Abstract
Although it has been recognized that echolocating bats may experience jamming from the signals of conspecifics, research on this problem has focused exclusively on time-frequency adjustments in the emitted signals to minimize interference. Here, we report a surprising new strategy used by bats to avoid interference, namely silence. In a quantitative study of flight and vocal behavior of the big brown bat (Eptesicus fuscus), we discovered that the bat spends considerable time in silence when flying with conspecifics. Silent behavior, defined here as at least one bat in a pair ceasing vocalization for more than 0.2 s (200 ms), occurred as much as 76% of the time (mean of 40% across 7 pairs) when their separation was shorter than 1 m, but only 0.08% when a single bat flew alone. Spatial separation, heading direction, and similarity in call design of paired bats were related to the prevalence of this silent behavior. Our data suggest that the bat uses silence as a strategy to avoid interference from sonar vocalizations of its neighbor, while listening to conspecific-generated acoustic signals to guide orientation. Based on previous neurophysiological studies of the bat's auditory midbrain, we hypothesize that environmental sounds (including vocalizations produced by other bats) and active echolocation evoke neural activity in different populations of neurons. Our findings offer compelling evidence that the echolocating bat switches between active and passive sensing to cope with a complex acoustic environment, and these results hold broad implications for research on navigation and communication throughout the animal kingdom.
Keywords: echolocation, jamming avoidance, passive listening, spatial hearing
Active sensing enables a wide range of animal species to orient and forage under conditions where light levels are low or absent (1). Self-produced acoustic or electric signals give rise to information about the environment that is used to guide a variety of behaviors. Echolocating animals produce ultrasonic signals and determine the direction, distance, and features of objects in the environment from the arrival time, amplitude, and spectrum of sonar reflections (2). Electric fish generate discharges from an electric organ in the tail, and sense the location and features of nearby objects from amplitude and phase changes in the electric field (3).
With the benefits of active sensing also come challenges, namely the potential for interference from signals produced by neighboring conspecifics. Past research has uncovered strategies by which echolocating bats and electric fish avoid jamming through active adjustments in the signals they produce to probe the environment. Wave-type weakly electric fish modify the electric organ discharge frequency, and pulse-type weakly electric fish change the timing of electric organ discharge to avoid interference from the signals of neighbors (3). In echolocating bats, spectral and/or temporal adjustments in the characteristics of sonar vocalizations, which yield acoustic separation between signals of conspecifics, have been reported in several species (4–6). None of the studies of jamming avoidance in bats measured the relative positions of animals when they made the reported vocal adjustments, and none have uncovered the finding that bats often go silent to minimize interference from the signals of conspecifics.
In bats, relative spatial position and flight direction influence the magnitude of acoustic interference from vocalizing conspecifics. Since spherical spreading loss and excess attenuation of ultrasonic frequencies produce a decrease in acoustic energy with distance (7), one would predict a negative correlation between interference level and inter-bat spatial separation. In addition, the sonar radiation pattern (8, 9) and receiver (10, 11) are directional. Thus, the angle between two bats' heading directions would also be expected to impact interference level and concomitant adjustments in sonar behavior.
Exploiting technological advances, we were able to quantitatively analyze strategies that an echolocating big brown bat, Eptesicus fuscus, uses to avoid signal interference when flying with a conspecific in a complex environment. Taking 3D high speed video and sound recordings, we quantified the relation between flight path and vocal behavior, and importantly, identified which bat produced each vocalization in a stream of calls. Analyses of these data led to the discovery that bats flying in pairs go silent for extended periods of time (over 0.2 s), covering distances of at least 0.6 m when flying at a speed of 3 m/s, and the prevalence of silent behavior depends on the flight pattern of the bats and the baseline similarity of their sonar calls.
Results
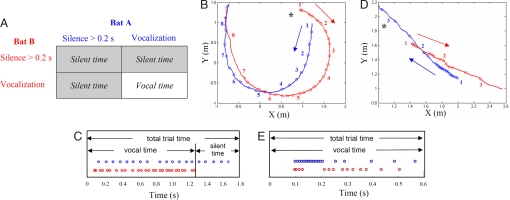
To examine how the echolocating bat changes its behavior in response to the presence of a conspecific, 8 big brown bats, E. fuscus, were trained to fly in pairs and compete for a single prey item in a laboratory flight room. In these experiments, bats exhibited a significant amount of silent time, defined as no vocalizations from at least one bat for over 0.2 s (200 ms), when paired together (28% of the time collapsed across all inter-bat separation distances; 40% of the time when the inter-bat separation was less than 1 m). However, bats almost never exhibited silent times longer than 0.2 s when flying alone (0.08%). Silent time indicates a period during an entire trial segment when one or both bats ceased vocalizing for more than 0.2 s, while vocal time refers to periods when both bats were continuously vocalizing (Fig. 1A). Total trial time is the duration of each analyzed trial and also the sum of vocal and silent times. Fig. 1 B and D shows examples from selected trials, illustrating that silent behavior is related to the flight configuration of paired bats (see Table 1 for definitions). Fig. 1 C and E shows the timing of each bat's vocalizations and the silent/vocal times for the 2 trials in Fig. 1 B and D. One bat stopped vocalizing at 1.28 s in the first example (Fig. 1 B and C); the total trial time for this trial is 1.8 s, vocal time is 1.28 s and silent time is 0.52 s. Both bats were continuously vocalizing for the entire trial in the second example; therefore, the total trial time is equal to the vocal time for this trial (Fig. 1 D and E). Across trials, the silent time ranged from 0.2 to 2.55 s, and the mean silent time duration was 0.5 s. The average flight speed of bats in this experiment was 3.23 m/s; therefore the estimated distance the bat flew in silence ranged from 0.6 to 8 m, with an average of 1.6 m. However, the bat neither collided with the other bat nor exhibited signs of disorientation during prolonged silent times.
Fig. 1.
Definitions of silent/vocal times and flight trajectories of paired bats in different flight configurations from selected trials. (A) Two possible behaviors: (1) Silence >0.2 s: the bat stopped vocalizing for more than 0.2 s; (2) Vocalization: the bat was vocalizing continuously, with intervals between 2 consecutive pulses always shorter than 0.2 s. Silent time is defined as when one or both bats went silent for over 0.2 s, and vocal time is defined as when both bats were vocalizing continuously. (B and D) Each circle represents an echolocation call and the asterisk marks the position of the target (tethered mealworm) in each trial. The number “1” beside each flight path represents the starting point and the time interval between successive numbers is 0.5 s. (B) Following flight for time indices “1–5” is 30–60° following flight and for time indices “6–8” is 0–30° following flight. The bat whose behavior is shown in red, stopped vocalizing after time index “7.” (D) Converging and diverging flights: before time index “2” is converging flight and after this point is diverging flight. The bat (data shown in blue) produced a series of short duration and short pulse interval calls (avoidance buzz) between time indices “1” and “2.” (C and E) The silent time, vocal time and total trial time. Each circle represents one vocalization and different colors represent different bats. Plots in B and C are based on one trial and plots in D and E are based on another trial.
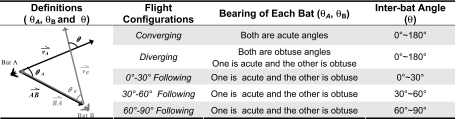
Table 1.
Paired bats' flight configurations, which were categorized by the bearing of each bat and the inter-bat angle
The diagram on the left shows the bearing of each bat (θA and θB) and the inter-bat angle (θ).
The results of this study were obtained by first training individual bats to intercept a tethered mealworm in a large laboratory flight room illuminated with dim, long-wavelength lighting (>650 nm). The floor was carpeted and the walls and ceiling were lined with acoustic foam (Sonex-1). Data recordings began after all bats learned the prey capture task. Three ultrasound sensitive microphones were placed on the floor to record echolocation calls, and 2 high-speed infrared sensitive video cameras were mounted in adjacent corners of the flight room to permit reconstruction of 3D flight paths. Synchronized sound recordings with a 16 microphone array on 3 walls of the room (8) were used to substantiate that all sonar calls produced by the bats were recorded. Call assignment to individual bats was accomplished through joint analysis of video position and sonar pulse travel times to the 3 microphones [Materials and Methods, supporting information (SI) Text, and Figs. S1 and S2].
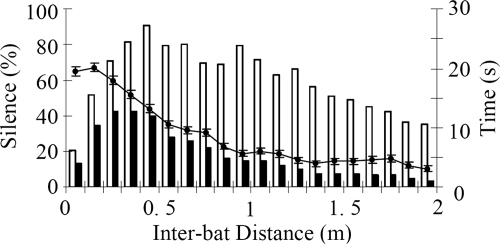
The occurrence of silent times appears to be related to the level of call interference, which is influenced by the distance between 2 bats. When paired bats flew less than a meter apart, silence was observed as much as 76% of the time, with a mean across 7 bat pairs of 40%. Fig. 2 shows that the percentage of silent time decreased as the inter-bat separation increased. The average percentage of time spent in silence was negatively correlated with the inter-bat distance (Pearson's r = −0.77, p < 0.0001). The correlation between distance and silent time was even stronger for inter-bat distance shorter than 1 m (Pearson's r = −0.99, p < 0.0001).
Fig. 2.
The relationship between the percentage of silent time and inter-bat distance. The percentage of silent time was calculated by dividing total duration of silent time by total trial time. Data, taken from a total of 441.27 s across 152 trials with 7 pairs of bats are included. Error bars represent standard errors of the mean. The percentage of silent time as a function of inter-bat distance (filled circles, left ordinate). The histogram shows on the right ordinate the total trial time (white) and duration of silent times (black).
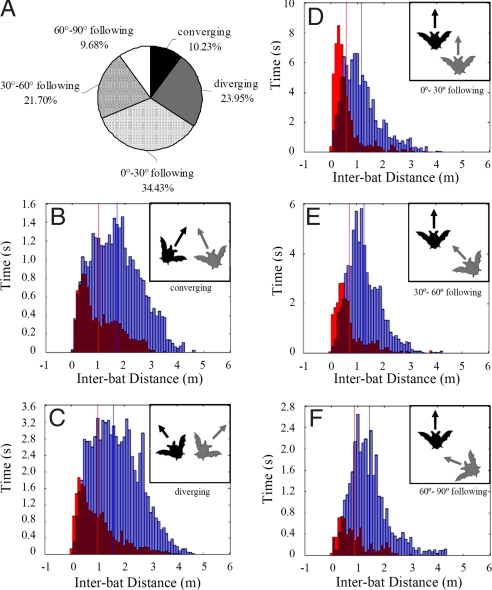
We used the bearing of each bat to describe the relative position of paired bats and defined 3 major flight patterns: converging, diverging, and following (Table 1). Following flight was the most common configuration, and we further divided this flight pattern into 3 groups according to the inter-bat angle. Fig. 3A shows that one-third of the time one bat in a pair flew behind the other bat in the same general direction (0–30° following) and another one-third of the time in different directions (30–60° and 60–90° following). The rest of the time both bats either flew toward or away from each other (converging or diverging), with the time paired bats spent in diverging flight double that of converging flight.
Fig. 3.
The proportion of each flight pattern and the inter-bat distance histogram of 5 different flight patterns. (A) Pie chart shows the percentage of time bats spent in each flight pattern. About 65% of the time one bat followed the other bat and more than half of the time, bats maintained an inter-bat angle smaller than 30°, which indicates that one bat was following another bat in a similar direction. (B–F) The x axis is the inter-bat distance (m) and the y axis represents the duration of silent and vocal times. Red bars represent the silent times and blue bars represent the vocal times at specified inter-bat distances. If the bat was silent more than vocal, the red bar exceeds the blue one. If the bat was vocal more than silent, the blue bars exceed the red. Overlap regions are indicated by purple bars. Note that the y axes in panels (B–F) do not display the same scales. (Upper Right Inset) The geometric configuration of each flight pattern. Arrows represent the flight direction of each bat. (B) When the bearing of both bats is at an acute angle, the flight pattern is referred to as converging flight. (C) When the bearing of both bats is at an obtuse angle, the flight pattern is referred to as diverging flight. (D–F) When the 2 bats are flying in the same general direction, the flight pattern is called following flight. We divided following flight into 3 separate patterns according to the inter-bat angle between paired bats. (D) Inter-bat angle between 0° and 30° is called 0–30° following. (E) Inter-bat angle between 30° and 60° is called 30–60° following. (F) Inter-bat angle between 60° and 90° is called 60–90° following.
Fig. 3B-F shows the distribution of inter-bat distance during silent and vocal times in various flight patterns. The y axis is the duration of silent (red bars) and vocal (blue bars) times at various inter-bat separations. The average inter-bat distance in silent times was always shorter than in vocal times, which shows that the silent behavior typically occurred when the spatial separation between 2 bats was small. Silent and vocal times were almost equivalent when 2 bats approached each other (converging flight) at short inter-bat distances (Fig. 3B). When paired bats flew away from each other (diverging flight), silent times were greater than vocal times only when the inter-bat distance was shorter than 0.4 m (Fig. 3C). In following flight, (0–30° inter-bat angle) and at short inter-bat distances, silent times were more frequent than vocal times (Fig. 3D). The ratio of silent to vocal times decreased as the inter-bat angle increased at the same inter-bat distance (Fig. 3D-F).
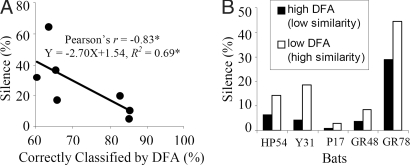
The similarity in call design, determined by discriminant function analysis (DFA), between one bat and another bat in a pair also influenced silent behavior. We compared the call designs of each bat in a pair and found that the incidence of silent behavior was related to differences between paired bats' echolocation call designs, i.e., duration, bandwidth, start/end frequencies and sweep rate of the frequency modulated call, when flying alone. Discriminant function analysis was applied to determine how well these 5 call features can correctly distinguish the echolocation calls from each individual. Calls that are correctly assigned to one bat in a pair can be classified as distinctly different from those of the other bat. A negative correlation was found between the silent behavior of a bat in a pair and the percentage of correct classifications by discriminant function analysis (Pearson's r = −0.83, p < 0.05). The similarity of call design between 2 bats in a pair reliably predicted the prevalence of silent behavior (Fig. 4A; R2 = 0.69, p < 0.05). When a bat was paired across different sessions with different individuals, it showed more silent behavior when paired with an individual whose signals were more similar to its own (Fig. 4B). The greater the similarity in call design between paired bats, the more silent behavior each exhibited.
Fig. 4.
The correlation between sonar signal DFA and silent behavior. (A) Each data point represents a pair of bats and a total of 7 pairs of bats were used in this study. Five call features (start/end frequency, duration, bandwidth, sweep rate) were used in discriminant function analysis (DFA) to classify calls from different individuals. The percentage correctly classified represents how well these 5 features could distinguish an individual's echolocation calls. The more separation in the time-frequency structures of a bat pair, the less silent behavior they showed in this study. * indicates p < 0.05. (B) An individual bat's silent behavior depended upon the similarity of its calls to that of the bat with which it was paired. Data shown for the 5 bats that were paired with more than 1 individual. Black bars represent the percentage silence of a bat when paired with another bat whose signals show a high DFA. White bars represent the percentage silence of a bat when paired with another bat whose signals show a low DFA. High DFA indicates low similarity between paired bats in call design, while low DFA indicates high similarity. All bats tested in multiple pairings showed an increase in silent behavior when paired with an individual whose signals were more similar to its own.
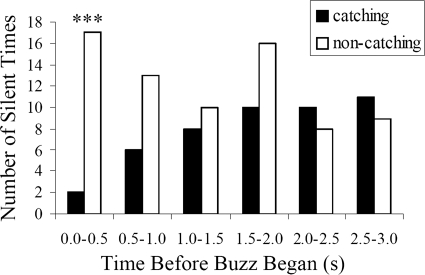
Only one prey item was presented in every trial, so only one individual captured the tethered prey. We examined the timing of silent behavior for the bat that caught the worm to determine whether silent behavior immediately preceded the feeding buzz that always accompanied prey capture. The bat that captured the prey showed significantly fewer silent times than the one who did not capture the worm during the last 0.5 s before the a start of the feeding buzz (χ2 = 11.8, p < 0.001), but there was no significant difference in silent behavior between the catching and non-catching bats during other time intervals (Fig. 5). The bat that caught the prey started its echolocation at least 0.24 s before initiating the feeding buzz.
Fig. 5.
The relationship between prey capture and silent behavior. The number of silent times before the feeding buzz for both the bat that captured the worm and the bat that did not capture the worm. The x axis is the time a silent time ended before the beginning of the feeding buzz and y axis is the number of silent times. Black bars represent the bat that caught the worm and white bars represent the bat that did not catch the worm. χ2 test is applied to examine the difference of silent behavior between these 2 bats and *** means p < 0.001.
Discussion
The results of this study suggest that echolocating big brown bats employ a surprising jamming avoidance strategy, silence. The relation between the occurrence of silent behavior and the spatial separation and heading of the paired bats indicates that one bat stopped vocalizing to avoid interference with another bat's echolocation (Figs. 2 and 3). This interpretation is bolstered by the observation that silent behavior is most prevalent in pairs of bats that produce similar echolocation calls when flying alone (Fig. 4).
Short inter-bat distance and/or small angular separation in heading directions of paired bats occurred most often with silent times. When 2 bats approached each other, the acoustic interference between them is greater than in the diverging flight configuration because the intensity of the bat's sonar vocalization is strongest directly in front of the animal (9). The bats did not show greater silent times in converging flight but instead produced a series of calls with short duration and short pulse interval (Fig. 1B, the path marked with blue between time indices “1” and “2”). This series of calls suggests an avoidance buzz, with intervals close to those observed in the feeding buzz (6), and we infer that the bat's vocal behavior in this situation served to increase its spatial resolution to avoid collision with the other bat. The ratio of silent to vocal times decreased as the inter-bat angle increased for a given inter-bat distance for pairs engaged in following flight, demonstrating that silent behavior is affected by the angular separation of paired bats' flight directions. Large angular separations in flight direction between bats reduce acoustic interference between individuals, as both sonar transmission and reception are directional (8–11).
The big brown bat produces individual-specific echolocation calls, which differ in the details of signal features (12), and personalized call design may help a bat to segregate its own signals from those of conspecifics (13). Psychophysical experiments have demonstrated that interference signals most similar to a bat's own call caused the most severe disruption to its target ranging performance (14). Bats using similar call design could interfere with each other's sonar target localization; therefore, increased silent behavior may serve to minimize disorientation.
Previous studies of acoustic communication in other animal species, such as birds (15) and frogs (16), have reported the use of temporal separation of signals to avoid acoustic interference from conspecifics or noise. In birds and frogs, social communication calls are sometimes interrupted, presumably to avoid acoustic interference, but this silent behavior would not disrupt spatial orientation in these animals. However, echolocating bats use vocalizations for both communication and spatial orientation. Our results suggest that the echolocating bat engages in silent behavior to avoid interference from conspecifics, and we speculate below on how the bat can orient without producing sonar cries.
Although bats can use spatial memory to reference the position of obstacles in a familiar flyway (17–19), we exclude the possibility that the silent bat oriented entirely by spatial memory in this study. It may be possible for a bat to use spatial memory instead of echolocation to avoid fixed obstacles; however, the unpredictable movement of a conspecific eliminated the possibility that the silent bat could rely on spatial memory to avoid in-flight collision. The bat's use of vision was excluded by the low level infrared lighting in the room, which fell outside the spectral sensitivity of the bat's retina (20).
Multiple sonar sources produce signal interference, but can also potentially provide useful information about the surroundings to other listening individuals (21, 22). There has been speculation that a silent bat can acquire information about the environment, locate another bat, and avoid collision by passively listening, rather than actively echolocating; however, this has not been previously addressed with quantitative kinematic and acoustic analysis. The big brown bat can passively localize sound sources (23), but its accuracy is not as high along the azimuthal axis (23, 24) and has not been measured along the range axis, compared with active echolocation (25). In addition, studies of other animal species, such as dolphins and birds, suggest that acoustic signals may aid in determining the relative distance between individuals (26, 27), but this has not been previously studied in bats. Even man-made radar systems, such as multistatic radar, passive radar and passively guided missiles, monitor signals generated by other sources to localize targets, suggesting that echolocating bats may do the same. Our finding that the bat could orient without producing echolocation calls suggests the possibility that vocalizations from other bats may convey spatial information to a silent animal about the position of obstacles in the dark. Moreover, our data shows that around 70% of silent times came from the trailing individual in following flight. This implies it may have used the leading bat's echolocation calls for spatial orientation.
A few studies with animals that are capable of active sensing have reported the use of passive sensing for stealth strategies, object discrimination, prey localization, and orientation when encountering conspecifics. For example, a subordinate electric fish ceases its electric organ discharge for longer than 0.2 s when passing by the territory of a dominant conspecific (28–31). Dolphins and whales can passively listen to echolocation signals of their companions to navigate and discriminate different objects (32–34). Gleaning bats, which take prey from substrate, can passively listen to prey-generated sound to localize their prey (35). Although it has been observed that active-sensing animals are capable of using passive sensing, little is known about whether aerial hawking bats, which rely heavily on echolocation for prey capture and navigation most of the time, can also use passive sensing for the same purposes.
Gleaning pallid bats, which echolocate but also rely on passive listening to prey-generated sounds for foraging, cannot process sonar and prey-generated sounds concurrently (36). Therefore, when listening to prey-generated sounds, a gleaning bat stops producing sonar vocalizations (35). Although the big brown bat is not known to rely on passive listening to find its prey, it may experience difficulty in simultaneously processing 2 auditory streams of information, similar to that reported for the pallid bat. When the disadvantage of echolocation outweighs the advantage, a bat may choose to cease echolocation and process only one auditory stream from conspecific-generated sounds for orientation.
The bat that caught the mealworm in each trial of this study stopped its vocalization less often (2/158 trials) than the other one (17/158 trials) in the 0.5 s before producing the feeding buzz, which suggests that echolocation is essential for prey localization. Feeding buzzes were recorded in all insect capture trials, indicating that the bat requires this series of self-generated calls with high repetition rate to accurately localize its prey. The bat might roughly localize the position of its prey by listening to conspecific-generated echoes, but more precise localization of the target is required for interception.
Neurophysiological recordings from the midbrain of the bat suggest that separate populations of neurons may be specialized for passive listening to acoustic signals in the environment and active listening to sonar echoes. Tonic and chopper neurons in the inferior colliculus of the little brown bat (Myotis lucifugus), with long integration times, long latencies, and robust responses to sinusoidal amplitude modulation, exhibit response characteristics that would be best suited for processing sounds generated in the environment; whereas onset neurons, with relatively short and stable response latencies, high best frequencies, and poor responses to sinusoidal amplitude modulation, would be best suited for processing self-generated echolocation signals and sonar returns (37).
Two populations of neurons have been identified in the superior colliculus of the big brown bat (E. fuscus), one that responds selectively to acoustic stimulation at a particular azimuth and elevation (2D neurons) and one that responds selectively to acoustic stimulation over restricted azimuth, elevation, and distance (3D neurons) (38). The population of 2D neurons responds to single frequency-modulated (FM) signals and would be well suited for passive localization of sound sources but not for precise distance measurements required for prey capture. The population of 3D neurons is selectively activated by a high amplitude FM sweep (pulse) followed by a weaker FM sweep (echo) and responds to pulse-echo pairs over a limited delay window. It is believed that echo-delay-tuned neurons are important for target ranging in bats (39–41), and activity of echo-delay-tuned neurons in the bat superior colliculus also depends on the azimuth and elevation of stimulation. The population of 3D neurons in the bat superior colliculus therefore exhibits response characteristics that could support active localization by echolocation. We hypothesize that environmental sounds, including vocalizations from other bats, evoke activity in 2D neurons, and echolocation evokes activity in 3D neurons. Two-dimensional localization is sufficient for estimates about the azimuth and elevation of a sound source (e.g., the position of another vocalizing bat), but precise 3D localization is required for prey capture.
This paper reports a newly discovered silence strategy used by echolocating bats to avoid call interference. Additionally, the silent bat can potentially trace another bat's position by passively listening to the other bat's vocalizations and resulting echoes. The silence strategy provides new perspectives on swarming and chasing behavior, as well as navigation and communication, in a variety of animals.
Materials and Methods
Animals.
Eight big brown bats, E. fuscus, were used in this study and formed 7 pairs. Five bats were tested in pairings with more than one individual bat. Bats were collected in Maryland and housed in a colony room at the University of Maryland, College Park. All experiments were approved by the University of Maryland's Institutional Animal Care and Use Committee.
Behavioral Experiments.
Experiments were run between June 2005 and August 2006. All bats were trained first in a large flight room (7 × 6 × 2.5 m) to catch tethered mealworms. Baseline (one bat alone) and two-bat data (paired bats competing for a single tethered prey item) were collected on 4 different days per pair. We analyzed 30 baseline trials/bat and 20 two-bat trials/pair. Light in the room was long-wavelength filtered (>650 nm), to prevent the bat from using visual cues for orientation and prey capture (20).
Data Recording.
Audio recordings were acquired by 3 ultrasound sensitive microphones (UltraSound Advice) on the floor, amplified (UltraSound Advice) and stored digitally (Wavebook, IOTech), sampled at 250 kHz/channel) and a 16 microphone array positioned on 3 walls of the room (8). Video recordings were taken by 2 high-speed cameras (Kodak MotionCorder Analyzer, Model 1000, 240 frames/s) mounted in 2 corners of the room, permitting off-line 3D reconstruction of the bats' flight paths. Audio and video recordings were simultaneously end-triggered after one bat made contact with the prey, and the preceding 8 s of data were stored. Audio and video data were later analyzed by 2 custom MATLAB programs.
Call Assignment.
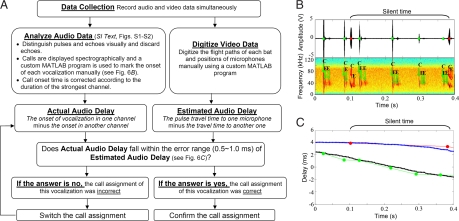
The assignment of sonar calls to individual bats flying in pairs was accomplished by following the steps outlined in Fig. 6. Briefly, 3 microphones (separated by 2 to 3 m) ensured pick-up of all sonar calls, and at least one microphone was always ≤2 m from each bat flying in the video-calibrated space. Time waveforms and spectrograms of the audio recordings from the 3 separate channels were examined to select 2 channels with good S:N. Spectrographic displays of signals in the selected channels were marked manually by the investigator using a custom MATLAB program, and signals in the unselected channel were also examined to ensure that no vocalizations were missed. The actual audio delay of each echolocation call was determined by computing the temporal offset of call onset times in 2 different channels from spatially separated microphones. Call duration recorded from the strongest channel was used to calculate onset of vocalization in the 2 other weaker channels (SI Text). Position data of each bat and the microphones were digitized (accuracy approximately 1.5 cm) by another custom MATLAB program and used for frame-by-frame measurement of 3D inter-bat separation and bat distances to the microphones. The estimated audio delay was the difference between the pulse travel times to 2 selected microphones, computed from sound travel time in air (346.65 m/s) and bat position data. The actual and estimated audio delays should be equal (within 0.5 to ≈1 ms margin of error) if the association between calls and bats was correct. We included the third microphone and the 16 microphone array to ensure detection of all sonar calls and to increase the reliability of assigning each call to the vocalizing bat. Details of call assignment, correction for onset of vocalizations and several trial examples are presented in SI Text and Figs. S1–S3. An animation of a selected trial is available at Movie S1.
Fig. 6.
A flow chart illustrating how each call in a stream was assigned to the vocalizing bat in a pair and an example of call assignment to different bats. (A) Steps for analyzing video and audio recordings. (B) Manual call assignment in one of the 3 channels. Letters “C” and “E” refer to sonar call and echo, respectively. Red (Bat A) and green (Bat B) dots in B and C mark vocalizations that belong to each of the 2 bats. (C) Comparison between actual and estimated audio delays. Blue (Bat A) and black (Bat B) lines in C are estimated audio delays, while red (Bat A) and green (Bat B) dots are actual audio delays. Note that estimated and actual delays for the 2 bats' vocalizations coincide.
Supplementary Material
Acknowledgments.
We thank Amaya Perez and Kari Titcher for help with the experiment and data analysis and Murat Aytekin, Ben Falk, Kaushik Ghose, Houng Li, Susanne Sterbing-D'Angelo, Hans-Ulrich Schnitzler, Nobuo Suga, Genevieve S. Wright, and an anonymous reviewer for helpful comments on this manuscript. This research was funded by NSF Grant IBN-0111973, NIH Grants R01-MH056366 and R01-EB004750 to CFM and the William Hodos dissertation fellowship and Ann G. Wiley dissertation fellowship (to C.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804408105/DCSupplemental.
References
- 1.Nelson ME, MacIver MA. Sensory acquisition in active sensing systems. J Comp Physiol A. 2006;192:573–586. doi: 10.1007/s00359-006-0099-4. [DOI] [PubMed] [Google Scholar]
- 2.Thomas JA, Moss CF, Vater M, editors. Echolocation in Bats and Dolphins. Chicago: Univ of Chicago Press; 2004. [Google Scholar]
- 3.Heiligenberg W. Neural Nets in Electric Fish. MA: MIT Press Cambridge; 1991. [Google Scholar]
- 4.Bates ME, Stamper SA, Simmons JA. Jamming avoidance response of big brown bats in target detection. J Exp Biol. 2008;211:106–113. doi: 10.1242/jeb.009688. [DOI] [PubMed] [Google Scholar]
- 5.Obrist MK. Flexible bat echolocation: The influence of individual, habitat and conspecifics on sonar signal design. Behav Ecol Sociobiol. 1995;36:207–219. [Google Scholar]
- 6.Ulanovsky N, Fenton MB, Tsoar A, Korine C. Dynamics of jamming avoidance in echolocating bats. Proc R Soc Lond B. 2004;271:1467–1475. doi: 10.1098/rspb.2004.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence BD, Simmons JA. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. J Acoust Soc Am. 1982;71:585–590. doi: 10.1121/1.387529. [DOI] [PubMed] [Google Scholar]
- 8.Ghose K, Moss CF. The sonar beam pattern of a flying bat as it tracks moving and stationary prey. J Acoust Soc Am. 2003;114:1120–1131. doi: 10.1121/1.1589754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartley DJ, Suthers RA. The sound emission pattern of the echolocating bat, Eptesicus fuscus. J Acoust Soc Am. 1989;85:1348–1351. doi: 10.1121/1.395684. [DOI] [PubMed] [Google Scholar]
- 10.Shimozawa T, Suga N, Hendler P, Schuetze S. Directional sensitivity of echolocation system in bats producing frequency-modulated signals. J Exp Biol. 1974;60:53–69. doi: 10.1242/jeb.60.1.53. [DOI] [PubMed] [Google Scholar]
- 11.Aytekin M, Grassi E, Sahota M, Moss CF. The bat head-related transfer function reveals binaural cues for sound localization in azimuth and elevation. J Acoust Soc Am. 2004;116:3594–3605. doi: 10.1121/1.1811412. [DOI] [PubMed] [Google Scholar]
- 12.Masters WM, Raver KAS, Kazial KA. Sonar signals of big brown bats, Eptesicus fuscus, contain information about individual identity, age and family affiliation. Anim Behav. 1995;50:1243–1260. [Google Scholar]
- 13.Masters WM, Jacobs SC, Simmons JA. The structure of echolocation sounds used by the big brown bat. Eptesicus fuscus: Some consequences for echo processing. J Acoust Soc Am. 1991;89:1402–1413. [Google Scholar]
- 14.Masters WM, Raver KAS. The degradation of distance discrimination in big brown bats (Eptesicus fuscus) caused by different interference signals. J Comp Physiol A. 1996;179:703–713. doi: 10.1007/BF00216134. [DOI] [PubMed] [Google Scholar]
- 15.Ficken RW, Ficken MS, Hailman JP. Temporal pattern shifts to avoid acoustic interference in singing birds. Science. 1974;183:762–763. doi: 10.1126/science.183.4126.762. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz JJ. Male calling behavior, female discrimination and acoustic interference in the Neotropical treefrog Hyla microcephala under realistic acoustic conditions. Behav Ecol Sociobiol. 1993;32:401–414. [Google Scholar]
- 17.Jensen ME, Moss CF, Surlykke A. Echolocating bats can use acoustic landmarks for spatial orientation. J Exp Biol. 2005;208:4399–4410. doi: 10.1242/jeb.01901. [DOI] [PubMed] [Google Scholar]
- 18.Mueller HC, Mueller NS. Sensory basis for spatial memory in bats. J Mamm. 1979;60:198–201. [Google Scholar]
- 19.Schnitzler HU, Moss CF, Denzinger A. From spatial orientation to food acquisition in echolocating bats. Trends Ecol Evol. 2003;18:386–394. [Google Scholar]
- 20.Hope GM, Bhatnagar KP. Electrical response of bat retina to spectral stimulation: Comparison of four microchiropteran species. Experientia. 1979;35:1189–1191. doi: 10.1007/BF01963279. [DOI] [PubMed] [Google Scholar]
- 21.Kuc R. Object localization from acoustic emissions produced by other sonars. J Acoust Soc Am. 2002;112:1753–1755. doi: 10.1121/1.1508792. [DOI] [PubMed] [Google Scholar]
- 22.Simmons JA, et al. Video/acoustic-array studies of swarming by echolocating bats. J Acoust Soc Am. 2004;116:2632. [Google Scholar]
- 23.Koay G, Kearns D, Heffner HE, Heffner RS. Passive sound-localization ability of the big brown bat (Eptesicus fuscus) Hear Res. 1998;119:37–48. doi: 10.1016/s0378-5955(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 24.Koay G, Heffner HE, Heffner RS. Audiogram of the big brown bat (Eptesicus fuscus) Hear Res. 1997;105:202–210. doi: 10.1016/s0378-5955(96)00208-0. [DOI] [PubMed] [Google Scholar]
- 25.Simmons JA. The resolution of target range by echolocating bats. J Acoust Soc Am. 1973;54:157–173. doi: 10.1121/1.1913559. [DOI] [PubMed] [Google Scholar]
- 26.Holland J, Dabelsteen T, Pedersen SB. Degradation of wren Troglodytes troglodytes song: Implications for information transfer and ranging. J Acoust Soc Am. 1998;103:2154–2502. [Google Scholar]
- 27.Mercado E, III, et al. Acoustic cues available for ranging by humpback whales. J Acoust Soc Am. 2007;121:2499–2502. doi: 10.1121/1.2717495. [DOI] [PubMed] [Google Scholar]
- 28.Hopkins CD. In: Electroreception. Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. New York: Springer-Verlag; 2005. pp. 264–289. [Google Scholar]
- 29.Moller P, Serrier J, Bowling D. Electric organ discharge displays during social encounter in the weakly electric fish Brienomyrus niger L (Mormyridae) Ethology. 1989;82:177–191. [PubMed] [Google Scholar]
- 30.Scheffel A, Kramer B. Electrocommunication and social behaviour in Marcusenius senegalensis (Mormyridae, Teleostei) Ethology. 1997;103:404–420. [Google Scholar]
- 31.Werneyer M, Kramer B. Intraspecific agonistic interactions in freely swimming mormyrid fish, Marcusenius macrolepidotus (South African form) J Ethol. 2002;20:107–121. [Google Scholar]
- 32.Gotz T, Verfuβ UK, Schnitzler HU. “Eavesdropping” in wild rough-toothed dolphins (Steno bredanensis)? Biol Lett. 2006;2:6–7. doi: 10.1098/rsbl.2005.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammers MO, Au WW. Directionality in the whistles of Hawaiian spinner dolphins (Stenella longirostris): A signal feature to cue direction of movement? Mar Mamm Sci. 2003;19:249–264. [Google Scholar]
- 34.Xitco MJ, Jr, Roitblat HL. Object recognition through eavesdropping: passive echolocation in bottlenose dolphins. Anim Learn Behav. 1996;24:355–365. [Google Scholar]
- 35.Russo D, Jones G, Arlettaz R. Echolocation and passive listening by foraging mouse-eared bats Myotis myotis and M blythii. J Exp Biol. 2007;210:166–176. doi: 10.1242/jeb.02644. [DOI] [PubMed] [Google Scholar]
- 36.Barber JR, Razak KA, Fuzessery ZM. Can two streams of auditory information be processed simultaneously? Evidence from the gleaning bat Antrozous pallidus. J Comp Phsyiol A. 2003;189:843–855. doi: 10.1007/s00359-003-0463-6. [DOI] [PubMed] [Google Scholar]
- 37.Condon CJ, White KR, Feng AS. Neurons with different temporal firing patterns in the inferior colliculus of the little brown bat differentially process sinusoidal amplitude-modulated signals. J Comp Physiol A. 1996;178:147–157. doi: 10.1007/BF00188158. [DOI] [PubMed] [Google Scholar]
- 38.Valentine DE, Moss CF. Spatially selective auditory responses in the superior colliculus of the echolocating bat. J Neurosci. 1997;17:1720–1733. doi: 10.1523/JNEUROSCI.17-05-01720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dear SP, Fritz J, Haresign T, Ferragamo M, Simmons JA. Tonotopic and functional organization in the auditory cortex of the big brown bat, Eptesicus fuscus. J Neurophysiol. 1993a;70:1051–1070. doi: 10.1152/jn.1993.70.5.1988. [DOI] [PubMed] [Google Scholar]
- 40.Dear SP, Simmons JA, Fritz J. A possible neuronal basis for representation of acoustic scenes in auditory cortex of the big brown bat. Nature. 1993b;364:620–623. doi: 10.1038/364620a0. [DOI] [PubMed] [Google Scholar]
- 41.Suga N, O'Neill WE. Neural axis representing target range in the auditory cortex of the mustache bat. Science. 1979;206:351–353. doi: 10.1126/science.482944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.