Abstract
Acute myocardial infarction (MI) due to coronary artery occlusion is accompanied by a pathological remodeling response that includes hypertrophic cardiac growth and fibrosis, which impair cardiac contractility. Previously, we showed that cardiac hypertrophy and heart failure are accompanied by characteristic changes in the expression of a collection of specific microRNAs (miRNAs), which act as negative regulators of gene expression. Here, we show that MI in mice and humans also results in the dysregulation of specific miRNAs, which are similar to but distinct from those involved in hypertrophy and heart failure. Among the MI-regulated miRNAs are members of the miR-29 family, which are down-regulated in the region of the heart adjacent to the infarct. The miR-29 family targets a cadre of mRNAs that encode proteins involved in fibrosis, including multiple collagens, fibrillins, and elastin. Thus, down-regulation of miR-29 would be predicted to derepress the expression of these mRNAs and enhance the fibrotic response. Indeed, down-regulation of miR-29 with anti-miRs in vitro and in vivo induces the expression of collagens, whereas over-expression of miR-29 in fibroblasts reduces collagen expression. We conclude that miR-29 acts as a regulator of cardiac fibrosis and represents a potential therapeutic target for tissue fibrosis in general.
Acute myocardial infarction (MI) due to coronary artery occlusion represents a major cause of morbidity and mortality in humans (1). The loss of blood flow to the left ventricular free wall of the heart after MI results in death of cardiomyocytes and impaired cardiac contractility. Scar formation at the site of the infarct and interstitial fibrosis of adjacent myocardium prevent myocardial repair and contribute to loss of pump function and susceptibility to arrhythmias (2). The extracellular matrix (ECM) is a dynamic microenvironment that contributes to adverse ventricular remodeling after MI, via activation of profibrotic pathways and matrix metalloproteinases that enhance collagenase activity (3). Phenotypically transformed fibroblast-like cells, termed myofibroblasts, are primarily responsible for fibrous tissue formation at the site of infarction (4). Thus, reversal of this process represents an important therapeutic target in post-MI management and heart failure.
Recently, we and others described key roles of microRNAs (miRNAs) in cardiac hypertrophy and heart failure, pointing to a new mode of regulation of cardiac disease (5–12). MiRNAs are short, ≈22-nucleotide RNAs that modulate gene expression by base pairing with the 3′ untranslated regions (UTRs) of mRNAs and inhibiting translation or promoting mRNA degradation (13). Targeting of a miRNA to a mRNA requires a “seed” sequence consisting of seven nucleotides at the 5′ end of the miRNA. Additional sequence complementarity influences target recognition, as does secondary structure of surrounding regions of the mRNA (14, 15). There are estimated to be as many as 1,000 miRNAs encoded by the human genome (16). Individual miRNAs can target numerous mRNAs and individual mRNAs can be targeted by multiple miRNAs, providing vast regulatory potential.
In an effort to identify miRs with potential roles in post-MI cardiac remodeling, we examined the effect of surgical MI in mice on the expression patterns of miRs in the heart. Here, we describe a collection of miRs, referred to as MImiRs, which are dysregulated in mouse and human hearts after MI. Among these MImiRs, we found miR-29 to be dramatically down-regulated in the region of the fibrotic scar after MI. We show that miR-29 acts in cardiac fibroblasts to negatively regulate a plethora of mRNAs encoding various collagens and other extracellular matrix proteins in vivo and in vitro. The down-regulation of miR-29 provides a mechanistic basis for cardiac fibrosis and suggests that strategies to enhance miR-29 expression may have therapeutic value in the setting of post-MI cardiac remodeling and other disorders associated with tissue fibrosis.
Results
Identification of MImiRs.
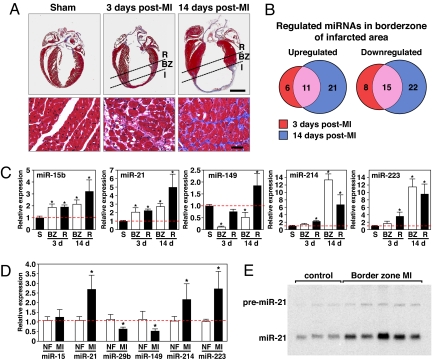
In an effort to identify miRNAs involved in post-MI remodeling, we induced MI by occlusion of the left coronary artery (LCA) and compared miRNA expression profiles in mouse hearts 3 and 14 days after MI in both the border zone of the infarcted region and the noninfarcted (remote) myocardium with the miRNA expression profile of sham-operated animals [Fig. 1 A and B, supporting information (SI) Fig. S1, and Tables S1 and S2]. Among 569 individual miRNAs represented on the microarrays, 40 miRNAs were significantly regulated in the border zone of the infarcted region 3 days after MI (17 miRNAs showed an increase in expression ≥2-fold and 23 miRNAs showed a ≥2-fold decrease in expression). In addition, 22 miRNAs appeared to be changed in the remote myocardium (12 miRNAs showed an increase in expression ≥2-fold, and 10 miRNAs showed a ≥2-fold decrease in expression) (Tables S1 and S2). Two weeks after MI, 69 miRNAs were regulated ≥2-fold in the border zone of the infarcted region, whereas 40 miRNAs showed a ≥2-fold change in the remote myocardium (Tables S1 and S2). The array data were confirmed by real-time PCR analysis, using miRNA-specific probes (Fig. 1C).
Fig. 1.
MiRNA profiling in response to MI. (A) Masson Trichrome staining of mouse heart sections shows early scar formation 3 days after MI, with myocyte hypertrophy, loss of myocytes, and collagen deposition. Fourteen days after MI, there is a thin stretched infarct that results in cardiac hypertrophy and interstitial fibrosis in the border zone of the infarcted region. I, infarct; BZ, borderzone; R, remote myocardium. (Lower) Higher magnification of the borderzone regions of the infarcted hearts and the comparable level of the sham operated heart. (Scale bars: Upper, 2 mm; Lower, 20 μm). (B) Microarray analysis reveals miRNAs are dynamically regulated in response to MI. Even after initial infarct healing, 14 days after MI, 11 miRs are overlappingly up-regulated, whereas 15 miRs are down-regulated. The number of miRNAs regulated ≥2-fold in each category is shown. (C) Real-time PCR analysis confirms the regulation of specific miRNAs in response to MI compared with sham operated animals (n = 3–4. S, sham; BZ, borderzone; R, remote. *, P < 0.05 compared with sham operated animals). (D) Real-time PCR analysis shows the regulation of miRNAs in human heart samples in response to MI compared with nonfailing hearts (n = 5–6, NF = nonfailing, MI = myocardial infarction). *, P < 0.05 compared with nonfailing hearts. (E) Northern blot analysis of three nonfailing human hearts and five human hearts after MI indicates a consistent increase in miR-21 in the borderzone of human heart samples in response to MI.
To examine the regulation of these miRNAs in human hearts, we obtained cardiac tissue from the border zone of the infarcted region from patients receiving cardiac transplant. Real-time PCR analysis confirmed that several of the regulated miRNAs in the murine MI model were regulated similarly in human hearts. MiR-21, miR-214, and miR-223 showed a striking increase in expression in the border zone of the infarct, whereas we found miR-29b and miR-149 to be significantly down-regulated (Fig. 1D). Northern blot analysis of miR-21 verified the real-time expression data (Fig. 1E). These results reveal a collection of miRNAs, which we refer to as MImiRs, which are dysregulated during cardiac remodeling in response to ischemia.
Down-regulation of miR-29 Expression After MI.
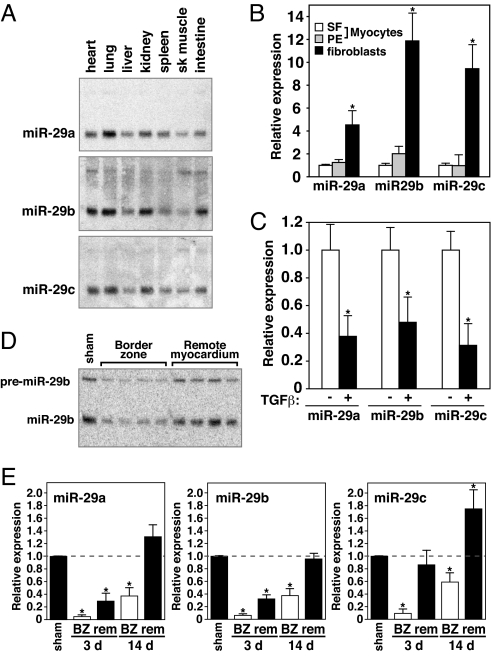
Among the MImiRs, all three members of the miR-29 family were down-regulated in response to MI. This miRNA family consists of three members expressed from two bicistronic miRNA clusters. MiR-29b-1 is coexpressed with miR-29a, whereas the second copy of miR-29b (miR-29b-2) is coexpressed with miR-29c. All family members share a conserved seed region and miR-29a and miR-29c differ by only one base from the miR-29b sequence (Fig. S2). Northern blot analysis of multiple mouse tissues indicated a comparable expression pattern for all three miR-29 family members with highest expression in the lung, kidney, and heart (Fig. 2A). By isolating cardiac myocytes and fibroblasts, we found that miR-29 was expressed preferentially in the fibroblast population. Compared with the expression level in cardiomyocytes, either kept in serum-free medium or stimulated with the hypertrophic agonist phenylephrine (PE), the level of expression of miR-29 family members was 5- to 12-fold higher in cardiac fibroblasts (Fig. 2B).
Fig. 2.
Down-regulation of miR-29 in the infarcted region after MI. (A) Northern blot analysis of mouse tissues indicates a large overlap in expression of all three miR-29 members, with highest expression in lung, heart, and kidney. Of the miR-29 members, miR-29b appeared most highly expressed in the heart. (B) Real-time PCR analysis indicated all three members of the miR-29 family to be highly expressed in fibroblasts compared with cardiomyocytes either under serum free conditions (SF) or stimulated with phenylephrine (PE). Comparable amounts of RNA were used in each reaction. *, P < 0.05 compared with untreated myocytes. (C) Real-time PCR analysis indicates that all three miR-29 family members are down-regulated in fibroblasts after exposure to TGFβ for 48 h. *, P < 0.05 compared with untreated fibroblasts. (D) Northern blot analysis on mouse cardiac tissue 3 days after MI shows a consistent down-regulation of miR-29 in response to MI compared with sham-operated animals. Down-regulation is more pronounced in the borderzone than in the remote myocardium. The Northern blot shows the level of miR-29b in four different animals both in the borderzone and the corresponding remote area. (E) Real-time analysis indicates all miR-29 members to be regulated in response to MI. Whereas the down-regulation is most pronounced in the border zone (BZ) of the infarct 3 days after MI, this down-regulation remains present even after initial infarct healing has taken place. (n = 3–4 per group. BZ, borderzone; rem, remote. *, P < 0.05 compared with sham operated animals.)
Cardiac fibrosis is a major aspect of the remodeling process after MI (17). The proliferation of fibroblasts and increased deposition of ECM components results in myocardial stiffness and diastolic dysfunction. Transforming growth factor beta (TGFβ) has been shown to play a dominant role in the production and deposition of collagens in the heart and induces a transformation of fibroblasts into myofibroblasts (18). Real-time PCR analysis on cardiac fibroblasts exposed to TGFβ revealed a decrease in miR-29 expression, suggesting that the decrease in miR-29 after MI might be TGFβ-regulated (Fig. 2C).
Northern blot analysis of miR-29b expression in both the border zone of the infarcted area and the remote myocardium in four different animals verified a consistent decrease in expression in response to MI. Compared with the baseline level and the expression in the remote myocardium, the level of miR-29b was consistently down-regulated in the border zone 3 days after MI (Fig. 2D). Real-time RT-PCR analysis further confirmed the decrease in expression of all three members of the miR-29 family within 3 days after MI. However, by day 14, when the infarct had healed and secondary remodeling was underway, miR-29 expression remained decreased in the border zone adjacent to the infarct (Fig. 2E).
MiR-29 Regulates the Expression of Fibrotic Genes.
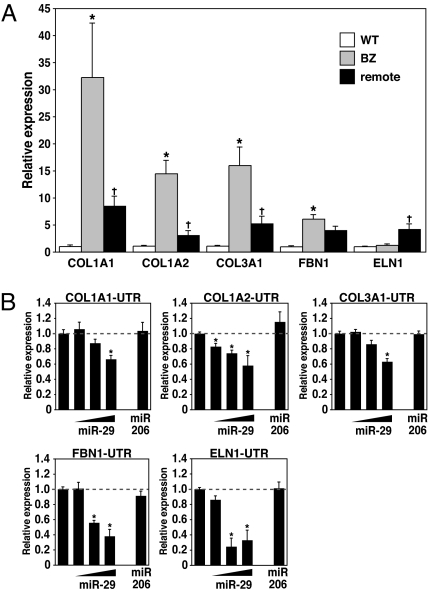
To begin to define the possible functions for miR-29 in the heart after MI, we made use of computational predictions to identify possible miR-29 targets. The Targetscan prediction program indicated an unexpectedly high number of fibrosis-related mRNAs encoding collagens, metallopeptidases, and integrins as possible targets for miR-29 (www.targetscan.org). To determine whether the down-regulation of miR-29 might regulate cardiac fibrosis, we focused on predicted targets implicated in ECM production in heart. Elastin (ELN), fibrillin 1 (FBN1), collagen type I, alpha 1 and 2 (COL1A1, COL1A2) and collagen type III, alpha 1 (COL3A1) all contain one or more conserved potential seed sequences for miR-29 (Fig. S3A).
Because miRNAs down-regulate the steady state levels, and the translation, of their target mRNAs (19, 20), we analyzed the expression of predicted miR-29 mRNA targets in cardiac samples 3 days after MI by real-time RT-PCR. As shown in Fig. 3A, the down-regulation of miR-29 correlated with the up-regulation of COL1A1, COL1A2, COL3A1, and FBN1 in the infarcted region. ELN expression was unchanged in the border zone but increased in the remote myocardium (Fig. 3A).
Fig. 3.
miR-29 regulates extracellular matrix protein mRNAs. (A) Real-time PCR analysis of predicted target genes in both the borderzone and remote myocardium 3 days after MI shows a decrease in miR-29 to correlate with an increase in collagens (COL1A1, COL1A2, and COL3A1) and fibrillin (FBN1), whereas there was no significant change in elastin (ELN1). (n = 3–4 per group. BZ, borderzone. *, P < 0.05 compared with sham operated animals, + P < 0.05 compared with BZ region). (B) COS cells were transiently transfected with luciferase reporters (100 μg) linked to the 3′UTR sequences of the indicated genes. Increasing amounts of the miR-29b-1/miR-29a cluster (25–50-100 μg) repress the expression of luciferase, whereas this decrease was absent when using an unrelated miR, miR-206 (50 μg). *, P < 0.05 compared with luciferase reporter alone.
Using a CMV-driven expression plasmid, we overexpressed miR-29b-1 and miR-29a in COS cells (Fig. S3B) with luciferase expression plasmids containing the 3′-UTRs of the predicted miR-29 targets. Increasing amounts of CMV-driven miR-29b-1/miR-29a resulted in a dose-dependent decrease in luciferase activity, whereas comparable amounts of a miR-206 expression plasmid, as a negative control, had no effect (Fig. 3B and Table S3), substantiating these mRNAs as targets for repression by miR-29.
Inhibition of miR-29 in Vivo Induces Collagen mRNA Expression.
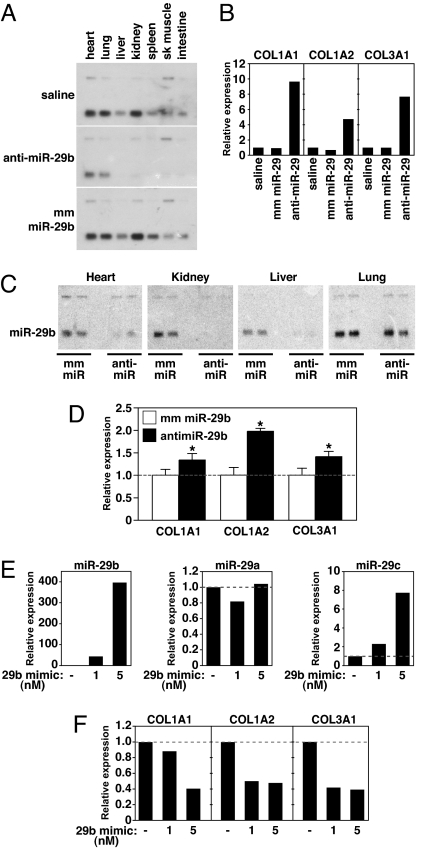
To further explore the potential role of miR-29 as a negative regulator of collagen expression, we knocked down miR-29b in vivo, using cholesterol-modified oligonucleotides (21, 22) complementary to the mature miRNA sequence of miR-29b (anti-miR-29b) and either saline or an oligonucleotide containing a four base mismatch (mm miR-29b) as a negative control (Fig. S4). Three days after a single tail vein injection of anti-miR-29b (80 mg/kg), we observed a dramatic diminution of miR-29b expression in all tissues examined (Fig. 4A). In contrast, a comparable dose of the mm miR-29b oligonucleotide had no effect on the expression level of miR-29b compared with the saline control. Knockdown by anti-miR-29b appeared to be specific to the mature miRNA, because the level of premiRNA remained comparable between anti-miR and mm treated animals. Although the knockdown in the liver and kidney appeared to be nearly complete, a low level of miR-29b remained detectable in the heart and lung (Fig. 4A).
Fig. 4.
miR-29 inhibition induces fibrosis in vivo. (A) Northern blot analysis showing knockdown of miR-29b expression three days after i.v. injection of 80 mg/kg of either anti-miR-29 or mm miR-29 or a comparable volume of saline. (B) Real-time PCR analysis of liver extracts reveals a pronounced increase in collagen expression in response to miR-29 knockdown, whereas this effect was absent after saline or mm injection. (C) Northern blot of the indicated tissues 3 weeks after i.v. injection with 80 mg/kg on two consecutive days of either anti-miR-29 or mm miR-29 oligonucleotide or a comparable volume of saline. miR-29 is nearly abolished in the liver, heart and kidney, whereas miR-29 levels in lung appear unaffected by anti-miR-29. (D) Real-time PCR analysis of heart extracts indicates an increase in cardiac collagen expression in response to miR-29 knockdown. (n = 2 per group, *, P < 0.05 compared with mm treated animals). (E) Cardiac fibroblasts were either left untreated or treated with 1 or 5 nM miR-29b mimic for 48 h. Real-time PCR analysis indicates an increase in miR-29b expression in fibroblasts two days after miR-29b mimic treatment, whereas miR-29a levels were unchanged and miR-29c levels only slightly increased. (F) Cardiac fibroblasts that were either left untreated or treated with 1 or 5 nM miR-29b mimic for 48 h show a decrease in expression of collagen genes in response to increased levels of miR-29b as determined by real-time PCR analysis.
Because the other miR-29 members share high sequence homology with miR-29b, we also examined the expression of miR-29a and -c in response to miR-29b anti-miR. Although we could detect a significant knockdown in liver and kidney (especially for miR-29c), cardiac expression did not appear to change (Fig. S5). Real-time PCR analysis indicated that miR-29b knockdown was sufficient to cause the up-regulation of collagen mRNAs in the liver, whereas this effect was absent in the mismatch controls (Fig. 4B). These data indicate that miR-29 indeed functions as a negative regulator of collagen gene expression in vivo and thereby influences collagen deposition and fibrosis.
To enhance cardiac knockdown, we injected 80 mg/kg of oligonucleotide on two consecutive days and collected tissues 3 weeks later. Northern blot analysis indicated complete knockdown of miR-29b in kidney and liver in response to anti-miR-29b compared with the expression level seen after mm miR-29b injection. Cardiac levels of miR-29b were also reduced, whereas the expression of miR-29b in lung appeared unaffected by anti-miR-29b (Fig. 4C). Collagen expression in the heart was modestly increased in response to miR-29b inhibition (Fig. 4D). We believe the greater induction of collagen expression in the liver compared with heart reflects, at least in part, the reduced efficiency of miR-29 knockdown in the heart by anti-miR-29b.
Down-Regulation of Collagen mRNA Expression with a miR-29 Mimic.
To determine whether overexpression of miR-29 was capable of reducing collagen expression, we exposed fibroblasts to a miR-29b mimic. The level of miR-29b expression in fibroblast cultures increased by as much as 400-fold after 3 days of exposure to miR-29b mimic (Fig. 4E). miR-29a expression was unaffected and miR-29c expression was increased only slightly by miR-29b mimic (Fig. 4E). Real-time PCR analysis indicated that the expression of collagen transcripts was diminished in response to miR-29b mimic (Fig. 4F). However, the magnitude of the decrease in collagen expression was modest compared with the increase in expression of miR-29b, suggesting that miR-29 levels are not the sole determinant of collagen mRNA expression (see Discussion).
Discussion
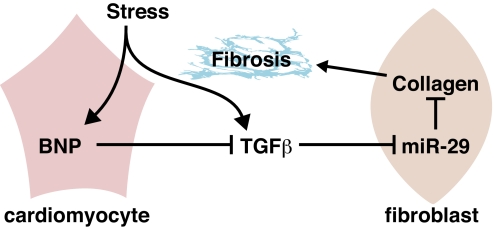
The results of this study reveal a collection of miRNAs that are dysregulated in the heart during post-MI remodeling. Among these MImiRs are members of the miR-29 family, which are predicted to function as inhibitors of numerous mRNAs involved in ECM production and fibrosis. Members of the miR-29 family are also down-regulated in response to pressure overload and chronic calcineurin signaling (9), pathological stimuli also associated with cardiac fibrosis. A model to account for the role of miR-29 as a regulator of fibrosis is shown in Fig. 5.
Fig. 5.
A model for the role of miR-29 in cardiac fibrosis. In response to cardiac stress, TGFβ is activated and triggers the down-regulation of miR-29 in cardiac fibroblasts and consequent up-regulation of the expression of collagens and other ECM proteins involved in fibrosis. At the same time, stress induces the expression of anti-fibrotic BNP, which is secreted by cardiomyocytes and counteracts the activation and thereby profibrotic function of TGFβ.
Our results show that miRNAs are dynamically regulated in different regions of the heart during post-MI remodeling. Several of these MImiRs were shown to be involved in hypertrophic cardiac remodeling (4–6, 8), including miR-15b, -21, -199, and -214, which are up-regulated, and miR-29c and -150, which are down-regulated. Because infarct healing is a dynamic process involving specific regional and temporal changes in cardiomyocyte hypertrophy, apoptosis, and fibrosis, the dynamic regulation of these miRNAs in different regions of the heart likely contributes to the many aspects of the remodeling response.
Control of Fibrosis by miR-29.
The precise mechanisms that lead to repression of miR-29 expression after MI and other forms of cardiac stress remain to be determined, but our results indicate that TGFβ, a major regulator of cardiac fibrosis (18), can repress miR-29 expression. In addition to collagen deposition at the site of tissue damage after MI, there is enhanced collagen deposition distal to the infarct, which causes diastolic stiffness (23). Because of its role in fibrosis, there have been numerous efforts to therapeutically target TGFβ. However, this has been problematic because of additional functions of TGFβ, for example, in the immune response. The ability of miR-29 to coordinately regulate such a broad collection of mRNAs with roles in fibrosis and ECM remodeling illustrates the potential of miRNAs to control complex cellular processes. Stress-provoked secretion of BNP from cardiomyocytes has been shown to counteract TGFβ signaling (24). In this regard, it seems feasible that signaling by BNP from cardiomyocytes to cardiac fibroblasts serves to modulate miR-29 expression.
In addition to targeting components of the ECM, miR-29b has been shown to be induced during type II diabetes and to repress insulin-stimulated glucose uptake in adipocytes (25). Other demonstrated targets of miR-29 include the DNA methyltransferases (DNMT)3A and -3B (de novo methyltransferases), two key enzymes involved in DNA methylation, that are frequently up-regulated in lung cancer and associated with poor prognosis (26). Thus, miR-29 appears to regulate multiple gene expression programs.
Although our results suggest a key role for miR-29 in the control of cardiac fibrosis, it is important to note that there is not a direct one-to-one stoichiometric relationship between the levels of miR-29 and collagen expression. For example, a few-fold decrease in miR-29 expression after MI was accompanied by up to a 20-fold increase in collagen mRNA expression, whereas a miR-29 antagomir induced collagens only slightly. In addition, although we found a striking increase in expression of collagens and fibrillin mRNAs in response to MI, which coincides with miR-29 down-regulation, expression of elastin, another miR-29 predicted mRNA target, was unaffected. These findings indicate that the presence of a miRNA binding site is not the sole determinant of mRNA targeting by miR-29 and suggest that additional regulatory steps are involved in the actions of miR-29 and in the control of fibrosis. We speculate that stress signals augment the actions of miR-29, as has been shown for other miRNAs. (reviewed in ref. 27). In addition, the moderate effect on collagen mRNA expression in response to miR-29 knockdown in the heart is very likely because of the relative inefficiency of cardiac knockdown, because the effect on collagen expression was much greater in the liver, where the knockdown was complete.
Therapeutic Opportunities.
Cardiac fibrosis, which results in stiffening of the ventricular walls, diminished contractility, and abnormalities in cardiac conductance, is a common consequence of numerous forms of heart disease, including pathological hypertrophy, volume overload, and MI (28). Fibrosis is also commonly associated with numerous other tissue disorders, such as liver, kidney, and lung disease. (reviewed in ref. 29) While this work was being completed, another study reported the involvement of miR-29c in fibrosis of nasopharyngeal carcinomas (30). In this case, down-regulation of miR-29 was postulated to contribute to tumor cell invasiveness and metastatic potential, which involve alterations in the ECM and cell migration.
Our results suggest that the down-regulation of miR-29 contributes to cardiac fibrosis and that strategies to maintain miR-29 expression may be beneficial in the settings of fibrotic diseases. In this regard, miR mimics might represent an effective means of elevating miR-29 expression and thereby diminishing fibrosis. Alternatively, pharmacological inhibitors to prevent the down-regulation of miR-29 expression during fibrosis also represent an intriguing therapeutic approach.
Materials and Methods
Surgical Procedures.
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center. Adult C57BL/6 male mice were subjected to myocardial infarction or sham surgery. See SI Materials and Methods for more detailed information.
Histological Analysis and RNA in Situ Hybridization.
Tissues used for histology were incubated in Krebs–Henselheit solution, fixed in 4% paraformaldehyde, sectioned, and processed for hematoxylin and eosin (H&E) and Masson's Trichrome staining or in situ hybridization by standard techniques (31).
Microarray for miRNAs.
For the miRNA microarrays, total RNA was prepared by using TRIzol, and RNA samples were analyzed by LC Sciences on their microarray platform with a probe set based on Sanger software, Version 9.0. See SI Materials and Methods for more details.
RNA Analysis.
Total RNA was isolated from cells, mouse and human cardiac tissue samples or isolated myocytes by using TRIzol reagent (Gibco/BRL). Cardiac tissue samples of border zone regions of anonymous humans diagnosed as having suffered a myocardial infarction were obtained by biopsy after catheterization. Cardiac tissue samples of left ventricles of anonymous humans diagnosed as having nonfailing hearts were obtained from Myogen. The Institutional Review Board at University of Texas Southwestern Medical Center in accordance with the Department of Health and Human Services considers this work to meet the criteria of exempt review. Equal loading was confirmed by staining Northern gels with ethidium bromide. Northern blot analyses to detect microRNAs were performed as described in ref. 9. To detect the level of miRNA, RT-PCR was performed by using the Taqman MicroRNA reverse Transcriptase kit (Applied Biosystems) according the manufacturer's recommendations. The expression of a subset of genes was analyzed by quantitative real-time PCR, using Taqman probes purchased from Applied Biosystems. Details can be found in SI Materials and Methods.
Cell Culture, Transfection and Luciferase Assays.
A 1793-bp genomic fragment encompassing miR-29b-1 and miR-29a coding region was amplified by PCR and ligated into pCMV6. Genomic fragments of the murine 3′UTR encompassing the miR-29 binding site(s) were PCR-amplified and ligated into the firefly luciferase (f-luc) reporter construct (pMIR-REPORTTM; Ambion) (see Table S3 for primer sequences). COS cells were transfected with Fugene 6 (Stratagene) according to manufacturer's instructions. The total amount of DNA per well was kept constant by adding the corresponding amount of expression vector without a cDNA insert. Forty-eight hours after transfection, cell extracts were assayed for luciferase expression, using the luciferase assay kit (Promega). Relative promoter activities are expressed as luminescence relative units normalized for β-galactosidase expression in the cell extracts.
Cardiac fibroblasts (CFs) were isolated as described in ref. 32. Myofibroblast differentiation was induced by changing the medium to low serum (2% FBS) with l-ascorbic acid (10 μg/μl) and administration of 10 ng/ml TGFβ1 for 48 h. See SI Materials and Methods for more details.
In Vivo miR-29b Modulation by Synthetic Oligonucleotide Treatment.
Chemically modified oligonucleotides comprising a sequence complementary to the mature miR-29b (anti-miR 29b) were used to inhibit miR-29b activity. All nucleosides were 2′-OMe modified, the 5′ terminal two and 3′ terminal four bases contained a phosphorothioate internucleoside bond, and the molecules contained 3′ cholesterol attached via a hydroxyprolinol linker. The miR-29b mimic is a double stranded construct consisting of guide and passenger strands. The guide strand contains 2′ F nucleosides at every pyrimidine residue, two 3′ terminal phosphorothioate linkages and is chemically phosphorylated on the 5′ terminus. The passenger strand contains two 5′ terminal 2′ OMe residues and two 3′ terminal phosphorothioate bonds. Cholesterol is attached to the 3′ end of the passenger strand through a hydroxyprolinol linker. Eight-week-old C57BL/6 male mice received either anti miR-29b, mismatch miR-29b or mimic of miR-29b at a dose of 80 mg/kg body weight or a comparable volume of saline through tail vein injection. Tissues were collected either 3 days or 3 weeks after treatment.
Animal Care.
All animal procedures were approved by the Institutional Animal Care and Use Committee at University of Texas Southwestern Medical Center.
Supplementary Material
Acknowledgments.
We thank T. McKinsey, J. Molkentin, M. Periasamy, and W. Pu for comments on the manuscript. Work in the laboratory of E.N.O. was supported by grants from the National Institutes of Health, the Donald W. Reynolds Clinical Cardiovascular Research Center, the Robert A. Welch Foundation, and the Sandler Foundation for Asthma Research and a Scientist Development Grant from the American Heart Association (to E.v.R.).
Footnotes
Conflict of interest statement: E.v.R., W.S.M., and E.N.O. are cofounders of MiRagen Therapeutics.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805038105/DCSupplemental.
References
- 1.Fox CS, et al. Increasing cardiovascular disease burden due to diabetes mellitus: The Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 2.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 3.Bouzeghrane F, Reinhardt DP, Reudelhuber TL, Thibault G. Enhanced expression of fibrillin-1, a constituent of the myocardial extracellular matrix in fibrosis. Am J Physiol Heart Circ Physiol. 2005;289:H982–991. doi: 10.1152/ajpheart.00151.2005. [DOI] [PubMed] [Google Scholar]
- 4.Powell DW, et al. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, et al. MicroRNAs are aberrantly expressed in hypertrophic heart: Do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 7.Tatsuguchi M, et al. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42:1137–1141. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thum T, et al. MicroRNAs in the human heart. A clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 9.van Rooij E, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA. 2006;103(48):18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda S, et al. Altered microRNA expression in human heart disease. Physiol Genomics. 2007;31:367–373. doi: 10.1152/physiolgenomics.00144.2007. [DOI] [PubMed] [Google Scholar]
- 11.van Rooij E, Olson EN. MicroRNAs: Powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Rooij E, Olson EN. microRNAs put their signatures on the heart. Physiol Genomics. 2007;31:365–366. doi: 10.1152/physiolgenomics.00206.2007. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Ambros V. MicroRNA pathways in flies and worms: Growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 15.Hammond SM. MicroRNAs as oncogenes. Curr Opin Genet Dev. 2006;16:4–9. doi: 10.1016/j.gde.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Berezikov E, et al. Phylogenetic shadowing and computational identification of human microRNA genes. Cell. 2005;120:21–24. doi: 10.1016/j.cell.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 17.McDonald K. Diastolic heart failure in the elderly: Underlying mechanisms and clinical relevance. Int J Cardiol. 2008;125:197–202. doi: 10.1016/j.ijcard.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 19.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 21.Krutzfeldt J, et al. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007;35:2885–2892. doi: 10.1093/nar/gkm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krutzfeldt J, et al. Silencing of microRNAs in vivo with “antagomirs.”. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 23.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1) Mol Genet Metab. 2000;71:418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 24.Kapoun AM, et al. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: Fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. 2004;94:453–461. doi: 10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- 25.He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3–L1 adipocytes. Mol Endocrinol. 2007;21:2785–2794. doi: 10.1210/me.2007-0167. [DOI] [PubMed] [Google Scholar]
- 26.Fabbri M, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung AK, Sharp PA. microRNAs: A safeguard against turmoil? Cell. 2007;130:581–585. doi: 10.1016/j.cell.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Diez J. Mechanisms of cardiac fibrosis in hypertension. J Clin Hypertens (Greenwich) 2007;9:546–550. doi: 10.1111/j.1524-6175.2007.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sengupta S, et al. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shelton JM, Lee MH, Richardson JA, Patel SB. Microsomal triglyceride transfer protein expression during mouse development. J Lipid Res. 2000;41:532–537. [PubMed] [Google Scholar]
- 32.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50:101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.