Abstract
E-proteins are a class of helix-loop-helix (HLH) proteins, which play multiple roles throughout lymphoid development. The DNA binding activities of the E-proteins are regulated by a distinct class of antagonistic HLH proteins, named Id1–4. Here we demonstrate that Id2 deficient mice in a C57BL/6 genetic background exhibit increased cellularity in the granulocyte/myeloid progenitor compartment and show significantly higher numbers of maturing neutrophils. Within 6 months of age, Id2 deficient mice succumbed from overwhelming granulocytosis. The disease closely mimicked the distinctive features of human chronic myeloid leukemia: leukocytosis with maturing neutrophils, splenomegaly, hepatomegaly, and myeloid infiltration into peripheral tissues, including spleen, liver, and lungs. Strikingly, forced Id2 expression in murine bone marrow cells substantially delayed the onset of myeloproliferative disease (MPD). Collectively, these studies show that suppression of E-protein activity interferes with the development of BCR-ABL-mediated MPD.
Keywords: Id2, Myeloproliferative disorder, chronic myeloid leukemia
Chronic myeloid malignancies can be subdivided into myelodysplastic syndromes (MDS), myeloproliferative diseases (MPD), and diseases with mixed myelodysplastic and myeloproliferative characteristics (1). Bone marrow derived from MDS patients show normal or increased cellularity. In contrast, bone marrow derived from MPD always shows hypercellularity. Furthermore, whereas the fraction of blasts in MDS varies between 1–20%, during the early stages of MPD the proportion of blasts (5%) is normal. In MPD myeloid developmental progression is relatively normal but increased myeloid cellularity can be observed.
The most common MPD is chronic myeloid leukemia (CML) (2). CML has been characterized by leukocytosis, with a significant increase in the number of maturing neutrophils and a triphasic clinical course with chronic, accelerated, and blast crisis stages (2). Both MPD as well as MDS are frequently associated with mutations in genes encoding tyrosine kinases (3). For example, the abelson tyrosine kinase (ABL) is activated by a chromosomal translocation involving the BCR gene product, resulting in a BCR-ABL hybrid gene product (4). Several lines of evidence point to BCR-ABL as the direct cause of CML. Enforced expression of BCR-ABL in murine hematopoietic stem cells activates a CML-like MPD in mice (5). Furthermore, treatment of transformed cells carrying the BCR-ABL translocation, with imatinib mesylate, a compound that is a competitive inhibitor of the ABL ATP binding pocket, leads to a block in proliferation and induction of apoptosis (6). Imatinib has recently been the treatment of choice in chronic phase CML but enthusiasm has been hampered since acquired resistance is frequently observed in advanced CML, arising from gene amplifications or point mutations, rendering BCR-ABL less responsive to the compound (7). Drug resistance of BCR-ABL is likely a problem that will persist even though new compounds that target the kinase activity of BCR-ABL are being generated and clinically tested. Therefore, identifying novel targets that contribute to the development of CML is an important issue.
The basic helix-loop-helix (bHLH) proteins can be categorized into distinct classes based on their biochemical and functional properties (8). Class I HLH proteins, also named E-proteins, are transcriptional regulators that interact with specific DNA sequences, named E2-box sites (9). They are widely expressed but not in a ubiquitous fashion. Class I HLH or E-proteins have the ability to interact with DNA either as homo- or as heterodimers. In vertebrate organisms, 4 E-proteins have been identified. They include E12, E47, HEB, and E2–2 (9). During lymphocyte development, E-proteins form either homodimers or heterodimers among themselves (10). Specifically, in developing B cells, E47 homodimers and E47/E2–2 heterodimers act to induce a B lineage program of gene expression (11, 12). In developing thymocytes, heterodimers of E47 and HEB act in concert to promote developmental progression and regulate cellular expansion (13–15). E2A proteins also act to restrain hematopoietic progenitor cells to develop into the myeloid and natural killer (NK) cell lineages (16, 17).
The DNA binding activities of E-proteins are regulated by another class of HLH proteins, named the Id (inhibitors of differentiation) gene products (8). Four members of the Id gene family are present in the mammalian genome, named Id1–4. Id proteins are HLH proteins but they lack a basic region and, upon interacting with E-proteins, antagonize their DNA binding activity (18). Numerous genetic studies have indicated that E-proteins are the critical Id targets during thymocyte development (8, 17, 19, 20). Id1 and Id2 have also been shown to play critical roles during human myeloid granulopoiesis (21). Specifically, inhibition of Id2 activity blocks the developmental progression of both the eosinophil and neutrophil lineages whereas enforced Id2 expression induced both eosinophil and neutrophil maturation (21).
Here we show that a deficiency in Id2 in a C57BL/6 genetic background leads to abnormalities in early hematopoiesis and to the development of MPD. Specifically, Id2 deficient mice showed increased cellularity within the granulocyte/macrophage progenitors (GMPs) compartment. Substantially higher numbers of maturing neutrophils were detected in the bone marrow and peripheral organs of Id2 deficient mice. Id2 deficient mice developed MPD within 6 months of age and died from overwhelming granulocytosis. In contrast, enforced expression of Id2 interfered with the ability of BCR-ABL to promote MPD. Based on these findings we propose that inhibition of E-protein activity in hematopoietic progenitors suppresses the development of MPD.
Results
Myeloid Development in Id2 Deficient Mice.
Previous studies have established a critical role for Id2 in NK, dendritic, and CD8 cell maturation (22–24). To examine in greater detail the potential roles of Id2 during hematopoiesis, Id2 heterozygous mice were back-crossed for 10 generations into the C57BL/6 background and Id2 null mutant mice were generated. The large majority (95%) of Id2 null mutant mice in a C57BL/6 background died within 1 week after birth. We note that the survival rate of newborn and adult Id2-ablated mice in a C57BL/6 background is distinct from that observed in previous studies in which Id2-deficient mice were examined within the context of a mixed 129/C57BL/6 background (22).
To examine whether Id2 plays a role in early hematopoiesis, bone marrow and peripheral blood was isolated from Id2+/− and Id2−/− 4 weeks old mice and analyzed using immunohistochemistry approaches as well as flow cytometry. May-Grunwald staining revealed an increase in the proportion of neutrophils in both the bone marrow and peripheral blood derived from Id2-ablated mice as compared to Id2+/− mice (Tables S1 and S2). Furthermore, the fraction of eosinophils in the peripheral blood was substantially increased in Id2-deficient mice (Table S2). Consistent with these observations, in Id2-ablated bone marrow, spleen, and peripheral blood, the fraction of maturing neutrophils was substantially increased (Fig. 1A).
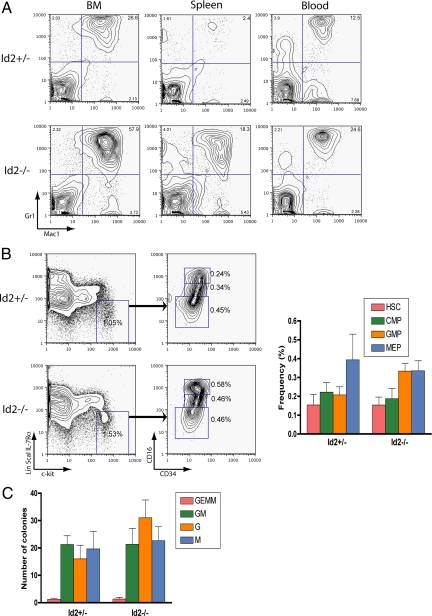
Fig. 1.
Increased neutrophil cellularity in Id2 deficient mice at 3–4 weeks after birth. (A) Bone marrow (BM), spleen, and peripheral blood derived from Id2 deficient mice show expanded myeloid population. Representative flow cytometric profile of hematopoietic cells isolated from bone marrow, spleen, and peripheral blood derived from Id2+/− and Id2−/− mice are shown. Cells were stained with fluorescein isothiocyanate-labeled Mac-1 and phycoerythrin-labeled Gr-1. Note that percentages of Mac-1+Gr-1+ cells are markedly higher in Id2 deficient populations. (B) Effects of Id2 deletion on the numbers of hematopoietic progenitors in adult mouse BM. Total BM cells from Id2+/− and Id2−/− mice were harvested and prepared for analysis by flow cytometry. Shown are representative staining profiles for erythromyeloid progenitors (lin−/Sca-1−/IL-7Rα−/c-kit+) that were gated according to their expression of FcγR and CD34 to obtain the CMP, MEP, and GMP populations. Note the increased proportion of GMPs in the BM of Id2−/− mice. HSC refers to hematopoietic stem cell. CMP refers to common myeloid progenitor. GMP refers to granulocytic/monocytic progenitor. MEP refers to megakaryocytic/erythroid progenitor. (C) Total BM cells (2 × 104) from Id2+/− and Id2−/− mice were plated in methylcellulose-containing media supplemented with the appropriate cytokines for each progenitor type. Standard morphologic criteria were used to score colonies. GEMM refers to granulocyte/erythrocyte, macrophage/megakaryocyte colony-forming units. GM refers to granulocyte, macrophage colony-forming units. G refers to granulocyte colony-forming units. M refers to macrophage colony-forming units.
To determine whether the loss of Id2 affects the development of myeloid progenitors, bone marrow cells were examined for the expression of Sca-1, c-kit, IL-7Rα, CD34 and FcγR by flow cytometry. Id2−/− mice exhibited a 2-fold increase in the relative proportion of GMPs (lin−/Sca-1−/IL-7Rα−/c-kit+/CD34+/FcγRhigh) (Fig. 1B). To further evaluate the number of myeloid and erythroid progenitors in the bone marrow of Id2−/− mice, we performed in vitro colony-forming unit (CFU) assays. In methylcellulose cultures, bone marrow derived from Id2−/− mice showed a slight increase in the number of myeloid (colony-forming unit granulocytes [CFU-G]) colonies as compared to bone marrow isolated from wild-type mice (Fig. 1C). Collectively, these data indicate that the fraction of GMPs is modestly elevated in Id2-deficient bone marrow as compared to Id2+/− bone marrow.
Development of Myeloproliferative Disorder in Id2 Deficient Mice.
Id2 deficient mice that survived beyond 4 weeks after birth were monitored and analyzed in detail. The surviving Id2 null mutant mice were generally runted and displayed distress and ruffled coats 1–3 months after birth. All of the mice that were monitored became moribund by 28 weeks of age. Upon necropsy, the Id2-ablated mice showed grossly enlarged spleens, with a complete destruction of the splenic architecture (Fig. 2A). WBC were found to often invade the liver (Fig. 2 A). WBC counts were substantially elevated (on average 17,000 WBC per μl) in Id2 deficient peripheral blood versus ≈9,000 WBC per μl in Id2 heterozygous mice (Table S2). In contrast, erythroid cellularity in peripheral blood cells was decreased and anemia was observed in all of the animals (Tables S1 and S2). To examine the phenotype of the WBC in greater detail, bone marrow, peripheral blood cells, and splenocytes were isolated from Id2−/− and Id2+/− mice. Cells were stained for the expression of Gr-1 and Mac-1 and analyzed by flow cytometry (Fig. 2B). In Id2-ablated mice, the bone marrow, spleen, and peripheral blood showed substantially increased numbers of mature myeloid cells as compared to Id2+/− mice (Fig. 2B).
Fig. 2.
Id2 deficiency promotes the development of distinct myeloproliferative disorder (MPD) in adult mice. (A) MPD in Id2 deficient mice as examined by hematoxylin and eosin staining. Histology shows massive invasion of matured granulocytes in the liver and bone marrow and destruction of the spleen architecture. (B) Development of MPD in 3-month-old Id2-deficient mice. Representative flow cytometric profile of hematopoietic cells isolated from bone marrow (BM), spleen, and peripheral blood derived from Id2+/− and Id2−/− mice are shown. Cells were examined for the presence of maturing myeloid cells using Mac-1 and Gr-1 staining. Note that percentages of Mac-1+Gr-1+ cells are markedly higher in cells isolated from Id2 deficient mice.
Myeloproliferative Disorder in Id2-Ablated Bone Marrow.
Since the majority of Id2 deficient mice died soon after birth, an extensive analysis using a large cohort of Id2 deficient mice was not possible. To generate a relatively large sample of mice carrying Id2 deficient hematopoietic progenitor cells, we isolated fetal livers from Id2 deficient and control genotypes. Fetal liver cells were subsequently injected into irradiated 129 hosts and monitored for distress over a 7-month period. Recipient mice that were transplanted with cells derived from Id2 ablated fetal livers developed symptoms of distress within 1 to 2 months post transplantation. All 12 recipients that were injected with Id2 deficient fetal liver cells became ill within 30 weeks, with the median age of morbidity being approximately 5 months (Fig. S1A; primary recipients). In contrast, all of the hosts transplanted with Id2+/− fetal liver remained healthy during this period (Fig. S1A; primary recipients).
At autopsy, the mice reconstituted with Id2−/− fetal liver cells showed marked splenomegaly and hepatomegaly. Histopathological analysis of the mice showed extensive infiltration of Gr-1+Mac-1+ cells in the spleen, bone marrow, lung, and liver (Fig. S1B). As expected, the proportion of Gr-1+Mac-1+ cells was substantially increased in Id2-deficient bone marrow, peripheral blood, and spleen as compared to Id2+/− mice (Fig. S1C). Hematological analysis also revealed a 2-fold increase in the number of WBCs (Tables S1 and S2).
To determine whether the MPD observed in Id2 deficient mice can be transferred to secondary recipients, bone marrow cells from diseased animals were transplanted into syngeneic recipients pretreated with a lethal dose of irradiation. In 8 secondary recipients, hematological disease developed with a median range of ≈35 weeks (Fig. S1A and D; secondary recipients). None of the recipients injected with Id2+/− bone marrow cells showed distress. All of the mice transplanted with Id2 deficient bone marrow cells showed extensive accumulations of maturing granulocytes in the bone marrow and peripheral organs (Fig. S1D). Collectively, these data show that the MPD phenotype observed in Id2-ablated mice can be transferred into irradiated recipient mice and that the defect is intrinsic to the hematopoietic lineage.
Forced Id2 Expression Interferes with the Development of BCR-ABL-Mediated Myeloproliferative Disorder.
The observations described above showed that in a C57BL/6 background, a deficiency in Id2 promotes the development of MPD. To determine whether forced Id2 expression interferes with the development of BCR-ABL-mediated MPD, we over-expressed Id2 in conjunction with p210 BCR-ABL in murine bone marrow cells that were transplanted into irradiated hosts. Specifically, retroviral constructs containing p210 BCR-ABL as well as an internal ribosomal entry site that allows the expression of either GFP or Id2 were generated, named p210+GFP and p210+Id2. Bone marrow was isolated from 5-fluorouracil-treated wild-type donors and transduced with virus encoding either p210+GFP or p210+Id2. Transduced bone marrow cells were injected into lethally irradiated BALB/c mice and monitored for distress. Five mice transplanted with bone marrow transduced with p210+GFP developed a fatal CML-like MPD within 4 weeks (Fig. 3A), as previously described (38, 39). As predicted the mice showed massive expansion of maturing myeloid cells in bone marrow, spleen, liver, and peripheral blood (data not shown). The peripheral leukocyte count at death was substantially elevated and composed predominantly of maturing neutrophils (Fig. 3B and data not shown).
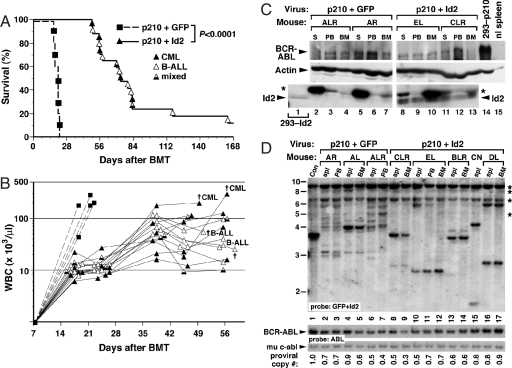
Fig. 3.
Co-expression of Id2 attenuates CML-like myeloproliferative disease induced by p210 BCR-ABL in mice. (A) Kaplan-Meier survival curve for recipients of bone marrow transduced with retrovirus co-expressing p210 BCR-ABL and GFP (dashed line, n = 5) or retrovirus co-expressing p210 and Id2 (solid line, n = 17). Individual mice are indicated by the symbols (squares = p210+GFP, triangles = p210+Id2), while the phenotype of the leukemia that each recipient developed is indicated by the shading (black = CML-like MPD, white = B-cell acute lymphoblastic leukemia (B-ALL), black/white = both diseases simultaneously). The difference in survival between the 2 cohorts was highly significant (P < 0.0001, Mantel-Cox test). The curve is a composite of 2 independent bone marrow transplantation experiments that gave similar results. (B) Scatter plot of peripheral blood leukocyte counts (y axis, logarithmic scale) versus time after transplantation (x axis) for a subset of the recipients in A. Symbols are the same as in panel A. The demise of several individual mice during this time interval and their leukemic diagnoses are indicated by the crosses. The sampling dates for several mice were altered on the graph by 1–2 days for clarity. (C) Western blot analysis of primary myeloid cell extracts from recipients of BCR-ABL-transduced BM. (Top) BCR-ABL levels, (middle) actin loading control, (bottom) Id2 levels. The pair of panels (p210+GFP and p210+Id2) for each antibody is from the same exposure of the same blot. Lane 1 is an extract from 293 cells transfected with an Id2 expression construct, demonstrating the position of Id2 protein. The asterisk indicates a non-specific band. Lanes 2–4 and 5–7 are extracts from spleen (S), peripheral blood (PB), and bone marrow (BM) from 2 representative mice with CML-like disease induced by p210+GFP retrovirus vector, while lanes 8–10 and 11–13 are from 2 representative mice with disease induced by p210+Id2 vector. ALR and AR refer to mice transplanted with hematopoietic progenitors expressing p210+GFP. EL and CLR refer to mice transplanted with hematopoietic progenitors expressing p210+Id2. Mouse EL was killed for analysis on day 29 posttransplant with moderate leukocytosis and splenomegaly, while mouse CLR died of CML-like leukemia. Lane 14 is an extract from 293 cells expressing p210 BCR-ABL, while lane 15 is an extract from normal mouse splenocytes. Note the considerable overexpression of Id2 protein (detectable here as 2 distinct species) in recipients of p210+Id2 virus-transduced marrow. (D) Southern blot analysis of proviral integration. Genomic DNA from spleen (spl), peripheral blood (PB), or BM of leukemic mice was analyzed by Southern blot with a mixture of GFP and Id2 probes to detect distinct proviral integration events (top panel), and subsequently with an ABL probe (bottom panels) to determine the total proviral content of each sample (40). The bands indicated by asterisks (right) arise from the murine Id2 gene. Con (lane 1) is control DNA from a cell line containing a single BCR-ABL provirus. Lanes 2–7 show the clonality of CML-like leukemia in p210+GFP recipients, while lanes 8–17 are representative leukemia samples from p210+Id2 recipients. ALR, AL and AR refer to mice transplanted with hematopoietic progenitors expressing p210+GFP. CLR, EL, BLR and DL refer to mice transduced with hematopoietic progenitors carrying p210+Id2.
Strikingly, none of the 17 recipients transplanted with hematopoietic progenitors that expressed p210+Id2 showed distress 4 weeks post injection (Fig. 3A). However, 3 months post transplantation, the majority of the reconstituted mice transplanted with bone marrow cells carrying p210+Id2 developed disease with a median survival period of 11 weeks (Fig. 3A). Several of the mice transplanted with hematopoietic progenitors expressing p210+Id2 developed MPD 5 weeks posttransplantation, with increased WBC (16–115 × 103/μl) and splenomegaly. However, the development of disease was substantially delayed, and only a minor fraction of the recipients died because of complications of CML-like disease (Fig. 3A). Rather, the large majority of p210+Id2 recipients developed B-cell acute lymphoblastoid leukemia (B-ALL) or a mixture of MPD and B-ALL (Fig. 3 A and B). We also observed that the bone marrow of mice transduced with the hematopoietic progenitors that expressed p210+Id2 showed an increase in the number of eosinophils similar as described for human CD34+ cells transduced with virus expressing Id2 (6.1 ± 1.3% for Id2-p210 BCR-ABL versus 0.3 ± 0.3% for BCR-ABL) (21).
To determine that bone marrow cells transduced with p210+Id2 express both proteins, cells were isolated and analyzed for protein expression using Western blotting. BCR-ABL p210 in bone marrow cells transduced with p210+Id2 virus was expressed at levels comparable to that of cells transduced with p210+GFP (Fig. 3C). As expected, Id2 expression was also readily detectable in extracts derived from cells transduced with p210+Id2 (Fig. 3C). We also examined the efficiency of p210+GFP and p210+Id2 viral transduction in different tissues by Southern Blot analysis of genomic DNA using GFP plus Id2 or ABL probes (Fig. 3D). The number of proviral copies was similar in p210+GFP and p210+Id2 transduced cells (Fig. 3D). Collectively, these observations indicate that the absence of CML-like MPD in p210+Id2 transduced bone marrow cells was not caused by lower levels of p210 expression and/or engraftment of BCR-ABL-expressing leukemic cells. Rather, enforced expression of Id2 delays the onset of BCR-ABL mediated MPD.
These data raised the possibility that BCR-ABL acts to promote the development of CML-like disease by modulating Id2 expression. To test this possibility directly, GMPs of normal mice were sorted and infected with empty vector “control” alone or BCR-ABL p210 retrovirus. Two days posttransduction, the GFP positive cells were sorted, mRNAs isolated and examined for Id1–3 expression by real-time PCR. Id1 expression was elevated as previously reported, Id3 levels were substantially lowered but Id2 abundance was only modestly reduced (ref. 25 and data not shown).
In summary, these data demonstrate that forced Id2 expression interferes with the development of BCR-ABL-induced CML-like disease. However, since BCR-ABL expression does not substantially interfere with Id2 expression in GMPs, the data suggest that BCR-ABL and Id2 do not act in a linear pathway to promote the development of MPD.
Discussion
Previous observations have indicated that the E-proteins act to modulate the development of the lymphoid and myeloid lineages (8). The E-proteins also act as proto-oncogenes and as tumor suppressors. In pro-B ALL, the E2A bHLH domain is replaced with a leucine zipper domain derived from HLF, generating an E2A/HLF fusion protein (26, 27). In pre-B ALL, the N-terminal E2A domains are fused to a homeodomain-containing protein (Pbx-1) resulting in the aberrant expression of E2A/Pbx-1 (27, 28). Both E2A/HLF and E2A/Pbx1 have been demonstrated to play critical roles in the development of pro-B and pre-B ALL, respectively. E2A and HEB also function as tumor suppressors since E2A- and HEB-deficient mice rapidly develop T cell lymphoma (29, 30). A significant fraction of patients with human acute T lymphoblastic leukemia carry chromosomal translocations that involve the TAL-1 and TAL-2 genes and there is now ample evidence indicating that the TAL gene products cause lymphoma by antagonizing E-protein mediated transactivation (31). Interestingly, forced Id2 expression in developing thymocytes promotes the development of T cell lymphoma with similar kinetics and characteristics as described for E2A-and HEB-ablated T cell malignancies (32). These data suggested that E2A and/or HEB are the critical targets for Id2-mediated lymphomagenesis. Here we demonstrate a role for Id2 as a potential tumor suppressor. Whereas Id2 acted to suppress the development of MPD, forced Id2 expression interfered with the development of BCR-ABL-mediated MPD.
A large number of studies have reported on the role of Id2 in hematopoiesis (8). However, this is the first study indicating a role for Id2 in suppressing the development of MPD. This brings into question as to why other studies failed to observe a role for Id2 in promoting myeloid hyperplasia. We would like to suggest that differences in the genetic backgrounds, C57BL/6 versus 129, likely are the cause for these apparent differences. The Id2 deficient mice that were generated in our studies were backcrossed 10 times into the C57BL/6 mice. It is these mice that develop MPD within a 3- to 6-month period. When the Id2 deficiency was backcrossed into the 129 strain, the development of MPD was not observed within this time frame. Hence, these observations suggest that modifier alleles must exist within the 129 and/or C57BL/6 background that modulate the potential activity of Id2 as a tumor suppressor.
The data also bring into question as to how a deficiency of Id2 contributes to the development of MPD. Previous studies have demonstrated that constitutive expression of Id2 in human hematopoietic progenitors acts to accelerate the developmental progression of neutrophils, whereas suppression of Id2 activity blocks neutrophil maturation (21). In contrast, we show here that Id2 overexpression impairs BCR-ABL-driven neutrophil production, suggesting that the mechanisms controlling normal and leukemic myelopoiesis are distinct.
E-proteins have been shown to control cell growth in developing hematopoietic cells. Both cell survival and cell cycle progression are modulated by E-protein activity. High levels of E2A promote cell death through a mechanism mediated by the Bcl-2 pathway (33). In contrast, low abundance of E2A activity in pro-B cells results in cell death, through a mechanism yet to be uncovered (33). Overexpression of E47 in fibroblasts and lymphoid cell lines has been shown to both promote and antagonize cell cycle progression (34–36). E47 has been shown to directly regulate cdk6, c-myc, p21, and cyclin D3 transcription, suggesting that E47 promotes cell cycle progression by controlling the expression of cell cycle regulators (37). The paradoxical effects of E47 on cell survival and cell cycle progression may relate to the differences in genes activated during developmental progression. Thus, it is conceivable that Id2 expression in myeloid progenitors acts to suppress the activity of E-proteins to modulate cellular expansion.
Another interesting possibility is that forced Id2 expression interferes with the development of BCR-ABL-induced MPD by suppressing the self-renewal activity of the leukemic stem cell. Consistent with this scenario are our recent observations indicating that E-protein activity is required to promote the stem cell self-renewal activity of HSCs (C. Semerad, E. Mercer, and C.M. Murre, unpublished observations). We favor this mechanism and it will be particularly important to determine whether E-protein activity is required to permit the aberrant self-renewal activity of GMPs that carry the BCR-ABL chromosomal translocation.
Our data raise the question whether BCR-ABL modulates Id2 expression to promote MPD. We consider this unlikely since Id2 levels were not substantially reduced in GMPs that express BCR-ABL. Interestingly, however Id1 abundance was increased whereas Id3 levels were substantially reduced in GMPs that express BCR-ABL. How the modulation of Id expression by BCR-ABL modulates E-protein activity is unclear and further studies will be necessary to establish the link between BCR-ABL and E-proteins. Which of the E-proteins, E12, E47, HEB or E2–2, is required to promote BCR-ABL-mediated MPD? The function of E-proteins in early hematopoiesis and their roles in leukemia remain to be elucidated. It will be particularly important, however, to address this issue and examine the ability of BCR-ABL to promote MPD in E2A, HEB, and E2–2 single- and compound-ablated backgrounds. Regardless of the precise mechanism by which forced expression of Id2 interferes with p210 BCR-ABL-mediated leukemic transformation, the data described here show that the E- and Id-proteomes are potentially appealing novel targets for the treatment of BCR-ABL-mediated MPD.
Materials and Methods
Viral Transduction of Bone Marrow.
To generate the p210+Id2 retrovirus vector, the GFP gene was removed from pMIG R1 (38) and a 1.5 kb murine ID2 cDNA inserted 3′ to the internal ribosome entry site (IRES), followed by introduction of the cDNA for p210 BCR-ABL in the position 5′ to the IRES. High-titer, replication-defective ecotropic retroviral stocks were generated by transient transfection of 293 cells using the kat system as described (39) and titered by Southern blot analysis of genomic DNA from cells transduced with serial dilutions of virus. Retroviral stocks were matched for titer and gave equivalent transduction efficiency in 3T3 cells (data not shown). Bone marrow transduction and transplantation was carried out using 5-fluorouracil-treated (200 mg/kg) male donor and lethally irradiated (900 cGy) female recipient BALB/c mice (Taconic Farms) as described (40, 41).
Supplementary Material
Acknowledgments.
We thank members of the Murre laboratory for stimulating discussion. We thank Andrea Schnitz for editing the manuscript. This study was supported by funds from the Leukemia and Lymphoma Society (to R.A.V.E.) and the National Institutes of Health (R.A.V.E. and C.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805073105/DCSupplemental.
References
- 1.van Etten RA, Shannon KM. JAKing up hematopoietic proliferation. Cancer Cell. 2004;6:547–552. doi: 10.1016/j.ccr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, et al. The biology of chronic myelogenous leukemia. N Eng J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 3.van Etten RA. Aberrant cytokine signaling in leukemia. Oncogene. 2007;26:6738–6749. doi: 10.1038/sj.onc.1210758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Klein A, van Kessel AG, Grosveld G, Bartram CR, Hagemeijer A, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukemia. Nature. 1982;300:765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- 5.Daley GQ, van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 6.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr/Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 7.Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–283. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- 8.Murre C. Helix-loop-helix proteins in lymphocyte development. Nat Immunol. 2005;6:1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 9.Massari ME, Murre C. Helix-loop-helix proteins: Regulators of transcription in eukaryotic organisms. Mol Cell Biol. 2000;20:429–40. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain G, Cravatt C, Loomans C, Alberola-Ila J, Hedrick SM, et al. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat Immunol. 2001;2:165–171. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- 11.Bain G, Maandag EC, Izon DJ, Armsen D, Kruisbeek AM, et al . E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang Y, Soriano P, Weintraub H . The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 13.Heemskerk MH, Blom B, Nolan G, Stegmann AP, Bakker AQ, et al. Inhibition of T cell and promotion of natural killer cell development by the dominant negative factor ID3. J Exp Med. 1997;186:1597–1602. doi: 10.1084/jem.186.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel I, Johns C, Bain G, Rivera RR, Murre C. Inhibition of T cell and promotion of natural killer cell development by the dominant negative helix loop helix factor Id3. J Exp Med. 2001;194:733–745. [Google Scholar]
- 15.Barndt RJ, Dai M, Zhuang Y. Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol Cell Biol. 2000;20:6677–6685. doi: 10.1128/mcb.20.18.6677-6685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikawa T, Kawamoto H, Goldrath AW, Murre C. E-proteins and Notch signaling cooperate to promote T cell lineage specification and commitment. J Exp Med. 2006;203:1329–1342. doi: 10.1084/jem.20060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benezra R, Davis RL, Lassar A, Tapscott S, Thayer M, et al. Id: A negative regulator of helix-loop-helix DNA binding proteins. Control of terminal myogenic differentiation. Cell. 1990;61:49–59. doi: 10.1111/j.1749-6632.1990.tb42359.x. [DOI] [PubMed] [Google Scholar]
- 19.Rivera RR, Johns CP, Quan J, Johnson RS, Murre C. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity. 2000;12:17–26. doi: 10.1016/s1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- 20.Yan W, Young AZ, Soares VC, Kelley R, et al . High incidence of T-cell tumors in E2A-null mice and E2A/Id1 double-knockout mice. Mol Cell Biol. 1997;17:7317–7327. doi: 10.1128/mcb.17.12.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buitenhuis M, van Deutekom HW, Verhagen LP, Castor A, Jacobsen SW, et al . Differential regulation of granulopoiesis by the basic helix-loop-helix transcriptional inhibitors Id1 and Id2. Blood. 2005;105:4272–4281. doi: 10.1182/blood-2004-12-4883. [DOI] [PubMed] [Google Scholar]
- 22.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, et al . Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 23.Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 24.Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, et al. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- 25.Nowicki MO, Pawlowski P, Fischer T, Hess G, Pawlowski T, et al. Chronic myelogenous leukemia molecular signature. Oncogene. 2003;22:3952–3963. doi: 10.1038/sj.onc.1206620. [DOI] [PubMed] [Google Scholar]
- 26.Look AT. E2A-HLF chimeric transcription factors in pro-B cell acute lymphoblastic leukemia. Curr Top Microbiol Immunol. 1997;220:45–53. doi: 10.1007/978-3-642-60479-9_3. [DOI] [PubMed] [Google Scholar]
- 27.Kamps MP, Murre C, Sun X, Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990;60:547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- 28.Nourse J, Mellentin JD, Galili N, Wilkinson J, Stanbridge E, et al. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990;60:535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- 29.Bain G, Engel I, Robanus Maandag EC, te Riele HPJ, Voland JR, et al. E2A deficiency leads to abnormalities in alphabeta T-cell development and to rapid development of T-cell lymphomas. Mol Cell Biol. 1997;17:4782–4791. doi: 10.1128/mcb.17.8.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neil J, Shank J, Cusson N, Murre C, Kelliher M. TAL1/SCL induces leukemia by inhibiting the transcriptional activity of E47/HEB. Cancer Cell. 2004;5:587–596. doi: 10.1016/j.ccr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Ferrando AA, Look AT. Gene expression profiling in T-cell acute lymphoblastic leukemia. Semin Hematol. 2003;40:274–280. doi: 10.1016/s0037-1963(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 32.Morrow MA, Mayer EW, Perez CA, Adlam M, Siu G. Overexpression of the Helix-Loop-Helix protein Id2 blocks T cell development at multiple stages. Mol Immunol. 1999;36:491–503. doi: 10.1016/s0161-5890(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 33.Engel I, Murre C . Ectopic expression of E47 or E12 promotes the death of E2A-deficient lymphomas. Proc Natl Acad Sci USA. 1999;96:996–1001. doi: 10.1073/pnas.96.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engel I, Murre C. E2A proteins enforce a proliferation checkpoint in developing thymocytes. EMBO J. 2004;23:202–211. doi: 10.1038/sj.emboj.7600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao F, Vilardi A, Neely RJ, Choi JK. Promotion of cell cycle progression by basic helix-loop-helix E2A. Mol Cell Biol. 2001;21:6346–6357. doi: 10.1128/MCB.21.18.6346-6357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peverali FA, Ramqvist T, Saffrich R, Pepperkok R, Barone MV, et al. Regulation of G1 progression by E2A and Id helix-loop-helix proteins. EMBO J. 1994;13:4291–4301. doi: 10.1002/j.1460-2075.1994.tb06749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz R, Engel I, Fallahi-Sichani, Petrie HT, Murre C. Gene expression patterns define novel roles for E47 in cell cycle progression, cytokine-mediated signaling, and T lineage development. Proc Natl Acad Sci USA. 2006;103:9976–9981. doi: 10.1073/pnas.0603728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pear WS, Miller JP, Xu L, Pui JC, Soffer B, et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 39.Million RP, Aster J, Gilliland DG, van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. Blood. 2002;99:4568–4577. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S, Ilaria RL, Million RP, Daley GQ, van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roumiantsev S, de Aos IE, Varticovski L, Ilaria RL, van Etten RA. The src homology 2 domain of Bcr/Abl is required for efficient induction of chronic myeloid leukemia-like disease in mice but not for lymphoid leukemogenesis or activation of phosphatidylinositol 3-kinase. Blood. 2001;97:4–13. doi: 10.1182/blood.v97.1.4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.