Abstract
Separase is an endopeptidase that separates sister chromatids by cleaving cohesin Rad21 during the metaphase-to-anaphase transition. Conditional expression of Separase in tetracycline-inducible diploid FSK3 mouse mammary epithelial cells with both p53 WT and mutant (Ser-233-234) alleles of unknown physiological significance develops aneuploidy within 5 days of Separase induction in vitro. Overexpression of Separase induces premature separation of chromatids, lagging chromosomes, and anaphase bridges. In an in vivo mouse mammary transplant model, induction of Separase expression in the transplanted FSK3 cells for 3–4 weeks results in the formation of aneuploid tumors in the mammary gland. Xenograft studies combined with histological and cytogenetic analysis reveal that Separase-induced tumors are clonal in their genomic complements and have a mesenchymal phenotype suggestive of an epithelial–mesenchymal transition. Induction of Separase resulted in trisomies for chromosomes 8, 15, and 17; monosomy for chromosome 10; and amplification of the distal region of chromosomes 8 and 11. Separase protein is found to be significantly overexpressed in human breast tumors compared with matched normal tissue. These results collectively suggest that Separase is an oncogene, whose overexpression alone in mammary epithelial cells is sufficient to induce aneuploidy and tumorigenesis in a p53 mutant background.
Keywords: breast cancer, oncogene, sister chromatid cohesion
Aneuploidy (aberrant chromosome number) is a hallmark feature of human malignancies (1, 2) and has also been proposed as a necessary event for tumorigenesis (2). Although there have been many proposed hypotheses, there is no general agreement as to why aneuploidy is so highly prevalent in cancer cells, and how it contributes to tumor progression (3, 4). Importantly, if aneuploidy forms an underlying cause of human cancer, it has not been fully substantiated. The mechanisms of aneuploidy also remain a fundamental unresolved problem in cancer biology.
To understand how aneuploidy might originate in mammalian tissues, we have focused on the elements that regulate chromosomal segregation, particularly those involved in sister chromatid cohesion and separation, because chromosome missegregation, for example during mitosis, can lead to aneuploidy. A key gene in our analysis is ESPL1, which encodes an endopeptidase called Separase that separates sister chromatids by cleaving cohesin Rad21/Mcd1/Scc1 during the metaphase to anaphase transition. The hypothesis we tested is that hormonal stimulation of the p53-null mouse mammary gland results in misexpression of the ESPL1 gene, thus promoting aneuploidy and breast cancer formation. Dysregulation of the mitotic machinery that helps maintain chromosomal stability in mammary cells can result in aneuploidy and subsequently, cancer formation. We focused on Separase for the following reasons that have important implications for breast cancer: (i) Separase plays a central role in promoting faithful chromosome segregation; (ii) our previous studies strongly indicated that hormonal stimulation of p53-null mice mammary gland results in overexpression of the ESPL1 and Separase protein, which may be a direct cause of aneuploidy (5); and (iii) siRNA-mediated knockdown of Separase and Separase deficient mouse embryonic fibroblasts results in genomic instability (6–8).
An evolutionarily conserved protein complex called cohesin and an endopeptidase named Separase play pivotal roles in the accurate segregation of sister chromatids into two daughter cells. Cohesion along the length of the sister chromatids is formed during DNA replication in S phase. Cohesion along the chromosomal arms is removed during prophase and from centromeric regions at the metaphase-to-anaphase transition when Separase is activated after its inhibitory chaperone securin is degraded (9, 10).
To understand how aberration in sister chromatid separation may contribute to chromosomal missegregation, we investigated the role of Separase overexpression in mouse mammary cells by using a mammary epithelial transplant model (11) as well as various biochemical and functional assays. Our results indicate that conditional overexpression of Separase alone in mammary epithelial cells with a p53 mutant background is sufficient to induce aneuploidy and tumorigenesis in vitro and in vivo.
Results
Conditional Expression of Mouse Separase (mSeparase) Results in Aneuploidy in Mouse Mammary Epithelial Cells.
To examine the direct effect of Separase expression on aneuploidy, we used an in vitro tissue culture system to overexpress Separase protein by conditionally expressing the ESPL1 gene by using a tetracycline-inducible system. Because of background polyploidy in most cultured mouse mammary cells, we used FSK-3 mouse mammary epithelial cells that are diploid to develop a tet-on cell line to examine if conditional expression of Separase can induce aneuploidy.
Separase expression was turned on in six of the seven mESPL1 stably transfected FSK3 clones that were tested by adding 1 μg/ml doxycycline for 24 h [supporting information (SI) Fig. S1A]. Only clone no. 24 showed no significant induction in the presence and absence of the drug. However, in four of these positive clones (nos. 31, 38, 40, and 94), Separase expression was leaky, because in the absence of doxycycline significant level of Separase expression was detected. In the empty vector control clone (FSK3 #64EV), no detectable Separase expression was seen in the absence or presence of the drug. Based on these results, we used clones nos. 50 and 62 as positive clones and clones nos. 24 and 64EV as negative controls for subsequent characterization. Spectral karyotyping (SKY) analysis of 10 metaphases from these clones showed normal diploid karyotypes before Separase induction using conventional G banding and SKY analysis (Fig. S1B).
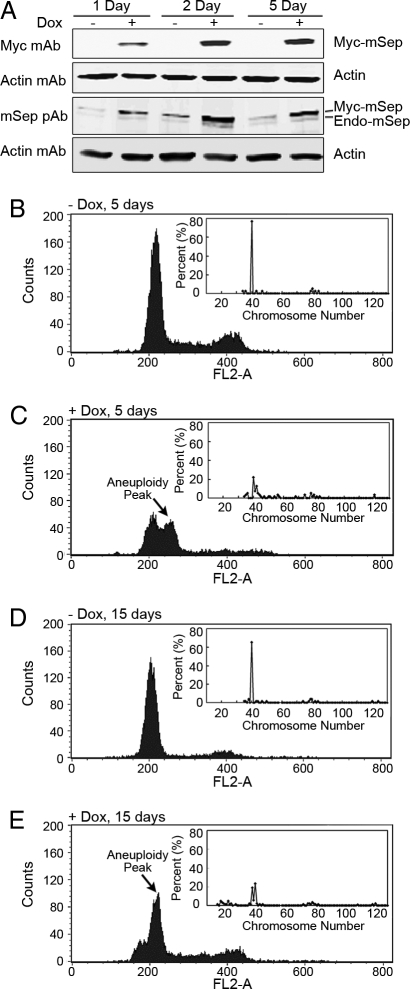
To monitor the ploidy after Separase induction, in a time course experiment, four of these clones (FSK3 #50, 62, 24, and 64EV) were cultured in the presence and absence of 1 μg/ml doxycycline for a period of 15 days. Cells were passaged every 5 days (total of three times) during this experiment. Cells treated with and without doxycycline were sampled every 24 h and analyzed by FACS. Similar results were obtained by using both clones nos. 50 (Fig. 1) and 62 (data not shown). Separase protein was measured on days 1, 2, and 5 by using epitope-tagged myc Ab and antibody against mouse Separase (Fig. 1A). As shown in Fig. 1A, induction of Separase in clone no. 50 resulted in an increase of Separase protein. In uninduced cells, myc-Separase protein could not be detected. The endogenous level of Separase protein in FSK3 cells appeared to be low (Fig. 1A). On days 5 and 15, chromosome numbers were measured by using conventional G banding techniques as described (5, 11). For each preparation, 40–85 metaphases from exponentially growing cells were examined, and total chromosome numbers were counted by using QUIPS Pathvysion software (Applied Imaging) (see Fig. 1 B–E Insets for chromosome distribution).
Fig. 1.
Conditional expression of Separase in FSK3 Tet-on cells develops gross aneuploidy. Development of aneuploidy in Separase-induced FSK3 Tet-on cells (clone no. 50) was assayed at 1, 2, and 5 days after doxycycline (1 μg/ml) treatment. (A) Separase protein was assayed by using Western blot (pAb, polyclonal antibody). (B–E) FACS analysis of the propidium iodide (PI) stained nuclei obtained from cells treated in the presence (Separase induced) or absence (noninduced) of doxycycline for a period of 5 and 15 days. Arrow indicates aneuploidy, shown as an additional peak. Inset shows distribution of chromosome number at that time point.
FACS analysis indicated that induction of Separase in both clones nos. 50 (Fig. 1 B–E) and 62 (data not shown), but not in the control clone 64EV (Fig. S2) and clone no. 24 (data not shown), resulted in a significant increase in aneuploidy shown as a distinct additional peak after 5 days of drug treatment (Fig. 1C). We did not observe this peak at earlier time points (1, 2, 3, and 4 day; data not shown). The aneuploidy peak rose further at the 15-day time point (Fig. 1E). The ploidy levels by FACS analysis were in agreement with the chromosome number distribution identified by conventional cytogenetic analysis in these cells (Fig. 1 C and E Inset). In Separase-induced cells, ≈20% of the metaphases analyzed were diploid, whereas ≈60% of the metaphases had >40 chromosomes (Fig. 1 C and E Inset). However, at the 15-day time point, 25% of the metaphases were also found to be hypodiploid, indicating both chromosome gain and loss after prolonged Separase induction in vitro. The chromosomes in the Separase-overexpressed cells are found to be thinner, suggesting premature sister chromatid separation (see Fig. 4A). FACS and G banding from the clone 64EV that contains the empty vector control (Fig. S2) and clone no. 24 (data not shown) with no detectable myc-Separase expression in the presence or absence of doxycycline had no detectable changes in chromosome number. These findings clearly indicate that overexpression of Separase alone can induce aneuploidy in euploid mouse mammary epithelial cells with a p53 mutant background in vitro.
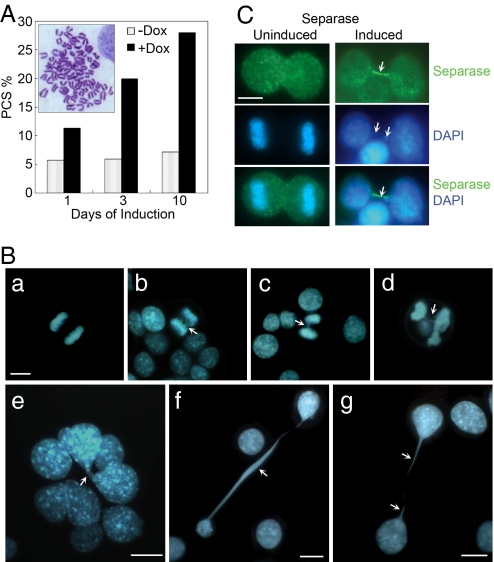
Fig. 4.
PCS, formation of anaphase bridge, and other nuclear abnormalities in Separase-induced FSK3 tet-on cells (clone no. 50). Separase was induced by treatment with 1 μg/ml doxycycline for the indicated time periods. (A) Two hundred G banded metaphase chromosome preparations were examined for PCS phenotype (see Inset) in Separase-uninduced and -induced cells at each time point. Data were presented as percentage PCS. (B) To examine anaphase bridges and other nuclear abnormalities after Separase induction, cells were fixed in cold methanol (−20°C) for 15 min and nuclear material was visualized by DAPI staining (blue fluorescence, B). Ba represents a normal anaphase, whereas B b–d represent lagging chromosome at metaphase and anaphase bridges (arrowheads). (C) Control and Separase-induced FSK3 cells were immunostained with our anti-Separase rabbit polyclonal antibody (Ab74553) and visualized by the addition of fluorescein-labeled goat anti-rabbit IgG (green fluorescence). The merger of blue and green fluorescence indicated colocalization of Separase and chromatin material between two dividing cells (arrowheads). (Scale bars, 10 μm.)
Oncogenic Activity of Separase in Vivo.
The following experiments were designed to investigate whether Separase overexpression in vivo can also result in aneuploidy formation and tumorigenesis. FSK3 mouse mammary cells are diploid and have no tumorigenic activity in vivo (12). We injected FSK3 Tet-on clones nos. 50 and 62 that are euploid (Fig. 2A) into the mouse mammary fat pads of 3-week-old WT mice. As a control empty vector, clone (FSK3 #64EV) with no detectable myc-Separase expression in the absence or presence of doxycycline was used.
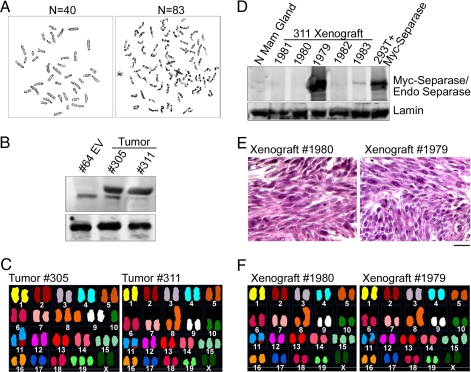
Fig. 2.
A representative metaphase spread from FSK3 Tet-inducible Separase clone no. 50: (A) before injecting to the mouse mammary fat pads showing diploid karyotypes (Left); sample karyotypes of tumor arise from the transplanted cells showing aneuploidy (Right) (n = number of chromosomes). (B and D) Western blot analysis of Separase protein levels in 305 and 311 tumors and 311 tumor-derived xenograft. Empty vector transplant (clone no. 64EV, B), and normal mammary gland (N Mam Gland, D) are shown as controls. (C) Multicolor spectral karyotypic analysis of Separase-induced tumors (305, 311), and (F) 311-xenografts 1979 and 1980. (C) (Left) A classified colored karyotype from tumor 305 showing translocation between chromosome 2 and 11 (T(2:11)), trisomies for chromosome 8, 15 and 19 (Ts8, Ts15, Ts19), and monosomy for chromosome 10 (Ms10). (Right) The spectral analysis identified a karyotype Rb (8, 8),Ts6,Der(11)T(2:11),Ts15 in genomic compliment of tumor 311, and (F) its xenografts. (E) Hematoxylin/eosin staining of the 1979, 1980 xenografts showing mesenchymal phenotype. (Scale bar, 50 μm.)
Induction of Separase in transplanted mammary cells develops tumors within a short period of 3–4 weeks. Five of the six transplants from each Tet-induced clones developed tumors (Table S1). Transplanted mice with clones nos. 50 and 62 without the drug treatment had no tumors at 8 weeks after transplant. Similarly, mice injected with the empty vector control cells had no signs of tumor in the absence or presence of the drug. Metaphase spread analysis of the tumor (no. 305) derived from FSK3 clone no. 50 and tumor (no. 311) derived from clone no. 62 indicated aneuploidy with chromosome number ranging from 39 to 83 (Fig. 2A Right) (modal 42) and from 37 to 81 (modal 41), respectively (Fig. 2C) (Table S1). Western blot analysis of the tumors nos. 305 and 311 indicated significantly higher levels of Separase protein compared to the empty vector transplanted clone (no. 64EV) (Fig. 2B). Histological analysis revealed these tumors have a mesenchymal phenotype. Western blot analysis indicted expression of a number of epithelial–mesenchymal transition (EMT) markers (13, 14), including missing full-length E-cadherin, but the presence of cleaved E-cadherin fragments and expression of smooth muscle actin (SMA) in these tumors. Absence of the above mentioned markers in parental FSK3 mammary epithelial cells suggested of EMT (data not shown). This mesenchymal phenotype is often seen in mouse mammary cells injected in vivo that have been established in culture and then undergo spontaneous or induced transformation.
Chromosomal Instability in Separase-Induced Tumors.
We used a combination of molecular cytogenetic methods, including comparative gene hybridization (CGH), SKY, and array CGH to identify the chromosomal instability in Separase-induced tumors (305 derived from clone nos. 50, 311 from clone no. 62, and 1979 and 1980 xenografts derived from 311 tumor). Several recurrent chromosomal changes were identified in both 305, 311 tumor cells, and 311 tumor xenografts.
SKY analysis of tumor 305 revealed a translocation between chromosomes 2 and 11 (Fig. 2C Left). In addition, trisomies for chromosomes 8, 15, and 19 and monosomy for chromosome 10 were noted in tumor genomic compliment–41–42,XX,Ts8, Ms10,Der(11)T(2:11),Ts15,Ts19. The spectral analysis identified a karyotype 41,XX,Rb (8, 8),Ts6,Der(11)T(2:11),Ts15 in genomic compliment of tumor 311 (Fig. 2C Right).
To examine the clonality of the Separase-induced tumors, we xenografted the epithelial cell preparations from 311 tumors into RAG2-SCID mice. After 2 weeks of injection, all five mice showed palpable tumors that grow very rapidly reaching a size of 1,500–2,000 mm3 in 2 weeks. Because of the heavy tumor burden, the animals were killed after 4 weeks of injection. However, no sign of tumors were observed in control FSK3 or clone no. 64 EV injected animals up to 16 weeks after injection, when the experiment was terminated and animals were killed. SKY and array CGH analysis of 311-derived xenografts (1979 and 1980) indicated identical cytogenetics profiles by SKY and array CGH as that of the parental line, indicating clonality of this tumor (Fig. 2F). Furthermore, as that of the parental 311 tumor, histological analysis revealed the xenografts having a mesenchymal phenotype (Fig. 2E). Western blot analysis of the xenografts indicated varying levels (high to very low) of Separase protein (Fig. 2D), suggesting Separase expression may not be critical at this stage for continued tumorigenesis.
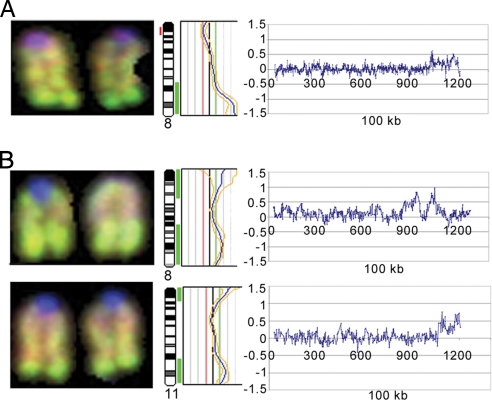
DNA extracted from tumors 305 and 311 was subjected to chromosomal and array CGH analysis to identify DNA copy number aberrations. CGH analysis revealed the gain of chromosome 15 in both tumors (data not shown). High-level amplification on telomeric region of chromosome 8 in tumor 305 (Fig. 3A) and chromosomes 8 and 11 in tumor 311 (Fig. 3B) were found. Subsequent array CGH analysis of the same tumors refined the amplifications on chromosomes 8 and 11 (Fig. 3). The amplified clones from chromosomes 8 and 11 are summarized in Table 1.
Fig. 3.
Conventional CGH and array CGH analysis of Separase-induced tumors. Partial CGH karyotypes for chromosome 8 from tumor 305 (A) and chromosomes 8 and 11 from tumor 311 (B). The corresponding ratio profiles showing high-level amplification at telomeric region. The vertical red bar on the left and green bars on the right of the ideogram indicate the threshold values of 0.80 and 1.20 for loss and gain, respectively. A log ratio for the same chromosome was generated by using high-resolution CGH arrays. The amplification identified by chromosomal CGH was confirmed by array CGH and identified several clones from the chromosomes 8 and 11 amplicon (Table 1).
Table 1.
Amplified clones from chromosomes 8 and 11 amplicons
| Tumor no. | Chromosome | Amplified clones | Map position | Known genes |
|---|---|---|---|---|
| 305 | 11 | RP23–207J12 | 1032.8 | None |
| RP23–443K17 | 1038.4 | NSF | ||
| RP23–365P14 | 1133.5 | SLC39A11 | ||
| RP23–284L12 | 1171.1 | None | ||
| 311 | 8 | RP23–167L14 | 936.7 | NUP93, HERPUD1, SLC12A3 |
| RP23–21J8 | 942.5 | DOK4, GPR56, GPR97 | ||
| RP23–59L13 | 1053.1 | DDX28, NFATC3 | ||
| 311 | 11 | RP23–452B17 | 1090.5 | Rsg9, Axin2 |
| RP23–151N19 | 1175.7 | TMC8, TK1, SYNGR2 | ||
| RP23–468M22 | 1193.6 | NPTX1 | ||
| RP23–183M12 | 1199.8 | AZI1 |
The clones on each chromosome are arranged on the basis of UCSC mapping positions.
Premature Separation of Sister Chromatids and Formation of Anaphase Bridges in Separase-Induced Fsk3 Tet-on Cells.
To assess the effect of overexpressing Separase on sister chromatid cohesion and the nature of defects causing aneuploidy in Separase-induced cells, we examined the level of premature chromatid separation (PCS) before exit from mitosis and formation of anaphase bridge, a form of genomic instability. PCS consists of separate and splayed chromatids with discernible centromeres and involves all or most chromosomes of a metaphase (Fig. 4A Inset). Using the above criteria, PCS was examined in metaphase chromosomes from Separase-induced FSK3#50 cells that were treated in the presence or absence of the doxycycline for 1, 3, and 10 days in in vitro cell culture using a protocol described for HeLa cell (15). As shown in Fig. 4A, PCS was observed in ≈10% of these cells within 1 day of Separase induction and can increase up to 30% within 10 days. The basal level of PCS was 3–5% in Separase-uninduced cells.
We also examined formation of anaphase bridge, a form of genomic instability (16), in 500 DAPI-stained nuclei of FSK3 #50 cells that were treated in the presence or absence of doxycycline for 5 days in in vitro cell culture. As shown in Fig. 4B, lagging chromosomes at anaphase and formation of anaphase bridges were observed in ≈2% of Separase-induced cells (Fig. 5B b–d). No bridges were observed in uninduced cells (Fig. 4Ba). In addition to anaphase bridges, a number of other nuclear abnormalities (large and multinucleated cells, 3%; defective cytokinesis that includes presence of chromatins in late telophase or cytokinesis, 22%) were observed in the Separase-induced cells (Fig. 4B e–g). In uninduced cells, the above defects are rare and ranged between 0.2% and 0.5%. Among these phenotypes in Separase-induced cells, the most prominent is the presence of chromatin between two dividing cells, seen up to 20–30% of the mitotic cells (Fig. 4B f and g). These cells were also immunostained by using Separase antibody and/or myc (9E10) antibody. Interestingly, approximately half of the DAPI-stained DNA forming the chromatin fibers was also stained with Separase (green) (Fig. 4C) as well as myc (9E10) (data not shown). These findings collectively suggest that hyperactive Separase results in prematurely segregated chromosomes and lagging chromosomes at anaphase.
Fig. 5.
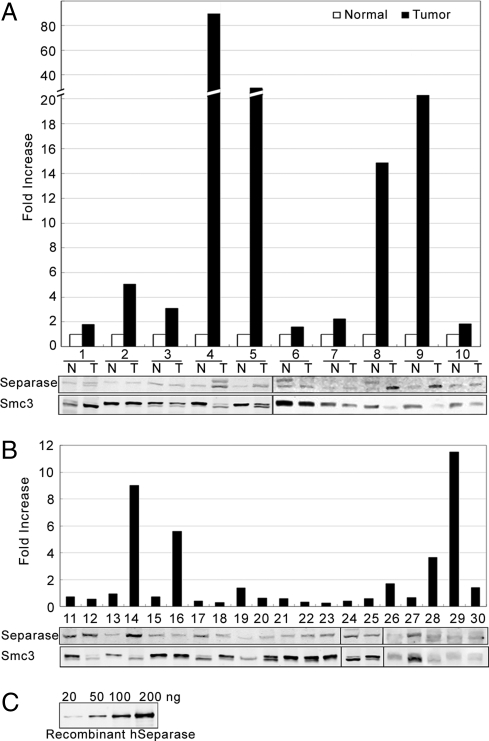
Western blot analysis of hSeparase protein in a panel of 30 human breast tumor specimens and 10 normal breast tissues. (A) Ten pairs of normal (N) and tumor (T) samples were probed with anti-hSeparase mAb. The bar graph represents expression of Separase in tumor specimens as fold increases over matched normal tissue after normalization to the expression of a cohesin protein Smc3 to compensate for loading control. Data were analyzed statistically by using paired tests comprising of paired t, rank-sum, and signed-rank tests, and expression of Separase in tumors was found to be significantly higher in tumors compared with normal (P < 0.03). (B) Expression of hSeparase in a panel of tumor samples with no matched control. The bar graph represents expression of Separase in tumor specimens as fold increases over average Separase expression in the 10 normal tissue samples shown in A after normalization to the expression of a cohesin protein Smc3 to compensate for loading control. Data were analyzed statistically by using unpaired tests and found to be significantly higher in Separase expression in tumors compared with normal (P < 0.02). (C) Assay of the detection limit for the hSeparase mAb using serially diluted recombinant hSeparase protein.
Overexpression of Separase Protein in Human Breast Cancer Samples.
We used Western blot analysis to assess the level of Separase protein expression in a panel of 30 human breast tumor specimens representing mostly infiltrating ductal carcinoma (Fig. 5 A and B). A set of 10 matched normal breast tissues was used as control (Fig. 5A). Compared with the controls, Separase was significantly overexpressed (2- to 90-fold) in 70% matched tumor specimens (P value 0.032). When all 30 tumor specimens were compared with the mean expression from the 10 normal breast tissues, Separase levels in tumors were also found to be highly significant with a P value of 0.019388. The level of Separase expression in the normal tissue is very low and occasionally beyond the detection limit of the antibody used. To assess the detection efficiency of the Separase antibody, we used serially diluted bacterially expressed recombinant human Separase (hSeparase) as a reference (Fig. 5C) and found that <20 ng of recombinant Separase cannot be efficiently detected by the hSeparase mAb used in our study. In the literature, there is no consensus among investigators for using any specific housekeeping protein to compensate for loading in Western blot analysis of breast tumor specimens due to varied cell types in the tumor (e.g., epithelial, mesenchymal, etc.). We have used Smc3 as a loading control for the following reasons: (i) Smc3 is a cohesin protein with no known role in breast tumorigenesis, and (ii) analysis of the publicly available microarray data indicated no significant variation of Smc3 gene expression in normal vs. breast tumors specimens.
Discussion
In the experiments described herein, we provide direct evidence that overexpression of Separase protein alone is sufficient to generate aneuploidy and facilitate tumorigenesis in immortalized mouse mammary epithelial cells with a p53 mutant background. Our studies also demonstrate that chromosomal instability, manifested as increased chromosomal number (aneuploidy), trisomy, translocation, and other chromosomal defects is associated with rapid tumor development. Overexpression of Separase induces premature separation of chromosomes, lagging chromosomes, and anaphase bridges. Up to 30% of PCS and formation of anaphase bridges from lagging chromosomes were observed in ≈2–5% of Separase-induced cells, whereas no bridges were observed in the uninduced cells. These findings suggest that hyperactive Separase results in prematurely segregated chromosomes and lagging chromosomes at anaphase. Increased PCS and formation of anaphase bridges are indicators of chromosomal instability and development of aneuploidy (18).
Conditional expression of Separase in the diploid FSK3 cells in vitro can induce aneuploidy within 5 days of Separase induction. Using an in vivo mouse mammary transplant model, we also demonstrated that turning on the expression of Separase for 3–4 weeks resulted in the formation of aneuploid tumors in the mammary gland. Until now, the role of overexpression of chromosomal cohesion and separation protein in the development of aneuploidy and tumorigenesis was not tested in vivo. We have previously reported that the hormonal (progesterone and estrogen) stimulation of p53-null mice mammary glands not only induces aneuploidy but also results in the overexpression of Separase, a key chromosomal separation protein, and Mad2, a mitotic checkpoint protein (5). FSK3 cells used in the present studies have both p53 WT and mutant (Ser-233-234) alleles (17). However, physiological significance of this p53 mutation is not presently known. Because overexpression of Separase either constitutionally or conditionally can cause aneuploidy independent of hormonal stimulation, Separase induction is therefore likely to be a key mechanism of hormone-induced aneuploidy in p53 null mammary epithelium (5).
To further assess how Separase induction results in aneuploidy, we analyzed the pattern of chromosomal imbalances by chromosomal CGH. Xenograft studies combined with cytogenetic analysis reveal the Separase-induced tumors are clonal in their genomic complements with trisomies for chromosome 8, 15, and 19, monosomy for chromosome 10. These studies also revealed two consistent chromosomal alterations in the Separase-induced tumor cells. The most consistent change occurred in the distal (telomeric) region of chromosomes 8 and 11. Subsequent array CGH analysis indicated an amplification of two BACs harboring genes such as NFATC3, DDX28 (chromosome 8) and RGS9, and AXIN2 (chromosome 11), with a suspected role in mammary tumorigenesis (19–21). The amplified region in chromosomes 8 and 11 is syntenic to human chromosomes 14q12 and 17q24.1, respectively. 17q23-q24 is a region that shows frequent loss of heterozygosity in breast cancer, neuroblastoma, and other tumors (22). Mutations in the genes (e.g., Axin2) in this region have been associated with colorectal cancer with defective mismatch repair (19, 22, 23). It is also interesting to note that recently Axin2/Wnt signaling pathway has been demonstrated to trigger the EMT that characterizes the tissue-invasive phenotype associated with the development and progression of human breast cancer (24). This finding is particularly relevant to our result of the mesenchymal phenotype of the Separase-induced tumors from the transplanted FSK3 mammary epithelial cells. Chromosomal alterations in Separase-induced tumors are clonal, because both the transplant (311) and its xenograft tumors had virtually identical karyotypes. Furthermore, it is apparent that once these clonal changes occur, level of Separase overexpression may not be critical and selected out to maintain tumorigenesis, and thus provides an example where oncogene addiction for tumor maintenance (25) may not hold true.
Presently, there is no known example of human mammary tumors resulting from Separase overexpression. Our studies clearly demonstrate that, compared with matched normal tissue, Separase protein is significantly overexpressed in human breast tumors (P < 0.03), which greatly strengthens the significance of our finding of the oncogenic activity of Separase in breast carcinogenesis using the mouse mammary model. It is interesting to note that analysis of publicly available microarray data from www.oncomine.org indicated that Separase transcript is overexpressed in a number of tumors, including brain, lungs, breast, ovarian, lymphoma, and sarcoma. Fourteen independent studies using breast cancer specimens indicated a strong Separase expression correlating with bad outcome (P < 0.00001). Two studies also identified that overexpression of Separase strongly correlates with p53 as well as BRCA1 mutations. These studies strengthen our hypothesis that Separase overexpression plays an important role in mammary carcinogenesis, and induction of Separase is likely to be a key mechanism of hormone-induced aneuploidy in p53-null mammary epithelium that we reported earlier (5). Furthermore, Separase misexpression provides an experimental model to study the molecular mechanisms of aneuploidy and mammary carcinogenesis.
Materials and Methods
Cell Culture.
A diploid immortal mouse mammary epithelial cell line (FSK3) was derived by in vitro passage of collagenase-treated BALB/c mammary ductal tissue explants and maintained as described (12, 26).
Tumor Specimens.
Thirty anonymized human breast tumor specimens and a set of 10 matched normal breast tissues were obtained from the tissue repository of the M. D. Anderson Cancer Center and the tumor bank of Lester and Sue Smith Breast Center at Baylor College of Medicine. All tissues in this study were obtained after Institutional Review Board-approved informed consent. Information on the samples provided by the tissue bank is summarized in Table S2.
Conditional Expression of mSeparase in Mouse Mammary Epithelial Cells.
Commercially available Tet-on gene expression systems (BD Biosciences) were used to set up FSK3 conditionally expressing myc-tagged mESPL1. FSK3 cells at passage 13 were transfected with pTetON + pTRE2hyg2-myc-mESPL1 or pTetON + pTRE2hyg2-myc empty vector. After neomycin (G418) and hygromycin selection, 100 clones from each of pTRE2-hyg2-myc and pTRE2hyg2-myc-mESPL1 transfected cells were picked, cultured, and expanded. The mSeparase protein expression of seven of these transfectants was detected by Western blot analysis by using myc-epitope tag 9E10 antibody and subsequently verified with commercially available Separase antiserum (Abnova) or homemade mSeparase polyclonal antisera. Four representative clones from mESPL1 transfected (named FSK3 #24, 50, 62) and one clone from pTRE2hyg2-myc vector (named FSK3 #64EV) were selected for further studies. The clone selection was based on the expression level of Separase protein.
Animal Model.
All mice were bred and maintained in a conventional mouse facility at Baylor College of Medicine. FSK3 Tet-on clones nos. 50 and 62 that are euploid but develop aneuploidy after Separase induction in vitro were injected into the cleared mouse mammary fat pads of 3-week-old BALB/c female p53 WT mice (27). As a control, empty vector clone (FSK3 #64EV) with no detectable Separase expression in the absence or presence of the doxycycline was used. For each clone, six mice were injected with one million cells in each fat pad. Three mice from each clone (total of six fat pads) were induced for a period of 3 weeks by the drug (1 mg of doxycycline/l mg of water), whereas three uninduced mice from the same group served as controls. Mammary fat pads were examined weekly by palpation for the formation of tumors. Two tumors specimens named 305 and 311 arising from clones 50 and 62, respectively, were subjected to cytogenetic analysis.
To study the clonality of the Separase-induced tumors that arose from the transplant studies, epithelial cell preparations from 311 tumor was xenografted to five RAG2 SCID mice. As a control, five mice were injected with empty vector clone (FSK3#64EV). Mice ages 6–8 weeks were transplanted by s.c. injection of 5 × 106 live cells into mammary gland, as described (28). Xenograft growth was measured weekly, and at the end of 1 month after injection, tumor-bearing mice were killed, and tumors specimens (1979, 1980, 1981, 1982, and 1983) were subjected to cytogenetic, histological, and Western blot analysis. The control mice were observed for 3 more months.
Cytogenetic Analysis.
Cells that were stably transfected with ESPL1 cDNA and vector control were analyzed for aneuploidy by using conventional G banding, SKY, CGH, and array CGH as reported (29, 30) (see SI Methods for details).
Array CGH.
The mouse genome tiling arrays consist of 35,000 BAC clones with unique sequences at both ends. A computer program was used to select clones with insert size in the range of 170–210 kb to achieve uniform array hybridization signals. The position information for all clones was obtained from the University of California, Santa Cruz (UCSC) database. The details of the array CGH protocol and the data analysis has been described (30, 31).
Western Blots.
Western blot analysis of the effect of hormone stimulation on the expression of mitotic proteins involved in sister chromatid cohesion and separation was performed as described (5).
Recombinant hSeparase.
Purification of full-length recombinant hSeparase was performed according to ref. 32 with modifications. cDNA coding for full-length hSeparase bearing 6-His at the C terminus was cloned into pGEX-4T vector (GE Lifesciences) and expressed in BL21 Escherichia coli cells as a GST fusion.
Statistical Analysis.
Separase expression in human breast tumor specimens was compared with the matched normal tissues by using a set of paired tests, including the paired t, rank-sum, and signed-rank test, wherever applicable. Rank-sum and signed-rank tests are more robust to departures from normality, particularly for small sample size. The unpaired one-sample t test was also performed to test whether Separase expression is statistically significant in tumor specimens where no matched normal tissue is available. For the unpaired analysis, the mean expression of Separase from the available 10 normal specimens was used to compare the tumors.
Supplementary Material
Acknowledgments.
This study was supported by grants from the National Cancer Institute (1RO1 CA109330) and the Susan G. Komen Foundation (BCTR0504113), to D.P.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801610105/DCSupplemental.
References
- 1.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 2.Duesberg P, Li R, Fabarius A, Hehlmann R. Aneuploidy and cancer: From correlation to causation. Contrib Microbiol. 2006;13:16–44. doi: 10.1159/000092963. [DOI] [PubMed] [Google Scholar]
- 3.Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Zimonjic D, Brooks MW, Popescu N, Weinberg RA, Hahn WC. Derivation of human tumor cells in vitro without widespread genomic instability. Cancer Res. 2001;61:8838–8844. [PubMed] [Google Scholar]
- 5.Pati D, et al. Hormone-induced chromosomal instability in p53-null mammary epithelium. Cancer Res. 2004;64:5608–5616. doi: 10.1158/0008-5472.CAN-03-0629. [DOI] [PubMed] [Google Scholar]
- 6.Waizenegger I, Gimenez-Abian JF, Wernic D, Peters JM. Regulation of human separase by securin binding and autocleavage. Curr Biol. 2002;12:1368–1378. doi: 10.1016/s0960-9822(02)01073-4. [DOI] [PubMed] [Google Scholar]
- 7.Kumada K, et al. The selective continued linkage of centromeres from mitosis to interphase in the absence of mammalian separase. J Cell Biol. 2006;172:835–846. doi: 10.1083/jcb.200511126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wirth KG, et al. Separase: A universal trigger for sister chromatid disjunction but not chromosome cycle progression. J Cell Biol. 2006;172:847–860. doi: 10.1083/jcb.200506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen-Fix O, Peters JM, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 10.Zachariae W, Nasmyth K. Whose end is destruction: Cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- 11.Goepfert TM, et al. Progesterone facilitates chromosome instability (aneuploidy) in p53 null normal mammary epithelial cells. FASEB J. 2000;14:2221–2229. doi: 10.1096/fj.00-0165com. [DOI] [PubMed] [Google Scholar]
- 12.Kittrell FS, Oborn CJ, Medina D. Development of mammary preneoplasias in vivo from mouse mammary epithelial cell lines in vitro. Cancer Res. 1992;52:1924–1932. [PubMed] [Google Scholar]
- 13.Hugo H, et al. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 14.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 2005;3:e86. doi: 10.1371/journal.pbio.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gisselsson D, et al. Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity. Proc Natl Acad Sci USA. 2000;97:5357–5362. doi: 10.1073/pnas.090013497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozbun MA, Medina D, Butel JS. p53 mutations in mouse mammary epithelial cells: Instability in culture and discordant selection of mutations in vitro versus in vivo. Cell Growth Differ. 1993;4:811–819. [PubMed] [Google Scholar]
- 18.Gisselsson D, Hoglund M. Connecting mitotic instability and chromosome aberrations in cancer–can telomeres bridge the gap? Semin Cancer Biol. 2005;15:13–23. doi: 10.1016/j.semcancer.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Salahshor S, Woodgett JR. The links between axin and carcinogenesis. J Clin Pathol. 2005;58:225–236. doi: 10.1136/jcp.2003.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siderovski DP, Strockbine B, Behe CI. Whither goest the RGS proteins? Crit Rev Biochem Mol Biol. 1999;34:215–251. doi: 10.1080/10409239991209273. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Chouinard L, Bonin M, Michel RN. NFATc3 deficiency may contribute to the development of mammary gland adenocarcinoma in aging female mice. Mol Carcinog. 2005;44:219–222. doi: 10.1002/mc.20136. [DOI] [PubMed] [Google Scholar]
- 22.Mai M, Qian C, Yokomizo A, Smith DI, Liu W. Cloning of the human homolog of conductin (AXIN2), a gene mapping to chromosome 17q23–q24. Genomics. 1999;55:341–344. doi: 10.1006/geno.1998.5650. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 24.Yook JI, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006:1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 25.Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21:3214–3231. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- 26.Medina D, Kittrell FS. Immortalization phenotype dissociated from the preneoplastic phenotype in mouse mammary epithelial outgrowths in vivo. Carcinogenesis. 1993;14:25–28. doi: 10.1093/carcin/14.1.25. [DOI] [PubMed] [Google Scholar]
- 27.Medina D. The mammary gland: A unique organ for the study of development and tumorigenesis. J Mammary Gland Biol Neoplasia. 1996;1:5–19. doi: 10.1007/BF02096299. [DOI] [PubMed] [Google Scholar]
- 28.Li XN, et al. Phenylbutyrate and phenylacetate induce differentiation and inhibit proliferation of human medulloblastoma cells. Clin Cancer Res. 2004;10:1150–1159. doi: 10.1158/1078-0432.ccr-0747-3. [DOI] [PubMed] [Google Scholar]
- 29.Rao PH, et al. Multicolor spectral karyotyping identifies new recurring breakpoints and translocations in multiple myeloma. Blood. 1998;92:1743–1748. [PubMed] [Google Scholar]
- 30.Cai WW, et al. Genome-wide detection of chromosomal imbalances in tumors using BAC microarrays. Nat Biotechnol. 2002;20:393–396. doi: 10.1038/nbt0402-393. [DOI] [PubMed] [Google Scholar]
- 31.Kallioniemi A, et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992;258:818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- 32.Holland AJ, Bottger F, Stemmann O, Taylor SS. Protein phosphatase 2A and separase form a complex regulated by separase autocleavage. J Biol Chem. 2007;282:24623–24632. doi: 10.1074/jbc.M702545200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.