Abstract
The semaphorin and plexin family of ligand and receptor proteins provides important axon guidance cues required for development. Recent studies have expanded the role of semaphorins and plexins in the regulation of cardiac, circulatory and immune system function. Within the immune system, semaphorins and plexins regulate cell–cell interactions through a complex network of receptor and ligand pairs. Immune cells at different stages of development often express multiple semaphorins and plexins, leading to multivariate interactions, involving more than one ligand and receptor within each functional group. Because of this complexity, the significance of semaphorin and plexin regulation on individual immune cell types has yet to be fully appreciated. In this work, we examined the regulation of T cells by semaphorin 6D. Both in vitro and in vivo T cell stimulation enhanced semaphorin 6D expression. However, semaphorin 6D was only expressed by a majority of T cells during the late phases of activation. Consequently, the targeted disruption of semaphorin 6D receptor–ligand interactions inhibited T cell proliferation at late but not early phases of activation. This proliferation defect was associated with reduced linker of activated T cells protein phosphorylation, which may reflect semaphorin 6D regulation of c-Abl kinase activity. Semaphorin 6D disruption also inhibited expression of CD127, which is required during the multiphase antigen-presenting cell and T cell interactions leading to selection of long-lived lymphocytes. This work reveals a role for semaphorin 6D as a regulator of the late phase of primary immune responses.
Keywords: immune regulation, lymphocyte selection, response maturation
The important regulatory functions of semaphorin (Sema) and plexin (Plex) family members within the immune system have only recently been appreciated (1–5). In our previous reports, we demonstrated that mature dendritic cells (DCs) express plexin-A1 (Plxna1), which regulates T cell activation (6, 7). Based on these observations, we postulated that T cells express a Sema protein that interacts with Plxna1 in a simple ligand–receptor pairing. However, recent publications have revealed that similar to the TNF, CD28, and B7 families, immune cells express numerous Sema and Plex ligand–receptor pairs with multiple proteins interacting within a functional group (8–12). For example, during DC and T cell interactions, combinations of the following known receptor–ligand pairs are expressed: Plxna1–Sema3A, Sema3A–Plxna4, Plxna1–Sema6D, and Plxna4–Sema6D. DCs express Plxna1 along with plexin-A4 (Plxna4), semaphorin 3A (Sema3A), and the coreceptor for Plxna1 and Plxna4, neuropilin-1 (Nrp1) (10, 13, 14). T cells express Sema3A, semaphorin 6D (Sema6D), and Plxna4 (5, 10, 14–16). These multiple Sema and Plex proteins mediate cell–cell contact between DCs and T cells. Moreover, the expression patterns also suggest the potential for homotypic pairings during T cell–T cell contact or DC–DC contact. Finally, the identification of Sema and Plex expression by multiple immune cell types increases the potential for complex cell–cell interactions beyond a simple DC and T cell model of regulation (11, 13). The multivariate interactions of Sema and Plex proteins are difficult to characterize, especially given that the majority of functional studies have relied on knockout mice with potential developmental disruptions in which multiple tissues, interactions, cell types, and maturation states may be affected (12). Elucidating the function of the individual Sema and Plex proteins will help to contextualize the total receptor–ligand interactions that occur during an immune response. Thus far, there have been no studies that directly examine the role of Sema6D in the immune system. Sema6D function has only been inferred by the examination of DCs that lack Plxna1 expression (6, 7, 15). In light of the Sema–Plex immune network complexity, we have characterized the role of Sema6D in T cells by using reagents that directly target Sema6D or the multiple Plex protein partners of Sema6D in the context of an intact immune system. Our studies uncover an unknown role of Sema6D in the late phase of a T cell primary immune response.
Results
In Vitro Activation of CD4+ T Cells Enhances Sema6D Expression.
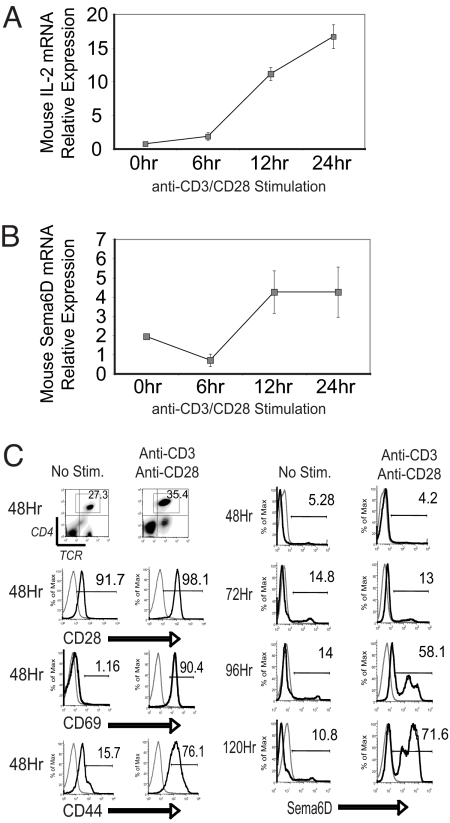
We examined the expression of Sema6D by CD4+ T cells after activation. Murine splenic CD4+ T cells were isolated and stimulated in vitro with plate-bound anti-CD3 and -CD28 antibody (CD3/CD28) over a 24-h period. T cell activation was monitored by IL-2 expression (Fig. 1A). We observed that the expression of Sema6D mRNA is decreased after initial activation, similar to previous observations (15). However, by 12–24 h after stimulation, Sema6D expression was increased over naïve levels (Fig. 1B). Public databases confirmed the expression of Sema6D in both human and mouse T cells and that CD3/CD28 stimulation enhances Sema6D expression over a 48-h period [supporting information (SI) Fig. S1]. Stimulation of CD4+ T cells by CD3/CD28 also produced an increased expression of Sema6D protein on the cell surface. Although mRNA levels were increased by 24 h, protein expression was not enhanced until 96–120 h after CD3/CD28 stimulation (Fig. 1C). The T cell activation was confirmed by enhanced expression of CD69 and CD44 by 48 h after stimulation. We also observed enhanced expression of Sema6D protein after CD3/CD28 stimulation of MOG-specific 2D2 TCR transgenic (Tg) T cells (Fig. S1D). Coculture of ovalbumin (OVA)-specific OTII TCR Tg T cells with OVA-loaded dendritic cells (DCs) also resulted in enhanced expression of Sema6D (data not shown).
Fig. 1.
Activation enhances Sema6D expression in CD4+ T cells. T cells were stimulated with plate-bound anti-CD3 and -CD28 antibodies. (A and B) Splenic CD4+ T cells were isolated by magnetic bead selection to a purity of >90%. RNA was isolated from cultures at 0, 6, 12, and 24 h after initiation and analyzed by quantitative PCR for IL-2 and Sema6D expression. The results are representative of three independent experiments. (C) Protein expression of CD28 and the activation markers CD69 and CD44 (black line) vs. an isotype control (gray line) was determined by FACS analysis on CD4+, TCR+ T cells cultured for 48 h. Sema6D protein expression was determined at 48, 72, 96, and 120 h after culture initiation. Black line, anti-Sem6D monoclonal Ab (R&D Systems); dotted line, isotype control. The results are representative of six independent experiments.
Although the plexin and semaphorin receptor–ligand interactions within the immune system appear to be complex, our previous work with Plxna1 led us to examine interactions with Sema6D. To identify cell–cell interactions mediated by Sema6D, we produced a Sema6D-Ig fusion protein (Fig. S2 A–C). Sema6D-Ig colocalized with Plxna1 on the cell surface of COS-7 cells and DCs. The association of Sema6D-Ig with COS-7 cells or DCs required Plxna1 expression (Fig. S2 D–F). Thus, during an immune response, DC Plxna1, along with other plexins, can participate in cell–cell interactions mediated by Sema6D.
Inhibition of Sema6D Reduces a Late Phase of T Cell Activation in Vitro.
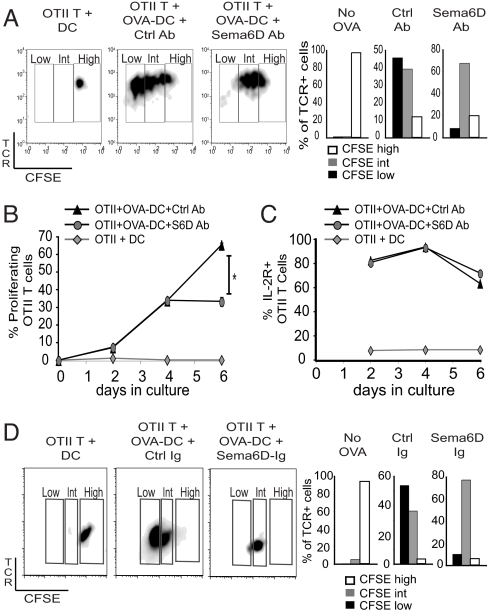
Previous studies using mice or cells deficient in a single Plex protein have suggested a possible role for Sema6D within the immune system. However, a definitive function of Sema6D cannot be determined from these studies given that multiple ligands and receptors are expressed that may interact with Plex or Sema proteins. To target Sema6D function directly, we used both an anti-Sema6D monoclonal antibody and a Sema6D-Ig fusion protein to block Sema6D interactions during T cell activation. OVA-specific CD4+ OTII TCR Tg Vβ5+ T cells, labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE), were cocultured with either OVA antigen-loaded DCs (OVA-DC) or unloaded DCs (DC) for 6 days. The extent of T cell proliferation was measured directly by analysis of CFSE dilution, in which an intermediate level of CFSE labeling (CFSEint) identifies early rounds of division, whereas a low level of CFSE labeling (CFSElow) identifies more extensive division occurring during later periods of proliferation. T cells not exposed to antigen through coculture with unloaded DC did not proliferate, remaining CFSEhigh (Fig. 2A). OTII T cells exposed to antigen through coculture with OVA-DC for 6 days proliferated for multiple divisions, indicated by CFSEint and CFSElow phenotypes (Fig. 2A). Treatment of the coculture with anti-Sema6D antibody inhibited the amount of cell division specifically during the late periods of proliferation, reducing the percentage of OTII T cells with a CFSElow phenotype but not a CFSEint phenotype. The inhibited cell division at day 6 after anti-Sema6D Ab treatment did not result from a defect in the rate of proliferation at earlier time points (Fig. 2B). The percentage of proliferating OTII T cells (diluted CFSE labeling) was compared between anti-Sema6D Ab- and control Ab-treated cocultures at days 2, 4, and 6. The rate of proliferation between the groups was similar from day 0 to day 4. However, proliferation was significantly reduced between days 4 and 6 by anti-Sema6D Ab (Fig. 2B). Moreover, anti-Sema6D Ab treatment did not overtly affect cell viability or T cell activation measured by expression of the IL-2 receptor (Fig. 2C). Thus, anti-Sema6D Ab treatment inhibited late stages of proliferation without affecting either early division or activation. We also examined the possibility that blocking Sema6D imprinted T cells with a defect that manifest only in later rounds of proliferation by delaying anti-Sema6D Ab treatment. OTII T cells were cocultured with OVA-DC, but anti-Sema6D treatment was delayed until day 4. Late rounds of proliferation, measured on day 6 as a CFSElow phenotype, were inhibited by the anti-Sema6D Ab treatment administered on day 4 (Fig. S3A). Thus, T cell proliferation can be inhibited after initial activation by a late administration of anti-Sema6D Ab. As an alternative method of Sema6D targeting, we also treated cocultures with a Sema6D-Ig fusion protein for 6 days. Similar to the anti-Sema6D Ab studies, Sema6D-Ig treatment did not affect initial T cell proliferation indicated by a CFSEint phenotype but did inhibit the late rounds of proliferation indicated by a CFSElow phenotype (Fig. 2D). Thus, blockade of Sema6D interactions with either an antibody or a fusion protein reduced late T cell proliferation.
Fig. 2.
Targeting Sema6D inhibits T cell proliferation at a late phase of activation. DCs that were loaded with whole OVA protein (OVA-DC) or unloaded (DC) were cocultured with purified OTII T cells in vitro. Before culture initiation, OTII T cells were labeled with CFSE. The dilution of CFSE was determined by flow cytometry for TCR+ cells gated from Vβ5+ and CD4+ cells at day 2, 4, or 6. (A–C) OVA-stimulated cultures were also treated with either an anti-Sema6D monoclonal antibody (R&D Systems) or a control antibody. (B) The percentage of proliferating cells was determined for TCR+ VB5+ OTII T cells. At day 6, a two-tailed t test of five samples for each treatment group was performed. The P value was <0.05. (C) IL-2 receptor expression was determined for TCR+ VB5+ OTII T cells. (D) Sema6D-Ig was used as an alternative to anti-Sema6D Ab treatment in vitro. The dilution of CFSE was determined by flow cytometry for TCR+ cells gated from Vβ5+ and CD4+ cells at day 6.
Inhibition of Sema6D Disrupts Endogenous T Cell Signaling.
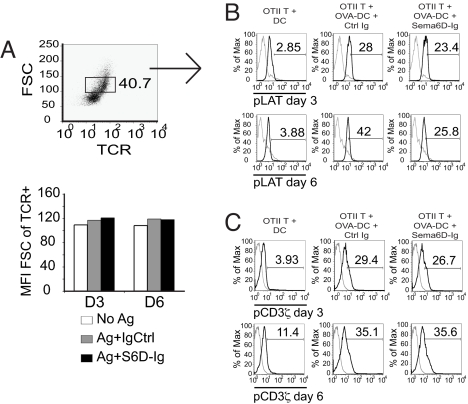
Given the abnormalities in T cell proliferation, we examined endogenous signaling pathways associated with activation, specifically phosphorylation of linker of activated T cells (LAT). We analyzed the pattern of phospho-LAT (pLAT) in OTII T cells stimulated with DCs at day 3 and day 6 of coculture by using phosphospecific flow cytometry (PhosphoFlow) techniques (17–19). Between the coculture groups, we specifically compared T cells of equivalent cell size, to enhance our resolution of activation-induced phosphorylation (Fig. 3A). We also set the detection of phosphorylation in relation to T cells cultured with unloaded DCs at both day 3 and day 6. We detected phosphorylation of LAT after stimulation with OVA-DC in ≈28% of T cells at day 3 and 42% of T cells at day 6 of coculture (Fig. 3B). The observed increase in the percentage of pLAT+ T cells is most likely derived from the coculture conditions, given the dynamic nature of the DC–T cell interactions. Treatment of the cocultures with Sema6D-Ig did not affect pLAT detection at day 3 but did profoundly inhibit the increased percentage of pLAT+ T cells observed at day 6 (Fig. 3B). The percentage increase in pLAT+ T cells between days 3 and 6 was ≈50% in control Ig-treated coculture compared with only 10% in the Sema6D-Ig treated group. Although Sema6D-Ig treatment affected pLAT, it did not affect phosphorylation of the TCR CD3ζ chain (pCD3ζ+), which is one of the first phosphorylation events associated with T cell activation (Fig. 3C). These effects of Sema6D-Ig on cell signaling may be explained by two recent reports. The first identified that Sema6D can activate Abl kinase within heart tissue (16). The second demonstrated that within T cells, Abl kinase regulates phosphorylation of LAT but not CD3ζ (20). We examined the activity of Abl kinase by analyzing the phosphorylation of an Abl kinase target, CrkL (pCrkL). Sema6D-Ig inhibited pCrkL at day 6 but not day 3 (Fig. S3B). Collectively, these observations demonstrate that Sema6D regulates specific T cell signaling pathways that include pLAT and may occur by a direct activation of Abl kinase at a late time point of T cell activation.
Fig. 3.
Targeting Sema6D inhibits endogenous T cell signaling at a late phase of activation. DCs that were loaded with whole OVA protein (OVA-DC) or unloaded (DC) were cocultured with purified OTII T cells in vitro. OVA-stimulated cultures were also treated with either the Sema6D-Ig fusion protein or a control Ig. PhosphoFlow analysis was done of LAT phosphorylation (pLAT) and CD3ζ phosphorylation (pCD3ζ) in T cells cocultured with DCs at days 3 and 6. (A) To reduce nonspecific autofluoresence impact on pLAT detection, TCR+ cells of similar cell size (FSC) were analyzed for each treatment group. (B) The detection of pLAT was determined in reference to T cells cocultured with unloaded DCs. Black line, anti-pLAT antibody; gray line, intracell isotype control. (C) The detection of pCD3ζ was determined in reference to T cells cocultured with unloaded DCs. Black line, anti-pCD3ζ antibody; gray line, intracell isotype control. Results are representative of three independent experiments.
In Vivo Activation of CD4+ T Cells Enhances Sema6D Expression.
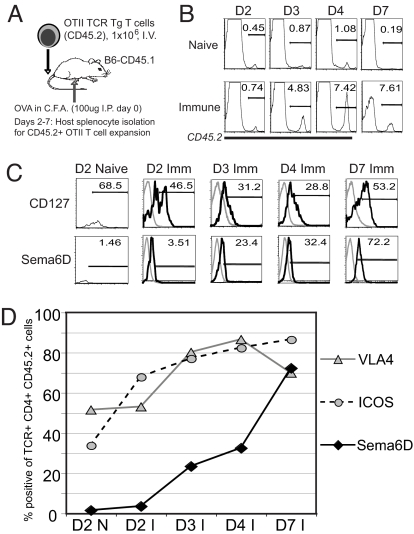
We examined the expression of Sema6D during the course of a primary response by tracking T cell activation in vivo with an adoptive transfer model (Fig. 4A). The in vivo activation of the OTII T cells was visualized as an expansion of donor CD45.2+, CD4+, and TCR+ T cells within the population of CD45.1+ congenic recipient splenocytes. Expansion of the OTII T cells was observed by day 3 after immunization and continued through day 7 (Fig. 4B). Expansion was correlated with a characteristic decrease in IL-7 receptor (CD127) expression by T cells during the early phases of the primary response (21–26). During the later phases between days 4 and 7 (Fig. 4C), the percentage of T cells expressing CD127 increased, similar to reported observations. The percentage of T cells expressing Sema6D increased with activation and reached a majority during the late phases of the response, similar to CD127 (Fig. 4C). By day 7, >70% of activated T cells expressed Sema6D. In contrast, by day 3, >70% of T cells expressed the activation-induced surface proteins, VLA4 (very late antigen 4) and ICOS (inducible costimulator) (Fig. 4D) (27–30).
Fig. 4.
Sema6D expression is enhanced by T cell activation in vivo. (A) OTII T cells (CD45.2+) were adoptively transferred to congenic (CD45.1+) recipients and the splenocytes analyzed at days 2, 3, 4, and 7 after injection and immunization. (B) The expansion of OTII T cells was measured by flow cytometry as the percentage of TCR+ CD4+ CD45.2+ cells in the isolated splenocyte population. (C) The expression of the IL-7 receptor (CD127) and Sema6D on the CD45.2+ OTII T cells was analyzed by flow cytometry at days 2, 3, 4, and 7. Black line, surface expression; gray line, isotype control. (D) The percentage of CD45.2+ OTII T cells expressing VLA4, ICOS and Sema6D was determined by flow cytometry at days 2, 3, 4, and 7. The expression values within the immune samples (I) are presented in relation to the expression observed at day 2 in the naïve samples (D2 N). Results are representative of three independent experiments.
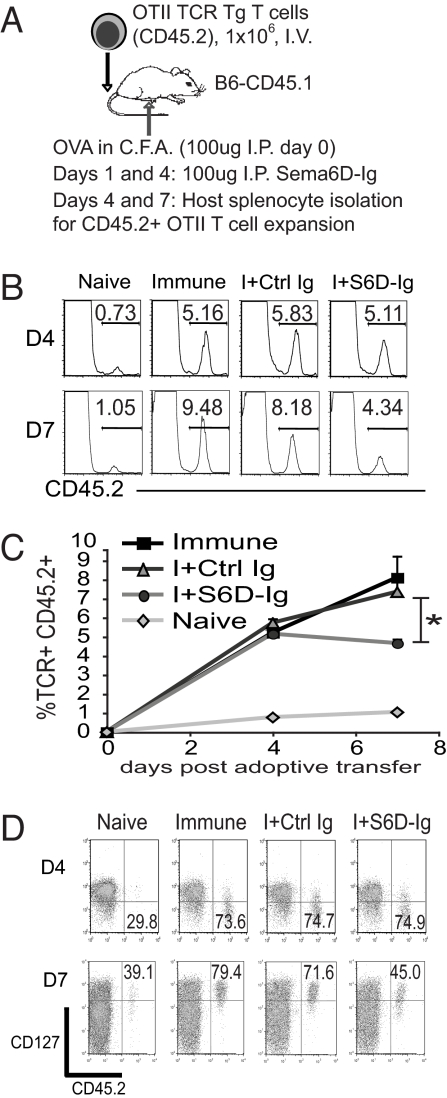
Sema6D Regulates T Cell Activation in Vivo.
We examined the impact of targeting Sema6D in vivo by using the OTII T cell adoptive transfer model (Fig. 5A). Congenic hosts that received OTII T cells were injected with Sema6D-Ig at days 1 and 4 after immunization. The expansion of the CD45.2+ OTII T cells was analyzed at both an early and late phase of the response, days 4 and 7, respectively. Sema6D-Ig treatment did not affect T cell activation at day 4 but did result in a significant reduction of expansion at day 7 (Fig. 5 B and C). We also examined the impact of Sema6D-Ig treatment on the expression of CD127 in vivo. T cell expression of CD127 was not affected at day 4. However, targeting Sema6D inhibited the increase of CD127 expression at day 7 (Fig. 5D). As an alternative methodology, we used a monoclonal antibody to target Sema6D in the context of a neuroinflammatory adoptive transfer model with MOG-specific 2D2 TCR Tg T cells (Fig. S4A). In this model, antigen is delivered by DCs injected directly into the CNS. Targeting of Sema6D by anti-Sema6D antibody resulted in a reduced expansion of 2D2 T cells in the CNS without a corresponding reduction in the percentage of 2D2 T cells detected in the periphery (Fig. S4 B–D). Thus, targeting Sema6D with either Sema6D-Ig or anti-Sema6D antibody resulted in reduced T cell activation in vivo.
Fig. 5.
Targeting Sema6D inhibits late-phase T cell activation in vivo. (A) OTII T cells (CD45.2+) were adoptively transferred to congenic (CD45.1+) recipients, and the splenocytes were analyzed at days 4, and 7 after injection and immunization. Recipients were treated with Sema6D-Ig or a control Ig at days 0 and 4. (B) The expansion of TCR+ CD4+ CD45.2+ OTII T cells was analyzed in vivo at days 4 and 7 after adoptive transfer. (C) The expansion of OTII T cells for eight replicate samples within each treatment group was compared. A two-tailed t test comparing the Sema6D-Ig-treated samples with either the immunized or control Ig groups returned a P value of <0.05. (D) The expression of CD127 on OTII T cells was determined by flow cytometry. At day 4, the percentage of the total CD45.2+ T cells with low CD127 expression is denoted. At day 7, the percentage of the total CD45.2+ T cells with high CD127 expression is shown. The results are representative of two independent experiments.
Discussion
We have observed that Sema6D regulates T cell activation during the late phases of an immune response. Recent studies have underscored the importance of long-lasting interactions between T cells and antigen presenting cells (31). Two-photon studies have identified multiple phases of interactions, which engender early proliferation and also regulate the late phases of a primary response (32–35). Disruption of these interactions, such as by loss of ICAM-1, can result in loss of a memory response even in the presence of initial activation (36). The effects of disrupting T cell interactions often are not observed until many days after initial activation (37). Our studies demonstrate that Sema6D regulates the late-phase activity of T cells during a primary immune response. Future studies will identify the interacting proteins that contribute to Sema6D regulation at this late phase. Our analysis of Sema6D association with DCs suggests that in the context of DC-T cell contact in vivo, Plxna1 and Sema6D probably mediate late-phase interactions. T cell contact with other cell types during the late phases of a primary immune response may be mediated by Sema6D interactions with other proteins.
The ability of Sema6D to regulate T cell activation involves modulation of endogenous signaling pathways. We have observed that disrupting Sema6D interactions results in an inhibition of LAT phosphorylation in T cells without a concomitant inhibition of CD3ζ phosphorylation. Corresponding to the inhibition of late-phase proliferation, Sema6D targeting primarily reduced late-phase pLAT signaling. Concomitantly, we also observed a late-phase disruption of CrkL phosphorylation with Sema6D-Ig treatment. Previous studies have reported that Sema6D can activate Abl kinase and that within T cells, Abl regulates phosphorylation of CrkL and LAT but not CD3ζ (16, 20). Given that Sema6D surface protein expression is not enhanced until the late phases of activation, it appears possible that the late-phase inhibition of pLAT may derive directly from a defect in Sema6D signaling by Abl kinase activity. However, the regulation of Abl kinase activity within T cells is complex, and future studies are required to elucidate fully the endogenous T cell signaling pathways regulated by Sema6D.
The in vivo expression pattern of Sema6D is similar to the pattern for CD127 expression during the late phases of an immune response. Previous studies have documented a decrease in CD127 expression by T cells during the early phases of a primary response. In the later phases, as T cell are selected into the memory pool, CD127 expression is increased. Inhibition of this late-phase increase in CD127 expression results in defective T cell memory (21, 24, 26). Intriguingly, we observed that Sema6D targeting results in the inhibition of CD127 expression by T cells during the late phase of an in vivo primary response. This may be caused, in part, by the activity of Abl kinase downstream of Sema6D. Abl kinase activity promotes IL-2 production, which is known to be required for CD127 expression during the late phase of a primary immune response (20–22, 38). Recent studies have demonstrated that inhibitors of Abl kinase, such as Imatinib, inhibit CD127 expression by T cells and memory responses while allowing initial phases of activation, including proliferation, to occur (39). However, in contrast to pan-T cell inhibitors, Sema6D targeting may specifically affect a distinct activated population of T cells. Our analysis of in vivo T cell activation demonstrated a differential expression pattern of Sema6D between T cell subsets, with only low expression detected on CD8+ T cells or unactivated T cells throughout the course of the primary response (data not shown). Thus, the regulation of T cell activity by Sema6D may be restricted to the activated CD4+ T cell compartment.
Given the complexity of the Sema–Plex network, future studies will be required to identify all of the functional interactions that regulate specific immune functions. Early studies using knockout models demonstrated a significant role for semaphorins and plexins in directing immune system activity. Recent observations of multiple receptor and ligand interactions, however, suggest a need for characterization of individual protein function. Studies using Plxna1-deficient DCs and knockout animals suggested that Sema6D, as a possible ligand, may regulate immune activation. Subsequent studies have revealed other ligands within the immune system for Plxna1, making indirect conclusions regarding ligand function difficult. In our studies directly examining the impact of Sema6D on T cells, we have observed an important role for Sema6D in the late phases of a primary immune response. The interacting partner for Sema6D at this late phase may be Plxna1 or some other protein yet to be identified. Future studies are required to comprehend this complex network and to determine the Sema–Plex combinations that can be manipulated as a treatment regime for human disease. This work has identified Sema6D as an important regulator of the late phase of a primary immune response, and thus, treatment modalities involving Sema6D may enhance vaccine development, reduce the impacts of autoimmunity, or improve immune-based cancer therapies.
Materials and Methods
Mice.
All experiments were performed with 8- to 12-week-old C57BL/6 mice from Jackson Laboratories or B6-Ly5.2/Cr (CD45.1) mice from the National Cancer Institute/National Institutes of Health. OT-II mice that express the OVA[323–339]-specific TCR transgene on the C57BL/6 background were generous gifts from M. Croft (La Jolla Institute for Allergy and Immunology, La Jolla, CA). All animal procedures were conducted in complete compliance with the National Institutes of Health guidelines and are approved by the IACUC of University of North Carolina, Chapel Hill.
SYBR Green Real-Time PCR.
SYBR Green quantitative PCR Rox mix (Abgene) was used for all quantitative PCR experiments. The following cycle conditions were used: (i) 50° for 2 min; (ii) 95° for 15 min; (iii) 95° for 15 s, 56–57° for 15–30 s, 72° for 15–30 s, repeat 40 times; (iv) dissociation curve. Target genes were calculated in reference to β-actin: forward, 5′-agggctatgctctccctcac-3′; and reverse, 5′-ctctcagctgtggtggtgaa-3′. The Sema6D primers used were: forward, 5′-cagaagcatgggagatggat-3′; and reverse, 5′-gccacccatgtcgtttttac-3′.
Cloning and Production of Mouse Sema6D-Ig Fusion Protein.
A full-length cDNA of isoform 6 of Sema6D (Sema6D-6) was isolated, cloned, sequenced, and verified as Sema6D. A cDNA fragment containing the extracellular region of Sema6D was subcloned into a modified pcDNA3.1 vector (Invitrogen) containing a human IgG1 fragment (Hinge-CH2-CH3). Generation of stable expression cells was performed with DHFR-Chinese hamster ovary cells (CHO/DG44; Invitrogen) selected in medium supplemented with 100 nM methotrexate (Sigma).
Flow Cytometry.
Detection of Sema6D was performed with a monoclonal anti-Sema6D antibody (R&D Systems). Phosphospecific flow cytometry was performed according to the techniques developed by Gary Nolan's group and adopted by BD PharMingen (19). Before surface staining, cell samples were fixed for 10 min at 37°C in 20× volumes of prewarmed (37°C) Phospho Fix buffer I (catalog no. 557870; BD PharMingen) or Cytofix/Cytoperm (catalog no. 54722; BD PharMingen). Cells were then stained for surface antigens followed by washing and permeabilization for 20–30 min in prechilled (−20°C) PhosFlow Perm buffer III (catalog no. 558050; BD Pharmingen). After permeabilization, cells were washed and incubated with a mixture of Fc receptor blocking antibodies for 15 min. The appropriate antibodies for detection of intracellular phosphospecific epitopes (BD PharMingen) were then added to each sample and incubated for an additional 30 min. Staining was quantified with a FACSCalibur (Becton Dickinson). Fluorescence signals were detected by four-decade logarithmic amplification; FSC and SSC were detected on a linear scale. Data were analyzed with FlowJo software (FlowJo).
In Vitro CD3/CD28 Stimulation and Coculture Conditions.
For stimulation of T cells, 5 μg/ml anti-mouse CD3 and anti-mouse CD28 were added in PBS to cell culture plates for overnight coating at 4°C. Primary T cells isolated from spleens were incubated at 1 × 106 cells per ml in 2 ml per well of a 6-well coated plate. For in vitro coculture stimulation, OTII TCR Tg (OVA-specific) T cells were incubated with immature DCs at a ratio of 5:1 in RPMI medium 1640. OTII T cells were isolated from the spleens of Tg mice and purified by negative selection with T enrichment columns (R&D Systems). Isolated T cells were labeled with CFSE (Molecular Probes) or unlabeled before culture. Murine bone marrow-derived DCs were isolated from bone marrow and grown in vitro for maturation as described (6, 7). For OVA loading, DCs were resuspended at a concentration of 1 × 106 cells in 1 ml of cRPMI with 10 μg/ml whole OVA protein. The final concentration of CFSE used for T cell labeling was 15 μM in RPMI medium 1640 with 10–20 million cells per ml.
Anti-Sema6D Antibody and Sema6D-Ig Fusion Protein Treatment.
Anti-Sema6D monoclonal Ab (R&D Systems) was used at a final concentration of 10 μg/ml for in vitro and ex vivo treatment of T cells. Anti-Sema6D Ab was given every 3rd day of in vitro culture. The Sema6D-Ig fusion protein was used at a final concentration of 5 μg/ml for in vitro treatment. In vivo, Sema6D-Ig was injected i.p. at a concentration of 100 μg of per mouse. Intraperitoneal injections were given at days 1 and 4 after adoptive transfer.
Adoptive Transfer and Immunization.
After isolation of OTII splenocytes from mice, RBCs were lysed by incubation with an ammonium chloride Tris solution. The percentage of Tg OTII T cells within a population was determined by staining 2 × 105 cells with anti-Vα2 and anti-Vβ5 in 5% bovine calf serum in balanced salt solution at 4°C and analyzed by a Becton Dickinson FACSCalibur. For each primary transfer, ≈1 × 106 CD45.2+ OTII T cells were injected by tail vein into B6-CD45.1 recipient mice. At the time of adoptive transfer, some recipients were also injected i.p. with 100 μg of OVA protein emulsified in Complete Freund's adjuvant. Typically, three mice were used per experimental group.
Supplementary Material
Acknowledgments.
We thank Dr. Edward J. Collins (University of North Carolina, Chapel Hill, NC) for graciously providing the CD8α construct used in these studies; Drs. Weiguo Zhang and Ann Marie Pendergast (Duke University, Durham, NC), for helpful discussions; Drs. Conti and W. Fleming for help with CD3/CD28 stimulation of splenic T cells; and Dr. R. J. Noelle and D. Gondek for their helpful comments. This work was supported by National Institutes of Health Grants AI29564 and DK38108 (to J.P.-Y.T.). B.P.O. is an Irvington Institute Fellow of the Cancer Research Institute. C.B.M. is a Juvenile Diabetes Foundation Postdoctoral Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803386105/DCSupplemental.
References
- 1.Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat Rev Immunol. 2003;3:159–167. doi: 10.1038/nri1003. [DOI] [PubMed] [Google Scholar]
- 2.Liu BP, Strittmatter SM. Semaphorin-mediated axonal guidance via Rho-related G proteins. Curr Opin Cell Biol. 2001;13:619–626. doi: 10.1016/s0955-0674(00)00260-x. [DOI] [PubMed] [Google Scholar]
- 3.Tamagnone L, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 4.Tamagnone L, Comoglio PM. To move or not to move? Semaphorin signalling in cell migration. EMBO Rep. 2004;5:356–361. doi: 10.1038/sj.embor.7400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyofuku T, et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eun SY, et al. Cutting edge: Rho activation and actin polarization are dependent on plexin-A1 in dendritic cells. J Immunol. 2006;177:4271–4275. doi: 10.4049/jimmunol.177.7.4271. [DOI] [PubMed] [Google Scholar]
- 7.Wong AW, et al. CIITA-regulated plexin-A1 affects T cell-dendritic cell interactions. Nat Immunol. 2003;4:891–898. doi: 10.1038/ni960. [DOI] [PubMed] [Google Scholar]
- 8.Keir ME, Sharpe AH. The B7/CD28 costimulatory family in autoimmunity. Immunol Rev. 2005;204:128–143. doi: 10.1111/j.0105-2896.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 9.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 10.Lepelletier Y, et al. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol. 2006;36:1782–1793. doi: 10.1002/eji.200535601. [DOI] [PubMed] [Google Scholar]
- 11.Watarai H, et al. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc Natl Acad Sci USA. 2008;105:2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 13.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto M, et al. Plexin-A4 negatively regulates T lymphocyte responses. Int Immunol. 2008;20:413–420. doi: 10.1093/intimm/dxn006. [DOI] [PubMed] [Google Scholar]
- 15.Takegahara N, et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- 16.Toyofuku T, et al. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat Cell Biol. 2004;6:1204–1211. doi: 10.1038/ncb1193. [DOI] [PubMed] [Google Scholar]
- 17.Krutzik PO, Hale MB, Nolan GP. Characterization of the murine immunological signaling network with phosphospecific flow cytometry. J Immunol. 2005;175:2366–2373. doi: 10.4049/jimmunol.175.4.2366. [DOI] [PubMed] [Google Scholar]
- 18.Krutzik PO, Irish JM, Nolan GP, Perez OD. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: Techniques and clinical applications. Clin Immunol. 2004;110:206–221. doi: 10.1016/j.clim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Krutzik PO, Nolan GP. Intracellular phosphoprotein staining techniques for flow cytometry: Monitoring single cell signaling events. Cytometry. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 20.Zipfel PA, Zhang W, Quiroz M, Pendergast AM. Requirement for Abl kinases in T cell receptor signaling. Curr Biol. 2004;14:1222–1231. doi: 10.1016/j.cub.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7Rα-expressing cells. J Exp Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dooms H, Abbas AK. Control of CD4+ T cell memory by cytokines and costimulators. Immunol Rev. 2006;211:23–38. doi: 10.1111/j.0105-2896.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 23.Kondrack RM, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 27.Nojima Y, Rothstein DM, Sugita K, Schlossman SF, Morimoto C. Ligation of VLA-4 on T cells stimulates tyrosine phosphorylation of a 105-kDa protein. J Exp Med. 1992;175:1045–1053. doi: 10.1084/jem.175.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu Y, van Seventer GA, Horgan KJ, Shaw S. Roles of adhesion molecules in T cell recognition: Fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 29.Coyle AJ, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 30.Hutloff A, et al. ICOS is an inducible T cell costimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 31.Bousso P, Robey EA. Dynamic behavior of T cells and thymocytes in lymphoid organs as revealed by two-photon microscopy. Immunity. 2004;21:349–355. doi: 10.1016/j.immuni.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Celli S, Garcia Z, Bousso P. CD4 T cells integrate signals delivered during successive DC encounters in vivo. J Exp Med. 2005;202:1271–1278. doi: 10.1084/jem.20051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell–dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Garcia Z, et al. Competition for antigen determines the stability of T cell–dendritic cell interactions during clonal expansion. Proc Natl Acad Sci USA. 2007;104:4553–4558. doi: 10.1073/pnas.0610019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henrickson SE, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule 1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Dolfi DV, et al. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. J Immunol. 2008;180:2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu JJ, Zhang N, He YW, Koleske AJ, Pendergast AM. Defective T cell development and function in the absence of Abelson kinases. J Immunol. 2007;179:7334–7343. doi: 10.4049/jimmunol.179.11.7334. [DOI] [PubMed] [Google Scholar]
- 39.Sinai P, et al. Imatinib mesylate inhibits antigen-specific memory CD8 T cell responses in vivo. J Immunol. 2007;178:2028–2037. doi: 10.4049/jimmunol.178.4.2028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.