Abstract
GABAergic synapses are crucial for brain function, but the mechanisms underlying inhibitory synaptogenesis are unclear. Here, we show that postnatal Purkinje cells (PCs) of GABAAα1 knockout (KO) mice express transiently the α3 subunit, leading to the assembly of functional GABAA receptors and initial normal formation of inhibitory synapses, that are retained until adulthood. Subsequently, down-regulation of the α3 subunit causes a complete loss of GABAergic postsynaptic currents, resulting in a decreased rate of inhibitory synaptogenesis and formation of mismatched synapses between GABAergic axons and PC spines. Notably, the postsynaptic adhesion molecule neuroligin-2 (NL2) is correctly targeted to inhibitory synapses lacking GABAA receptors and the scaffold molecule gephyrin, but is absent from mismatched synapses, despite innervation by GABAergic axons. Our data indicate that GABAA receptors are dispensable for synapse formation and maintenance and for targeting NL2 to inhibitory synapses. However, GABAergic signaling appears to be crucial for activity-dependent regulation of synapse density during neuronal maturation.
Keywords: gephyrin, Purkinje cell, synaptogenesis
The identification of the factors that are critical for the assembly, maturation, and stabilization of synaptic connections remains a central puzzle in developmental neuroscience. Studies on cultured neurons have been invaluable for uncovering the molecular mechanisms of synaptogenesis (1, 2). Unfortunately, such studies can be influenced by varying experimental conditions, and their results often have not been replicated by in vivo analyses of gene knockout (KO) mice (3). As an alternative approach, gene deletion techniques allow investigating the in vivo function of genes that are believed to be critical for synapse formation (4–8). However, the premature death of newborn mutant mice has often prevented long-term analyses of synapse maturation and function. In the present study, we took advantage of a KO model that survives up to adulthood without a major phenotype, despite extensive loss of GABA-mediated function in specific populations of neurons.
Specifically, we investigated the in vivo effects of a selective silencing of GABAergic transmission on the formation and long-term stability of GABAergic synapses, which provide the major inhibitory control over neuronal activity in the brain (9). By analogy to glutamatergic synapses, assembly of GABAergic synapses is believed to involve selective trans-synaptic interactions between adhesion molecules and cytosolic interactions with scaffolding proteins (2). Neuroligin-2 (NL2) is a postsynaptic adhesion molecule that localizes at GABAergic synapses and triggers synapse formation by interacting with presynaptic neurexins (10, 11). NL2 belongs to a family of related proteins also comprising NL1, NL3, and NL4 (12). Importantly, NL1 is targeted to glutamatergic synapses (13, 14), suggesting that different NLs may play an important role in specifying distinct types of synapse and in determining a balance between neuronal excitation and inhibition (15, 16). The mechanisms responsible for the differential targeting of NL1 to glutamatergic synapses and NL2 to GABAergic synapses are unclear, although there is evidence that such specificity could arise either from intracellular interactions with postsynaptic scaffolds (15) or from selective extracellular binding of NL splice variants with neurexin isoforms (17, 18). However, the binding partners of NL2 at GABA synapses are not known.
The present study had two major goals. First, we wanted to assess in vivo whether the localization of NL2 at GABAergic synapses depends on GABAA receptors and on the integrity of the postsynaptic apparatus, a major component of which is the scaffolding molecule gephyrin (19). Second, we aimed to understand to what extent synapse formation requires GABA-mediated neurotransmission. To address these issues, we exploited the fact that cerebellar Purkinje cells (PCs) of GABAA receptor α1 subunit (GABAAα1) KO mice lack postsynaptic GABAergic activity (20, 21). We have shown previously that in PCs of GABAAα1 KO mice perisomatic synapses are present in normal numbers, whereas dendritic synapses are strongly reduced, and GABAergic axons make mismatched synapses with dendritic spines (21). Here, we demonstrate that NL2 clusters at synapses lacking GABAA receptors and gephyrin, but does not occur at mismatched synapses. In addition, we provide evidence that signaling through GABAA receptors plays an important role in regulating synapse number during postnatal synaptogenesis.
Results
Synaptic Localization of NL2 Is Independent of GABAA Receptors.
In PCs of wild-type (WT) mice, immunolabeling for NL2 had a punctate pattern that overlapped precisely with that of GABAAγ2 (Fig. 1A1 and A2). In GABAAα1 KO mice, there were no γ2-positive clusters on PCs (Fig. 1B1), reflecting the absence of GABAA receptors in these neurons (20, 21). In contrast, the labeling pattern for NL2 at perisomatic synapses was indistinguishable from that seen in WT animals (Fig. 1B2; mean ± SEM puncta/100 μm of somatic membrane: WT, 35.3 ± 1.3, n = 30 cells; KO, 33.3 ± 1.6, n = 36 cells; unpaired t test, P = 0.3298). Moreover, NL2 puncta were opposed to GAD65-positive boutons (Fig. 1C), and preembedding electron microscopy revealed the selective presence of NL2-immunoreactivity at postsynaptic specializations (Fig. 1D).
Fig. 1.
NL2 clusters at inhibitory synapses in the absence of GABAA receptors. (A1 and A2) NL2 and GABAAγ2 colocalize precisely at perisomatic synapses in WT PCs. (B1 and B2) In GABAAα1 KO, NL2 puncta outline PCs despite the complete absence of GABAA receptors. (C) Triple immunofluorescence shows that NL2 (red) clusters opposite GAD65-positive boutons (green) on a Car8-positive PC (blue) from a mutant mouse. (D) ImmunoGold labeling for NL2 at a perisomatic synapse (arrowhead) of a P30 mutant mouse. (Scale bars: A–C, 10 μm; D, 200 nM).
In the molecular layer (ML) of GABAAα1 KO mice, there was a strong reduction in the density of postsynaptic aggregates containing GABAA receptors and gephyrin [Fig. 2 and supporting information (SI) Table S1], in line with previous observations (22). The remaining receptors contained both GABAAγ2 (Fig. 2B) and GABAAα3 (Fig. S1), and likely were located at synapses between interneurons (22 and data not shown). In contrast, NL2 was affected less severely (Table S1) and, as a consequence, many NL2-positive puncta did not colocalize with GABAA receptors or with gephyrin (Fig. 2B). Immunolabeling with carbonic anhydrase VIII (Car8), a marker of PCs (SI Materials and Methods and Fig. S2), revealed that such puncta were distributed along PC dendrites (Fig. 2C). Moreover, triple-labeling with GAD65 demonstrated that NL2-positive puncta were associated with inhibitory synapses (Fig. 2E), and this was confirmed by electron microscopy (Fig. 2 F and G). Notably, NL2 colocalized with dystroglycan at postsynaptic sites lacking GABAA receptors (Fig. 2D and Fig. S3), whereas gephyrin was found only in a small proportion of such synapses (Fig. 2C and Table S1). These findings indicate that the postsynaptic localization of NL2 in PCs does not require GABAA receptors or gephyrin.
Fig. 2.
Reduced GABAergic innervation of PC dendrites in GABAAα1 KO mice and absence of NL2 from heterologous synapses. (A and B) Confocal images of the iML of WT (A) and GABAAα1 KO mice (B) after triple labeling for NL2, gephyrin and GABAAγ2. Note in WT that NL2 is precisely colocalized with gephyrin and GABAA receptors (triple labeling results in white puncta). In contrast, in mutant numerous puncta are immunopositive for NL2, but lack gephyrin and GABAA receptors. Occasionally, gephyrin clusters are positive for NL2 but not for GABAAγ2 (arrowheads). (C and D) Punctate distribution of NL2 along the dendrites of Car8-positive PCs in GABAAα1 KO. Note that gephyrin is found only seldom at NL2-positive sites (C, arrowheads), whereas α-dystroglycan colocalizes extensively with NL2 (D). (E) NL2 clusters in front of GAD65-positive boutons along PC dendrites of GABAAα1 KO. (F and G) In GABAAα1 KO mice, NL2 localizes at symmetric synapses (arrowheads) on the dendrites of both PCs (F) and interneurons (G). (H) Representative images of PC dendritic segments of the eML and iML after double labeling for Car8 and NL2 in WT and GABAAα1 KO mice. (I) Quantitative analysis showing the reduced density of NL2 clusters in PC dendrites of GABAAα1 KOs (gray) compared with WT (white). Error bars, SEM. (**, P < 0.0001, Mann–Whitney test; n ranges from 67 to 92 dendritic segments). (J) The eML/iML ratio of NL2 cluster density is significantly lower in GABAAα1 KO compared with WT. Standard deviations of ratio values (error bars) were determined with the error propagation formula (*, P < 0.001, unpaired t test). (K) GAD65-positive boutons (blue) establish heterologous contacts with dendritic spines of PCs (red labeling for mGluR1α). NL2 (green) clusters opposite GABAergic axons (arrowheads), but is not present in spines. (L) NL2 (green) localizes at the site of contact (arrowhead) between a GAD65-positive bouton (blue) and a Car8-labeled PC dendrite (red). Several spines, which appear “embedded” in the GAD bouton, do not show any labeling for NL2. (Scale bars: A–D, 6 μm; E, 4 μm; F and G, 200 nm; K and L, 2 μm).
In GABAAα1 KO mice, synapses containing NL2 but no GABAA receptors were prevalent in the internal part of the ML (iML) and, conversely, synapses containing both NL2 and α3-GABAA receptors were more abundant in the external part of the ML (eML; Fig. S1 and Table S1). To assess whether this reflected an uneven distribution of inhibitory synapses along the dendritic arborization of PCs, we calculated the density of NL2 clusters along Car8-positive dendrites (Fig. 2H). In both genotypes, cluster density was significantly higher in dendritic segments of the iML compared with the eML (Fig. 2I), but this difference was considerably more pronounced in mutant mice (Fig. 2J). Accordingly, in GABAAα1 KO mice NL2 clusters on PC dendrites were reduced by 35.6% in the iML and by 52% in the eML. Importantly, labeling for Car8 did not disclose any obvious alteration in the general morphology of PC dendrites (Fig. 4H and data not shown), and the thickness of the ML was identical in WT and α1 KO (164.9 ± 4.9 and 163.7 ± 3 μm, respectively; mean ± SEM, n = 17 and 26 from four mice; unpaired t test, P = 0.8312). These data indicate that the loss of GABAA receptors from PCs causes a reduction in the number of GABAergic synapses and that the residual synapses are distributed according to an inside-out gradient along PC dendrites.
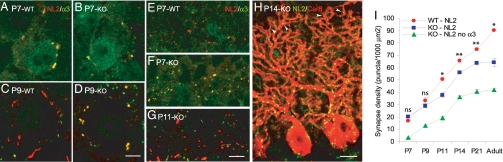
Fig. 4.
Loss of GABAA receptors reduces inhibitory synaptogenesis in the ML. (A and B) P7: double labeling for NL2 and GABAAα3 reveals the presence of α3-GABAA receptors in PCs of WT (A) and GABAAα1 KO (B). (C and D) P9: immunoreactivity for GABAAα3 is still visible at perisomatic synapses in GABAAα1 KO (D), but is completely absent from WT PCs (C). (E–G) Double labeling for NL2 and GABAAα3 in the iML. At P7, synapse density is similar in WT (E) and GABAAα1 KO (F), and virtually all synapses in the mutant are immunopositive for GABAAα3. At P11 (G), most synapses in the iML of mutant mice lack α3-GABAA receptors. (H) Double labeling shows the distribution of NL2 puncta on Car8-positive PCs of a P14 GABAAα1 KO mouse. Note that NL2-positive synapses are distributed along the entire dendritic arborization of PCs, reaching the most apical dendritic compartments (arrowheads). These distal dendrites are formed after P11, when PCs lose GABAA receptors. (I) Synapse density was calculated in the iML, just above the PCL. The density of NL2-positive puncta increases progressively from P7 to adult in both WT and KO. However, starting from P11 synapse density is lower in KO compared with WT littermates (two-way ANOVA, *, P < 0.05; **, P < 0.001). Note that the density of synapses expressing NL2 but no α3-GABAA receptors also increases significantly from P11 to adult (two-way ANOVA, P < 0.001). (Scale bars: A–D, 5 μm; E–G, 7 μm; H, 15 μm).
NL2 Is Not Present at Mismatched Synapses with PC Spines.
We next asked whether innervation by GABAergic axons is sufficient for recruiting NL2 at mismatched synapses on PC spines, which are characterized by a postsynaptic density typical of excitatory synapses and contain δ2 glutamate receptors (21). We performed triple labeling for NL2, GAD65, and the metabotropic glutamate receptor mGluR1α, which is selectively localized in PC spines (23). Some of the GAD65-positive boutons were unusually large in GABAAα1 KO mice and made contacts with multiple spines, as shown by electron microscopy (21). However, immunolabeling for NL2 was not associated with the spines of PCs (Fig. 2K). Similarly, in sections triple labeled for NL2, GAD65, and Car8, NL2 aggregated selectively at contact sites on the dendritic shaft of PCs, but not in spines contacting GAD65 boutons (Fig. 2L). In agreement with this observation, we did not detect NL2 immunoreactivity at mismatched synapses with preembedding electron microscopy (data not shown). We conclude that in vivo GABAergic axons face NL2 only when they make synapses with the appropriate postsynaptic targets.
Purkinje Cells of GABAAα1 KO Mice Express Transiently GABAA Receptors During Development.
During the first postnatal week, GABAergic axons first target the cell body of PCs, and then make synapses with their dendrites as the latter grow toward the pial surface until the end of the third postnatal week (24). Therefore, we hypothesized that a developmentally regulated process could be responsible for the inside-out gradient in the distribution of GABAergic synapses along PC dendrites in GABAAα1 mutants. Although adult PCs express exclusively GABAAα1 (25), PCs have been reported to express also α3 subunit mRNA in the first postnatal week (26). This led us to postulate that PCs of GABAAα1 KO mice could express transiently α3-GABAA receptors during early postnatal development and that this could be responsible for the differences in synapse density in PC dendrites of the eML compared with those located closer to the cell body.
To test this hypothesis, we made voltage-clamp recordings from PCs located in lobule V of the vermis. At P7 (Fig. 3A), spontaneous inhibitory postsynaptic currents (sIPSCs) recorded in the presence of 1 mM kynurenic acid were observed in PCs of both WT (n = 11 cells) and GABAAα1 KO mice (n = 17 cells), although they occurred at a lower frequency in mutant animals (3.67 ± 1.18 Hz in WT, 1.26 ± 0.34 Hz in KO, mean ± SEM; Mann–Whitney test, P < 0.05). In all cells tested, these currents were abolished by the GABAA receptor antagonist SR-95531 (gabazine, 20 μM; Fig. 3A). The mean amplitude of miniature events (mIPSCs) recorded in the presence of 1 μM tetrodotoxin (TTX) was significantly smaller in GABAAα1 KO mice compared with WT littermates, whereas their decay time-constant was prolonged in PCs of mutants (Table S2). These findings are consistent with several other studies showing that loss of GABAAα1 results in a marked reduction of IPSC frequency and amplitude, and in a prolongation of postsynaptic responses (27–30).
Fig. 3.
Developmentally regulated loss of GABAA receptors in PCs of GABAAα1 KO mice. (A) Spontaneous GABAergic activity recorded in the presence of 1 mM kynurenic acid from WT and KO PCs at P7. Bath addition of 20 μM gabazine totally abolishes spontaneous activity in PCs of both genotypes. (B) Lack of spontaneous GABAergic activity in PCs from P13 GABAAα1 KO mice compared with WT. Exogenous application of 10 μM muscimol evokes a large inward current in WT PCs, but has no effect in KO (middle). Gabazine (20 μM) abolishes both miniature and agonist-evoked responses in WT PCs (bottom). No changes are detectable in the recording baseline.
In slices prepared from older mice (P11–P14), sIPSCs were present in all WT PCs (n = 7), but were absent from PCs (n = 12) of GABAAα1 KOs (Fig. 3B). Similarly, bath application of the GABAA receptor agonist muscimol (10 μM) evoked large postsynaptic currents in WT PCs, but had no effect in those of KO. Moreover, application of gabazine did not disclose any shift in the holding current, arguing against a tonic activation of GABAA receptors (Fig. 3B).
These findings were supported by immunocytochemistry. At P7, PCs of both WT and GABAAα1 KO mice expressed GABAAα3 (Fig. 4 A and B), although in WT animals many PCs were positive only for GABAAα1 (data not shown). By P9, no immunolabeling for GABAAα3 was detected in WT PCs (Fig. 4C), whereas in mutants the large majority of PCs remained immunopositive for this subunit (Fig. 4D). Thereafter, immunolabeling for GABAAα3 declined progressively, and was undetectable after P11. Thus, the combined electrophysiological and immunocytochemical analysis supports the conclusion that PCs of GABAAα1 KO mice express α3-GABAA receptors transiently during postnatal development.
Loss of GABAA Receptors Impairs Synapse Formation.
What is the contribution of GABAA receptors during synaptogenesis? To address this question, we monitored synapse density in the ML of GABAAα1 KO and WT mice throughout development. This analysis was done on confocal images taken in lobule V just above the PC layer. To verify that NL2 clusters at developing synapses, we initially analyzed postnatal WT mice and found that most NL2 puncta colocalized with GABAA receptors and gephyrin (Fig. S4 and Table S3). Notably, at all developmental stages practically all GABAA receptor clusters contained NL2. Moreover, in KO animals NL2 puncta lacking GABAA receptors were found opposite GAD65 boutons (Fig. S4). These data indicate that NL2 aggregates represent synaptic sites and that clustering of NL2 is one of the earliest detectable signs of synapse formation.
At P7, the density of NL2-positive puncta was similar in GABAAα1 KO and WT mice (Fig. 4 E, F, and I), and >90% of these postsynaptic sites were also immunopositive for GABAA receptors. Therefore, at the onset of synaptogenesis PCs express functional GABAA receptors, and synapses form at a normal density. Starting from P11, synapses containing NL2 but no GABAA receptors became evident in the ML of GABAAα1 KOs (Fig. 4G). In addition, the total density of GABAergic synapses became significantly lower compared with WT mice (Fig. 4I). Despite this, there was a progressive increase in synapse density in mutant mice from P11, when PCs lose inhibitory postsynaptic responses, to adulthood (two-way ANOVA, post-hoc Tukey test, F = 144.925, P < 0.001). Strikingly, the density of synapses lacking GABAA receptors also increased significantly during this period (two-way ANOVA, post-hoc Tukey test, P < 0.001). In addition, new synapses were formed on the apical portion of PC dendrites as the ML expanded toward the pial surface, as shown by double labeling for NL2 and Car8 at P14 (Fig. 4H). Gephyrin clusters were not visible in such nascent synapses, excluding the possibility that this scaffolding molecule is expressed transiently at synapses that lack GABAA receptors. These results indicate that PCs retain their synaptogenic potential even after the loss of GABAA receptors. Thus, absence of GABAA receptor-mediated neurotransmission reduces, but does not completely abolish, synapse formation in PCs.
Discussion
The present data demonstrate that the synapse-specific localization of NL2 in cerebellar PCs does not depend on the GABAA receptor-gephyrin complex. However, NL2 was not found at mismatched synapses on PC spines, suggesting that innervation by GABAergic axons may be necessary, but not sufficient, for recruiting NL2 at postsynaptic sites. Notably, loss of GABAA receptors during postnatal development resulted in a noticeable decrease in the density of inhibitory synapses, suggesting that synapse number is regulated by an activity-dependent mechanism during the period of synaptogenesis.
GABAergic Signaling Determines Correct Number of Inhibitory Synapses.
A key finding of the present study is that PCs express transiently α3-GABAA receptors at a time when they start receiving inhibitory synapses. Notably, expression of the α3 subunit was protracted in GABAAα1 KOs compared with WT littermates, a finding that may be explained by reduced turnover of α3-GABAA receptors in neurons lacking the α1 subunit. Similarly, it has been shown that ablation of the adult acetylcholine receptor ε subunit causes a retention of the fetal γ subunit at the neuromuscular junction (31). From a developmental perspective, the transient expression of the α3 subunit in PCs of GABAAα1 KO mice enabled us to assess which aspects of synapse development are strictly dependent on GABAA receptors. We show that inhibitory synapses form at a normal density during the first 10 days of postnatal development, when PCs display GABAergic activity. Subsequently, loss of GABAA receptors causes a slowdown in the formation of inhibitory synapses and the appearance of aberrant connections with PC spines (21). This temporal sequence of events results in a gradient in the distribution of GABAergic synapses on PC dendrites, with fewer synapses being formed on the apical dendritic domains.
Our results extend previous in vitro studies showing that activity blockade during the period of synaptogenesis selectively reduces the number of inhibitory synapses (32–34) by demonstrating that GABAA receptors are critical in regulating this process. This conclusion is also supported by RNA interference experiments, showing that disruption of postsynaptic GABAA receptor clusters leads to a nearly complete loss of GABAergic innervation (35, 36). However, our results differ from those on cultured neurons in that a substantial number of “silent” synapses are assembled in PCs lacking GABAA receptors. In particular, synapses on PC dendrites located in the eML, which represent 48% of those seen in WT (Fig. 2I), are formed after GABAA receptors have disappeared. Different lines of evidence suggest that such silent synapses are stable. First, the pattern of perisomatic innervation was indistinguishable in PCs of WT and GABAAα1 KO mice, as documented by immunolabeling for NL2 (Fig. 1) and electron microscopy (21). Second, synapse density was rather constant in both the iML and eML of different animals (Table S1 and data not shown), and, third, silent synapses have been observed so far in mice aged >7 months. Our findings are in agreement with those of a recent study (37) in which knockdown of GAD67 in cortical interneurons impaired inhibitory synapse formation during the peak period of synaptogenesis, but had little effect on the maintenance of full-grown synapses. We conclude that GABAA receptors are dispensable for synapse formation and maintenance, but they play an important role in regulating synapse number and in shaping the pattern of connectivity with the appropriate dendritic targets.
This conclusion is in conflict with the fact that inhibitory synaptogenesis was normal in the spinal cord of KO mice lacking the vesicular inhibitory amino acid transporter, Viaat (8). However, Viaat mutants die between E18.5 and birth, thus precluding the analysis of brain regions in which synaptogenesis occurs postnatally. In addition, nonvesicular release of GABA (38) could contribute to maintain a level of receptor activation sufficient for sustaining proper synapse development in Viaat KOs.
How do GABAA receptors regulate the morphogenesis of inhibitory synapses? We propose that GABAergic signaling may be required to stabilize transient axodendritic contacts into permanent synapses. According to this view, local activation of GABAA receptors may be the rate-limiting factor in determining whether a synaptic contact is stabilized or eliminated during development (39). Thus, under normal conditions active synapses are strengthened, whereas mismatched synapses on spines are removed. This competitive, activity-dependent process is disrupted in PCs of GABAAα1 KOs, leading to incomplete elimination of mismatched synapses and impaired formation of inhibitory synapses on the dendritic shaft. This model is consistent with the observation that mismatched synapses are normally present, albeit at low density, during cerebellar development (21).
The mechanisms linking GABAergic activity to synapse maturation remain to be determined. Signaling through GABAA receptors may result in the local release of trophic factors, such as BDNF, which promote the maturation of inhibitory synapses (32–34, 40–42), and/or act as protective signals that prevent synapse elimination (43). An attractive hypothesis is that immature synapses are stabilized by an activity-dependent process that requires the combined action of specific NL isoforms (16, 44). This idea is congruent with our present results, and with recent data showing impaired synaptic transmission and/or reduced inhibitory synapse density in NL2 KO mice (16) and α-neurexin triple-KO mice (7). Thus, the formation of proper number of inhibitory synapses may depend on combined GABAergic activity and transsynaptic NL/neurexin interactions.
Synaptic Clustering of NL2 Is Independent of GABAA Receptors and Gephyrin.
The selective localization of NL1 and NL2 at excitatory and inhibitory synapses, respectively (11, 13), has lead to the idea that these adhesion molecules may contribute to specify distinct types of synapse in an activity-dependent manner (15, 16). Two potential mechanisms have been proposed to explain the synapse-specific localization of NLs: (1) intracellular interactions with scaffolding molecules or other postsynaptic proteins may recruit different NL isoforms to glutamatergic or GABAergic synapses (15); (2) synapse specificity may arise from selective extracellular interactions between distinct NL-neurexin isoform pairs (17, 18). Our present data demonstrate that NL2 is not expressed ectopically in dendritic spines establishing mismatched synapses, indicating that innervation by GABAergic axons per se is not sufficient for recruiting NL2 at postsynaptic sites. Although it cannot be excluded that spines may represent a non permissive environment for NL2, this appears unlikely, because overexpression of PSD-95 in cultured neurons causes a shift in the distribution of NL2 from inhibitory to excitatory synapses (15). The present results argue against a simple model in which presynaptic input determines the type(s) of NLs which are expressed in the postsynaptic plasma membrane. More likely, our data are compatible with a model according to which neurons have an intrinsic capability of targeting NL2 to inhibitory synapses, and that such a mechanism is not dependent on synaptic activity.
One attractive idea is that NL2 organizes the GABAergic postsynaptic membrane via interactions with GABAA receptors (44). This is supported by previous analyses revealing impaired GABAA receptor clustering or decreased IPSC amplitudes in NL2 KO and NL1–3 triple KO mice (6, 16). Our results do not exclude the existence of functional interactions between NL2 and GABAA receptors, however they clearly demonstrate that in PCs the synaptic localization of NL2 does not depend on GABAA receptors. Similarly, gephyrin is not necessary for the stabilization of postsynaptic NL2 clusters. Given that NL2 accumulates at GABAergic synapses early during development (Fig. S4), our observations support the idea that clustering of NL2 is one of the very first steps in postsynaptic differentiation, and that this adhesion molecule may contribute to stabilize GABAA receptor clusters at developing synapses (6, 16). It should be noted that the synapses investigated in this study differ from several other types of GABAergic synapse in that they express a molecular complex containing dystroglycan and dystrophin (Fig. S3). We have shown that dystroglycan colocalizes with NL2 at postsynaptic sites lacking GABAA receptors (Fig. 2D and Fig. S3). Notably, it has been reported that dystroglycan binds to neurexin (45) and indirectly interacts with NL2 through the scaffold molecule S-SCAM (46). Although deletion of dystroglycan in cultured neurons does not affect inhibitory synaptogenesis (47), it might be informative to investigate the possible redundancy of transneuronal signals mediated by the neurexin-dystroglycan and neurexin-NL adhesion systems at GABAergic synapses.
In conclusion, our results support the idea that inhibitory synaptogenesis is a multistep process which involves distinct cellular events. GABAA receptors are largely dispensable for initial synapse formation and for recruiting NL2 at postsynaptic sites. However, transmission mediated by GABAA receptors appears to be crucial for the activity-dependent stabilization of young synapses. By recruiting gephyrin and its interacting partners, GABAA receptors may also play a structural role and contribute to the assembly of the postsynaptic specialization.
Methods
Animals.
GABAAα1 KO mice have been generated on a mixed C57BL/6J-129/Sv/SvJ genetic background (27). The present analyses were performed on WT and homozygous KO mice derived from heterozygous breeding pairs at the University of Turin. The experiments were designed according to the European Community Council Directive 86/609/EEC for care and use of experimental animals and approved by the Bioethical Committee of Turin University.
Immunofluorescence and Confocal Microscopy.
Postnatal and adult (>P40) mice were anesthetized with intraperitoneal ketamine-xylazine 1:1 (0.1 ml/kg) and decapitated. The cerebellar vermis was cut in parasagittal slabs of ≈1 mm, which were fixed by immersion in ice-cold paraformaldehyde (4% in 0.1 M phosphate buffer, PB) for 20–30 min. Double and triple immunofluorescence was performed on cryostat sections as described in ref. 48. See SI Materials and Methods for antibody specification and characterization. For quantitative analyses, confocal images were segmented using a manual threshold and processed with the colocalization module of the Imaris software (Bitplane). Puncta were then quantified by a particle detection algorithm (National Institutes of Health Image J). Dendritic cluster density was determined by counting manually NL2 clusters contacting the smooth surface of PC dendritic profiles randomly selected in iML and eML.
Electron Microscopy.
Preembedding ImmunoGold was performed in WT and GABAAα1 KO mice using Fluoronanogold-Fab conjugated secondary antibodies. For details, see SI Materials and Methods.
Electrophysiology.
Whole-cell recordings were made from PCs located in lobule V of the cerebellar vermis at a holding voltage of −70 mV. Spontaneous and miniature events were analyzed with Mini Analysis (Synaptosoft). For details, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank G. Homanics (University of Pittsburgh, Pittsburgh) for providing GABAAα1 KO mice and M. Del Giudice (University of Torino, Torino, Italy) for help with statistics. This work was supported by Compagnia di San Paolo, Regione Piemonte (Ricerca Scientifica Applicata A218 and Ricerca Sanitaria Finalizzata 2006), and MIUR PRIN (to M.S.P.); Swiss National Science Foundation Grant 31-63901.00 (to J.M.F.); and a grant from the Cure Autism Now foundation (to F.V.). F.B. is the recipient of a fellowship from Fondazione CRT (Progetto Lagrange).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802390105/DCSupplemental.
References
- 1.Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 2.Craig AM, Graf ER, Linhoff MW. How to build a central synapse: Clues from cell culture. Trends Neurosci. 2006;29:8–20. doi: 10.1016/j.tins.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudanova I, Tabuchi K, Rohlmann A, Sudhof TC, Missler M. Deletion of alpha-neurexins does not cause a major impairment of axonal pathfinding or synapse formation. J Comp Neurol. 2007;502:261–274. doi: 10.1002/cne.21305. [DOI] [PubMed] [Google Scholar]
- 4.Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 5.Varoqueaux F, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci USA. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varoqueaux F, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Missler M, et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 8.Wojcik SM, et al. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 10.Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 12.Ichtchenko K, et al. Neuroligin 1: A splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 13.Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 15.Levinson JN, El-Husseini A. Building excitatory and inhibitory synapses: Balancing neuroligin partnerships. Neuron. 2005;48:171–174. doi: 10.1016/j.neuron.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Chubykin AA, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Graf ER, Kang Y, Hauner AM, Craig AM. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J Neurosci. 2006;26:4256–4265. doi: 10.1523/JNEUROSCI.1253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Kralic JE, et al. Genetic essential tremor in gamma-aminobutyric acidA receptor alpha1 subunit knockout mice. J Clin Invest. 2005;115:774–779. doi: 10.1172/JCI23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritschy JM, Panzanelli P, Kralic JE, Vogt KE, Sassoe-Pognetto M. Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAA receptors containing the alpha1 subunit in Purkinje cells. J Neurosci. 2006;26:3245–3255. doi: 10.1523/JNEUROSCI.5118-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kralic JE, et al. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J Comp Neurol. 2006;495:408–421. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka J, et al. Gq protein alpha subunits Galphaq and Galpha11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur J Neurosci. 2000;12:781–792. doi: 10.1046/j.1460-9568.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 24.Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- 25.Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takayama C, Inoue Y. Transient expression of GABAA receptor alpha2 and alpha3 subunits in differentiating cerebellar neurons. Brain Res Dev Brain Res. 2004;148:169–177. doi: 10.1016/j.devbrainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Vicini S, et al. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vicini S, Ortinski P. Genetic manipulations of GABAA receptor in mice make inhibition exciting. Pharmacol Ther. 2004;103:109–120. doi: 10.1016/j.pharmthera.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Lagier S, et al. GABAergic inhibition at dendrodendritic synapses tunes gamma oscillations in the olfactory bulb. Proc Natl Acad Sci USA. 2007;104:7259–7264. doi: 10.1073/pnas.0701846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein PA, et al. Prolongation of hippocampal miniature inhibitory postsynaptic currents in mice lacking the GABA(A) receptor alpha1 subunit. J Neurophysiol. 2002;88:3208–3217. doi: 10.1152/jn.00885.2001. [DOI] [PubMed] [Google Scholar]
- 31.Missias AC, et al. Deficient development and maintenance of postsynaptic specializations in mutant mice lacking an ‘adult’ acetylcholine receptor subunit. Development (Cambridge, UK) 1997;124:5075–5086. doi: 10.1242/dev.124.24.5075. [DOI] [PubMed] [Google Scholar]
- 32.Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seil FJ, Drake-Baumann R. TrkB receptor ligands promote activity-dependent inhibitory synaptogenesis. J Neurosci. 2000;20:5367–5373. doi: 10.1523/JNEUROSCI.20-14-05367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- 35.Li RW, et al. Disruption of postsynaptic GABA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J Neurochem. 2005;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- 36.Fang C, et al. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chattopadhyaya B, et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y, Wang W, Diez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 40.Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 41.Marty S, Wehrle R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 44.Huang ZJ, Scheiffele P. GABA and neuroligin signaling: Linking synaptic activity and adhesion in inhibitory synapse development. Curr Opin Neurobiol. 2008;18:1–7. doi: 10.1016/j.conb.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugita S, et al. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2002;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sumita K, et al. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J Neurochem. 2007;100:154–166. doi: 10.1111/j.1471-4159.2006.04170.x. [DOI] [PubMed] [Google Scholar]
- 47.Lévi S, et al. Dystroglycan is selectively associated with inhibitory GABAergic synapses but is dispensable for their differentiation. J Neurosci. 2002;22:4274–4285. doi: 10.1523/JNEUROSCI.22-11-04274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider Gasser EM, et al. Immunofluorescence in brain sections: Simultaneous detection of presynaptic and postsynaptic proteins in identified neurons. Nat Protoc. 2006;1:1887–1897. doi: 10.1038/nprot.2006.265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.