medium; Erk, extracellular signal-regulated kinase.
References
- 1.Garcia, J. G., Liu, F., Verin, A. D., Birukova, A., Dechert, M. A., Gerthoffer, W. T., Bamberg, J. R., and English, D. (2001) J. Clin. Investig. 108689 -701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaphorst, K. L., Chiang, E., Jacobs, K. N., Zaiman, A., Natarajan, V., Wigley, F., and Garcia, J. G. (2003) Am. J. Physiol. 285L258 -L267 [DOI] [PubMed] [Google Scholar]
- 3.Xu, M., Waters, C. L., Hu, C., Wysolmerski, R. B., Vincent, P. A., and Minnear, F. L. (2007) Am. J. Physiol. 4C1309 -C1318 [DOI] [PubMed] [Google Scholar]
- 4.Peng, X., Hassoun, P. M., Sammani, S., McVerry, B. J., Burne, M. J., Rabb, H., Pearse, D., Tuder, R. M., and Garcia, J. G. (2004) Am. J. Respir. Crit. Care Med. 1691245 -1251 [DOI] [PubMed] [Google Scholar]
- 5.Lee, J. F., Zeng, Q., Ozaki, H., Wang, L., Hand, A. R., Hla, T., Wang, E., and Lee, M. J. (2006) J. Biol. Chem. 28129190 -29200 [DOI] [PubMed] [Google Scholar]
- 6.Murata, N., Sato, K., Kon, J., Tomura, H., Yanagita, M., Kuwabara, A., Ui, M., and Okajima, F. (2000) Biochem. J. 352809 -815 [PMC free article] [PubMed] [Google Scholar]
- 7.Argraves, K. M., and Argraves, W. S. (2007) J. Lipid Res. 482325 -2333 [DOI] [PubMed] [Google Scholar]
- 8.Bielawski, J., Szulc, Z. M., Hannun, Y. A., and Bielawska, A. (2006) Methods (Amst.) 39 82-91 [DOI] [PubMed] [Google Scholar]
- 9.Giaever, I., and Keese, C. R. (1993) Nature 366591 -592 [DOI] [PubMed] [Google Scholar]
- 10.Giaever, I., and Keese, C. R. (1991) Proc. Natl. Acad. Sci. U. S. A. 887896 -7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kataoka, N., Iwaki, K., Hashimoto, K., Mochizuki, S., Ogasawara, Y., Sato, M., Tsujioka, K., and Kajiya, F. (2002) Proc. Natl. Acad. Sci. U. S. A. 9915638 -15643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, M. J., Evans, M., and Hla, T. (1996) J. Biol. Chem. 27111272 -11279 [DOI] [PubMed] [Google Scholar]
- 13.Taha, T. A., Argraves, K. M., and Obeid, L. M. (2004) Biochim. Biophys. Acta 1682 48-55 [DOI] [PubMed] [Google Scholar]
- 14.Vogel, C., Bauer, A., Wiesnet, M., Preissner, K. T., Schaper, W., Marti, H. H., and Fischer, S. (2007) J. Cell. Physiol. 212236 -243 [DOI] [PubMed] [Google Scholar]
- 15.Wachtel, M., Frei, K., Ehler, E., Bauer, C., Gassmann, M., and Gloor, S. M. (2002) Biochem. J. 367873 -879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park, K. S., Kim, M. K., Lee, H. Y., Kim, S. D., Lee, S. Y., Kim, J. M., Ryu, S. H., and Bae, Y. S. (2007) Biochem. Biophys. Res. Commun. 356239 -244 [DOI] [PubMed] [Google Scholar]
- 17.Wu, J., Spiegel, S., and Sturgill, T. W. (1995) J. Biol. Chem. 27011484 -11488 [DOI] [PubMed] [Google Scholar]
- 18.Lee, M. J., Thangada, S., Paik, J. H., Sapkota, G. P., Ancellin, N., Chae, S. S., Wu, M., Morales-Ruiz, M., Sessa, W. C., Alessi, D. R., and Hla, T. (2001) Mol. Cell 8 693-704 [DOI] [PubMed] [Google Scholar]
- 19.Osinde, M., Mullershausen, F., and Dev, K. K. (2007) Neuropharmacology 521210 -1218 [DOI] [PubMed] [Google Scholar]
- 20.Lee, M. J., Thangada, S., Claffey, K. P., Ancellin, N., Liu, C. H., Kluk, M., Volpi, M., Sha'afi, R. I., and Hla, T. (1999) Cell 99301 -312 [DOI] [PubMed] [Google Scholar]
- 21.Hubsch, A. P., Casas, A. T., and Doran, J. E. (1995) J. Lab. Clin. Med. 126548 -558 [PubMed] [Google Scholar]
- 22.Cockerill, G. W., McDonald, M. C., Mota-Filipe, H., Cuzzocrea, S., Miller, N. E., and Thiemermann, C. (2001) FASEB J. 151941 -1952 [DOI] [PubMed] [Google Scholar]
- 23.Thiemermann, C., Patel, N. S., Kvale, E. O., Cockerill, G. W., Brown, P. A., Stewart, K. N., Cuzzocrea, S., Britti, D., Mota-Filipe, H., and Chatterjee, P. K. (2003) J. Am. Soc. Nephrol. 141833 -1843 [DOI] [PubMed] [Google Scholar]
- 24.Emancipator, K., Csako, G., and Elin, R. J. (1992) Infect. Immun. 60596 -601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine, D. M., Parker, T. S., Donnelly, T. M., Walsh, A., and Rubin, A. L. (1993) Proc. Natl. Acad. Sci. U. S. A. 9012040 -12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cockerill, G. W., Rye, K. A., Gamble, J. R., Vadas, M. A., and Barter, P. J. (1995) Arterioscler. Thromb. Vasc. Biol. 151987 -1994 [DOI] [PubMed] [Google Scholar]
- 27.Nordestgaard, B. G., Wootton, R., and Lewis, B. (1995) Arterioscler. Thromb. Vasc. Biol. 15 534-542 [DOI] [PubMed] [Google Scholar]
- 28.McVerry, B. J., Peng, X., Hassoun, P. M., Sammani, S., Simon, B. A., and Garcia, J. G. (2004) Am. J. Respir. Crit. Care Med. 170987 -993 [DOI] [PubMed] [Google Scholar]
- 29.Bohm, S. K., Grady, E. F., and Bunnett, N. W. (1997) Biochem. J. 3221 -18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jo, E., Sanna, M. G., Gonzalez-Cabrera, P. J., Thangada, S., Tigyi, G., Osborne, D. A., Hla, T., Parrill, A. L., and Rosen, H. (2005) Chem. Biol. 12703 -715 [DOI] [PubMed] [Google Scholar]