Abstract
Dendritic cells (DCs) from HIV-1-infected individuals display numeric and functional defects, and recent evidence suggests that HIV-1 can both directly and indirectly activate DCs in vitro. However, the in vivo activation state and compartmentalization of DC subsets during HIV-1 infection remain poorly understood. We evaluated phenotypic and functional characteristics of myeloid and plasmacytoid DCs (mDC, pDC) directly ex vivo in peripheral blood and lymphoid tissue from HIV-1-infected and seronegative individuals. Analysis of a wide range of chemokine receptors and activation/maturation markers on circulating DCs from viremic, HIV-1-infected donors revealed a phenotype indicative of partial activation. Yet, blood DCs from viremic subjects still achieved full maturation when stimulated in vitro. In addition, blood pDCs from viremic individuals had a reduced capacity to migrate to CXCL12 in vitro. Total numbers of both DC subsets were increased in lymph nodes of asymptomatic, untreated HIV-1 infected subjects, consistent with DC accumulation in the lymphoid compartment. Lymph node DCs also expressed high levels of CD40 in the absence of increases of other typical activation/maturation markers. Atypical activation and depletion of DCs in blood with accumulation in lymphoid tissue may contribute to HIV-associated chronic immune activation and T cell dysfunction.
Keywords: Dendritic cells, HIV-1, lymph nodes, CD40
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1)-associated CD4+ T cell depletion in infected humans is associated with chronic, generalized immune activation, increased T cell exhaustion and apoptosis1-7. The level of immune activation may be a better predictor of CD4+ T cell depletion than viral load, even in the setting of antiretroviral therapy8-10. However, the fundamental mechanisms underlying HIV-1-associated immune activation remain poorly understood. Recent evidence suggests that HIV-1 may both directly and indirectly activate DCs11-13. The migratory nature of activated DCs might then allow them to interact closely with T cells in lymphoid tissue, thereby inducing T cell activation and aiding in the spread of HIV-1. Thus, HIV-1-associated DC activation may be a major factor contributing to the state of chronic T cell activation and dysfunction.
DCs are potent antigen-presenting cells that sense the presence of pathogens and serve as a link between innate and adaptive immune systems14, 15. They play a crucial role in priming MHC class II- and class I-restricted T cell responses, and they also function in the innate immune system by recognizing pathogen-associated molecular patterns (PAMPs) on microbial pathogens via pattern recognition receptors, such as Toll-like receptors (TLRs). DCs exist in tissues and blood in an immature state, but upon encounter with invading microbes, microbial antigens, or upon exposure to pro-inflammatory cytokines, DCs take up antigen and undergo a highly regulated maturation process16. DC maturation results in up-regulation of MHC and co-stimulatory molecules (CD80/CD86, CD40) and production of cytokines (IL-12, IFN-α) that allow for efficient T cell activation. Ultimately, activated DCs migrate to lymphoid organs, where they home to T cell areas and stimulate naïve, antigen-specific T cells to proliferate.
The two main subsets of human DCs, the mDC subset and the pDC subset, differ in morphology, phenotype, and function. Circulating blood pDCs and mDCs have been shown to have different patterns of TLR expression (eg. mDCs express TLR4 whereas pDCs express TLR9)17, 18, to have different migratory properties19 and to produce distinct cytokine profiles upon stimulation20-22. Taken together, these studies suggest that the activation state of the DC, the type of activation signal it encounters, and the cytokine environment all may contribute to the type and extent of T cell activation induced by DCs23-25.
Given their role in bridging innate and adaptive immunity and in priming antigen-specific T cell responses, DCs likely play an important role in HIV-1 pathogenesis. During both acute and chronic infection, both mDCs and pDCs have been shown to be depleted from the blood26-32, a finding associated with high viral loads26-29, 33-37 and directly correlated with CD4+ T cell counts in both adults26, 36 and children37. The depletion of blood pDCs has been associated with HIV-1 disease progression and development of opportunistic infections32. Although most studies found that antiretroviral therapy at least partially restored mDC and/or pDC subsets26, 30, 31, 35, 37, others have shown that abnormalities persist despite treatment34, 38.
In addition to numeric defects observed in the blood, functional defects and an altered phenotype have also been reported for both DC subsets from HIV-1-infected subjects, both in vitro and in vivo. We and others have reported that in viremic HIV-1-infected individuals there is an increased expression of CD8626, 39 and CD4026 on blood DCs and that costimulatory molecule expression correlated directly with viral load26. Modulation of chemokine receptor expression33 that persisted despite treatment38 has also been shown on freshly isolated blood DCs from HIV-1-infected subjects. The downstream consequences of altered blood DC activation on T cell function in the setting of HIV-1 infection remain poorly understood.
We hypothesized that HIV-1 replication in vivo advances the maturation state of circulating pDCs and mDCs, leading to their accelerated migration to and retention in lymphoid tissues and their observed depletion from the blood. Therefore, to more fully understand the activation profile of DC subsets in the setting of HIV-1 infection, we first used multi-parameter flow cytometry techniques to simultaneously compare expression of a wide range of maturation/activation markers and chemokine receptors on blood DC subsets in freshly isolated PBMC from both treated and untreated HIV-1-infected individuals relative to seronegative subjects. Additionally, functional impairment of DCs by HIV-1 infection in vivo was assessed by determining the in vitro ability of blood DCs from HIV-1-infected donors to migrate to specific chemokines and to reach full maturation in response to additional exogenous stimuli. Lastly, to explore the etiology of blood DC depletion in the setting of HIV-1 infection, the frequencies and phenotypic profiles of DC subsets in matched blood and lymph nodes from untreated, HIV-1-infected subjects and seronegative subjects were assessed.
MATERIALS AND METHODS
Study Population
For blood DC studies, 29 HIV-1-infected subjects who received their care through the University of Colorado Health Sciences Center (UCHSC) Infectious Diseases Group Practice were studied. These subjects were divided into two clinical cohorts based upon their treatment status: highly active antiretroviral therapy (ART)-treated with viral suppression (Suppressed) or untreated with plasma viremia (Viremic). Inclusion criteria for the Suppressed group (n=15) included receiving a combination of three or more antiretroviral agents for at least 4 months with suppression of plasma viral load to <200 copies HIV-1 RNA per milliliter of plasma at the time of screening (median CD4+ T cell count 644 cells/μl; range: 209-1400). Viremic subjects (n=14) were either ART naïve or had not been on treatment for at least one year at the time of screening with median viral load of 59200 copies HIV-1 RNA per milliliter of plasma (range 8900-440000) and CD4+ T cell count of 416 cells/μl (range: 180-900). HIV-1 seronegative (SN) subjects (n=17) were normal healthy adult volunteers self-identifying as HIV-1 uninfected.
For studies involving lymph nodes, palpable superficial inguinal lymph nodes generally located adjacent to the superficial saphenous vein were collected. Inguinal lymph nodes were collected from HIV-1 seropositive subjects (n=10) recruited from the infectious disease clinics at the University of Colorado Hospital and Denver Health and Hospitals Association based on the following criteria: 18 years or older, medically stable, CD4 count of ≥ 300 cells/μl, no history of AIDS, and either antiretroviral naïve or off therapy for 3 months. All HIV-1 seropositive subjects had been infected for greater than 1 year. This cohort had a median viral load of 8255 copies HIV-1 RNA per milliliter of plasma (range 735-181970) and a median CD4+ T cell count of 677 cells/μl (range: 365-960). Inguinal lymph nodes were also collected from HIV-1 seronegative individuals (n=6) recruited through the University of Colorado Hospital from patients scheduled to undergo surgery in the inguinal region. For both seropositive and seronegative individuals, blood samples were collected at the same time as collection of lymph nodes.
All study subjects participated voluntarily and gave informed consent. The study was approved by the Colorado Multiple Institutional Review Board (COMIRB) at UCHSC.
Collection and preparation of PBMC
Blood from HIV-1 seropositive and seronegative donors was collected in vacutainers containing sodium heparin, and peripheral blood mononuclear cells (PBMC) were isolated using standard Ficoll-Hypaque (GE Healthcare Bioscience, Piscataway, NJ) density gradient centrifugation. Total PBMC were then immediately stained with a panel of antibodies to determine direct surface expression of DC maturation and chemokine receptor molecules, were assessed for migratory ability, or were cultured overnight to determine maturation phenotype after in vitro stimulation, as described below.
Collection and preparation of lymph node cells
Inguinal lymph nodes from both HIV-1 seropositive and HIV-1 seronegative individuals were removed under local anesthesia and processed within 1 hour. Excess fat was removed from the lymph node and the total lymph node was weighed. The lymph node was then minced in PBS (Invitrogen, Carlsbad, CA) to obtain a single cell suspension. Lymph node cells were then either immediately stained to determine surface molecule expression or used in migration assays as described below.
Flow cytometry
Eight-parameter flow cytometry was performed on freshly isolated PBMC, disaggregated lymph node cells, or on cultured PBMC using a FACS Aria flow cytometer (BD Biosciences, San Jose, CA). DCs were identified by using the following monoclonal antibody panel: FITC-labeled anti-lineage cocktail (CD3/CD14/CD16/CD19/CD20/CD56; BD Biosciences), FITC-labeled anti-CD34 (BD Biosciences), APCCy7-labeled anti-HLA-DR (BD Biosciences), PECy5-labeled CD11c (BD Biosciences) and APC-labeled CD123 (IL-3R; Miltenyi, Auburn, CA). DC subsets were defined as follows: mDCs were Lineage− CD34− HLA−DR+ CD123low CD11c+, and pDCs were Lineage− CD34− HLA−DR+ CD11c− CD123high. Expression of various surface molecules on the DC were then determined using appropriate combinations of the following antibodies: PE-labeled CXCR3 (BD Biosciences), PE-labeled CXCR4 (BD Biosciences), PE-labeled CD83 (BD Biosciences), PECy7-labeled CCR5 (BD Biosciences), biotinylated CD86 (BD Biosciences) followed by streptavidin-labeled ECD (Beckman Coulter, Fullerton, CA), biotinylated CD40 (Ancell, Bayport, MN) followed by streptavidin-labeled ECD, or purified mouse anti-human CCR7 (BD Biosciences) followed by biotinylated anti-mouse IgM and streptavidin-labeled ECD. Background fluorescence was removed by using a fluorescence-minus-one (FMO) control determined by measuring the expression of each individual fluorescent antibody of interest in combination with the fluorescent antibodies used to identify the DCs in the absence of other fluorescent antibodies40. All incubations were performed at 4°C for 20 minutes and cells were fixed with 2% paraformaldehyde. Analysis was performed using DIVA software Version 4.1.1 (BD Biosciences) on total cells.
Four-color analysis was performed on total PBMC to determine DC migration using a FACSCalibur (BD Biosciences). The following monoclonal antibody panel was used: FITC-labeled anti-lineage cocktail, FITC-labeled anti-CD34, PE-labeled anti-CD11c, PerCp-labeled anti-HLA-DR (BD Biosciences) and APC-labeled CD123. All incubations were performed at 4°C for 20 minutes and cells were fixed with 2% paraformaldehyde. Analysis was performed on total cells using CellQuest software (BD Biosciences).
Culture and maturation of blood dendritic cells
In vitro stimulation of specific DC subsets in unfractionated PBMC was achieved by ligating TLRs that are differentially expressed on each DC subset. Lipopolysaccharide (LPS) was used to stimulate TLR4 expressed on mDCs, whereas CpG oligonucleotides were used to stimulate TLR9, restricted to the pDC subset41. PBMC were isolated as described above and then resuspended in RPMI 1640 medium (Invitrogen) with 10% human AB serum (Gemini Bioproducts, West Sacramento, CA) at 2 × 106 cells/ml and cultured at 37°C, 5% CO2 without stimuli or with either LPS from Salmonella minnesota (10μg/ml; Sigma-Aldrich, St Louis, MO) or with a combination of CpG oligodeoxynucleotide 2006 (5'-GGGGGACGATCGTCGGGGGG-3') and CpG oligodeoxynucleotide 2216 (5'-TCGTCGTTTTGTCGTTTTGTCGTT-3') (3μg/ml of each; Invitrogen). CpG oligodeoxynucleotide sequences were selected based on their ability to induce maturation of pDC (ODN 2006) and production of high amounts of IFN-α (ODN 2216)42. After 18-24 hours cells were collected and stained with DC-specific markers and maturation/activation markers as described above.
Migration assays
Evaluation of migratory properties of pDCs and mDCs in the blood of HIV-1-seropositive and seronegative subjects was performed using a modified transmigration assay43. This assay utilized unfractionated PBMC, therefore avoiding unintentional in vitro maturation effects during purification, and increased total input PBMC concentration in order to account for decreased frequencies of blood DCs measured in PBMCs of HIV-infected subjects26-36. Briefly, individual chemokines were diluted to final concentrations in RPMI 1640 + 10% human AB serum (CXCL12 200 ng/ml; CXCL-10 1μg/ml; CCL5 100 ng/ml; CCL21 100 ng/ml; (Peprotech, Rocky Hill, NJ)) and added in duplicate to the bottom wells of a 24-well Costar transwell plate (Corning Inc, Corning, NY). Duplicate wells of media alone without chemokine were used as controls. PBMCs were resuspended at 1.5-2 × 107 cells/ml in RPMI + 10% human AB serum and 100μl added to the top wells and incubated for 2 hours at 37°C, 5% CO2. Cells in the bottom wells were then harvested by pipetting and residual cells collected by rinsing the underside of the filter with cold PBS and 10mM EDTA. Cells were washed and stained with the appropriate antibodies as described above. In addition, 5 × 104 beads (Bangs Laboratories, Fishers, IN) were added to each tube to normalize the number of DCs between each sample prior to acquisition. An average of 160 (2-1406) migrating mDCs and 39(0-583) pDCs were evaluated per experiment.
To quantitate the number of mDCs and pDCs in each sample, the number of DCs collected was divided by the number of beads collected and then multiplied by the total number of input beads. Duplicate results were averaged and used to calculate the migration index, where the migration index was calculated as the number of DC migrated divided by the number of input DC multiplied by 100. Net migration was defined as the percentage of DCs migrating to chemokine minus the percent migrating in media alone.
Statistical analysis
Non-parametric statistics were used due to small samples sizes. Differences between seronegative and seropositive subjects were evaluated using Mann-Whitney U tests, and assessments of paired variables were performed using the Wilcoxon signed-rank test. For secondary comparisons between multiple groups, Kruskal-Wallis test was used and, given an overall p<0.05, pairwise comparisons were conducted using Dunn's multiple comparison adjustment. The Spearman correlation test was performed to analyze associations between DC surface marker expression and clinical parameters (CD4 count, viral load) and considered significant if r>0.3 and P<0.05. Data analysis was performed using GraphPad Prism statistical software.
RESULTS
Maturation markers on peripheral blood mDCs and pDCs are altered in the setting of untreated chronic HIV-1 infection
Blood DC subsets were enumerated, and the surface expression of a range of markers known to be involved in DC maturation/activation and migration or in co-stimulation of T cells14 assessed using the gating strategy detailed in Fig. 1. Both mDCs (Lineage− CD34− HLA-DR+ CD123low CD11c+) and pDCs (Lineage− CD34− HLA-DR+ CD11c− CD123high) as a fraction of total PBMC were measured in the blood of 28 chronically HIV-1-infected individuals and 17 seronegative subjects. Consistent with previous investigations26-36, the percentage of both mDC and pDC in total PBMC were significantly reduced in all HIV-1-infected subjects (mDC median percentage=0.119, range 0.033-0.257; pDC median percentage=0.093, range 0.031-0.374), compared to seronegative control subjects (mDC median percentage=0.211, range 0.078-0.413, P<0.0005; pDC median percentage=0.223, range 0.088-0.592, P<0.0003). To determine the impact that ART may have had on DC numbers, control subjects (n=17) were also compared to HIV-infected treated (suppressed, n=15) and untreated (viremic, n=13) subjects. Percentages of both mDC and pDC were significantly lower in viremics (mDC median percentage=0.107, range 0.061-0.257; pDC median percentage=0.074, range 0.034-0.216), than in the seronegative group (p<0.05 and p<0.001, respectively). The percentage of mDCs or pDCs were also significantly lower in PBMCs from treated HIV-infected subjects (mDC median percentage=0.141, range 0.033-0.180; pDC median percentage=0.143, range 0.031-0.374) than from seronegative subjects (P<0.01 and P<0.05 respectively). In contrast to some previous reports, no significant correlations between the percentage of either DC subset in peripheral blood and either CD4 count or viral load were demonstrated.
FIGURE. 1.
Gating strategy to assess expression of various surface markers on DC subsets in blood and lymph nodes. Multi-parameter flow techniques were used to simultaneously assess expression of chemokine receptors (CCR5, CCR7, CXCR3, CXCR4) and costimulatory/activation markers (CD86, CD83, CD40) on both myeloid (mDC) and plasmacytoid (pDC) subsets. Total DCs, for both PBMC and lymph nodes, were defined as Lineage− CD34− HLA-DR+ and then further divided into pDC (CD11c− CD123high) and mDC (CD11chigh CD123low) subsets. Subsequent analysis of expression of chemokine and costimulatory/activation markers by these subsets was assessed. Net Mean Fluorescence Intensity (MFI) was determined using a fluorescence minus one (FMO) control for each fluorescent antibody. Profiles shown are representative of seronegative donors.
To address the possibility that HIV-1 infection altered blood DC activation/maturation phenotype, expression levels of various markers were measured directly ex vivo and comparisons were made between blood DCs from seronegative donors with those from HIV-1-infected individuals, both untreated and treated. Despite evaluating a wide range of DC activation/maturation markers, only CD40 expression differed between seronegative and viremic DC subsets (Table 1). Although both subsets expressed higher levels of this activation molecule compared to expression on seronegative DC subsets, statistical significance was achieved only for the mDC subset comparison (Fig. 2A). CD40 levels on both DC subsets from treated subjects were similar to seronegative donors (Table 1, Fig. 2A). Furthermore, expression of CD40 by mDC and pDC correlated positively with viral load within the viremic cohort (Fig. 2B). Median expression levels of CD86 and CD83 were also higher on blood DCs from viremic subjects, although these differences did no achieve statistical significance. These findings suggest that HIV-1 replication in vivo may induce only partial activation rather than full maturation of DC subsets.
TABLE 1.
Expression of various chemokine and costimulatory/activation markers on blood DC subsets from seronegative donors and from HIV-1-infected donors either untreated (viremic) or receiving ART (suppressed)
| Seronegative (n=17) | Viremic (n=13) | Suppressed (n=15) | ||
|---|---|---|---|---|
| mDC | Overall Pa | |||
| CXCR4 | 361 (80-1929) | 754 (58-1778) | 639 (187-2457) | 0.43 |
| CCR7 | 535 (94-1054) | 365 (3-924) | 346 (24-1021) | 0.23 |
| CCR5 | 2720 (1409-5558) | 4723 (1291-9050) | 3086 (1457-5472) | 0.48 |
| CXCR3 | 545 (137-3002) | 959 (78-2380) | 382 (157-1357) | 0.72 |
| CD86 | 2115 (628-3528) | 2373 (1293-3480) | 1751 (321-3452) | 0.45 |
| CD40 | 2406 (1873-6350) | 4809 (2683-6370)P<0.05 | 2746 (1022-5221) | 0.01 |
| CD83 | 237 (24-4880) | 376 (30-3360) | 69 (14-834) | 0.12 |
| pDC | ||||
| CXCR4 | 1645 (90-4864) | 1560 (148-5568) | 837 (550-4572) | 0.80 |
| CCR7 | 470 (112-2282) | 596 (11-2324) | 360 (94-1649) | 0.63 |
| CCR5 | 1990 (652-4578) | 2870 (572-7600) | 2620 (843-4577) | 0.50 |
| CXCR3 | 2682 (836-7800) | 2536 (408-4463) | 1456 (702-3174) | 0.23 |
| CD86 | 389 (124-1547) | 462 (190-991) | 379 (2-873) | 0.62 |
| CD40 | 1660 (589-4277) | 2443 (1156-6287) | 1787 (962-5532) | 0.06 |
| CD83 | 222 (64-3583) | 324 (30-1685) | 108 (1-647) | 0.11 |
Expression of each surface marker was assessed on myeloid DC (mDC) and plasmacytoid DC (pDC) from seronegative donors (n=17), viremic donors (n=13) and suppressed donors (n=15). Values are expressed as Mean Fluorescence Intensity (MFI) minus FMO control (detailed in Fig. 1) with median (range) shown. Statistical analysis was performed using
Kruskall-Wallis test with pairwise comparisons to the control group conducted for P<0.05 using Dunn's Multiple comparison adjustment.
FIGURE 2.
Expression of CD40 on myeloid (mDC) and plasmacytoid (pDC) DC subsets within freshly isolated peripheral blood and relationship to viral load. A) Expression of CD40 on mDC and pDC from seronegative (n=17), viremic (n=13) and suppressed (n=12) donors. Viral load is positively correlated with expression of B) CD40. Statistical analysis was performed using Kruskall-Wallis (KW) test for comparisons between multiple groups with pairwise comparisons conducted when P<0.05 using Dunn's multiple comparison adjustment and Spearman correlation test was performed for correlations. Values are expressed as Mean Fluorescence Intensity (MFI) minus FMO control as described in Fig. 1.
DCs from HIV-1-infected subjects achieve full maturation after in vitro stimulation
We reasoned that the partial activation state of DCs in freshly-isolated blood PBMC from viremic HIV-1-infected individuals might alter their ability to achieve full maturation with additional stimulation in vitro. To test this hypothesis, PBMC from 9 seronegative subjects and 11 viremic, HIV-1-infected subjects were cultured with TLR-specific ligands, and DC subset expression of multiple surface markers was then evaluated by flow cytometry. Changes in expression levels were determined using paired analysis of expression before and after stimulation. As in shown in Table 2, CXCR4, CCR7, CD86, CD40 and CD83 expression increased whereas CCR5 decreased on mDCs from viremic subjects following LPS stimulation. Similarly, pDC from viremic donors increased CXCR4, CCR7, CD86, CD40 and CD83 expression following CpG stimulation. Similar trends were observed in expression of maturation markers on DCs from seronegative donors (data not shown).
TABLE 2.
Changes in expression of maturation markers on DC subsets from viremic donors in response to a potent stimulus in vitro.
| mDC |
pDC |
|||||
|---|---|---|---|---|---|---|
| Stimulation: | Before | After | Trend/Pa | Before | After | Trend Pa |
| CXCR4 | 456 (79-1857) |
1489 (548-6587) |
↑P=0.001 | 1004 (237-5703) |
4490 (533-30945) |
↑P=0.01 |
| CCR7 | 424 (132-1090) |
2762 (566-11412) |
↑P=0.001 | 526 (399-2441) |
3253 (882-8966) |
↑P=0.001 |
| CCR5 | 3449 (1451-9358) |
905 (576-4660) |
↓P=0.01 | 2550 (599-7683) |
758 (358-2467) |
↓P=0.002 |
| CXCR3 | 519 (129-2148) |
694 (205-7145) |
↔ | 1993 (450-4598) |
886 (335-2659) |
↓P=0.002 |
| CD86 | 2615 (1445-3633) |
4860 (1135-22573) |
↑P=0.04 | 640 (278-1096) |
4005 (1796-18432) |
↑P=0.002 |
| CD40 | 4966 (2809-6523) |
7592 (2642-24381) |
↑P=0.01 | 3118 (1241-6389) |
12451 (5519-30437) |
↑P=0.002 |
| CD83 | 280 (62-2124) |
2512 (374-6376) |
↑P=0.001 | 410 (90-1810) |
1840 (506-8359) |
↑P=0.001 |
Maximum fluorescence intensity (MFI) was assessed on myeloid DC (mDC) and plasmacytoid (pDC) DC in PBMC from viremic donors (n=11) before and after 18-24 hours of stimulation with TLR-specific stimuli (mDC: LPS; pDC: CpG) in vitro. Trends are shown with an arrow indicating a statistically significant increase (↑), decrease (↓) or no change (↔) in expression of each respective marker after stimulation relative to expression before stimulation as determined by
Wilcoxon signed rank test. Values shown are median (range) MFI values.
Although no differences were observed with in vitro maturation trends between DCs from viremic and control subjects, it was possible that the level to which each maturation marker was modulated in response to stimulation might differ (i.e. DCs from viremic subjects might not achieve complete maturation). However, when the absolute magnitude of expression detected for each surface marker following stimulation was compared, no significant differences were observed between the extent to which mDCs and pDCs from viremic subjects upregulated or downregulated markers relative to seronegative donors (data not shown). Thus, these findings suggest that both pDCs and mDCs from viremic, HIV-1-infected subjects can achieve full maturation (relative to seronegative donors) following in vitro TLR stimulation, despite an altered phenotype at baseline.
The ability of pDCs to migrate in response to CXCL12 is reduced in HIV-1-infected subjects
In addition to its effects on DC maturation phenotype, we hypothesized that HIV-1 infection might alter the chemotactic profile of blood DCs, thereby leading to increased migration from the blood to lymphoid organs or inflamed tissues and possibly explaining the decreased numbers of both DC subsets observed in the blood of HIV-1-infected individuals. A transwell migration assay was used to determine the ability of DC subsets in PBMC from a subset of seronegative and HIV-1-infected subjects to migrate in response to various chemokines, including CXCL12, CCL5, CCL21, and CXCL-10, based on documented DC expression of the relevant chemokine receptors (CXCR4, CCR5, CCR7, and CXCR3, respectively). When lower total PBMC numbers were obtained from some donors, a more limited panel of chemokines were investigated, thus there is some variation in the number of donors evaluated using each specific chemokine.
pDCs migrated to a greater extent than did mDCs to the CXCR4 ligand, CXCL12, in PBMCs from seronegative subjects (n=12; Fig. 3A) perhaps reflective of the higher expression levels of CXCR4 on pDC relative to mDC (Table 1). Further, net migration of mDCs was significantly greater than pDCs to CCL5, again possibly reflecting the higher expression of CCR5 on the mDC subset in seronegative donors (n=10; Fig. 3A, Table 1). However, despite a much greater expression of CXCR3 on pDCs than mDCs (Table 1), mDCs migrated better to CXCL-10, a CXCR3 ligand, than did pDCs (n=10, Fig. 3A). Additionally, mDCs displayed a greater migratory response to CCL21 than did pDCs (n=12), despite both DC subsets expressing similar levels of CCR7 in the normal donor cohort (Fig. 3A, Table 1). Thus, in agreement with previous studies44, migratory patterns of mDC and pDC from seronegative donors to specific chemokines did not always parallel expression of the relevant chemokine receptor, and DC chemotaxis for a number of chemokine receptor/chemokine ligand pairs was sometimes uncoupled for the pDC subset.
FIGURE 3.
Migratory capacity of blood DCs from HIV-1-infected and seronegative subjects. A transwell migration assay was used to determine the ability of DC subsets to migrate to various chemokines. Values are displayed as net migration (percentage of DCs migrating to a chemokine minus the percent migrating to media alone). A) Comparisons of myeloid DC (mDC) and plasmacytoid DC (pDC) for seronegative donors (CXCL12 n=12; CCL5 n=10; CCL21 n=12; CXCL-10 n=10). B) Comparisons of mDC and pDC for viremic donors (CXCL12 n=16; CCL5 n=10; CCL21 n=15; CXCL-10 n=9). C) Net migration to CXCL12 for pDC from viremic donors compared to pDC from seronegative and suppressed donors. Statistical analysis was performed using Mann-Whitney for comparisons between mDC and pDC and Kruskall-Wallis (KW) test for comparisons between multiple groups with pairwise comparisons conducted when P<0.05 using Dunn's multiple comparison adjustment.
Similar migratory patterns in response to chemokines were observed between mDC and pDC in PBMC from viremic subjects, with one notable exception (Fig. 3B). The migratory response of pDC to CXCL12 was significantly lower compared to the corresponding response of mDC to CXCL12 (Fig. 3B; n=16). Further comparisons demonstrated that pDCs from viremic subjects had a significantly reduced ability to migrate to CXCL12 compared to pDCs from seronegative donors (Fig. 3C). pDCs from suppressed donors appeared to have an increased ability to migrate in response to CXCL12 relative to DCs from viremic donors although this migratory ability did not achieve normal levels (Fig. 3C). This dramatic reduction in pDC migratory response to CXCL12 in viremic subjects was associated with only minimal differences in blood pDC CXCR4 expression between viremic and seronegative donors (Table 1). No other significant differences in migratory response to chemokines of either DC subset were found between seronegative and viremic subjects.
DCs are increased in absolute number and have an altered maturation phenotype in lymph nodes from HIV-1-infected subjects
Decreased numbers of DCs in the blood of HIV-1-infected subjects suggested that DCs were either dying or migrating from the blood to other anatomical sites, such as to lymphoid tissue, the site of the majority of active HIV-1 replication45. Thus, to elucidate DC subset numbers within the lymph node and to determine whether DCs within the lymphoid tissue also displayed altered phenotypes, lymph node cells and paired PBMC samples were obtained from a cohort of untreated, asymptomatic HIV-1-infected subjects (n=10) and from seronegative subjects (n=6), and an extensive phenotypic analysis of DC subsets was performed on cells from a subset of those subjects (n=9 HIV-1-infected; n=5 seronegative).
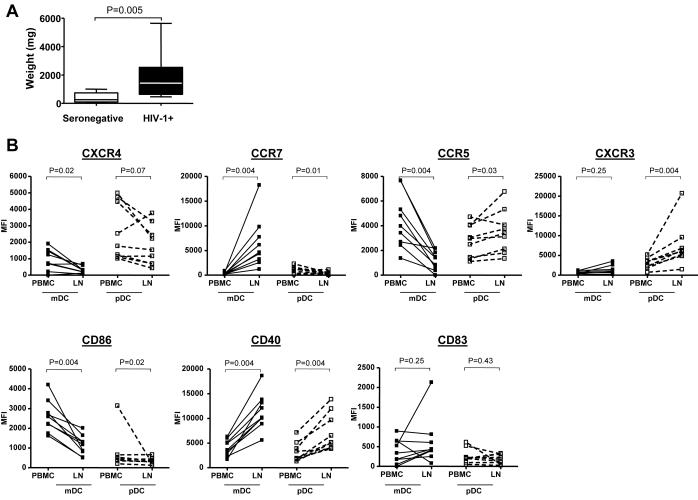
DC subsets were initially enumerated in PBMC and disaggregated lymph node tissue and results compared between subject groups (Table 3). The number of DCs in lymphoid tissue may vary depending on the size and cellularity of the lymph node tissue obtained. Thus, DCs in lymph nodes were expressed as fractions of DCs per total lymphoid cells, as number of DCs per gram of lymph node tissue, and to account for lymph node size, as the absolute number of DCs per entire lymph node. Reflecting the trends of the larger cohort, pDCs and mDCs in PBMCs from the HIV-1-infected lymph node donors showed typical depletion relative to those from seronegative lymph node donors (although the decreased pDC percentage did not achieve statistical significance, P=0.12). Although median percentages and numbers of DCs per gram of tissue for both mDCs and pDCs in lymphoid tissue from HIV-1-infected donors were not significantly different than their counterparts in lymph nodes from seronegative donors (Table 3), there was in fact an accumulation of both DC subsets when the absolute number of each DC subset within the whole lymph node was calculated (Table 3). This finding largely resulted from the significantly larger size of the lymph nodes obtained from HIV-1-infected subjects (Fig. 4A).
TABLE 3.
Frequency and number of DC subsets in PBMC and lymph nodes
|
% mDC in PBMC |
% mDC in LN |
mDC(106)/g LN |
Total mDC (106) per LN |
|
|---|---|---|---|---|
| SN (n=6) | 0.281 (0.140-0.380) |
0.191 (0.054-0.289) |
0.414 (0.117-0.579) |
0.063 (0.036-0.302) |
| HIV-1+ (n=10) |
0.129 (0.063-0.233) |
0.157 (0.024-0.565) |
0.333 (0.081-1.29) |
0.494 (0.126-1.80) |
| aP value | P=0.002 | P=1 | P=0.96 | P=0.008 |
|
% pDC in PBMC |
% pDC in LN |
pDC(106)/g LN |
Total pDC (106) per LN |
|
| SN (n=6) | 0.220 (0.040-0.441) |
0.444 (0.042-0.731) |
0.708 (0.051-2.71) |
0.194 (0.016-0.775) |
| HIV-1+ (n=10) |
0.089 (0.045-0.228) |
0.171 (0.077-0.371) |
0.438 (0.079-2.61) |
0.466 (0.057-3.59) |
| aP value | P=0.12 | P=0.43 | P=0.64 | P=0.07 |
Values shown are frequency of myeloid (mDC) and plasmacytoid (pDC) DC subsets as a percentage of total cells in peripheral blood (PBMC) and total disaggregated lymph nodes (LN) as well as DC subsets (106) per gram of LN tissue and absolute number of DC subsets (106) per LN from seronegative (SN; n=6) and untreated, HIV-1-infected (HIV-1+; n=10) donors. Results are displayed as median (range) with statistical analysis performed using
Mann-Whitney to compare seronegative donor values with HIV-1-infected donors
FIGURE 4.
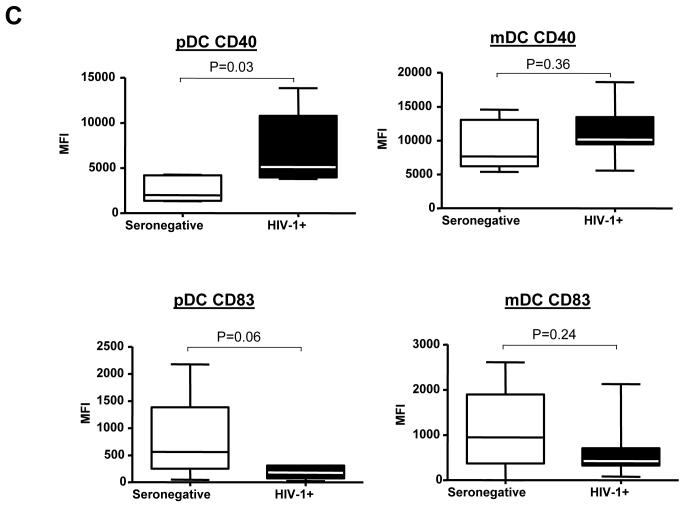
Comparison of lymph node weights and expression of surface markers on DC subsets from blood and lymph nodes from HIV-1-infected donors. A) Differences in overall weights (mg) of lymph nodes were compared between seronegative and HIV-1-infected (HIV-1+) subjects and statistical analysis performed using Mann-Whitney test. B) and C) Multi-parameter flow techniques were used to assess the expression of various chemokine receptors (CCR5, CCR7, CXCR3, CXCR4) and costimulatory/activation markers (CD86, CD83, CD40) on both myeloid DC (mDC) and plasmacytoid DC (pDC) subsets from freshly isolated peripheral blood (PBMC) and lymph nodes (LN) from 9 HIV-1-infected donors as shown in Fig.1. B) Changes in expression of markers by mDC and pDC within PBMC and lymph nodes (LN). Values are expressed as Mean Fluorescence Intensity (MFI) minus FMO control as described in Fig. 1. Statistical analysis was performed using Wilcoxon signed rank test. C) Expression of CD40 and CD83 on pDC and mDC in the lymph nodes of seronegative and HIV-1-infected (HIV-1+) donors. Values are expressed as Mean Fluorescence Intensity (MFI) minus FMO control (detailed in Fig. 1.). Statistical analysis was performed using Mann-Whitney test.
Extensive phenotypic analysis was performed on DCs in PBMC and lymph nodes from HIV-infected and seronegative donors. Fig. 1 shows the gating strategy used to define mDC and pDC subsets in the lymph node of a representative seronegative donor. Paired analysis was first performed to compare the expression of each surface marker on blood and lymph node DC subsets from the HIV-1-infected donors (Fig. 4B). Expression of CXCR4 on both mDCs and pDCs in the lymph node was lower than on DCs from PBMCs. In contrast, CCR7 expression was higher on lymph node mDC, but lower on lymph node pDC relative to PBMC expression. Additionally, CCR5 expression was lower on lymph node mDC, but higher on lymph node pDC compared to their blood counterparts. CXCR3 expression did not differ between lymph node and PBMC for mDCs, but for pDCs lymph node expression was higher. CD40 expression levels were higher on lymph node than on blood DCs, whereas CD86 expression was lower on lymph node mDC and pDC relative to PBMC, in these HIV-infected subjects. There was little difference in CD83 expression between the two compartments for either DC subset. The trends in DC expression between blood and lymph node in HIV-infected subjects generally mirrored those differences observed between lymph node and PBMC DCs in seronegative donors (data not shown).
What may be more clinically important than relative differences in surface marker expression between PBMC and lymph node DCs is whether the phenotype of lymph node DCs differed between HIV-1-infected and seronegative subjects. A direct comparison of surface marker expression levels on lymph node DCs between infected and seronegative subjects showed two major differences for both DC subsets: a relative increase in expression of CD40 and a decrease in CD83 expression on lymph node DCs from HIV-1-infected versus seronegative subjects (Table 4, Fig.4C). These differences only reached statistical significance for higher CD40 expression on lymph node pDCs from HIV-1 infected donors and trended towards significance for pDC expression of CD83. Expression levels of chemokine receptors and CD86 were similar on lymph node DC subsets in seronegative and HIV-1-infected subjects. These results show that DCs in lymph nodes from HIV-1-infected individuals display changes in phenotype relative to seronegative donor lymph node DCs consistent with atypical activation.
TABLE 4.
Expression of various chemokine receptors and maturation/activation markers on DC subsets within lymph nodes of seronegative and HIV-1-infected subjects
| SN (n=5) | HIV-1+ (n=9) | ||
|---|---|---|---|
| mDC | Overall Pa | ||
| CXCR4 | 341 (47-438) | 291 (55-676) | P=0.52 |
| CCR7 | 4224 (1284-5664) | 4605 (1192-18223) | P=0.36 |
| CCR5 | 1773 (1404-2798) | 1411 (0-2176) | P=0.30 |
| CXCR3 | 1237 (474-1683) | 998 (142-2004) | P=1 |
| CD86 | 901 (670-1578) | 1171 (497-2004) | P=0.80 |
| CD40 | 7645 (5369-14536) | 10142 (5565-18621) | P=0.36 |
| CD83 | 951 (4-2613) | 423 (78-2128) | P=0.24 |
| pDC | |||
| CXCR4 | 1694 (478-2950) | 1529 (416-3765) | P=1 |
| CCR7 | 244 (70-6730) | 417 (0-1073) | P=1 |
| CCR5 | 4158 (2223-5924) | 3411 (1327-6758) | P=0.24 |
| CXCR3 | 6750 (2087-8069) | 6025 (1444-20697) | P=1 |
| CD86 | 276 (240-758) | 303 (120-661) | P=1 |
| CD40 | 2006 (1328-4269) | 5115 (3791-13847) | P=0.03 |
| CD83 | 559 (46-2178) | 180 (23-325) | P=0.06 |
Expression of each surface marker was assessed on myeloid DC (mDC) and plasmacytoid DC (pDC) from seronegative donors (SN; n=5) and HIV-1-infected subjects (HIV-1+; n=9). Values are expressed as Mean Fluorescence Intensity (MFI) minus FMO control (detailed in Fig. 1) with median (range) shown. Statistical analysis was performed using
Mann-Whitney to compare seronegative values with those from HIV-1-infected individuals.
DISCUSSION
Recent evidence suggests that the advent of generalized, chronic activation of the immune system during HIV-1 infection may be largely responsible for CD4+ T cell depletion and progression to AIDS6. In fact, the degree of immune activation has been shown to be a better predictor of the rate of disease progression than plasma viral load8. Since DCs may activate T cells through both innate and adaptive immune mechanisms14, 15, and since HIV-1 has been shown to directly and indirectly activate DCs in vitro11-13, we hypothesized that HIV-1-associated activation of DCs in vivo might contribute to the observed generalized immune activation associated with chronic infection. Earlier reports had suggested that blood DCs from HIV-1-infected individuals displayed altered maturation profiles26, 33, 39, although these reports either did not address specific blood DC subsets or were limited to a small number of putative maturation markers. We sought to investigate the effects of HIV-1 infection on DCs in vivo by undertaking a comprehensive analysis of both blood and lymph node DC phenotype and function in HIV-1 infected individuals.
A major finding of this study is that untreated HIV-1 infection is associated with an atypical phenotype indicative of partial activation of both subsets of blood dendritic cells, characterized by upregulation of CD40 (statistical significance achieved for mDC only) in the absence of significant upregulation of other maturation markers such as CCR7 and CD83. Furthermore , the in vitro migratory capacity of pDCs to CXCL12, a ligand for CXCR4, was markedly reduced in HIV-1-infected subjects. Previous work has shown that blood pDC matured in vitro with CD40 ligand lost their ability to migrate to CXCL12 while acquiring a capacity to migrate towards a CCL19, a CCR7-specific chemokine44. The lack of a significant increase in CCR7 expression and an inability of pDC from HIV-1-infected individuals to acquire migratory ability to CCL21, the CCR7-specific chemokine used in this study, goes further to suggest that the pDC from HIV-1-infected subjects are only partially activated. However, both mDC and pDCs from HIV-1-infected individuals could still modulate expression of various activation/maturation markers in response to TLR-stimulation to similar levels as those induced in mDC and pDC from seronegative subjects. This HIV-associated upregulation of CD40 and altered migratory ability of blood DCs in HIV-1-infected individuals could contribute to immune dysfunction by altering DC trafficking patterns and inducing inappropriate T cell stimulation.
The finding that circulating DCs in the setting of HIV-1 viremia have partial activation phenotypes may reflect the limitations of studying a compartment in which DCs only transiently reside. If blood DCs were induced through interactions with HIV-1 or associated factors to migrate to or be retained in lymphoid organs upon reaching full maturation, then the DCs that remain in the blood in this setting could be those that have failed to achieve a sufficient maturation state. This hypothesis is supported by the observation that blood DCs in viremic subjects are only partially activated and have an altered migratory capacity. Likewise, if HIV-1-associated DC maturation were to induce DCs to be retained in lymphoid tissue, an accumulation of mature DCs might be expected in lymph nodes from HIV-1-infected subjects. Since lymphoid tissue is a major site of DC/T cell interactions14, 15 and is a major site of HIV-1 replication45, accumulation of activated DCs in the lymphoid compartment could contribute significantly to generalized T cell activation.
To test this hypothesis, we evaluated the number and maturation phenotype of DCs in lymphoid tissue of untreated HIV-1-infected subjects and a control group of seronegative donors. Although we observed no significant changes in either percentages of each DC subset or the number of DC per gram of tissue in lymph nodes of HIV-1-infected subjects relative to seronegatives, the total numbers of DCs per lymph node was increased in association with larger lymph node sizes in HIV-1-infected subjects. This finding of larger lymph nodes in HIV-infected subjects is in keeping with reports of lymph node hyperplasia in earlier stages of chronic infection46. This novel finding of DC accumulation in the lymphoid compartment of asymptomatic, chronically HIV-1-infected subjects may also indicate a contribution of DCs to the hyperplastic state of lymph nodes in early to mid-stage HIV-1 disease. A previous study, using only two-color immunohistochemical staining with an inability to distinguish between mDC and pDC subsets, had demonstrated an accumulation of total DC numbers within lymph nodes of individuals with acute HIV-1 infection47. A more recent study using flow cytometry to identify specific DC subsets demonstrated a marked decrease in both pDC and mDC proportions in the lymph nodes of a small cohort of subjects (n=3) with advanced, chronic HIV-1 infection48, an observation similar to that observed in simian AIDS49. Although not acutely infected, our lymph node patient cohort had less advanced disease than those of Biancotto et al48, with a median CD4 count of 677 cells/μl and viral load of 8255 copies HIV-1 RNA/ml plasma. Our findings highlight that even in early to mid stages of chronic HIV-1 infection, accumulation of pDCs and mDCs in lymphoid compartments occurs in association with lymphoid hyperplasia. The lack of DC accumulation in lymphoid tissue during late stage chronic infection may indicate that lymph node DCs are either gradually killed or that destruction of lymph node architecture prevents recruitment or retention of DCs. Thus, the loss of DCs observed in the blood during various stages of HIV-1 infection may result from increased accumulation within lymphoid organs during early disease and then subsequent increases in DC death50 combined with defects in DC homeostasis and incomplete replacement by DC progenitors in later stages of HIV-1 infection.
Since the lymph node is one of the main sites of interaction between DCs and T cells, it was important to ascertain whether DCs in lymphoid tissues from HIV-1-infected donors displayed altered phenotypes that might influence the type and magnitude of the T cell response. We addressed the relevant importance of these phenotypic changes as a response to HIV-1 infection by comparing the level of surface marker expression on lymph node DCs from HIV-1-infected donors to those on lymph node DCs from seronegative donors. CD40 was more highly expressed on mDCs and pDCs from HIV-1-infected donor lymph nodes than from seronegative donor lymph nodes, a finding consistent with increased activation, although this difference only achieved statistical significance for pDCs. This is in contrast to changes in CD40 expression on circulating blood DCs, where CD40 expression was elevated to a greater extent on mDCs than pDCs. CD83 levels were lower on lymph node DCs from HIV-1-infected donors despite the higher CD40 expression, suggestive of incomplete or atypical activation. It is interesting to note that although levels of CD86 on both DC subsets were lower in the lymph node than in the blood in HIV-infected subjects, we found no significant difference in lymph node DC CD86 expression between HIV-1-infected and seronegative donors. This may imply that lower CD86 levels reflect typical lymph node DC phenotype. Thus, despite accumulation of DCs within the lymph nodes HIV-1-infected individuals, these DCs did not display a fully mature phenotype, but rather one indicative of partial activation.
A number of possible mechanisms could explain the unusual phenotype of blood and lymph node DCs observed in viremic individuals. DCs may conceivably be activated in multiple ways in the setting of HIV-1 replication: either directly or indirectly by HIV-112, 13, by microbial products such as LPS that may be released into systemic circulation through a breakdown of the gut mucosal barrier51, through interactions with activated T cells, or by inflammatory cytokines14, 15. It has been also been shown that some HIV-1 antigens only partially activate DC or actually inhibit DC maturation52-57. Thus, the profile of incomplete or partial DC activation may result from a weak stimulatory signal or a combination of signals, some activating and some suppressive. The fact that blood DCs could fully mature following strong stimulation in vitro supports the idea that DCs are incompletely activated in vivo during HIV-1 infection.
The biological significance of increased CD40 expression on blood and lymph node DCs without changes in other markers typical of full maturation is unclear, but this finding has numerous possible consequences. DCs with a partially activated phenotype may fail to generate new antigen-specific T cell responses while maintaining the ability to non-specifically activate T cells and induce apoptosis, possibly via production of inflammatory cytokines48, 58-60. Additionally, lymph node DCs from HIV-1-infected subjects with a semi-mature phenotype have been shown to induce regulatory T cells61.
Signaling through CD40 has been shown to increase the survival of DCs cultured from cord blood progenitor cells62 and may lead to upregulation of an intracellular innate anti-viral factor in monocyte-derived DCs that is shown to play a role in the inhibition of HIV-1 replication63. Thus, mDCs with high CD40 expression may have a survival advantage and a reduced ability to support HIV-1 replication, resulting in their accumulation in lymphoid tissue. Conversely, activation of pDCs through CD40-CD40L interaction has been shown to induce viral replication resulting in cell death and increased transmission of HIV-1 to T cells64, 65. Clearly, the balance between these multiple complex interactions in different DC subsets, the timing at which they occur and the ultimate contribution of an accumulation of lymph node DCs with high levels of CD40 expression to HIV-1 replication and T cell activation in vivo remains to be determined.
To our knowledge, this is the first study that has extensively assessed a wide range of surface markers involved in DC activation and maturation on blood and lymph node DCs from HIV-1-infected subjects. In light of the intriguing finding that DCs with an altered phenotype do indeed accumulate in the lymphoid compartment of patients with chronic, asymptomatic HIV-1 disease, studies are now underway to determine the contribution these DCs may make to generalized T cell activation and proliferation. Additionally, given the differences in lymph node DC numbers observed in early to mid-stage HIV-1 infection versus in late-stage/AIDS, further studies to evaluate DC function and compartmentalization at different stages of HIV-1 disease are warranted
ACKNOWLEDGEMENTS
We thank the physicians, staff and patients in the Infectious Diseases Group Practice at the University of Colorado Health Sciences Center and the University of Colorado Hospital, for their assistance and participation in our study. We thank Dr. Mark R. Nehler, Associate Professor in the Division of Vascular Surgery, Department of Surgery and Dr. David A. Fullerton, Professor and Head of Division of Cardiothoracic Surgery, Department of Surgery, for surgical assistance in obtaining seronegative lymph nodes. We thank Laura Ingoldby for excellent technical assistance and the Colorado CFAR Immunology Core for assistance with flow cytometry.
Sources of support: This work was supported by NIH grants R01 AI065275 (C.W.), R21 HD051450 (EC), and P01 AI55356 (EC) and was facilitated by the infrastructure and resources provided by the Colorado Center for AIDS Research (AI054907).
REFERENCES
- 1.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006 Sep 21;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 2.Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RP, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992 Jul 10;257(5067):217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 3.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006 Oct 2;203(10):2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selliah N, Finkel TH. Biochemical mechanisms of HIV induced T cell apoptosis. Cell Death Differ. 2001 Feb;8(2):127–136. doi: 10.1038/sj.cdd.4400822. [DOI] [PubMed] [Google Scholar]
- 5.Silvestri G, Fedanov A, Germon S, et al. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J Virol. 2005 Apr;79(7):4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silvestri G, Feinberg MB. Turnover of lymphocytes and conceptual paradigms in HIV infection. J Clin Invest. 2003 Sep;112(6):821–824. doi: 10.1172/JCI19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006 Oct;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 8.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999 Apr;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 9.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. Aids. 2003 Sep 5;17(13):1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 10.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000 Jan 1;95(1):249–255. [PubMed] [Google Scholar]
- 11.Meier A, Alter G, Frahm N, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded toll-like receptor ligands. J Virol. 2007 Aug;81(15):8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beignon AS, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005 Nov;115(11):3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonteneau JF, Larsson M, Beignon AS, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004 May;78(10):5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998 Mar 19;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 16.Huang Q, Liu D, Majewski P, et al. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001 Oct 26;294(5543):870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 17.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001 Nov;31(11):3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001 Sep 17;194(6):863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penna G, Vulcano M, Sozzani S, Adorini L. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Hum Immunol. 2002 Dec;63(12):1164–1171. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 20.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000 Oct;1(4):305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 21.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006 Mar 15;107(6):2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 22.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999 Jun 11;284(5421):1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 23.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000 Oct;1(4):311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 24.Patterson S. Flexibility and cooperation among dendritic cells. Nat Immunol. 2000 Oct;1(4):273–274. doi: 10.1038/83644. [DOI] [PubMed] [Google Scholar]
- 25.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000 May 1;164(9):4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 26.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003 Jan 1;187(1):26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 27.Donaghy H, Pozniak A, Gazzard B, et al. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001 Oct 15;98(8):2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 28.Feldman S, Stein D, Amrute S, et al. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001 Nov;101(2):201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 29.Grassi F, Hosmalin A, McIlroy D, Calvez V, Debre P, Autran B. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. Aids. 1999 May 7;13(7):759–766. doi: 10.1097/00002030-199905070-00004. [DOI] [PubMed] [Google Scholar]
- 30.Killian MS, Fujimura SH, Hecht FM, Levy JA. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. Aids. 2006 Jun 12;20(9):1247–1252. doi: 10.1097/01.aids.0000232231.34253.bd. [DOI] [PubMed] [Google Scholar]
- 31.Pacanowski J, Kahi S, Baillet M, et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001 Nov 15;98(10):3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 32.Soumelis V, Scott I, Gheyas F, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001 Aug 15;98(4):906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 33.Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. Aids. 2005 Feb 18;19(3):261–271. [PubMed] [Google Scholar]
- 34.Chehimi J, Campbell DE, Azzoni L, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002 May 1;168(9):4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 35.Finke JS, Shodell M, Shah K, Siegal FP, Steinman RM. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004 Nov;24(6):647–652. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt B, Fujimura SH, Martin JN, Levy JA. Variations in plasmacytoid dendritic cell (PDC) and myeloid dendritic cell (MDC) levels in HIV-infected subjects on and off antiretroviral therapy. J Clin Immunol. 2006 Jan;26(1):55–64. doi: 10.1007/s10875-006-8401-3. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Fu J, Zhao Q, et al. Differential restoration of myeloid and plasmacytoid dendritic cells in HIV-1-infected children after treatment with highly active antiretroviral therapy. J Immunol. 2006 May 1;176(9):5644–5651. doi: 10.4049/jimmunol.176.9.5644. [DOI] [PubMed] [Google Scholar]
- 38.Almeida M, Cordero M, Almeida J, Orfao A. Persistent abnormalities in peripheral blood dendritic cells and monocytes from HIV-1-positive patients after 1 year of antiretroviral therapy. J Acquir Immune Defic Syndr. 2006 Apr 1;41(4):405–415. doi: 10.1097/01.qai.0000209896.82255.d3. [DOI] [PubMed] [Google Scholar]
- 39.Jones GJ, Watera C, Patterson S, et al. Comparative loss and maturation of peripheral blood dendritic cell subpopulations in African and non-African HIV-1-infected patients. Aids. 2001 Sep 7;15(13):1657–1663. doi: 10.1097/00002030-200109070-00008. [DOI] [PubMed] [Google Scholar]
- 40.Tung JW, Parks DR, Moore WA, Herzenberg LA, Herzenberg LA. New approaches to fluorescence compensation and visualization of FACS data. Clin Immunol. 2004 Mar;110(3):277–283. doi: 10.1016/j.clim.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004 Feb;16(1):27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Kerkmann M, Rothenfusser S, Hornung V, et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003 May 1;170(9):4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 43.de la Rosa G, Longo N, Rodriguez-Fernandez JL, et al. Migration of human blood dendritic cells across endothelial cell monolayers: adhesion molecules and chemokines involved in subset-specific transmigration. J Leukoc Biol. 2003 May;73(5):639–649. doi: 10.1189/jlb.1002516. [DOI] [PubMed] [Google Scholar]
- 44.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol. 2001 Aug 15;167(4):1862–1866. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- 45.Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 46.Pantaleo G, Graziosi C, Fauci AS. The role of lymphoid organs in the pathogenesis of HIV infection. Semin Immunol. 1993 Jun;5(3):157–163. doi: 10.1006/smim.1993.1019. [DOI] [PubMed] [Google Scholar]
- 47.Lore K, Sonnerborg A, Brostrom C, et al. Accumulation of DC−SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. Aids. 2002 Mar 29;16(5):683–692. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- 48.Biancotto A, Grivel JC, Iglehart SJ, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007 May 15;109(10):4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007 Jun 1;178(11):6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 50.Meyers JH, Justement JS, Hallahan CW, et al. Impact of HIV on Cell Survival and Antiviral Activity of Plasmacytoid Dendritic Cells. PLoS ONE. 2007;2:e458. doi: 10.1371/journal.pone.0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 52.Fantuzzi L, Purificato C, Donato K, Belardelli F, Gessani S. Human immunodeficiency virus type 1 gp120 induces abnormal maturation and functional alterations of dendritic cells: a novel mechanism for AIDS pathogenesis. J Virol. 2004 Sep;78(18):9763–9772. doi: 10.1128/JVI.78.18.9763-9772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izmailova E, Bertley FM, Huang Q, et al. HIV-1 Tat reprograms immature dendritic cells to express chemoattractants for activated T cells and macrophages. Nat Med. 2003 Feb;9(2):191–197. doi: 10.1038/nm822. [DOI] [PubMed] [Google Scholar]
- 54.Majumder B, Janket ML, Schafer EA, et al. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J Virol. 2005 Jul;79(13):7990–8003. doi: 10.1128/JVI.79.13.7990-8003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Messmer D, Jacque JM, Santisteban C, et al. Endogenously expressed nef uncouples cytokine and chemokine production from membrane phenotypic maturation in dendritic cells. J Immunol. 2002 Oct 15;169(8):4172–4182. doi: 10.4049/jimmunol.169.8.4172. [DOI] [PubMed] [Google Scholar]
- 56.Muthumani K, Hwang DS, Choo AY, et al. HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int Immunol. 2005 Feb;17(2):103–116. doi: 10.1093/intimm/dxh190. [DOI] [PubMed] [Google Scholar]
- 57.Hodges A, Sharrocks K, Edelmann M, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007 Jun;8(6):569–577. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- 58.Herbeuval JP, Nilsson J, Boasso A, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A. 2006 May 2;103(18):7000–7005. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez B, Lederman MM, Jiang W, et al. Interferon-alpha differentially rescues CD4 and CD8 T cells from apoptosis in HIV infection. Aids. 2006 Jun 26;20(10):1379–1389. doi: 10.1097/01.aids.0000233571.51899.ab. [DOI] [PubMed] [Google Scholar]
- 60.Luft T, Maraskovsky E, Schnurr M, et al. Tuning the volume of the immune response: strength and persistence of stimulation determine migration and cytokine secretion of dendritic cells. Blood. 2004 Aug 15;104(4):1066–1074. doi: 10.1182/blood-2003-12-4146. [DOI] [PubMed] [Google Scholar]
- 61.Krathwohl MD, Schacker TW, Anderson JL. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J Infect Dis. 2006 Feb 15;193(4):494–504. doi: 10.1086/499597. [DOI] [PubMed] [Google Scholar]
- 62.Bjorck P, Banchereau J, Flores-Romo L. CD40 ligation counteracts Fas-induced apoptosis of human dendritic cells. Int Immunol. 1997 Mar;9(3):365–372. doi: 10.1093/intimm/9.3.365. [DOI] [PubMed] [Google Scholar]
- 63.Pido-Lopez J, Whittall T, Wang Y, et al. Stimulation of cell surface CCR5 and CD40 molecules by their ligands or by HSP70 up-regulates APOBEC3G expression in CD4(+) T cells and dendritic cells. J Immunol. 2007 Feb 1;178(3):1671–1679. doi: 10.4049/jimmunol.178.3.1671. [DOI] [PubMed] [Google Scholar]
- 64.Fong L, Mengozzi M, Abbey NW, Herndier BG, Engleman EG. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J Virol. 2002 Nov;76(21):11033–11041. doi: 10.1128/JVI.76.21.11033-11041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt B, Scott I, Whitmore RG, et al. Low-level HIV infection of plasmacytoid dendritic cells: onset of cytopathic effects and cell death after PDC maturation. Virology. 2004 Nov 24;329(2):280–288. doi: 10.1016/j.virol.2004.08.016. [DOI] [PubMed] [Google Scholar]