Abstract
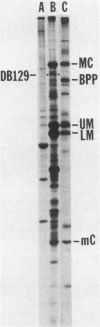
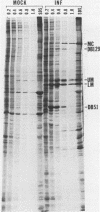
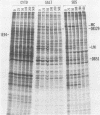
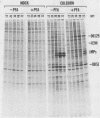
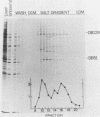
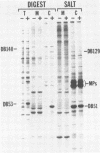
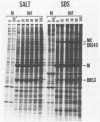
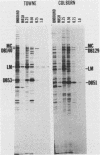
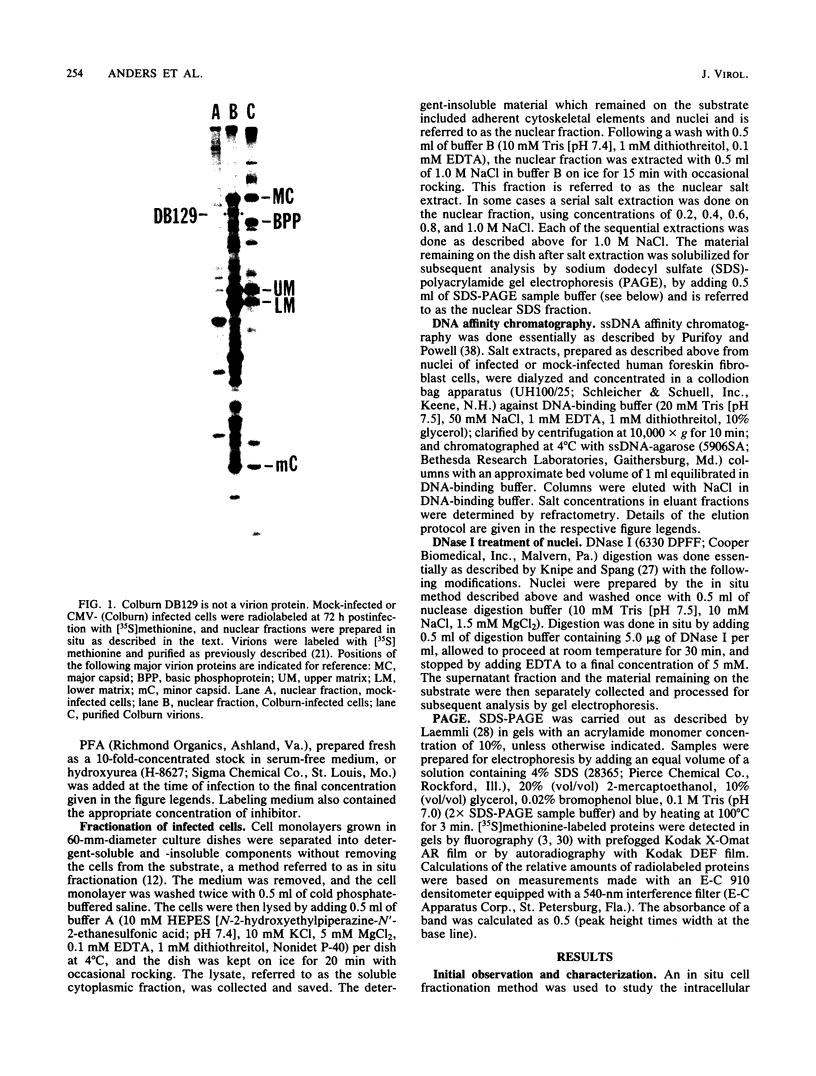
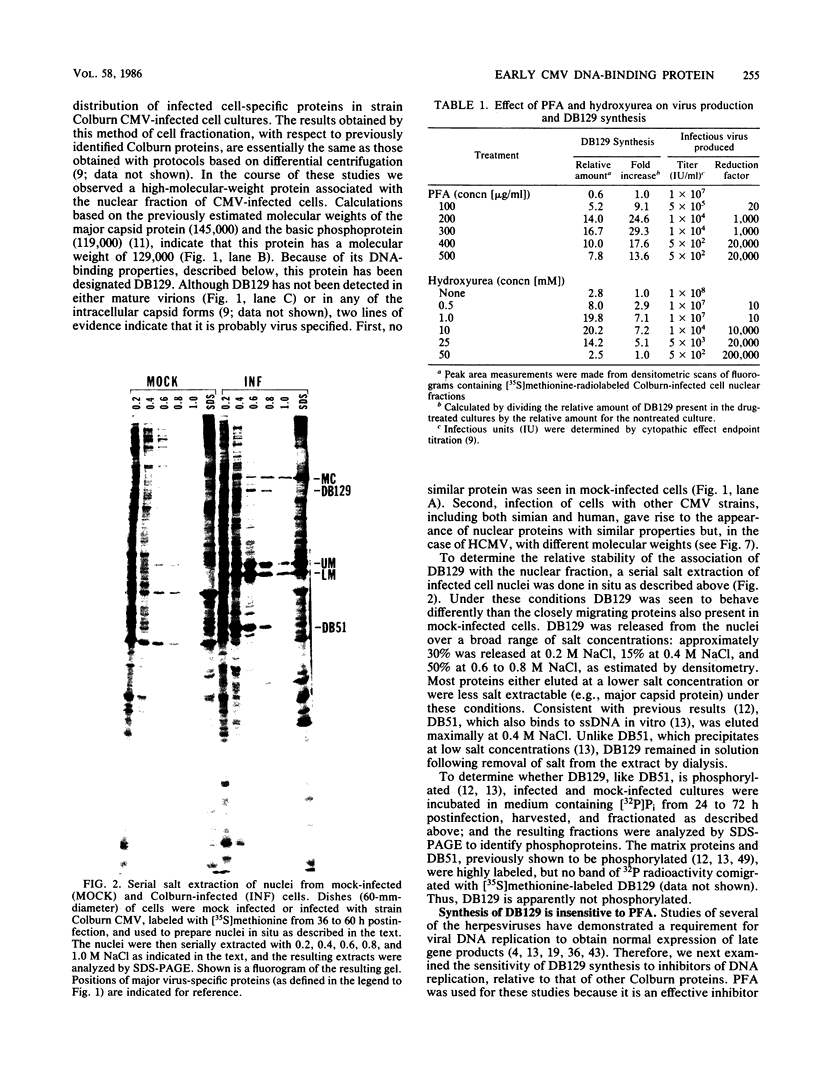
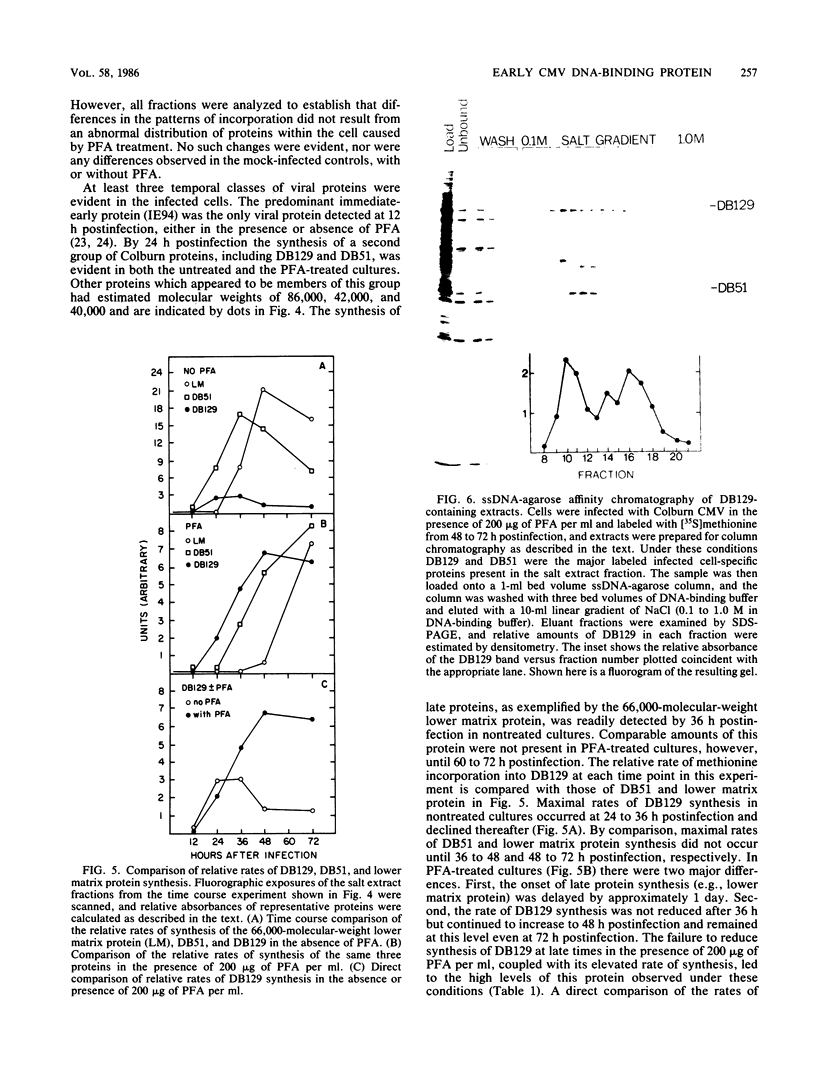
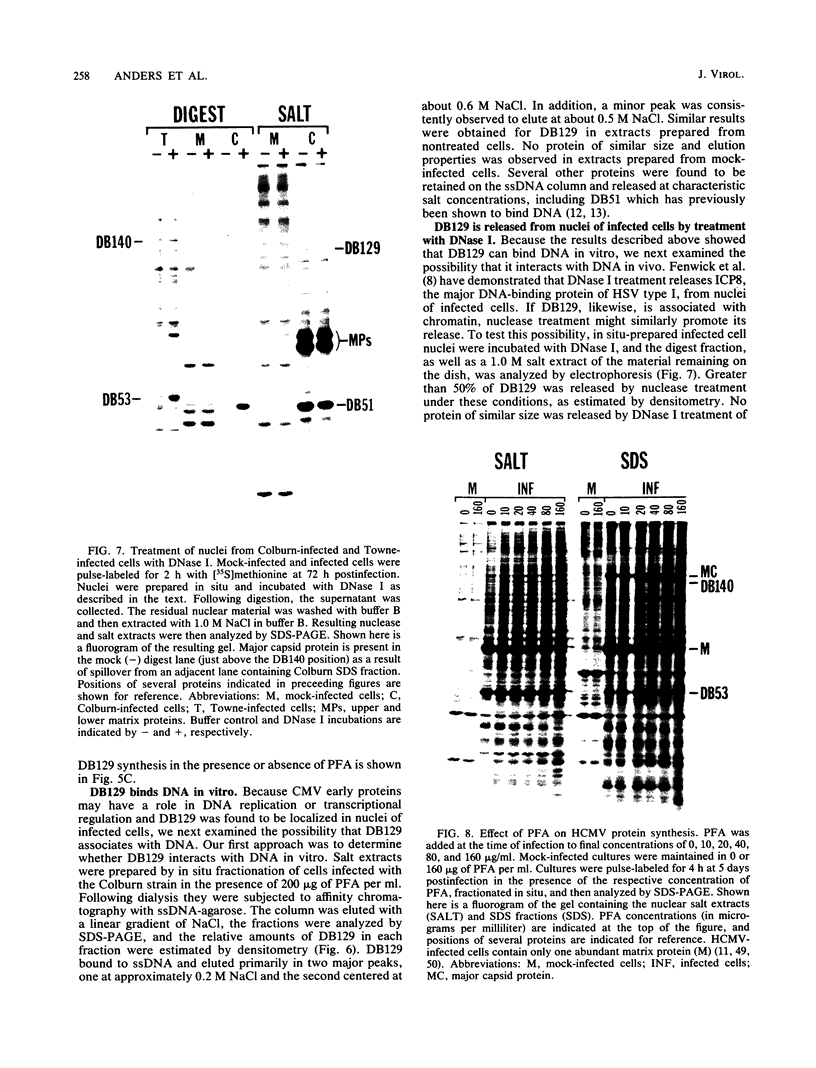
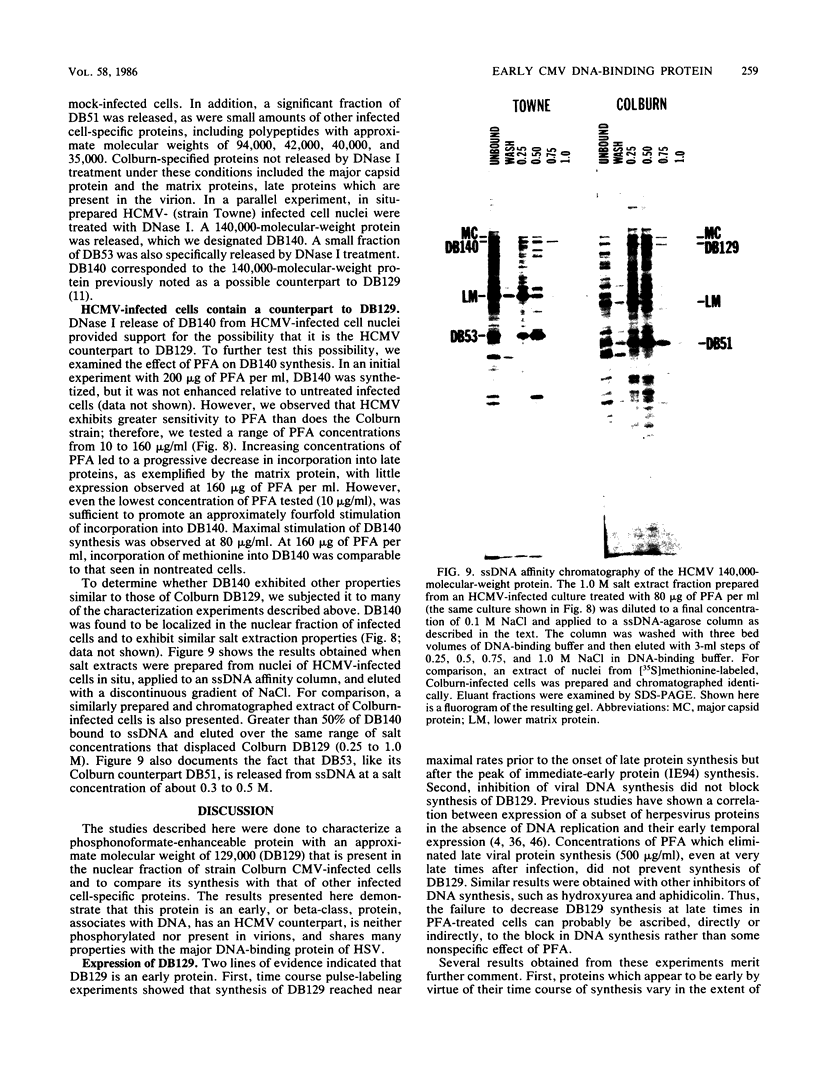
We characterized a DNA-binding protein with an approximate molecular weight of 129,000 (DB129) which is present in the nuclei of cytomegalovirus- (strain Colburn) infected cells, but not in virus particles. Results of two types of experiments demonstrated that DB129 is a member of the early class of herpesviral proteins. First, time course pulse-labeling experiments showed that its synthesis begins after that of the immediate-early protein IE94, but prior to the appearance of late viral proteins, and was reduced at late times. Second, in the presence of inhibitors of viral DNA replication, DB129 continued to be made and accumulated to elevated levels. A second set of experiments showed that DB129 bound to single-stranded DNA in vitro and was eluted by a NaCl gradient in two peaks, one at about 0.2 M and the second at about 0.6 M. A similar pattern of release was observed when infected-cell nuclei were serially extracted with increasing NaCl concentrations. In addition, treatment of nuclei with DNase I selectively released DB129, along with a small but significant fraction of another DNA-binding protein, DB51. These results suggest that DB129 is associated with DNA in vivo and that it interacts directly with single-stranded DNA. It was also shown that cells infected with human cytomegalovirus (strain Towne) contain a slightly larger counterpart to DB129, which was designated DB140. Similarities between these proteins and the major DNA-binding protein of herpes simplex virus are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss G. J., Marsden H. S., Hay J. Herpes simplex virus proteins: DNA-binding proteins in infected cells and in the virus structure. Virology. 1975 Nov;68(1):124–134. doi: 10.1016/0042-6822(75)90154-3. [DOI] [PubMed] [Google Scholar]
- Blanton R. A., Tevethia M. J. Immunoprecipitation of virus-specific immediate-early and early polypeptides from cells lytically infected with human cytomegalovirus strain AD 169. Virology. 1981 Jul 15;112(1):262–273. doi: 10.1016/0042-6822(81)90631-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Conley A. J., Knipe D. M., Jones P. C., Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981 Jan;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Specific targets for antiviral drugs. Biochem J. 1982 Jul 1;205(1):1–13. doi: 10.1042/bj2050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M., Blankenship M. L., Brown G. D., Kaplan A. S. Size and complexity of human cytomegalovirus DNA. Virology. 1978 Sep;89(2):643–646. doi: 10.1016/0042-6822(78)90209-x. [DOI] [PubMed] [Google Scholar]
- Elion G. B. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):9–17. doi: 10.1093/jac/12.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J., Petkevich J. M. On the association of virus proteins with the nuclei of cells infected with herpes simplex virus. J Gen Virol. 1978 Jun;39(3):519–529. doi: 10.1099/0022-1317-39-3-519. [DOI] [PubMed] [Google Scholar]
- Gibson W. Immediate-early proteins of human cytomegalovirus strains AD 169, Davis, and Towne differ in electrophoretic mobility. Virology. 1981 Jul 15;112(1):350–354. doi: 10.1016/0042-6822(81)90641-3. [DOI] [PubMed] [Google Scholar]
- Gibson W., Murphy T. L., Roby C. Cytomegalovirus-infected cells contain a DNA-binding protein. Virology. 1981 May;111(1):251–262. doi: 10.1016/0042-6822(81)90669-3. [DOI] [PubMed] [Google Scholar]
- Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983 Jul 30;128(2):391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- Gibson W. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology. 1981 Jun;111(2):516–537. doi: 10.1016/0042-6822(81)90354-8. [DOI] [PubMed] [Google Scholar]
- Gibson W., van Breemen R., Fields A., LaFemina R., Irmiere A. D,L-alpha-difluoromethylornithine inhibits human cytomegalovirus replication. J Virol. 1984 Apr;50(1):145–154. doi: 10.1128/jvi.50.1.145-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Mutations in the major DNA-binding protein gene of herpes simplex virus type 1 result in increased levels of viral gene expression. J Virol. 1983 Sep;47(3):478–486. doi: 10.1128/jvi.47.3.478-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgstrand E., Eriksson B., Johansson N. G., Lannerö B., Larsson A., Misiorny A., Norén J. O., Sjöberg B., Stenberg K., Stening G. Trisodium phosphonoformate, a new antiviral compound. Science. 1978 Sep 1;201(4358):819–821. doi: 10.1126/science.210500. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol. 1975 Dec;16(6):1560–1565. doi: 10.1128/jvi.16.6.1560-1565.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Kilpatrick B., Lakeman A., Alford C. A. Genetic analysis of a cytomegalovirus-like agent isolated from human brain. J Virol. 1978 Jun;26(3):718–723. doi: 10.1128/jvi.26.3.718-723.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmiere A., Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983 Oct 15;130(1):118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- Jacob R. J. DNA labeled during phosphonoacetate inhibition and following its reversal in herpesvirus infected cells. Arch Virol. 1984;79(3-4):221–240. doi: 10.1007/BF01310813. [DOI] [PubMed] [Google Scholar]
- Jeang K. T., Chin G., Hayward G. S. Characterization of cytomegalovirus immediate-early genes. I. Nonpermissive rodent cells overproduce the IE94K protein form CMV (Colburn). Virology. 1982 Sep;121(2):393–403. doi: 10.1016/0042-6822(82)90177-5. [DOI] [PubMed] [Google Scholar]
- Jeang K. T., Gibson W. A cycloheximide-enhanced protein in cytomegalovirus-infected cells. Virology. 1980 Dec;107(2):362–374. doi: 10.1016/0042-6822(80)90304-9. [DOI] [PubMed] [Google Scholar]
- Kilpatrick B. A., Huang E. S. Human cytomegalovirus genome: partial denaturation map and organization of genome sequences. J Virol. 1977 Oct;24(1):261–276. doi: 10.1128/jvi.24.1.261-276.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Quinlan M. P., Spang A. E. Characterization of two conformational forms of the major DNA-binding protein encoded by herpes simplex virus 1. J Virol. 1982 Nov;44(2):736–741. doi: 10.1128/jvi.44.2.736-741.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Spang A. E. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol. 1982 Jul;43(1):314–324. doi: 10.1128/jvi.43.1.314-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFemina R. L., Hayward G. S. Replicative forms of human cytomegalovirus DNA with joined termini are found in permissively infected human cells but not in non-permissive Balb/c-3T3 mouse cells. J Gen Virol. 1983 Feb;64(Pt 2):373–389. doi: 10.1099/0022-1317-64-2-373. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Knipe D. M. Thermolabile in vivo DNA-binding activity associated with a protein encoded by mutants of herpes simplex virus type 1. J Virol. 1983 Jun;46(3):909–919. doi: 10.1128/jvi.46.3.909-919.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinbach S. S., Casto J. F. Identification and characterization of deoxyribonucleoprotein complexes containing the major DNA-binding protein of herpes simplex virus type 1. Virology. 1983 Dec;131(2):274–286. doi: 10.1016/0042-6822(83)90496-8. [DOI] [PubMed] [Google Scholar]
- Leinbach S. S., Casto J. F., Pickett T. K. Deoxyribonucleoprotein complexes and DNA synthesis of herpes simplex virus type 1. Virology. 1984 Sep;137(2):287–296. doi: 10.1016/0042-6822(84)90220-4. [DOI] [PubMed] [Google Scholar]
- Littler E., Purifoy D., Minson A., Powell K. L. Herpes simplex virus non-structural proteins. III. Function of the major DNA-binding protein. J Gen Virol. 1983 May;64(Pt 5):983–995. doi: 10.1099/0022-1317-64-5-983. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Maeno K., Yoshida S. Characterization of human cytomegalovirus-induced DNA polymerase and the associated 3'-to-5', exonuclease. Virology. 1983 Jan 30;124(2):221–231. doi: 10.1016/0042-6822(83)90339-2. [DOI] [PubMed] [Google Scholar]
- O'Hare P., Honess R. W. Identification of a subset of herpesvirus saimiri polypeptides synthesized in the absence of virus DNA replication. J Virol. 1983 Apr;46(1):279–283. doi: 10.1128/jvi.46.1.279-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Littler E., Purifoy D. J. Nonstructural proteins of herpes simplex virus. II. Major virus-specific DNa-binding protein. J Virol. 1981 Sep;39(3):894–902. doi: 10.1128/jvi.39.3.894-902.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. DNA-binding proteins induced by herpes simplex virus type 2 in HEp-2 cells. J Virol. 1976 Aug;19(2):717–731. doi: 10.1128/jvi.19.2.717-731.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Weir A. C. Interaction with nucleic acids and stimulation of the viral DNA polymerase by the herpes simplex virus type 1 major DNA-binding protein. J Virol. 1984 Dec;52(3):727–733. doi: 10.1128/jvi.52.3.727-733.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman C., Jr, Smith S. H., Drach J. C., Klayman D. L. Antiviral activity of 2-acetylpyridine thiosemicarbazones against herpes simplex virus. Antimicrob Agents Chemother. 1981 Apr;19(4):682–685. doi: 10.1128/aac.19.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Mocarski E. S., Thomsen D. R. DNA of human cytomegalovirus: size heterogeneity and defectiveness resulting from serial undiluted passage. J Virol. 1979 Jul;31(1):231–239. doi: 10.1128/jvi.31.1.231-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J Virol. 1978 Jun;26(3):686–701. doi: 10.1128/jvi.26.3.686-701.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J Virol. 1977 Sep;23(3):751–767. doi: 10.1128/jvi.23.3.751-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. J., Banks L. M., Purifoy D. J., Powell K. L. Interactions between herpes simplex virus DNA-binding proteins. J Gen Virol. 1984 Nov;65(Pt 11):2033–2041. doi: 10.1099/0022-1317-65-11-2033. [DOI] [PubMed] [Google Scholar]
- Wahren B., Oberg B. Inhibition of cytomegalovirus late antigens by phosphonoformate. Intervirology. 1980;12(6):335–339. doi: 10.1159/000149093. [DOI] [PubMed] [Google Scholar]
- Wahren B., Oberg B. Reversible inhibition of cytomegalovirus replication by phosphonoformate. Intervirology. 1980;14(1):7–15. doi: 10.1159/000149156. [DOI] [PubMed] [Google Scholar]
- Weiner D., Gibson W., Fields K. L. Anti-complement immunofluorescence establishes nuclear localization of human cytomegalovirus matrix protein. Virology. 1985 Nov;147(1):19–28. doi: 10.1016/0042-6822(85)90223-5. [DOI] [PubMed] [Google Scholar]
- Weiner D., Gibson W. Identification of a primate cytomegalovirus group-common protein antigen. Virology. 1981 Nov;115(1):182–191. doi: 10.1016/0042-6822(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Weiner D., Gibson W. Phosphorylation, maturational processing, and relatedness of strain Colburn matrix proteins. Virology. 1983 Aug;129(1):155–169. doi: 10.1016/0042-6822(83)90403-8. [DOI] [PubMed] [Google Scholar]