Abstract
In a search for small molecule antagonists of heparan sulfate, we examined the activity of bis-2-methyl-4-amino-quinolyl-6-carbamide, also known as surfen. Fluorescence-based titrations indicated that surfen bound to glycosaminoglycans, and the extent of binding increased according to charge density in the order heparin > dermatan sulfate > heparan sulfate > chondroitin sulfate. All charged groups in heparin (N-sulfates, O-sulfates, and carboxyl groups) contributed to binding, consistent with the idea that surfen interacted electrostatically. Surfen neutralized the anticoagulant activity of both unfractionated and low molecular weight heparins and inhibited enzymatic sulfation and degradation reactions in vitro. Addition of surfen to cultured cells blocked FGF2-binding and signaling that depended on cell surface heparan sulfate and prevented both FGF2- and VEGF165-mediated sprouting of endothelial cells in Matrigel. Surfen also blocked heparan sulfate-mediated cell adhesion to the Hep-II domain of fibronectin and prevented infection by HSV-1 that depended on glycoprotein D interaction with heparan sulfate. These findings demonstrate the feasibility of identifying small molecule antagonists of heparan sulfate and raise the possibility of developing pharmacological agents to treat disorders that involve glycosaminoglycan–protein interactions.
Keywords: antithrombin, glycosaminoglycans, growth factors, viral infection, angiogenesis
A variety of proteins bind to the glycosaminoglycan (GAG) heparan sulfate (HS), including extracellular matrix proteins, proteases involved in coagulation and their inhibitors, lipases and apolipoproteins, as well as chemokines, cytokines, and various growth factors. These interactions affect cell proliferation, motility, and differentiation and play prominent roles in development and physiology (1–3). Sometimes these interactions go awry and cause or exacerbate pathophysiological conditions such as inflammation, tumor growth and angiogenesis, and infection (4). Agents that block the interaction of HS with its binding partners could therefore prove useful as therapeutic candidates.
Three categories of agents that antagonize protein interactions with heparin and HS have been described. The first group consists of other proteins or polypeptides containing clusters of positively charged amino acid residues that bind to the negatively charged sulfate and carboxyl groups. These antagonists include the heparin-neutralizing agent protamine (5) and lactoferrin (6), as well as synthetic peptides rich in lysine and arginine (7–10). The second class consists of heparin mimics, for example, sucrose octasulfate, suramin, pentosan polysulfate, and dextran sulfates, which presumably occupy the heparin-binding sites in proteins (11, 12). The third group consists of metabolic inhibitors that alter the biosynthesis of HS, such as sodium chlorate, an inhibitor of the universal sulfate donor 3′-phospho-adenyl-5′-phosphosulfate (PAPS) (13), and β-d-xylosides, which act as artificial primers of GAG biosynthesis (14, 15).
Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) was first described in 1938 as an excipient for the production of depot insulin (16). Subsequent studies have shown that surfen can block C5a receptor binding (17) and lethal factor (LF) produced by anthrax (18). It was also reported to have modest heparin-neutralizing effects in an oral feeding experiments in rats (19), but to our knowledge, no further studies involving heparin have been conducted, and its effects on HS are completely unknown. Presumably, surfen interacts with heparin to block its ability to bind and activate antithrombin (AT), but this mechanism of action has not been established.
Here, we demonstrate that surfen binds to heparin, low molecular weight heparins, and to HS and other GAGs. Binding neutralizes the ability of the various heparins to activate AT and inactivate Factor Xa. Surfen also blocks sulfation and degradation of GAG chains in vitro and alters cellular responses dependent on HS such as growth factor binding and activation, cell attachment, viral infection by HSV, and angiogenesis. These observations illustrate the ability of a small molecule to directly interfere with key biological processes that involve HS and suggest the possibility of designing more selective agents to target specific HS–protein interactions.
Results
Surfen Binds to GAGs in Solution.
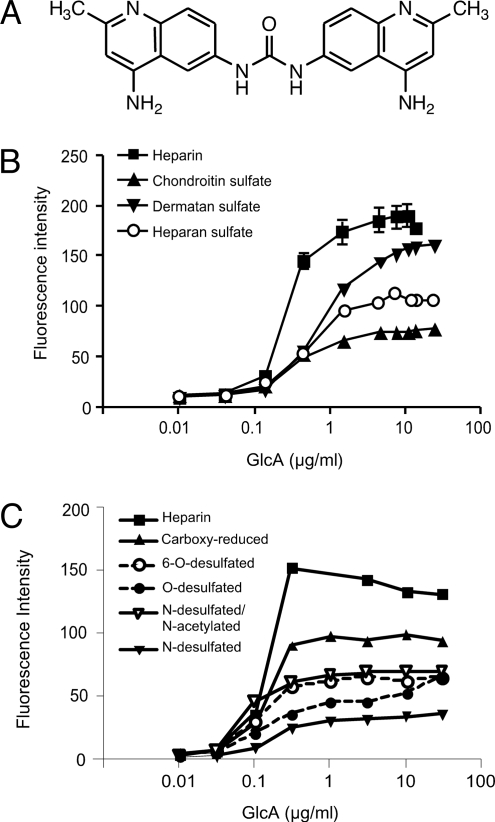
Fig. 1A shows the structure of surfen (Mr = 372.4). In aqueous buffers, surfen has a UV spectrum showing three distinct absorption peaks at 220, 260, and 340 nm, with molar extinction coefficients (εM) of 10,500, 74,400, and 14,500, respectively. Surfen exhibits weak fluorescence in neutral aqueous solutions with an emission maximum at 488 nm and an excitation maximum of 340 nm. However, adding heparin to the solution caused a large increase in emission intensity that rose proportionally to the amount of heparin added and eventually saturated (Fig. 1B). Under these conditions (3 μM surfen, 2 μg/ml heparin), the ratio of surfen to disaccharide subunits in heparin was ≈1 (the average disaccharide has a mass of 500 Da).
Fig. 1.
Surfen binds to GAGs. (A) Surfen, bis-2-methyl-4-amino-quinolyl-6-carbamide. (B) Binding of surfen to GAGs was determined by fluorimetry. A fixed amount of surfen (3 μM) was titrated with variable amounts of the indicated GAGs, and the emission at 488 nm was measured. Data for heparin is given as mean values ± range. (C) Binding to desulfated heparins. Each GAG was tested at least twice in separate experiments.
Assuming that the enhanced fluorescence was because of binding, we evaluated surfen's interaction with other GAGs by fluorimetry. As shown in Fig. 1B, the extent of binding increased in the order heparin > dermatan sulfate > HS > chondroitin sulfate (CS), which nearly parallels the relative number of sulfate groups along the chain [2.4 sulfates/disaccharide in heparin, 1.2 in dermatan sulfate (porcine intestinal mucosa), 0.85 in HS (porcine mucosa), and 0.95 in CS (shark cartilage)]. Surfen's interaction with heparin appeared to depend on charge based on diminished binding to N-desulfated, N-desulfated/N-acetylated, O-desulfated, 6-O-desulfated, and carboxyl-reduced forms of heparin (Fig. 1C). Because the extent of binding varied among different types of desulfated heparins (e.g., compare N-desulfated/N-acetylated heparin with O-desulfated heparin), it is tempting to speculate that surfen might align along the chain in register with specifically sulfated disaccharide subunits, perhaps by alignment of the protonated quinoline rings with negatively charged sulfate groups and carboxyl groups of the uronic acids. Further studies are needed, however, to determine the structure of these complexes.
Surfen Neutralizes Heparin and Low Molecular Weight Heparins.
Based on these binding data, we examined whether surfen would counteract the capacity of heparin and low molecular weight heparins to activate AT. AT is a plasma serine protease inhibitor (serpin) that covalently inactivates several coagulation enzymes including thrombin and factors Xa, IXa, XIa, and XIIa. Heparin increases the rate of inactivation by several orders of magnitude by binding and inducing a conformational change in AT and, in the case of thrombin, by acting as a template to approximate AT with the protease (20). To determine its potency for neutralizing different heparin preparations, the amount of heparin neutralized by a fixed amount of surfen was determined by using a Factor Xa inhibition assay (Table 1). One milligram surfen neutralized 74 and 87 units of two different preparations of unfractionated heparin. Surfen also antagonized the ability of heparin to prolong the time needed for plasma to form a fibrin clot (activated partial thromboplastin time) [supporting information (SI) Table S1 and SI Text]. Surfen neutralized dalteparin, tinzaparin, and enoxaparin, three types of low molecular weight heparins produced by chemical or enzymatic cleavage of native heparin as well as the synthetic pentasaccharide, fondaparinux. Although the capacity to neutralize the various preparations varied, neutralization by surfen followed the same trend as protamine, a clinically used heparin antagonist. Surfen had no effect on Factor Xa activity per se or clotting of blood in the absence of added heparin, consistent with the idea that its mode of action involved binding to heparin rather than a target protein.
Table 1.
Neutralization of heparin's capacity to inhibit Factor Xa
| Heparin | Units | Protamine | Surfen |
|---|---|---|---|
| Unfractionated heparin (ES) | USP | 101.3 ± 1.6 | 86.6 ± 0.6 |
| Unfractionated heparin (SPL) | USP | 95.3 ± 5.1 | 74.1 ± 6.4 |
| Dalteparin (LMWH) | anti-Xa | 108.4 ± 1.5 | 100.1 ± 7.3 |
| Tinzaparin (LMWH) | anti-Xa | 58.5 ± 2.3 | 64.8 ± 1.6 |
| Enoxaparin (LMWH) | anti-Xa | 80.6 ± 1.4 | 48.8 ± 1.8 |
| Fondaparinux (pentasaccharide) | μg | 3.7 ± 1.2 | 3.5 ± 0.9 |
ES, Elkins-Sinn; SPL, Scientific Protein Laboratories. Data are mean ± SEM and represent the amount of heparinoid that was neutralized by 1 mg of protamine or surfen. One USP unit of the standard (ES) unfractionated heparin inhibits the equivalent amount of Xa as: 1.0 USP units SPL, 2.5 anti-Xa units Dalteparin, 2.0 anti-Xa units Tinzaparin, 2.1 anti-Xa units Enoxaparin, and 1.9 μg Fondaparinux.
Surfen Affects Sulfation of Heparin in Vitro and Inhibits Degradation by Heparin Lyases.
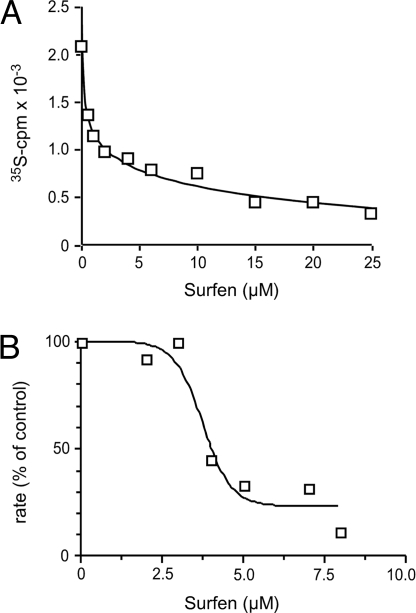
To test whether surfen could inhibit enzymes that interact with heparin, we examined its effect on one of the O-sulfotransferases that modifies the chains during biosynthesis. Incubation of recombinant uronyl 2-O-sulfotransferase with [35S]PAPS and 2-O-desulfated heparin yielded robust incorporation of 35S into product in the absence of surfen (Fig. 2A). However, adding surfen inhibited transferase activity with an IC50 of ≈2 μM. Surfen also inhibited enzymatic depolymerization of chains mediated by heparinases. In these experiments, we incubated HS with heparin lyases I, II, and III in the presence or absence of surfen and monitored production of disaccharides by UV absorbance (A232) (Fig. 2B). Surfen inhibited digestion of the chains with an IC50 of ≈4 μM. Surfen also inhibited enzymatic depolymerization of unfractionated heparin by lyases and CS by chondroitinase ABC (data not shown). These findings are consistent with the idea that surfen binds to the chains and prevents binding of the enzymes.
Fig. 2.
Surfen blocks uronyl 2-O-sulfotransferase and heparin lyase activity. (A) Surfen was mixed at various concentrations with 2-O-desulfated heparin, and the extent of transfer of 35SO4 from [35S]PAPS mediated by recombinant uronyl 2-O-sulfotransferase was measured. The data are single point determinations, but the experiment was performed three times with comparable results. (B) HS (100 μg/ml) was incubated with surfen. After addition of 1 mU/ml of heparin lyases I, II, and III, production of disaccharides was monitored by the change of absorbance at 232 nm. The rates were determined by using GraphPad Prism. Results are represented as a percentage of the rates measured in the absence of surfen. The experiment was performed three times.
Surfen Inhibits FGF2 Binding and Signaling.
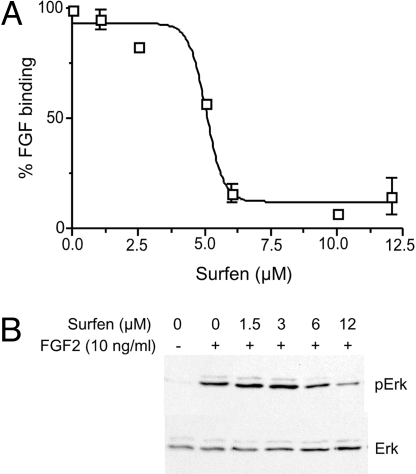
HS is structurally related to heparin but contains fewer sulfate groups per disaccharide, and it exists almost exclusively attached to protein cores of proteoglycans, which cells either display on the plasma membrane or secrete into the extracellular matrix. To test whether surfen can interact with HS in this setting, we examined its ability to antagonize biological processes dependent on HS. In CHO cells, the binding of FGF2 provides an indirect measure of HS on the cell surface, because the cells produce very few high affinity signal-transducing FGF receptors (21). As shown in Fig. 3A, surfen inhibited FGF2 binding in a dose-dependent manner with an IC50 of ≈5 μM. At 10 μM surfen, ≈95% inhibition was achieved, but for reasons not fully understood, increasing the concentration >20 μM resulted in reduced inhibitory activity. Thus, in all subsequent studies, the concentration of surfen was maintained at ≤20 μM.
Fig. 3.
FGF2 binding and signaling is inhibited by surfen. (A) Biotinylated FGF2 was added to CHO cells, and binding was measured by using flow cytometry. Surfen was added 10 min before the assay. Each point represents the average of 3–5 separate determinations. Results are presented as the percentage of binding obtained in the absence of surfen. The mean values ± SEM are given. (B) FGF2 was added to endothelial cells and after 10 min, the levels of Erk and Erk phosphorylation were determined by Western blot analysis. Band intensities were quantitated by densitometry. The experiment was repeated twice.
The cationic dyes methylene blue, toluidine blue, and alcian blue are used to measure GAGs in extracts and tissues, but none of them blocked FGF binding to cell surface HS when added to cells, even at 20 μM, the highest concentration tested (data not shown). Thus, surfen has unique binding properties that distinguish it from these other types of GAG-binding molecules.
Signaling by FGF2 via the FGF receptor tyrosine kinase (FGFR) depends on formation of ternary complexes between FGF, FGFR, and HS. Once the ternary complex is formed, the receptor can dimerize, autophosphorylate, and initiate an intracellular signaling cascade that activates the MAP kinase pathway, ultimately leading to phosphorylation of Erk. Surfen at 6 μM and 12 μM decreased FGF2-induced Erk phosphorylation in transformed endothelial cells by 55% and 86%, respectively (Fig. 3B). Complete abrogation of phosphorylation did not occur, most likely because activation of even a small percentage of receptors can lead to amplification of steps downstream of the receptor. In contrast to these findings, surfen had no effect on EGF-stimulated Erk phosphorylation, which does not depend on HS (data not shown). Also, increasing the level of FGF in the assays described above to concentrations that bypass the requirement for HS allowed signaling to occur. These findings suggest that surfen did not affect activation of the respective receptor tyrosine kinases or downstream kinases involved in Erk phosphorylation.
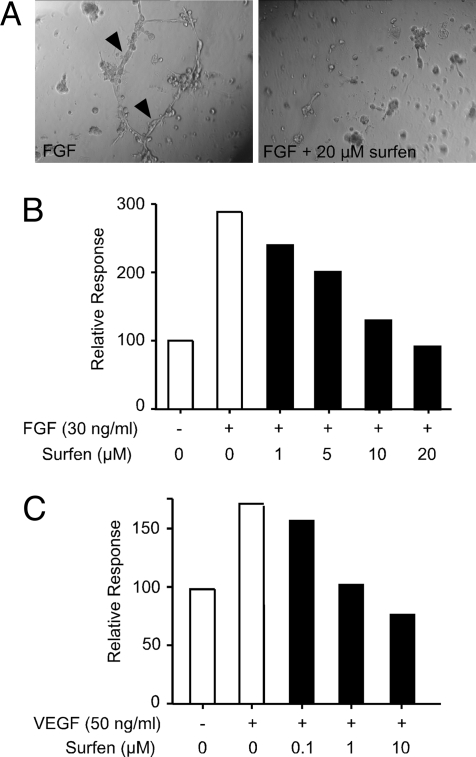
The ability of surfen to antagonize FGF2 binding and signaling suggested that it might block more complex biological processes, for example, growth or differentiation dependent on growth-factor signaling. To study this problem, we examined the ability of surfen to inhibit branching of primary mouse lung endothelial cells (formation of an anastomosing network of hollow tubes resembling angiogenesis) (Fig. 4A, arrowheads) when cultured on Matrigel and stimulated with FGF2 or VEGF165. Quantification of tube length showed that surfen inhibited tube formation in response to FGF2 with an IC50 of ≈5 μM, with complete inhibition achieved at 20 μM (Fig. 4B), but did not seem to affect cell viability per se (Fig. 4A Right). Surfen even more potently blocked angiogenic sprouting dependent on VEGF165, another proangiogenic heparin binding growth factor. To exclude the possibility that surfen inhibited cell proliferation, we incubated CHO cells in monolayer culture with increasing concentrations of surfen. As shown in Fig. S1, surfen had no effect on cell division and viability per se.
Fig. 4.
Surfen blocks endothelial tube formation. (A) Shown are phase-contrast images of endothelial cell sprouting on Matrigel in the presence and absence of 20 μM surfen. (Left) Arrowheads denote tubular connections. Note that surfen does not affect cell viability (Right). (B) Surfen inhibits FGF2 stimulated sprouting by endothelial cells. Response is measured in length (mm) of tubular connections formed. Data are normalized to negative control (no FGF stimulation). Each value represents multiple measurements made in a single well (see Materials and Methods). The experiment was repeated twice with comparable results. (C) Sprouting dependent on VEGF165. The analysis was done the same way as in B.
HS-Mediated Cell Attachment and Virus Infection Is Inhibited by Surfen.
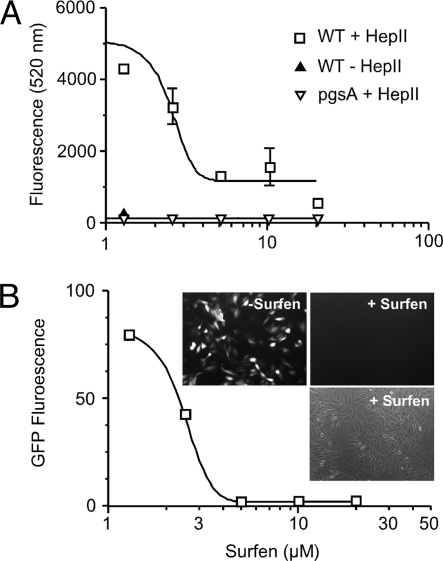
Cell adhesion to extracellular matrix is a multistep process that involves cell attachment to extracellular matrix proteins, cell spreading, and formation of focal adhesion plaques that depend in part on HS. To test whether surfen can inhibit cell attachment, CHO cells were loaded with 10 μM calcein acetoxymethyl ester (AM), a fluorescent dye, incubated with increasing concentrations of surfen, and then added to wells precoated with the HS-binding domain of fibronectin (Hep-II) (22). After 45 min, the wells were washed, and fluorescent cells bound to the plate were measured in a fluorescent plate reader. Surfen inhibited cell attachment in a dose-dependent manner with an IC50 of 3 μM, with complete inhibition of attachment occurring at 5 μM (Fig. 5A). For comparison, a CHO cell mutant lacking GAGs (pgsA) does not attach to wells coated with Hep-II.
Fig. 5.
Surfen inhibits cell attachment and viral infection. (A) Calcein AM-loaded cells were challenged to adhere to the heparin-binding domain (Hep-II) of fibronectin coated on 96-well plates. The number of adherent cells was determined by fluorimetry. Data are represented as mean values ± SEM. (B) Recombinant HSV-1, which expresses GFP in infected cells, was added to CHO cells transduced with 3-O-sulfotransferase-3A. After 12 h, GFP fluorescence was measured by flow cytometry and by fluorescence microscopy (Inset). The third image in the Inset represents a brightfield phase-contrast image of cells incubated with surfen.
HSV-1 can use HS for viral entry into some host cells via interaction with three HS-binding viral glycoproteins, gC, gB, and gD. Interaction of gC with HS is primarily responsible for adherence of the virus (23), whereas gB and gD play crucial roles in the fusogenic machinery facilitating viral entry (24). The HS modifying enzyme, glucosaminyl 3-O-sulfotransferase-3A (3ST-3A), generates a binding site for gD (25). To test whether surfen is able to neutralize viral infection dependent on this modification, CHO cells expressing 3ST-3A (CHO3.3A) were infected with a recombinant HSV-1 isolate, d106, which expresses GFP in infected cells (26). When the cells were exposed to virus in the presence of surfen, a dose-dependent reduction in infection occurred as measured by flow cytometry (Fig. 5B) or by fluorescence microscopy (Fig. 5B Inset). Complete inhibition of infection occurred at surfen concentrations ≥5 μM.
Discussion
In this report, we show that surfen binds to GAGs and neutralizes the anticoagulant activity of both unfractionated and low molecular weight heparins. Additionally, surfen antagonizes enzymes that act on heparin and HS as well as activities associated with HS, such as binding and signaling by FGF2, infection by HSV-1, cell attachment to a heparin-binding domain of fibronectin, and VEGF- and FGF-stimulated endothelial sprouting. These findings demonstrate the feasibility of identifying low molecular weight antagonists of GAG function. Currently available compounds used to block GAGs include high molecular weight cationic proteins, such as protamine, or polypeptides rich in lysine and arginine residues (10). A handful of synthetic heparin-binding molecules have been described (27) including peptidic foldamers (28), phenyl boronates (29), polyamidoamine dendrimers (30), calixarenes (31), and relatively high molecular weight dyes (32). In contrast to surfen, these agents have high ED50 values, and few of them have been tested or demonstrated efficacy for neutralizing HS in a cellular setting.
The exact mechanism by which surfen interacts with heparin and HS remains to be established. The data presented here suggest that ionic interactions between the positively charged, highly polarizable aminoquinoline moieties of surfen and the negatively charged sulfate and carboxyl groups in heparin/HS constitute the major binding interactions. Some preferential interaction may take place with different charged groups in the chain or different segments of the chain, depending on the extent of sulfation (Fig. 1). Possibly, surfen could induce secondary structures in heparin/HS, which is known to exist in a helical conformation in solution (33). Preliminary crystallographic data suggest that the disubstituted urea core of surfen could also interact with negative charges on the chain, and that surfen can form stacked structures that could in part explain the enhanced fluorescence of surfen when bound to the chains (Tor and Esko, unpublished data).
Surfen was first described as an excipient for generating soluble forms of insulin to improve dosing accuracy. On s.c. injection, surfen dissociates, and insulin precipitates to form a depot under the skin allowing for its slow release into the circulation. Used as an excipient for depot insulin in diabetic patients in Europe for decades, it appears to have low toxicity under these conditions. Subsequently, surfen was shown to exhibit antibacterial (34, 35) as well as trypanocidal (36) properties. Surfen has also been described as a potent competitive inhibitor of anthrax LF (with a Ki of 0.5 μM) (18) and has activity in National Cancer Institute Yeast anticancer drug screening assays (PubMed compound 71166). Whether any of these activities are related to its capacity to bind GAGs is a fruitful area for further study. Although one report (19) suggests that rats fed surfen at high doses over a prolonged period may develop lymphosarcoma and lesions suggestive of nutritional deficiency, toxicology studies in mice showed that surfen and a variety of derivatives are well tolerated by all routes of administration (37).
Protamine sulfate is the clinical standard antidote for heparin overdosing that can occur in patients undergoing anticoagulation therapy. Protamine is a heterogeneous family of proteins isolated from fish sperm and can elicit adverse, sometimes lethal, anaphylactic reactions in as many as 10% of patients. Consequently, a number of attempts have been made to design a potent, yet less toxic, substitute for clinical use with limited success (38). Although it is unlikely that surfen could fulfill this need because of its relatively high IC50 value, it serves as a starting point for the design and synthesis of more potent analogs. Based on its ability to block HS-dependent activities, it is tempting to suggest that surfen or its derivatives also could be used therapeutically for treating disorders exacerbated by unwanted HS interactions such as tumor growth and angiogenesis, chronic inflammation, or infection (39).
Materials and Methods
Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) was obtained from the Open Chemical Repository in the Developmental Therapeutic Program at the National Cancer Institute (NSC12155). Surfen binds avidly to plastic, necessitating the use of glass vessels, precoating all plasticware with serum before use, or presaturating vessels with a solution containing surfen. Surfen stock solutions were prepared in dimethyl sulfoxide at 30 mM and stored in glass containers under argon at −20°C in the absence of light. Fresh aqueous solutions were prepared as needed. When experiments involved plasticware, vessels were preincubated with serum-containing medium or presaturated with a solution of surfen.
Unfractionated heparin (sodium salts from porcine intestinal mucosa) was obtained from Elkins-Sinn and from Scientific Protein Laboratories. Tinzaparin low molecular weight heparin (LMWH) was from Pharmion, dalteparin LMWH was from Amersham Pharmacia, enoxaparin LMWH was from Aventis, and fondaparinux pentasaccharide was from Sanofi-Synthelabo. Desulfated heparins were from Neoparin or prepared as described (40). HS (from porcine mucosa) was from Neoparin, shark cartilage CS A was from Sigma, and porcine intestinal mucosa dermatan sulfate was from Celsus Laboratories. FGF2 was from Invitrogen and VEGF165 was obtained from R&D Systems.
Fluorimetry.
A solution of 3 μM surfen was prepared in PBS and titrated with increasing amounts of heparin, HS, dermatan sulfate, CS, or desulfated heparins. Fluorescence spectra were measured on a Perkin Elmer LS 50B luminescence spectrometer with excitation at 340 nm. Emission was monitored at 350–700 nm, and the maximum intensities at 488 nm were determined.
Coagulation Assays.
Human AT III (0.66 μg per well, Enzyme Research Laboratories), human Factor Xa (0.02 μg per well, Enzyme Research Laboratories), and heparinoids at various concentrations were incubated at room temperature for 10 min in 96-well microtiter plates in 150 μl of a solution containing 25 mM Hepes (N- [2-hydroxyethyl] piperazine-N′- [2-ethanesulfonic acid]), 150 mM NaCl, and 0.1% BSA (pH 7.5). Chromogenic substrate specific to Factor Xa (S-2765, 25 μg in 50 μl, Diapharma) was added and incubated for 15 min. Acetic acid (50 μl of a 20% solution) was added to stop reaction, and absorbance was measured at 405 nm. A curve was created with standard unfractionated heparin (Elkins-Sinn), by using a range of 0.125 × 10−3 to 1.0 × 10−3 USP units per well. A heparinoid concentration with Xa inhibition of ≈0.9 × 10−3 units per well was selected for measurement of neutralization. Protamine sulfate or surfen was added at final concentrations sufficient to neutralize ≈0.4 × 10−3 units per well of each heparinoid. Protamine and surfen had no direct effect on Factor Xa activity measured in the absence of heparin.
Enzyme Assays.
Recombinant CHO HS 2-O-sulfotransferase containing a protein A tag was prepared and assayed as described (41). Surfen was added at the indicated concentrations, and the incorporation of 35S from [35S]PAPS was determined.
Heparin lyases were assayed by incubating a solution of 100 μg/ml HS in 40 mM ammonium acetate buffer (pH 8) containing 3.3 mM CaCl2 and surfen. After the addition of 1 mU/ml of heparin lyase I, II, and III, absorbance at 232 nm was measured in a Beckman Coulter DU 640 spectrophotometer. The values were plotted and slopes (rates) were calculated by using GraphPad Prism. The rate was normalized to that obtained in the absence of surfen.
FGF2 Binding and Signaling.
WT CHO cells were grown in F12 growth medium (Invitrogen) to confluence, lifted with 5 mM EDTA, washed, and then incubated with 1–12 μM surfen in PBS/0.1% BSA on ice for 10 min. Biotinylated FGF2 was added (1:1000) and incubated for 30 min on ice. Cells were washed, and bound biotinylated FGF2 was detected by using streptavidin-PE-Cy5 (1:1000, PharMingen) and flow cytometry (FacsCalibur, BD Biosciences). Data were analyzed by using FlowJo Analytical Software (Tree Star Inc.). Results are represented as the extent of binding compared with a sample incubated in the absence of surfen.
For signaling assays, immortalized mouse lung endothelial cells were cultured in 12-well plates as described (42). The medium was exchanged for opti-MEM (Invitrogen) for 4 h before the addition of surfen. Cells were incubated for 10 min at 37°C then FGF2 (10 ng/ml) was added, and 10 min later, the cells were put on ice, washed with ice-cold PBS, and lysed by using radioimmunoprecipitation assay buffer. Protein concentration was determined by using the Bio-Rad Protein Assay before Western blot analysis. Antiphospho Erk and antitotal Erk antibodies (Cell Signaling Technology) were used at 1:1,000 dilutions. Secondary goat anti-rabbit HRP antibody (Bio-Rad, Eureka, CA) was used at 1:25,000. HRP was detected by using SuperSignal West Pico Chemiluminescent substrate (Pierce Biotechnology) according to the manufacturer's directions. Band intensities were quantitated by densitometry.
Cell Adhesion and Viral Infection Assays.
Untreated NUNC 96-well ELISA plates were coated with 15 μg/ml of the heparin-binding domain of mouse fibronectin (Hep-II) overnight at room temperature. Wells were blocked for 2 h with 5% BSA in PBS. CHO cells were harvested with 5 mM EDTA, washed, and then labeled with 10 μM calcein AM (Invitrogen) in media for 30 min at room temperature. Cells were centrifuged at 800 × g, resuspended in varying concentrations of surfen in PBS with 0.1% BSA, and incubated for 10 min on ice. Approximately 60,000 cells were added per well and allowed to adhere for 45 min at 37°C. The solution was removed, and the plate was washed vigorously three times with PBS. Fluorescence of adherent cells was measured in a fluorescent plate reader (excitation at 495 nm, emission at 520 nm). Data were graphed by using Graph Pad Prism software (sigmoidal dose-response with variable slope).
Recombinant d106 HSV-1 was propagated and purified as described (26). Infection was measured by using CHO3.3A cells that express two copies of the human HS 3-O-sulfotransferase 3A introduced by retroviral transduction (25). The virus was absorbed to cells at ≈80% confluence for 1 h with periodic rocking to ensure even distribution of the viral supernatant and to avoid drying out of the cells. After one hour, 1 ml of growth medium was added, and the cells were cultured under normal conditions for 12 h. The level of GFP expression was assessed by both fluorescence microscopy and flow cytometry. The effect of surfen on infection was determined by pretreating CHO3.3A cells 30 min before viral adsorption and throughout the incubation.
Angiogenesis Assay on Reconstituted Extracellular Matrix.
Primary murine lung endothelial cells were isolated from 16-week-old C57BL/6 mice (42) and cultured on the surface of polymerized Matrigel (BD Biosciences) in the presence or absence of 30 ng/ml recombinant FGF2 or 50 ng/ml VEGF165 and the indicated concentrations of surfen. After 24 h, the degree of endothelial sprouting over the gel surface was measured by determining the net length of endothelial processes viewed under phase contrast light microscopy. For each well, lengths of processes per ×100 field were averaged (3 fields per well) and normalized to baseline response (sprouting by untreated endothelia in the absence of growth factor) (39).
Supplementary Material
Acknowledgments.
We thank Dr. Neil DeLuca (University of Pittsburgh School of Medicine) for providing recombinant HSV-1 isolate d106, Dr. Patrick Shaklee (Scientific Protein Laboratories) for providing unfractionated heparin, and Dr. John. R. Couchman (University Of Copenhagen) for providing the Hep-II protein. This work was supported by National Institutes of Health Grants CA112278 and CA46462 (to J.D.E.), Hematology/Coagulation/Chemistry Core and Analytical Glycobiology Core Grant HL57345, U.S. Veteran's Administration Research Career Development Award (to M.M.F.), and training Grant CA67754 (to M.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805862105/DCSupplemental.
References
- 1.Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: The sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 2.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 3.Gorsi B, Stringer SE. Tinkering with heparan sulfate sulfation to steer development. Trends Cell Biol. 2007;17:173–177. doi: 10.1016/j.tcb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Portmann AF, Holden WD. Protamine sulphate, heparin, and blood coagulation. J Clin Invest. 1949;28:1451–1458. doi: 10.1172/JCI102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hekman A. Association of lactoferrin with other proteins, as demonstrated by changes in electrophoretic mobility. Biochim Biophys Acta. 1971;251:380–387. doi: 10.1016/0005-2795(71)90126-7. [DOI] [PubMed] [Google Scholar]
- 7.Morad N, Ryser HJ, Shen WC. Binding sites and endocytosis of heparin and polylysine are changed when the two molecules are given as a complex to Chinese hamster ovary cells. Biochim Biophys Acta. 1984;801:117–126. doi: 10.1016/0304-4165(84)90219-8. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs SM, Raines RT. Pathway for polyarginine entry into mammalian cells. Biochemistry. 2004;43:2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schick BP, Maslow D, Moshinski A, San Antonio JD. Novel concatameric heparin-binding peptides reverse heparin and low-molecular-weight heparin anticoagulant activities in patient plasma in vitro and in rats in vivo. Blood. 2004;103:1356–1363. doi: 10.1182/blood-2003-07-2334. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Rabenstein DL. Interaction of heparin with two synthetic peptides that neutralize the anticoagulant activity of heparin. Biochemistry. 2006;45:15740–15747. doi: 10.1021/bi061346a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X, Hsu BT, Rees DC. Structural studies of the binding of the anti-ulcer drug sucrose octasulfate to acidic fibroblast growth factor. Structure. 1993;1:27–34. doi: 10.1016/0969-2126(93)90006-3. [DOI] [PubMed] [Google Scholar]
- 12.Botta M, Manetti F, Corelli F. Fibroblast growth factors and their inhibitors. Curr Pharm Des. 2000;6:1897–1924. doi: 10.2174/1381612003398528. [DOI] [PubMed] [Google Scholar]
- 13.Baeuerle PA, Huttner WB. Chlorate–a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986;141:870–877. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz NB, Galligani L, Ho P-L, Dorfman A. Stimulation of synthesis of free chondroitin sulfate chains by β-D-xylosides in cultured cells. Proc Natl Acad Sci USA. 1974;71:4047–4051. doi: 10.1073/pnas.71.10.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fritz TA, Lugemwa FN, Sarkar AK, Esko JD. Biosynthesis of heparan sulfate on beta-D-xylosides depends on aglycone structure. J Biol Chem. 1994;269:300–307. [PubMed] [Google Scholar]
- 16.Umber F, Stoerring FK, Foellmer W. Erfolge mit einem neuartigen Depot Insulin ohne Protaminzusatz (Surfen-Insulin) Klinische Wochenschrift. 1938;17:443–446. [Google Scholar]
- 17.Lanza TJ, et al. Substituted 4,6-diaminoquinolines as inhibitors of C5a receptor binding. J Med Chem. 1992;35:252–258. doi: 10.1021/jm00080a008. [DOI] [PubMed] [Google Scholar]
- 18.Panchal RG, et al. Identification of small molecule inhibitors of anthrax lethal factor. Nat Struct Mol Biol. 2004;11:67–72. doi: 10.1038/nsmb711. [DOI] [PubMed] [Google Scholar]
- 19.Hunter DT, Jr, Hill JM. Surfen: A quinoline with oncogenic and heparin-neutralizing properties. Nature. 1961;191:1378–1379. doi: 10.1038/1911378a0. [DOI] [PubMed] [Google Scholar]
- 20.Huntington JA. Mechanisms of glycosaminoglycan activation of the serpins in hemostasis. J Thromb Haemost. 2003;1:1535–1549. doi: 10.1046/j.1538-7836.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- 21.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 22.Mahalingam Y, Gallagher JT, Couchman JR. Cellular adhesion responses to the heparin-binding (HepII) domain of fibronectin require heparan sulfate with specific properties. J Biol Chem. 2007;282:3221–3230. doi: 10.1074/jbc.M604938200. [DOI] [PubMed] [Google Scholar]
- 23.Herold BC, WuDunn D, Soltys N, Spear PG. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spear PG. Herpes simplex virus: Receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 25.Shukla D, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 26.Hobbs WE, II, DeLuca NA. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J Virol. 1999;73:8245–8255. doi: 10.1128/jvi.73.10.8245-8255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JRG, et al. Identification of inhibitors of heparin-growth factor interactions from combinatorial libraries of four-component condensation reactions. Bioorg Med Chem. 2001;9:825–836. doi: 10.1016/s0968-0896(00)00317-5. [DOI] [PubMed] [Google Scholar]
- 28.Choi S, et al. The design and evaluation of heparin-binding foldamers. Angew Chem Int Ed Engl. 2005;44:6685–6689. doi: 10.1002/anie.200501279. [DOI] [PubMed] [Google Scholar]
- 29.Wright AT, Zhong Z, Anslyn EV. A functional assay for heparin in serum using a designed synthetic receptor. Angew Chem Int Ed Engl. 2005;44:5679–5682. doi: 10.1002/anie.200501437. [DOI] [PubMed] [Google Scholar]
- 30.Bai S, Thomas C, Ahsan F. Dendrimers as a carrier for pulmonary delivery of enoxaparin, a low-molecular weight heparin. J Pharm Sci. 2007;96:2090–2106. doi: 10.1002/jps.20849. [DOI] [PubMed] [Google Scholar]
- 31.Mecca T, Consoli GM, Geraci C, La Spina R, Cunsolo F. Polycationic calix[8]arenes able to recognize and neutralize heparin. Org Biomol Chem. 2006;4:3763–3768. doi: 10.1039/b608887b. [DOI] [PubMed] [Google Scholar]
- 32.Sloand EM, Kessler CM, McIntosh CL, Klein HG. Methylene blue for neutralization of heparin. Thromb Res. 1989;54:677–686. doi: 10.1016/0049-3848(89)90132-1. [DOI] [PubMed] [Google Scholar]
- 33.Mulloy B, Forster MJ. Conformation and dynamics of heparin and heparan sulfate. Glycobiology. 2000;10:1147–1156. doi: 10.1093/glycob/10.11.1147. [DOI] [PubMed] [Google Scholar]
- 34.Iensch H. Neue chemotherapeutika der 4-amino-chinolin-reihe. Angew Chem Int Ed Engl. 1937;50:891–902. [Google Scholar]
- 35.Peng C, Daniels TC. The synthesis of some 6-N-Substituted amido derivatives of 4,6-diaminoquinaldine and a study of their in vitro antibacterial activity. J Am Pharm Assoc Am Pharm Assoc. 1956:3703. [Google Scholar]
- 36.Goble FC. Chemotherapy of experimental trypanosomiasis; trypanocidal activity of certain bis (2-methyl-4-amino-6-quinolyl) amides and ethers. J Pharmacol Exp Ther. 1950;98:49–61. [PubMed] [Google Scholar]
- 37.Goble FCaHJO. Observations on the toxicity of certain trypanocidal quinaldines and aromatic diamidines. Antibiotics and chemotherapy. 1952;II:581–589. [PubMed] [Google Scholar]
- 38.Schulman S, Bijsterveld NR. Anticoagulants and their reversal. Transfus Med Rev. 2007;21:37–48. doi: 10.1016/j.tmrv.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Fuster MM, et al. Genetic alteration of endothelial heparan sulfate selectively inhibits tumor angiogenesis. J Cell Biol. 2007;177:539–549. doi: 10.1083/jcb.200610086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang LC, Brown JR, Varki A, Esko JD. Heparin's anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J Clin Invest. 2002;110:127–136. doi: 10.1172/JCI14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai XM, Esko JD. An animal cell mutant defective in heparan sulfate hexuronic acid 2-O-sulfation. JBiolChem. 1996;271:17711–17717. doi: 10.1074/jbc.271.30.17711. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.