Abstract
Autoimmune regulator (Aire)-deficient mice and humans have circulating autoantibodies against a multitude of organs and multiorgan autoinflammatory infiltrates. It is not known to what extent autoantibodies or their source, B lymphocytes, are required for disease onset or progression. We show in this research that B cells must be present for Aire-deficient mice to develop fulminant infiltrates. We found no evidence that autoantibodies were directly pathogenic; rather, B cells appeared to play a critical early role in T cell priming or expansion. A therapeutic reagent directed against B cells, Rituximab, induced remission of the autoimmune disease in Aire-deficient mice, raising the hope of applying it to human patients with autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED).
Keywords: autoimmunity, B lymphocytes, tolerance, autoantibody
As T and B lymphocytes differentiate, random rearrangement of their antigen-specific receptor genes results in a diverse repertoire of surface receptors, including occasional receptors capable of recognizing the body's own constituents. To keep autoimmunity at bay, tolerance-induction mechanisms operate on lymphocytes as they differentiate in the thymus and bone marrow and after they emerge into the periphery.
Multiple mechanisms of tolerance induction operate on differentiating thymocytes (1). Stromal cells of the hematopoetic lineage (i.e., macrophages and dendritic cells) purge the repertoire of a number of self-reactive specificities, especially thymocytes that recognize ubiquitous or bloodborne antigens. A highly specialized stromal cell population, medullary epithelial cells (MECs), expresses a broad spectrum of peripheral tissue antigens (PTAs). Peptides derived from the PTAs are presented to thymocytes percolating through, driving clonal deletion of those whose T cell receptors (TCRs) are triggered within the appropriate range of affinity/avidity (2). Expression of many, although not all, PTAs is controlled by the transcriptional autoimmune regulator (Aire) (2, 3). In the absence of Aire, clonal deletion of self-reactive thymocytes is compromised (4, 5), and autoimmunity ensues (3, 6).
Mice and humans with a mutant Aire gene show autoimmunity to a multitude of organ-specific antigens. Aire-defective humans develop autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), which manifests as an autoimmune attack of the adrenal and parathyroid glands, stomach, liver, reproductive organs, etc. (7). Aire-deficient mice also develop a multiorgan autoimmune pathology in which targets and severity vary with the genetic background, becoming rapidly lethal on some but not other backgrounds (3, 6, 8, 9). In both the human and murine disorders, chronic inflammation of multiple organs is accompanied by autoantibodies against proteins made specifically by these organs (3, 6, 8, 10). The presence of autoantibodies in APECED patients is potentially of predictive value because they precede clinical disease and organ destruction (10, 11). In mice, a similar correlation exists between development of autoantibodies and of chronic inflammation in particular organs (8).
The Aire/anti-organ-autoantibody/anti-organ-infiltrate circle was recently closed; class-switched antibodies specific for interphotoreceptor retinoid-binding protein (IRBP) and glycosylated glycoprotein (Mucin 6) were found to result from the failure of Aire-deficient MECs to tolerize thymocytes to these eye and stomach PTAs, with the consequent activation of cognate B and T lymphocytes in the periphery and inflammation of the organs harboring these antigens (12, 13).
However, it is not known how B lymphocytes and the autoantibodies they produce relate to the autoimmune process. Self-reactive B cells may be involved as important participants in the disease or as mere bystanders of the autoimmune attack. This question is of practical importance given the recent advances in immunotherapy targeting B cells in a growing number of autoimmune diseases (14). We show here that B cells are obligate early participants in the inflammatory pathology of Aire-deficient mice and provide proof of principle that anti-B-cell therapy has promise for APECED patients.
Results
B Cell Deficiency Protects Aire-Null Mice from Their Characteristic Autoimmunity.
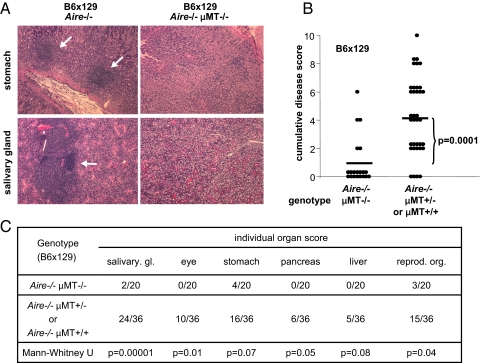
To investigate the contribution of B cells to the autoimmune pathology that develops in Aire-knockout mice, we introduced mutations causing a severe block in B-cell differentiation. First, we crossed Aire−/− mice with B-cell-deficient (μMT)−/− animals, whose B-cell differentiation is arrested at the pre-B-cell stage due to a mutated transmembrane domain in the immunoglobulin M (IgM) surface receptor (15). Aire−/−μMT−/− mice on a (B6 × 129) F2 genetic background were monitored for 20 weeks, whereupon histological sections from a diversity of organs were surveyed, yielding a cumulative disease-severity score that factored in the intensity of inflammatory infiltrates plus the number of organs affected in each mouse. As expected, Aire−/− mice with a standard B-cell compartment developed strong inflammation of many organs; in contrast, infiltrates were weaker and rarer in littermates homozygous for the μMT mutation (in addition to being Aire−/−) (Fig. 1 A–C). As depicted in Fig. 1C, the retina was always intact in Aire−/−μMT−/− double-mutant mice, which was significantly different from what was found with standard Aire−/− animals (p = 0.01). There was also significantly less inflammation of the salivary glands (p = 0.00001) and reproductive organs (p = 0.04) in the absence of B cells. Whereas there was a trend toward reduced inflammation of the stomach, pancreas, and liver in mice lacking B cells, this did not quite reach statistical significance (p = 0.07, 0.05, and 0.08, respectively). These results suggest that infiltration of different organs relies more or less critically on the participation of B cells at some stage of the autoimmune process.
Fig. 1.
Blocking B cell differentiation in Aire−/− mice results in protection from autoimmunity. (A) Infiltrates in stomach and salivary gland of (B6 × 129) F2 Aire−/− (Left Images) and Aire−/−μMT−/− (Right Images) animals. Arrows denote lymphoid infiltrates (all 10× objective). (B) H&E-stained sections were scored for the severity of inflammation. The cumulative disease score was the sum of individual organ scores and was lower on average in Aire−/−μMT−/− (Left Column, n = 20) than in Aire−/− mice (Right Column, n = 36) (p = 0.0001). (C) Inflammation of the indicated organs was scored and compared in Aire−/−μMT−/− and Aire−/− mice.
Previous reports have raised the possibility that an absence of B cells merely delays the onset of autoimmunity (16). Given the protracted course of disease in the Aire−/− mouse on a mixed B6 × 129 genetic background, we attempted to circumvent this potential issue by performing a similar cross on the nonobese diabetic (NOD) background, where the Aire deficiency results in early and severe autoimmune disease, with wasting of most animals beginning between 5 and 15 weeks of age (6, 8). Multiorgan inflammation is always extensive in this strain, with exocrine pancreatitis and complete destruction of the pancreatic parenchyma dominating the pathology.
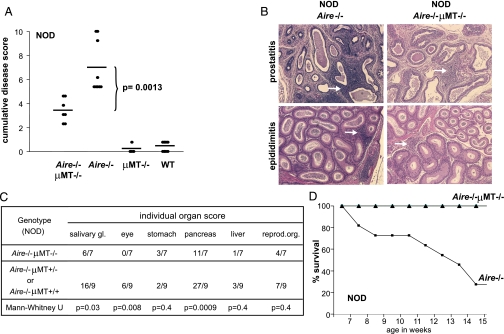
The severity of inflammation differed greatly between NOD-background Aire−/−μMT−/− and Aire−/− mice (Fig. 2 A–C). Within a given target organ, the same tissues were infiltrated, but often with a change in the severity and organization of the infiltrating cells; in general, Aire−/−μMT−/− mice had a modest and diffuse infiltrate, rather than the massive compact front of infiltrating cells usual for single-deficient Aire−/− animals. As was the case for (B6 × 129) F2 mice, the eye was always spared in NOD. Aire−/−μMT−/− animals; salivary glands and pancreata also tended to have milder inflammation in NOD mice lacking B cells (Fig. 2C). Whereas standard Aire-knockout mice on the NOD background developed severe wasting and gradually succumbed, all B-cell-deficient NOD. Aire−/− mice maintained their body weight and were alive at the end of the 20-week follow-up period (Fig. 2D).
Fig. 2.
B cells are responsible for lethal immunopathology in NOD.Aire−/− mice. (A) Cumulative disease scores (as defined in Fig. 1B) for Aire−/−μMT−/− (n = 7), Aire−/− (n = 9), μMT−/− (n = 4) and wild-type (n = 14) mice on the NOD background. Aire−/−μMT−/− compared with Aire−/− mice (p = 0.0013). (B) Infiltrates of prostates and epididimi of NOD.Aire−/− (Left Images) and NOD.Aire−/−μMT−/− mice (Right Images) (all 10× objective). Arrows indicate lymphoid infiltrates, severe in NOD.Aire−/− mice, but mild and diffuse in NOD.Aire−/−μMT−/− mice. (C) Inflammation scores were compared for individual organs in Aire−/−μMT−/− (n = 7) and Aire−/− (n = 9) mice on the NOD background. (D) Survival curves of Aire−/−μMT−/− (▴, n = 7) and Aire−/− (■, n = 11) mice on the NOD background.
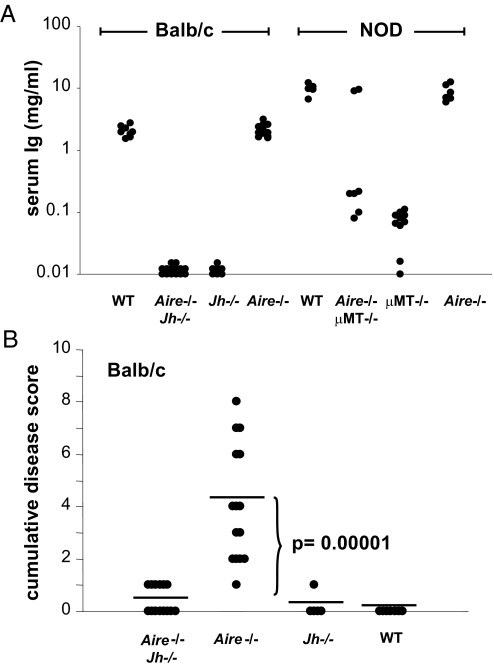
Thus, B-cell-deficient mice seemed substantially protected from the chronic inflammation provoked by the absence of Aire. We wondered whether the residual disease in Aire−/−μMT−/− mice might reflect the occasionally incomplete block of B cell differentiation in μMT−/− mice that has been observed on some genetic backgrounds (17). Therefore, we crossed Aire−/− animals with another line having a more robust block in B cell differentiation. Jh−/− mice lack the junctional region of the immunoglobulin (Ig) heavy chain and are thereby incapable of assembling a surface B-cell receptor, leading to a block in B-cell maturation at the pro-B to pre-B-transition (18). We analyzed autoimmunity in Aire−/−Jh−/− double-knockout mice, this time on the BALB/c background, compared with Aire−/− littermate controls. As expected, mice homozygous for the Jh mutation completely lacked B cells (data not shown) and Igs (Fig. 3A). Again, the autoimmune disease was considerably weaker in Aire−/− mice that lacked B cells (Fig. 3B), although a slight residual disease was still present. In brief, these results indicate that B cells are obligate players in the organ-specific autoimmunity of Aire-deficient mice.
Fig. 3.
Inflammation is abrogated by a complete block in B cell-differentiation. (A) Serum IgG levels of BALB/c wild-type (n = 8), Aire−/−Jh−/− (n = 13), Jh−/− (n = 7), and Aire−/− (n = 11) mice were assayed by ELISA and were compared with levels in the sera of NOD WT (n = 5), Aire−/−μMT−/− (n = 7), μMT−/− (n = 10), and Aire−/− mice (n = 6). (B) Sections of organs were stained by H&E, scored for the severity of inflammation, and accumulated for each mouse to yield an individual disease score. Average disease scores were compared for Aire−/−Jh−/− (n = 14), Aire−/− (n = 15), Jh−/− (n = 5), or wild-type (n = 7) mice on the BALB/c background; Aire−/−Jh−/− compared with Aire−/− mice (p = 0.00001).
No Detected Impact of Maternal Antibodies.
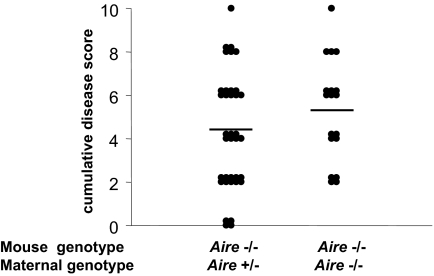
In the NOD model of autoimmune diabetes, which is also dependent on B lymphocytes, maternally transferred antibodies promote disease (19). Thus, we asked whether maternal Igs, transmitted from an Aire−/− mother, could influence the timing and severity of chronic inflammation in her Aire−/− offspring. The answer was that no significant influence occurred (p = 0.23) (Fig. 4).
Fig. 4.
No influence of maternally transferred Ig on inflammation in susceptible Aire−/− offspring. Organ inflammation due to Aire-deficiency was scored and summed in H&E-stained organ sections of 20-week-old (B6 × 129) F2 Aire−/− offspring of Aire−/− (n = 18) or Aire+/− mothers (n = 36).
Where B Cells Impact on the Autoimmune Process.
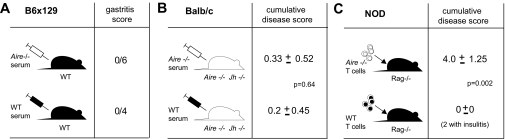
We questioned whether antibodies from Aire−/− mice might have a direct pathogenic effect. As an initial test, serum from 20-week-old diseased B6 × 129 Aire−/− mice was transferred to normal recipients twice weekly for 7 weeks. No disease was observed in any of the recipients (Fig. 5A). Next, a complementation assay was performed: Serum from Aire−/− mice was transferred weekly for 8 weeks into Aire−/−Jh−/− mice, which have a contingent of improperly tolerized T cells but do not develop disease due to the absence of B cells. If autoantibodies serve, for example, by direct toxicity, to augment the effector function of T cells in the target organs, such a protocol should recapitulate the Aire−/− phenotype. However, at the end of 8 weeks, no signs of inflammation were detected (Fig. 5B).
Fig. 5.
Not Aire−/− serum, but Aire−/− T cells, transfer multiorgan inflammation. (A) Wild-type (B6 × 129) F2 mice were injected with Aire−/− or wild-type serum from 20-week-old (B6 × 129) F2 donors. Inflammation was assayed by H&E-staining of stomach sections from recipient mice. (B) Inflammation was assayed by H&E-staining of organ sections from Aire−/−Jh−/− recipient mice injected weekly for 8 consecutive weeks with Aire−/− serum (n = 6) or PBS (n = 5). (C) NOD.Rag−/− mice transferred with T cells from Aire−/− (n = 15) or Aire+/+ (n = 4) mice were assessed for the presence of inflammation by H&E-staining of organs affected in the donor NOD.Aire−/− mice.
These findings suggested that the production of autoantibodies was unlikely to be the critical contribution of B lymphocytes to the pathogenic process. Rather, they were probably operating earlier, for example by facilitating T cell priming and/or expansion through their capacity for focused antigen-presentation. To test this notion, we determined whether T cells, once primed in the presence of B cells in Aire−/− mice, were able to mediate multiorgan inflammation on their own. Indeed, CD4+ plus CD8+ T cells sorted from 7-week-old NOD.Aire−/− mice could transfer multiorgan inflammatory disease to alymphoid NOD.Rag−/− mice, whereas this was not the case for T cells from wild-type donors (Fig. 5C). The absence of B cell contamination in T cell-transferred mice was confirmed by the absence of circulating Ig in the recipients <1% of normal serum immunoglobulin G (IgG) and IgM levels (data not shown)]. Parallel results have been reported by Niki et al. (9), who showed that NOD.Aire−/− T cells infiltrated the exocrine pancreas when transferred into lymphopenic NOD.scid recipients. Thus, the critical role of B cells in the autoimmune disease of Aire−/− mice is an early one: They are not directly pathogenic and are even dispensable at the effector phase.
Therapeutic B Cell Intervention.
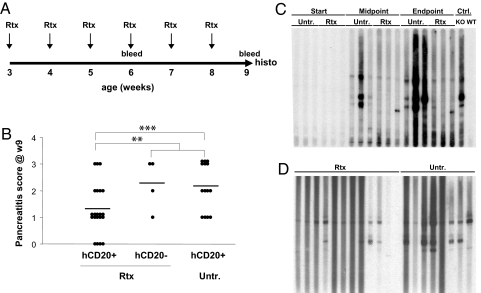
The important contribution of B cells to the autoimmune pathology of Aire-deficient mice prompted us to ask whether disease could be ameliorated by immunotherapy targeted at this cell-type, an approach that might be applicable to APECED patients. To tackle this question, we exploited a mouse line carrying a transgene encoding the human CD20 (huCD20) molecule (20); treatment of such mice with an anti-huCD20 monoclonal antibody (mAb), Rituximab, used in the clinic allows convenient preclinical exploration of anti-B cell therapies (21). The huCD20 transgene was introduced into the NOD.Aire−/− line through 11 generations of backcross. The mice were treated with repeated doses of Rituximab (1 mg weekly), starting at 3 weeks of age leading to a 60% reduction in circulating B cells, but no durable reduction in splenic marginal zone or follicular B cells (data not shown) (Fig. 6A). Pilot studies had demonstrated that exocrine pancreatitis was already initiated in NOD.Aire−/− mice at 3 weeks (M. Guerau-de-Arellano, C.B., and D.M., unpublished observations), so this protocol represents intervention in a disease already initiated but not fully developed.
Fig. 6.
B-cell-targeted immunotherapy ameliorates disease in Aire−KO NOD mice. (A) Schematic of Rituximab treatment. (B) Pancreatitis severity (null, 1 = mild infiltration, 2 = moderate infiltration with functional exocrine tissue, or 3 = severe infiltration with destruction of whole exocrine tissue) was scored on H&E sections of pancreata from Rituximab-treated and untreated mice. Transgene (tg)+ treated mice (n = 21) compared with: 1, tg+ untreated mice (n = 14, p = 0.013); 2, treated tg− mice plus untreated tg+ mice (n = 18, p = 0.03). (C) Immunoblot of pancreatic extract probed with serum from Rituximab-treated or untreated NOD.Aire−/− mice and with control reference sera; each lane shows autoantibody reactivity of individual mice. (D) Immunoblots of pancreas antigen detected at the end of the Rituximab-treatment by serum autoantibodies from treated or untreated NOD.Aire−/− mice.
Rituximab treatment had a marked effect on the survival rate; all treated mice lived to the end of the experiment, whereas 28% of the untreated animals had to be killed due to wasting disease (p = 0.01). Inflammation was assayed histologically one week after the last dose of mAb, focusing on the pancreas, the organ most consistently infiltrated in NOD.Aire−/− mice. The extent of inflammation and tissue destruction was significantly reduced in the Rituximab-treated animals (mean pancreatitis scores 1.28 versus 2.17, p = 0.03) (Fig. 6B). No modifications in the average titer of total serum IgG were noted in the treated animals (data not shown). Immunoblotting of pancreas extracts revealed that Rituximab-treated mice had a partial reduction in serum autoantibodies, fewer antigen bands being detectable (Fig. 6 C and D) and densitometric quantitation yielding an average integrated band intensity 1.83-fold lower than that of untreated controls (p = 0.0065).
When Rituximab therapy was started later in the course of the disease at 5 weeks of age, survival and wasting were still significantly improved (supporting information (SI) Fig. S1) (p = 0.04 for survival), but the histological score did not differ significantly from that of untreated mice: Average score was 2.16 in six treated mice versus 2.6 in five untreated mice (p = 0.6). Hence, there may be a time-window when inflammatory lesions are most susceptible to remission, consistent with the finding that B cells are operating early in the disease course to promote T cell priming and/or expansion.
Discussion
The major conclusion of these studies is that B cells are required for fulminant multiorgan inflammation in Aire−/− mice. They also provide proof-of-principle that anti-B-cell therapy may be an option for addressing the autoimmune disease in AIRE-deficient APECED patients.
The mild disease in Aire−/−μMT−/− mice is likely to reflect residual B cell function in μMT−/− mice, as described in previous studies (17), because it disappeared mainly in the Aire−/−Jh−/− animals. Although the residual disease of Aire−/−μMT−/− mice may be too rare to support a strong conclusion, it is interesting that not all target organs were equally affected: An intact B cell compartment was required for retinopathy, whereas inflammation of the salivary glands, reproductive organs, and pancreas could develop, albeit mildly, in the context of a compromised B cell compartment. The variable requirements for B cells for the occurrence of immunopathology in individual organs might be due to differential accessibility of the relevant antigen(s), with immunologically privileged sites like the eye being least accessible. Other potential influences include the organ's vascular properties and resident leukocyte populations.
How do B cells promote pathology in Aire-deficient mice? They probably do not express Aire themselves (22), although this fact has been debated of late (23), so the effect is likely to be an indirect one. Furthermore, the representations of B-cell subsets and the serum concentrations of Ig classes were unchanged at disease onset and during most of the disease course in Aire−/− adults compared with wild-type littermates [(3, 24) and our unpublished data]. B cells can have a variety of functions: They produce Igs, release immunomodulatory cytokines, regulate lymphoid tissue neogenesis and lymphangiogenesis, present antigen, and influence the activities of dendritic cells and T cells (25–29). Of these functions, the production of Igs is central to certain other autoimmune disease models, for example, transfer of self-reactive Igs triggers the immediate onset of inflammatory immunopathology in arthritis, lupus nephritis, oophoritis, pemphigus, etc. (30–33). Maternal Igs have been suggested to play a different role in type-1 diabetes by contributing to the eventual activation of islet-reactive T cells in the offspring. Neither of these activities was detected in our exploration of the role of B cells in the Aire−/− mouse model, suggesting that pathogenic Igs are not the primary mechanism in this case.
However, B cells can influence T cell function through a variety of means (21, 34–38). It has been suggested that they are involved in regulating the activation status and number of effector T lymphocytes based on observations of abnormal CD4+ T cell responses in B cell-deficient mice (36, 37). B cells can bind antigen with high affinity and are more effective than dendritic cells and macrophages at capturing and presenting rare antigens (39, 40). An impact of B cells via antigen presentation and T cell priming and/or expansion has been invoked to explain the B-cell dependence of autoimmune diabetes in the NOD mouse (16, 41), although more recently it was suggested that B cells in this context might also play a role in controling regulatory T cells (21), which may or may not be a reflection of the same molecular process(es). Our results in the Aire−/− setting are consistent with an effect of B cells on the priming/expansion of autoreactive T lymphocytes: T cells alone can transfer multiorgan autoimmunity in an effector phase that appears to be B-cell independent.
Whatever the mechanism, it is exciting that B-cell-directed intervention with a mAb already used in the clinic had a significant effect on early disease progression in Aire−/− mice. The fact that the treatment did not work at 5 weeks is consistent with a role for B cells in T cell priming/expansion, and raises the possibility of early-stage intervention in the analogous human context. Thus, APECED joins the list of T cell-mediated autoimmune diseases for which the exploration of the clinical efficacy of Rituximab seems warranted. This is a welcome development in the case of APECED because therapeutic options have so far been rather limited, confined to hormone replacement, anti-fungal agents, and immunosuppressive therapy with corticosteroids, azathioprine, cyclosporine A, or methotrexate (42), which treat the symptoms (metabolic abnormalities, immunodeficiency) rather than the cause.
Materials and Methods
Mice and Sera.
The sources and genotyping strategies for all mutant mouse strains are described in the SI Materials and Methods. Sera were obtained from tail bleeds or through cardiac puncture of euthanized mice.
Treatments.
Rituximab was provided by Genentech. One milligram Rituximab was administered intraperitoneally (ip) in 6 weekly doses to 3- or 5-week-old mice. Animals were killed 1 week after the last dose. Serum was administered ip in 2 weekly doses, 200 μl each, for a duration of 7 weeks to 6-week-old wild-type (B6 × 129) F2 recipient mice. One-week-old BALB/c.Aire−/−Jh−/− mice received weekly ip injections of 200 μl of serum for a duration of 8 weeks.
Histology.
Tissues were fixed in Bouin's solution and embedded with paraffin. Sections were stained with hematoxylin and eosin (H&E) and scored blindly by two independent investigators. Infiltration for each organ is indicated by zero (none), 1 (trace/mild), 2 (moderate), or 3 (severe). Individual organ scores were summed to yield a cumulative disease score. Images were acquired on a Zeiss Axiophot2 microscope and were processed with Spot Advanced imaging software (Diagnostic Instruments).
Western Blotting.
Protein was extracted from NOD mouse pancreata in a Dounce homogenizer, each mg of tissue being ground in 20 μl of sample buffer (62.5 mM Tris-HCl, pH6.8, 25% glycerol, 2% SDS, 1% bromophenol blue, and 5% β-mercaptoethanol). Extracted tissue protein was treated as described in the SI Materials and Methods.
Serum IgG Measurements.
ELISA plates were coated with 5 μg/ml goat anti-mouse IgG (heavy and light chains, Jackson ImmunoResearch) at 4°C overnight. Unbound Ig was removed by washing three times with PBS containing 1% BSA and once with PBS alone. Sera were tested as described in the SI Materials and Methods.
Statistical Analysis.
A two-tailed Mann–Whitney rank sum U test assessed statistical significance.
Supplementary Material
Acknowledgments.
We thank Dr. Wenyu Jiang for scoring of samples, Joyce LaVecchia and Girijesh Buruzula for flow cytometry, and Vanessa Tran for mouse rearing. This work was supported by the National Institutes of Health Grant R01 DK60027 and Young Chair funds to D.M. and C.B. and by Joslin's Diabetes and Endocrinology Research Center cores (P30 DK36836). I.G. received fellowships from the National Institutes of Health (T32 CA09382 and F32 AI62010).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806874105/DCSupplemental.
References
- 1.Siggs OM, Makaroff LE, Liston A. The why and how of thymocyte negative selection. Curr Opin Immunol. 2006;18:175–183. doi: 10.1016/j.coi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 4.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance immunity. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Liston A, et al. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey C, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 7.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91:2843–2850. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 8.Jiang W, et al. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niki S, et al. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. J Clin Invest. 2006;116:1292–1301. doi: 10.1172/JCI26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23:327–364. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, et al. DRB1*04 and DQ alleles: expression of 21-hydroxylase autoantibodies and risk of progression to Addison's disease. J Clin Endocrinol Metab. 1999;84:328–335. doi: 10.1210/jcem.84.1.5414. [DOI] [PubMed] [Google Scholar]
- 12.Devoss J, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavanescu I, et al. Loss of Aire-dependent thymic expression of a peripheral tissue antigen renders it a target of autoimmunity. Proc Natl Acad Sci USA. 2007;104:4583–4587. doi: 10.1073/pnas.0700259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 16.Serreze DV, et al. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 17.Hasan M, et al. Incomplete block of B cell development and immunoglobulin production in mice carrying the muMT mutation on the BALB/c background. Eur J Immunol. 2002;32:3463–3471. doi: 10.1002/1521-4141(200212)32:12<3463::AID-IMMU3463>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 19.Greeley SA, et al. Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat Med. 2002;8:399–402. doi: 10.1038/nm0402-399. [DOI] [PubMed] [Google Scholar]
- 20.Gong Q, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 21.Hu CY, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubert FX, et al. A specific anti-Aire antibody reveals Aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol. 2008;180:3824–3832. doi: 10.4049/jimmunol.180.6.3824. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki E, et al. Expression of AIRE in thymocytes and peripheral lymphocytes. Autoimmunity. 2008;41:133–139. doi: 10.1080/08916930701773941. [DOI] [PubMed] [Google Scholar]
- 24.Hassler S, et al. Aire-deficient mice develop hematopoetic irregularities and marginal zone B-cell lymphoma. Blood. 2006;108:1941–1948. doi: 10.1182/blood-2006-04-019679. [DOI] [PubMed] [Google Scholar]
- 25.Chesnut RW, Grey HM. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981;126:1075–1079. [PubMed] [Google Scholar]
- 26.Harris DP, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 27.Tumanov A, et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- 28.Angeli V, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 29.NgoVN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setiady YY, Samy ET, Tung KS. Maternal autoantibody triggers de novo T cell-mediated neonatal autoimmune disease. J Immunol. 2003;170:4656–4664. doi: 10.4049/jimmunol.170.9.4656. [DOI] [PubMed] [Google Scholar]
- 31.Korganow AS, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 32.Anhalt GJ, et al. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- 33.Vlahakos DV, et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992;41:1690–1700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- 34.Williams GS, Oxenius A, Benoist C, Mathis D. CD4+ T cell responses in mice lacking MHC class II molecules specifically on B cells. Eur J Immunol. 1998;28:3763–3772. doi: 10.1002/(SICI)1521-4141(199811)28:11<3763::AID-IMMU3763>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 35.Crawford A, et al. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 36.Bouaziz JD, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- 38.Yan J, et al. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 39.Janeway CA, Jr, Ron J, Katz ME. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987;138:1051–1055. [PubMed] [Google Scholar]
- 40.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 41.Wong FS, et al. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes. 2004;53:2581–2587. doi: 10.2337/diabetes.53.10.2581. [DOI] [PubMed] [Google Scholar]
- 42.Perheentupa J. APS-I/APECED: the clinical disease and therapy. Endocrinol Metab Clin North Am. 2002;31:295–320. doi: 10.1016/s0889-8529(01)00013-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.