Abstract
In this study, electroencephalography (EEG) was used to examine the relationship between two leading hypotheses of cognitive aging, the inhibitory deficit and the processing speed hypothesis. We show that older adults exhibit a selective deficit in suppressing task-irrelevant information during visual working memory encoding, but only in the early stages of visual processing. Thus, the employment of suppressive mechanisms are not abolished with aging but rather delayed in time, revealing a decline in processing speed that is selective for the inhibition of irrelevant information. EEG spectral analysis of signals from frontal regions suggests that this results from excessive attention to distracting information early in the time course of viewing irrelevant stimuli. Subdividing the older population based on working memory performance revealed that impaired suppression of distracting information early in the visual processing stream is associated with poorer memory of task-relevant information. Thus, these data reconcile two cognitive aging hypotheses by revealing that an interaction of deficits in inhibition and processing speed contributes to age-related cognitive impairment.
Keywords: aging, working memory, inhibitory deficit, distraction, attention
Cognitive impairment associated with normal aging impacts multiple domains [e.g., attention, working memory (WM) and episodic memory (1)], prompting a search for underlying neural mechanisms that might account for such widespread deficits. Two of the leading cognitive aging hypotheses are the “processing speed hypothesis,” in which performance deficits are attributed to a decline in processing speed (2), and the “inhibitory deficit hypothesis,” which proposes that impairment in diverse cognitive abilities are the result of an inability to reduce interference from task-irrelevant information (3). Despite widespread behavioral evidence, physiological data characterizing the neural underpinnings of these age-related deficits, and notably the interactions between them, are limited.
A recent functional magnetic resonance imaging (fMRI) study supports the presence of an age-related top-down modulation deficit in inhibitory control (4). Top-down modulation is the neural process that underlies our ability to focus on relevant information and ignore irrelevant distractions via both the enhancement and suppression of sensory cortical activity (5, 6). The fMRI data revealed that, although older adults were able to enhance visual cortical activity for relevant information to the same extent as younger individuals, they were unable to adequately suppress activity associated with irrelevant information, and this suppression deficit correlated with their impaired WM performance (4).
The current study is directed at exploring the relationship between the inhibitory deficit and processing speed hypothesis, a goal that necessitates obtaining high temporal resolution neural data to dissect the time-course of age-related processing changes. Because of the vascular nature of the fMRI blood oxygen-dependent signal (BOLD) signal, neural events cannot be resolved on the time scale of milliseconds. Therefore, the previous fMRI study was unable to evaluate the precise timing of top-down modulation changes with age. Here, we study a group of younger (n = 20, 19–33 years of age) and older participants (n = 26, 60–72 years of age) engaged in the same selective WM paradigm while neural activity was recorded with 64-channel EEG to enable detailed temporal evaluation.
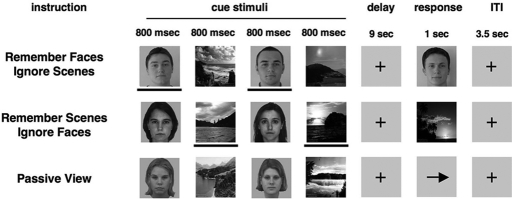
The cognitive paradigm consists of three tasks in which the presentation of visual information is balanced, whereas task demands are varied (Fig. 1) (6). During each trial, participants observe sequences of two faces and two natural scenes presented in a randomized order with the following instructions for the three tasks: (i) remember faces and ignore scenes, (ii) remember scenes and ignore faces, or (iii) passively view faces and scenes without attempting to remember them. In the two delayed-recognition WM tasks, visual processing of the stimuli during the cue period requires selective attention and thus permits the dissociation of distinct physiological measures of top-down enhancement and suppression of relevant and irrelevant stimuli relative to activity generated while passively viewing the stimuli.
Fig. 1.
Experimental paradigm. In the WM task response periods, a face or scene stimulus was presented corresponding to the relevant stimulus class, and participants were required to report with a button press whether the stimulus matched one of the previously presented stimuli. In the passive view response period, an arrow was presented, and participants were required to make a button press indicating the direction of the arrow. The lines below the stimuli are used to highlight task-relevance in this illustration and were not present in the actual task.
Previous aging EEG research has largely focused on changes in P300 parameters for relevant stimuli, revealing an increase in peak latency that suggested a generalized decline in processing speed with aging (7). A few studies have also assessed age-related changes in EEG measures for irrelevant stimuli and revealed hallmarks of inhibitory impairment (8–10). However, these studies did not evaluate the selectivity of age-related changes to suppression or the relationship between alterations in suppression and reductions in processing speed. We addressed this in the current study by examining measures of both top-down enhancement and suppression in younger and older adults at different time points during the period of stimulus encoding.
Results
Behavioral Measures.
Both recognition accuracy (hits + correct rejections/total possible items) and response times for the face and scene WM tasks were compared across age groups. The older adults exhibited a significantly reduced accuracy for both faces and scenes [faces: younger = 94.9% (SD = 3.6), older = 88.2% (SD = 10.3); P = 0.005; scenes: younger = 89.7% (SD = 5.1), older = 83.0% (SD = 9.1); P < 0.002]. The higher variance in the older age group suggested heterogeneity of the population and motivated the subgroup analysis described in the next section. There were no significant differences in response time across age groups [faces: younger = 1,192 ms (SD = 291 ms), older = 1,280 ms (SD = 294 ms); P > 0.05; accuracy for scenes: younger = 1,309 ms (SD = 298 ms), older = 1,362 ms (SD = 306 ms); P > 0.05].
Posterior EEG Measures.
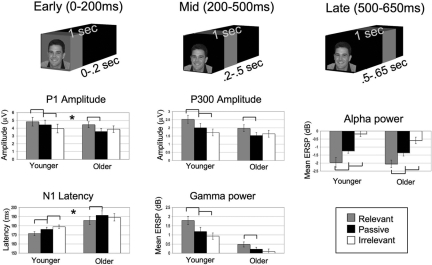
Five posterior EEG measures that have previously been shown to be modulated by attention and associated with visual processing were identified in the literature spanning the time frame that participants viewed the cue stimuli: P1 (50–150 ms) (11), N1 (120–220 ms) (6, 11), induced gamma synchronization (200–300 ms) (12), P300 (300–500 ms) (13), and induced alpha desynchronization (500–650 ms) (14). All data presented here are for EEG signals time-locked to the onset of the three types of face stimuli viewed during the cue period (“relevant,” “irrelevant,” and “passive”). Face stimuli were selected for this analysis, as opposed to scene stimuli, because of the presence of face-selective EEG measures at 100 ms (P1) (15) and 170 ms (N1) (16) after stimulus onset.
Our data analysis revealed significant overall top-down modulation independent of age (main effect of task) for all five EEG measures: P1 amplitude (F1,35 = 17.28, P < 0.001), N1 latency (F1,36 = 21.16, P < 0.001), gamma band synchronization (F1,37 = 24.51, P < 0.001), P300 amplitude (F1,37 = 12.7, P < 0.001), and alpha band desynchronization (F1,35 = 77.03, P < 0.001). Within-group t tests showed that younger participants exhibited both significant enhancement (relevant vs. passive) and suppression (passive vs. irrelevant) for all of these measures, such that there were three distinct levels for P1 amplitude, N1 latency, gamma power, P300 amplitude, and alpha power (all P values < .05) [Figs. 2 and 3, supporting information (SI) Fig. S1]. For these same comparisons, older participants exhibited only two distinct levels, such that there was significant enhancement but not suppression for each measure, except for the latest measure in the time-course, alpha desynchronization (500–650 ms), which displayed both significant enhancement and suppression (all P values < .05) and no between group difference (P > 0.05) (Figs. 2 and 3, Fig. S1). There were also significant task-independent latency increases with age (main effect of age) for N1 (F1,36 = 6.38, P < 0.05) and P300 (F1,32 = 4.6, P < 0.05), supporting reports in the literature of generalized slowing of processing speed with aging (7).
Fig. 2.
EEG data revealing an age-related deficit in top-down suppression in the earliest measures: P1 amplitude and N1 latency. All within-group t tests are designated as significant by brackets (P < 0.05). The asterisk denotes that only P1 amplitude and N1 latency revealed a significant age × task interaction plus a significant across-group suppression deficit. Error bars indicate standard error of the mean.
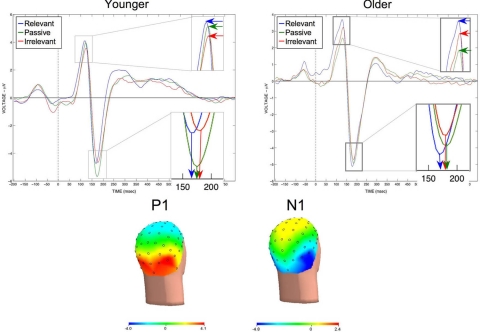
Fig. 3.
Grand averaged Event Related Potentials (ERPs) time-locked to the onset of the three types of face stimuli (relevant, passive, and irrelevant). (Left) Younger participant data shows the largest P1 amplitude and earliest N1 latency for the relevant faces, followed by passive faces and then irrelevant faces. (Right) Older participant data shows that the relationship of the P100 amplitude and N1 latency of the ERP for irrelevant faces compared with passive and relevant faces has shifted, such that there is a selective-suppression deficit (see Fig. 1 for quantitative results). Topographical voltage maps show the distribution of the P1 and N1 to extrastriate areas. Although it may seem counterintuitive that the N1 amplitude is largest for the passive view condition, this may be a result of face processing across trials being most consistent in the passive viewing task compared with the memory tasks (time-locked consistency across trials would lead to a larger ERP amplitude). Because enhancement and suppression were not significant in the younger subjects, the N170 amplitude was not used as a measure for across-age group comparisons.
Further evaluation of these measures uncovered a significant age × task interaction for P1 amplitude (F1,35 = 4.42, P < 0.05), N1 latency (F1,36 = 3.80, P < 0.05), and gamma synchronization (F1,37 = 3.57, P < 0.05). Across age-group t tests of modulation indices revealed a significant age-related, suppression deficit only in the earliest of these measures, P1 amplitude (P < 0.01) and N1 latency (P < 0.005), which occurred in the setting of preserved enhancement.
In addition to these neural changes, the behavioral performance of the older population was impaired on the WM recognition tasks, such that they exhibited reduced accuracy compared with younger participants. Because faces were supposed to be ignored during the scene WM task, failed suppression of faces, as documented by the neural data, may be associated with impairments in WM accuracy for scenes. To determine whether the neural suppression deficit was related to the age-related WM performance deficit, we assessed whether performance subgroups of older participants (median split into lower- and higher- performance on the scene WM task) exhibited differential suppression deficits of face stimuli relative to younger adults. The lower-performing, older subgroup exhibited a reduced N1 latency suppression index compared with the younger adults (P < 0.05), whereas the higher-performing, older subgroup did not show a significant suppression deficit (P > 0.05). This supports the relationship between inadequate suppression and impaired working memory performance in older adults. The same performance split of the older population by face WM accuracy did not show a significant difference in N1 latency enhancement index between the older adult subgroup and the younger population (P > 0.05), further suggesting WM performance impairment in lower-performing older adults is the result of a selective deficit in suppressing irrelevant information.
The focus of the above analysis was directed at EEG measures for face stimuli; the analyses of scene stimuli did not reveal significant enhancement or suppression of P1, N1, or P300 amplitude/latency or gamma power in young adults, and thus did not provide necessary markers of modulation to explore top-down changes in the older population. However, analysis of the induced alpha desynchronization (500–650 ms) for scene stimuli revealed significant suppression in both the younger (P < 0.005) and older (P < 0.05) adults, with no between group difference (P = 0.421), supporting the finding of a relative preservation of suppression later in the time course of signal processing.
Frontal EEG Measures.
To further investigate the basis of the sensory suppression deficit, we examined age-related differences in EEG signals from frontal electrodes. The frontal cortex is believed to be a source of top-down control of sensory cortical activity during goal-directed behavior and modulates extrastriate cortex activity as early as 100 ms (17), the time range of the aging suppression deficit. We focused on frontal midline theta, which has been localized to the medial prefrontal cortex (18), because there is extensive evidence of increased spectral power during mental effort, such as heightened attention required for short-term memory encoding (18). Theta bursting was greatest 100 ms to 200 ms after stimulus onset, in the same time frame that the suppression deficit was observed in posterior electrodes. Evaluation of frontal midline theta for face stimuli revealed a generalized increase in power with age (main effect of age: F1,36 = 13.95, P = 0.001) (Fig. 4 and Fig. S2). We also observed an age × task interaction (F1,36 = 6.80, P < 0.005), such that younger participants showed significantly higher theta power for “relevant” vs. “irrelevant” stimuli (P = 0.001), whereas the older population showed no difference (P = 0.81). A nonsignificant trend was observed for frontal midline theta associated with scene stimuli (young, P = 0.076; older, P = 0.692)
Fig. 4.
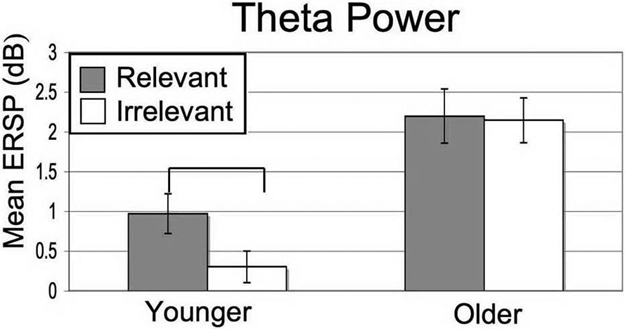
Frontal midline theta power for relevant and irrelevant face stimuli in both age groups. Only the younger adults exhibited a significant difference in theta power based on task-relevance of the stimuli (P < 0.001). Error bars indicate standard error of the mean.
Discussion
This study provides electrophysiological evidence of an age-related, selective deficit in top-down suppression, which manifests as early as 100 ms in the visual processing stream. Although an early, age-related, suppression deficit has been reported (8, 10, 19), these studies did not elucidate whether the deficit was selective to the processing of irrelevant stimuli and whether it was selective to early stages. The identification of EEG measures in young adults that spanned the time course of stimulus viewing and were modulated for both relevant and irrelevant information relative to passive viewing, offered powerful temporal markers to explore the influence of aging on the enhancement and suppression of visual information. The preservation of significant enhancement for each of the five measures in older adults is evidence of the selectivity of the top-down deficit for suppression. The presence of intact suppression later in the time-course of visual processing demonstrates that suppression ability is not abolished with normal aging, but rather delayed to a later processing stage, thus revealing an interaction between deficits in suppression and processing speed in older adults. This delay in processing is reminiscent of the “load-shift” model of cognitive aging recently described by Velanova et al. for memory retrieval (20).
The presence of a significant N1 latency suppression deficit occurring only in the lower-performing older adults suggests that a suppression deficit within 200 ms after a stimulus is viewed introduces sufficient interference from irrelevant information to impair WM recognition for relevant information presented in the same trial. This finding offers physiological support for the Hasher et al. (21) theoretical framework of the influence of inhibitory control over the contents of working memory. Of interest, performance impairment exists despite successful later suppression, implying that significant interference by irrelevant information overwhelms a limited WM capacity very rapidly, and is unable to be successfully compensated by intact, later processing stages.
In addition to yielding important temporal information related to an age-related, processing deficit, this EEG study replicates two core findings previously obtained using fMRI (4), but now with a direct electrical measure of neural activity. Namely, the current study confirms the selectivity of a top-down modulation deficit in suppression and the relationship between sensory suppression of irrelevant information and WM performance in aging. This is a vital replication of a previous finding, because the BOLD signal is a blood flow correlate of neural activity and interpretations of fMRI changes as reflecting neural changes must be made with caution in an older population with potential accompanying vascular alterations (22).
The finding of a generalized increase in frontal midline theta power with age suggests that older participants invest more overall effort in performing the task, perhaps as a compensation for an undertaking that was more difficult for them. The presence of increased frontal activity with aging as reflecting compensation is a well described phenomena in the aging fMRI literature (23, 24). The absence of a difference in midline theta power for relevant vs. irrelevant stimuli in the older population suggests that in addition to increased overall effort with aging, excessive attention is directed toward processing irrelevant stimuli early in the time-course of stimulus viewing.
This study reconciles the inhibitory deficit and the processing speed hypothesis of cognitive aging in three principle ways. First, it demonstrates the coexistence of physiological hallmarks of impaired neural suppression (i.e., P1 amplitude and N1 latency suppression deficits) and generalized processing speed decline (overall increases in N1 and P300 latency—main effects of age) in a population of older adults. Second, ERP latency measures, which are classically used to evaluate general declines in neural processing speed with age (7), are also altered in a manner selective to the suppression of irrelevant information, thus establishing a direct interaction between alterations in neural processing speed and suppression. Last, the data reveal that failure to suppress activity associated with irrelevant information is limited to the early stages of visual processing. In the context of frontal midline theta and performance data, this suggests that the delay in the older individual's ability to employ top-down suppression via a withdrawal of attention results in interference from irrelevant information sufficient to cause WM impairment. Thus, normal aging is associated with a dynamic interaction between deficits in neural processing speed and sensory inhibition, such that both hypotheses explain cognitive deficits in aging.
Materials and Methods
Participants.
Twenty healthy young individuals (mean age: 23.1 years; range 19–30 years; 10 males) and twenty-six healthy older individuals (mean age: 65.7 years; range 60–72 years, 13 males) gave consent to participate in the study. All participants were screened to ensure that they were healthy, had no history of neurological, psychiatric, or vascular disease, were not depressed, and were not taking any psychotropic or hypertensive medications. All participants had normal to corrected vision and were right handed, although two participants used their left hands for some activities. One younger and four older participants were excluded because there were too few usable segments of EEG data as the result of recording artifacts (<65 segments), leaving 19 younger subjects and 22 older subjects.
Neuropsychological Testing.
Participants in the older age group were administered 11 neuropsychological tests of executive and memory function, and were found to be cognitively intact (within two standard deviations) relative to normative values for age-matched controls. Neuropsychological testing was performed on a separate day from EEG and included the following tests: MMSE (25), Logical Memory I, Verbal Paired Associates I, and Visual Reproduction II [all from the Weschler Memory Scale Revised (26)], the Long-Delay Free Recall measure from the California Verbal Learning Test [CVLT (27)], Modified Wisconsin Card Sorting Test [WCST (28)], Controlled Oral Word Association Test (“FAS”) (29), Mental Arithmetic Test (26), Mental Control Test (30), and Digit Span Test (30). Nine of the older participants were not tested with this battery.
Experimental Procedures.
The cognitive paradigm was composed of three different conditions. Each condition consisted of the same basic temporal design, such that they all required viewing four images: two faces and two scenes presented in randomized order, each being displayed for 800 ms (200-ms ISI), followed by a nine-second delay period in which the images were to be remembered and mentally rehearsed. After the delay, a third image appeared (Probe). The subject was asked to respond with a button press (as quickly as possible without sacrificing accuracy) whether the third image (Probe) matched one of the previous images. The tasks differed in the instructions given at the beginning of each run. For the Face Memory condition, the participants were asked to remember only the face stimuli and to ignore the scene stimuli. Correspondingly for the Scene Memory condition, participants were asked to remember only the scene stimuli and ignore the faces. When the probe image appeared, it was composed of a face in the face memory conditions, or a scene in the scene memory conditions. In the Passive View, participants were instructed to relax and view the stimuli without trying to remember them. Instead of a probe image, an arrow was presented where participants were required to make a button press indicating the direction of the arrow. The task was presented in 3 separate runs (20 trials each) with each of the three conditions in random order. Conditions and stimuli were counterbalanced across participants
Stimuli.
The stimuli consisted of grayscale images of faces and natural scenes. All face and scene images were novel across all conditions and across all runs of the experiment. Images were 225 pixels wide and 300 pixels tall (14 × 18 cm) and subtended ≈5 by 6° of visual angle (participants were ≈172 cm from the screen). The face stimuli consisted of a variety of neutral-expression male and female faces across a large age range. The sex of the face stimuli was held constant within each trial, and each stimulus was used in only one trial.
EEG Recording.
Participants were seated in an armchair in a dark, sound-attenuated room and were monitored by camera during all tasks. The screen was ≈125 cm from the participants' eyes. Data were recorded during three runs of 20 trials for each of the three conditions, resulting in 60 trials per condition with 120 EEG segments (2 stimuli/trial).
Neural data were recorded with a BioSemi ActiveTwo 64-channel EEG acquisition system in conjunction with BioSemi ActiView software (CortechSolutions). Signals were amplified and digitized at 1,024 Hz with a 16-bit resolution. All electrode offsets were <20 kΩ. Anti-aliasing filters were used and data were band-pass filtered between 0.01–100 Hz during data acquisition. Trials with excessive peak-to-peak deflections, amplifier clipping, or excessive high frequency (EMG) activity were excluded before analysis.
Data Analysis.
Preprocessing was conducted through Analyzer software (Brain Vision, LLC). The raw EEG-data were referenced to an average reference off-line and were segmented into epochs beginning 200 ms before stimulus onset and ending 800 ms after stimulus onset (−200–0 baseline corrected). Eye-movements and artifacts were removed through an independent components analysis (ICA) and a voltage threshold of ±50 μV. Epochs were then cleaned of trials with excessive peak-to-peak deflections, amplifier clipping, or other artifacts.
Face and scene trials were then separately segmented and averaged (epochs to repeated stimuli were not included in the average to prevent motor movement contamination in the epochs). Only encoding-period segments from correct trials were considered. Across-subject event related potentials (ERPs) statistics were calculated using amplitudes and latencies obtained from each subject [with extreme outliers removed using an interquartile range exclusion factor—resulting in variation in the degrees of freedom for different measures (31)] and event-related spectral perturbations (ERSPs) were calculated using EEGLAB's wavelet decomposition from the timef function (32). Peak amplitudes were selected based on an area calculated using an 8-ms window centered around the peak amplitude deflection (±4 msec).
Electrode Selection.
To select electrodes for statistical analyses, we combined responses to all stimuli of one class (i.e., faces) that were viewed throughout the experiment, and chose the posterior electrode with the largest response at the group level. The P1 component was identified as the first positive deflection appearing between 50 and 150 ms after stimulus onset at electrode P10. The N1 component was identified at posterior sites as the maximal negative peak between 120 and 220 ms after stimulus onset at electrode P10. The P300 component was identified at posterior sites as the maximal positive peak between 300 and 500 ms after stimulus onset at P0z. Gamma activity (30–50 Hz) was measured from 100 to 2–00 ms at Pz. Alpha band (8–12 Hz) analyses were performed at electrode P08 with a time window of 500–650 ms to capture alpha desynchronization. Frontal Theta (4–7 Hz) was highest at electrode F2, and was evaluated in a window from 100–200 ms.
Statistical Analyses.
Analyses of Variance (ANOVA) were performed on all ERP and spectral components at the electrode of interest, as described above, with both latency and amplitude analyzed. A 2-way ANOVA (3 × 2) for task (Relevant, Passive, Irrelevant) × age (Young and Old) was performed. Post-hoc analysis consisted of both within-group, paired-sample t tests (two-sided) and across-group, unpaired t tests of modulation indices (enhancement = relevant - passive; suppression = passive - irrelevant) (P < 0.05, Greenhouse-Geisser correction).
Supplementary Material
Acknowledgments.
This work was supported by the National Institutes of Health (A.G., M.D., and R.K.) and American Federation for Aging Research (A.G.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806074105/DCSupplemental.
References
- 1.Craik FI, Salthouse TA. Handbook of Aging and Cogntion II. Mahwah, NJ: Erlbaum; 2000. [Google Scholar]
- 2.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 3.Hasher L, Zacks RT. In: The Psychology of Learning and Motivation. Bower GH, editor. New York, NY: Academic; 1988. pp. 193–225. [Google Scholar]
- 4.Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- 5.Pinsk MA, Doniger GM, Kastner S. Push-pull mechanism of selective attention in human extrastriate cortex. J Neurophysiol. 2004;92:622–629. doi: 10.1152/jn.00974.2003. [DOI] [PubMed] [Google Scholar]
- 6.Gazzaley A, Cooney JW, McEvoy K, Knight RT, D'Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cognit Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- 7.Pfefferbaum A, Ford JM, Wenegrat BG, Roth WT, Kopell BS. Clinical application of the P3 component of event-related potentials. I. Normal aging. Electroencephalogr Clin Neurophysiol. 1984;59:85–103. doi: 10.1016/0168-5597(84)90026-1. [DOI] [PubMed] [Google Scholar]
- 8.Alain C, Woods DL. Age-related changes in processing auditory stimuli during visual attention: Evidence for deficits in inhibitory control and sensory memory. Psychol Aging. 1999;14:507–519. doi: 10.1037//0882-7974.14.3.507. [DOI] [PubMed] [Google Scholar]
- 9.West R, Alain C. Age-related decline in inhibitory control contributes to the increased Stroop effect observed in older adults. Psychophysiology. 2000;37:179–189. [PubMed] [Google Scholar]
- 10.Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex. 1997;7:63–69. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- 11.Gomez Gonzalez CM, Clark VP, Fan S, Luck SJ, Hillyard SA. Sources of attention-sensitive visual event-related potentials. Brain Topogr. 1994;7:41–51. doi: 10.1007/BF01184836. [DOI] [PubMed] [Google Scholar]
- 12.Gruber T, Muller MM, Keil A, Elbert T. Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clin Neurophysiol. 1999;110:2074–2085. doi: 10.1016/s1388-2457(99)00176-5. [DOI] [PubMed] [Google Scholar]
- 13.Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Muller MM, Keil A. Neuronal synchronization and selective color processing in the human brain. J Cognit Neurosci. 2004;16:503–522. doi: 10.1162/089892904322926827. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann MJ, Ehlis AC, Ellgring H, Fallgatter AJ. Early stages (P100) of face perception in humans as measured with event-related potentials (ERPs) J Neural Transm. 2005;112:1073–1081. doi: 10.1007/s00702-004-0250-8. [DOI] [PubMed] [Google Scholar]
- 16.Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cognit Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- 18.Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. NeuroImage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Fabiani M, Low KA, Wee E, Sable JJ, Gratton G. Reduced suppression or labile memory? Mechanisms of inefficient filtering of irrelevant information in older adults. J Cognit Neurosci. 2006;18:637–650. doi: 10.1162/jocn.2006.18.4.637. [DOI] [PubMed] [Google Scholar]
- 20.Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for Frontally Mediated Controlled Processing Differences in Older Adults. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- 21.Hasher L, Zacks RT, May CP. In: Attention and performance, XVII, Cognitive regulation of performance: Interaction of theory and application. Gopher D, Koriat A, editors. Cambridge, MA: MIT Press; 1999. pp. 653–675. [Google Scholar]
- 22.D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 23.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 24.Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- 27.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual. San Antonio, TX: The Pyshcological Corporation; 1987. [Google Scholar]
- 28.Hart RP, Kwentus JA, Wade JB, Taylor JR. Modified Wisconsin Card Sorting Testin elderly normal, depressed and demented patients. Clinical Neuropsychologist. 1988;2:49–56. [Google Scholar]
- 29.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City: University of Iowa; 1976. [Google Scholar]
- 30.Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 31.Tukey JW. Exploratory data analysis. Menlo Park, CA: Addison-Wesley; 1977. [Google Scholar]
- 32.Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.