Abstract
Neuroblastoma accounts for nearly 15% of all pediatric cancer-related deaths. We have previously shown that gastrin-releasing peptide (GRP) stimulates neuroblastoma growth, and that its cell surface receptor, GRP-R, is overexpressed in advanced-stage human neuroblastomas; however, the effects of GRP/GRP-R on tumorigenesis and metastasis in vivo are not clearly elucidated. In the present study, we found that GRP-R knockdown in the aggressive cell line BE(2)-C induced cell morphology changes, reduced cell size, decreased cell proliferation, and inhibited DNA synthesis, corresponding to cell cycle arrest at G2/M phase. Activated Akt, a crucial regulator of cell survival and metastasis, was down-regulated by GRP-R silencing. In addition, expression of p-p70S6K and its downstream target molecule S6, key regulators of protein synthesis and cell metabolism, were also significantly decreased by GRP-R silencing. GRP-R knockdown also up-regulated the expression of tumor suppressor PTEN, the inhibitor of the PI3K/Akt pathway. Furthermore, silencing GRP-R as well as GRP in BE(2)-C cells suppressed anchorage-independent growth in vitro. Conversely, overexpression of GRP-R in less aggressive SK-N-SH neuroblastoma cells resulted in soft agar colony formation, which was inhibited by a GRP-blocking antibody. Moreover, GRP-R deficiency significantly delayed tumor growth and diminished liver metastases in vivo. Our findings demonstrate that GRP and GRP-R have important oncogenic properties beyond their established mitogenic functions. Therefore, GRP-R may be an ideal therapeutic target for the treatment of aggressive neuroblastomas.
Keywords: Akt, RNAi, anchorage independence, metastasis
Advanced-stage neuroblastoma in children remains highly lethal with mortality rates exceeding 50% (1). Because of their neuroendocrine lineage, neuroblastomas can produce a variety of peptides that contribute to the classic clinical symptoms and are related to tumor prognosis, including gastrin-releasing peptide (GRP) (2). GRP and its receptor, GRP-R, are known to be up-regulated in various cancers, including undifferentiated neuroblastoma (3), small cell lung carcinoma (4), and prostate cancer (5). We have previously shown that GRP is secreted by neuroblastoma cells and acts as an autocrine growth factor to promote proliferation (3). We have also found that stable transfection of GRP-R, a member of the G protein coupled receptor family, causes an increase in the binding capacity for its ligand GRP to stimulate a constitutive cellular growth rate in SK-N-SH neuroblastoma cells (6).
GRP/GRP-R signaling functionally correlates with aberrations in neuroblastoma behavior including cell cycle progression and angiogenesis. We have found that GRP treatment induces G1-S phase progression (7). We have also shown that bombesin, the amphibian equivalent of GRP, increases the vascularization of neuroblastoma xenografts in vivo by the up-regulation of vascular endothelial growth factor (8). These processes were shown to be mediated in part by the phosphatidylinositol 3-kinase (PI3K)/Akt survival pathway (7, 8). Correspondingly, GRP-R overexpression up-regulates Akt activation in neuroblastoma cells and we also found that the ratio of Akt, in comparison to its negative regulator PTEN, was increased in human malignant neuroblastomas (6). This finding is especially relevant because a recent study has shown that Akt activation correlates with poor prognosis in primary neuroblastoma (9).
The mitogenic actions of GRP in tumor cells have been well-established; however, another less known property of GRP/GRP-R is its morphogenic capability (10). Morphogenesis is an important step for cell motility during the development of the invasive nature of various cancers, including breast and colon (11, 12). In addition to its growth factor functions, we have also noted morphological alterations in neuroblastoma cells that overexpress GRP-R (6). Therefore, GRP/GRP-R may be involved in regulating multiple steps of tumorigenesis. The molecular mechanisms responsible for GRP-mediated tumor aggressiveness and metastatic potential are not clearly defined. The purpose of our current investigation was to elucidate, in broader detail, the oncogenic effects of GRP-R in relation to neuroblastoma survival, invasive potential, and metastasis development.
In this study, we report that down-regulation of GRP-R reversed the aggressive phenotype of human neuroblastoma cell line BE(2)-C, decreased cell proliferation, inhibited DNA synthesis, and induced cell cycle arrest at G2/M phase in vitro. GRP-R silencing also significantly blocked neuroblastoma tumorigenicity by reducing colony formation in vitro, and inhibiting xenograft growth and liver metastasis in vivo. We also observed, at the molecular level, that cell survival mediator Akt and its downstream targets p70S6K and S6 are regulated by GRP-R in human neuroblastoma. Our results further demonstrate that GRP-R is a clinically relevant therapeutic target in human neuroblastomas.
Results
Stable Knockdown of GRP-R Inhibits Neuroblastoma Cell Growth and Down-Regulates the PI3-K/Akt Pathway.
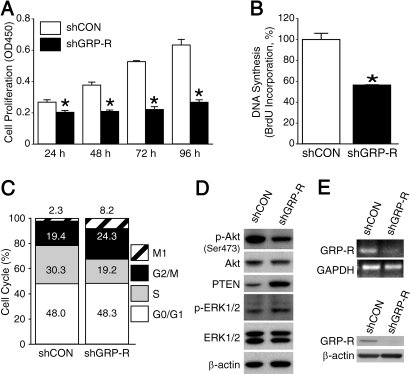
We have shown that GRP acts as an autocrine growth factor for neuroblastoma cells (3); this effect appears to depend on ligand binding to GRP-R (3, 8). We wanted to confirm the importance of GRP-R in mediating neuroblastoma growth; therefore, we measured the effect of GRP-R silencing on the cell proliferative capacity. We used short hairpin RNA (shRNA) vectors to establish stable knockdown of GRP-R in BE(2)-C cells and confirmed the knockdown with reverse transcription-PCR (RT-PCR) and Western blot analysis (Fig. 1E). Over a time course, the BE(2)-C cells expressing GRP-R shRNA (shGRP-R) displayed stagnant growth and significantly reduced proliferation in comparison with control shRNA (shCON) cells, whose growth rate steadily increased between each time point (Fig. 1A). In addition, we measured the amount of BrdU incorporation in shGRP-R cells and found that DNA synthesis was decreased to 57% compared with control cells (Fig. 1B). This reduction corresponded with a 37% decrease of shGRP-R cells in S phase of the cell cycle and an increase of cells arrested in G2/M phase, in comparison to shCON cells (Fig. 1C). We confirmed that the transfected control cells did not change the endogenous protein levels of GRP-R as well as the proliferative capacity when compared to native BE(2)-C cells [supporting information (SI) Fig. S1]. These data show that GRP-R plays a vital role in maintaining neuroblastoma cellular proliferation.
Fig. 1.
GRP-R silencing inhibits BE(2)-C cell proliferation and down-regulates the PI3-K/Akt pathway. (A) BE(2)-C cells expressing either shGRP-R or shCON were plated 1 × 104 cells per well and cell proliferation was measured (mean ± SEM; *, P < 0.004 vs. shCON). (B) DNA synthesis was analyzed by measuring the BrdU incorporation (mean ± SEM; *, P < 0.0001 vs. shCON). (C) Stably transfected cells (1 × 106 cells/well) were plated and analyzed for the percentage of cells in different cell cycle phases. (D) Western blot analysis was performed with the indicated antibodies in BE(2)-C cells after stable transfection. (E) GRP-R knockdown was confirmed with RT-PCR and Western blot analyses.
Our previous studies found that GRP/GRP-R could activate the PI3K/Akt survival pathway (7, 8). We also found that GRP-R overexpression enhanced the levels of phosphorylated (p-)Akt, and decreased the levels of PTEN, a tumor suppressor that negatively regulates the PI3K/Akt pathway (6). Furthermore, we determined that the ratio of Akt/PTEN was increased in poorly differentiated human neuroblastoma tissue samples (6). Since Akt activation regulates numerous oncogenic processes and correlates with poor prognosis in primary human neuroblastomas (9); we next assessed the role of GRP-R knockdown on p-Akt and PTEN expression. shGRP-R significantly decreased p-Akt (Ser-473) expression without notably affecting the levels of total Akt (Fig. 1D). Additionally, PTEN was up-regulated in cells expressing shGRP-R. Therefore, PI3K pathway activation appears to be regulated by GRP-R; on the other hand, activated ERK1/2 protein levels remained relatively unchanged with shGRP-R expression (Fig. 1D).
GRP-R Silencing Negatively Regulates Cell Size and Decreases p-p70S6K and S6 Expression.
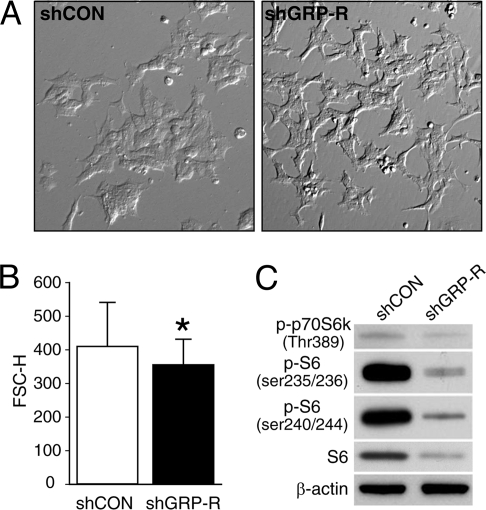
Cell viability is a product of cell size and metabolic activity (13, 14). Therefore, we next delineated the effects of GRP-R inhibition on tumor cell size. The expression of shGRP-R in BE(2)-C cells resulted in physiologically smaller cells, when compared to shCON cells, as assessed by phase-contrast microscopy (Fig. 2A). The cell sizes were quantitatively determined by FACS analysis. Despite the variability due to the wide distribution of size, an analysis of >100,000 cells per group confirmed that shGRP-R cells were significantly smaller than control cells (Fig. 2B). Similar results were also shown in transiently transfected cells with GRP-R small interfering RNA (siRNA) (data not shown). We then investigated the expression of two downstream targets of Akt that regulate cell size, p70S6K and S6 (14, 15). When activated, the kinase p70S6K phosphorylates ribosomal protein S6, which in turn initiates translation of essential proteins (15). As shown in Fig. 2C, both p-p70S6K and p-S6 were appreciably decreased in shGRP-R cells; interestingly, we also noted a significant decrease in total S6 levels. These results demonstrate that regulators of cell size depend on GRP-R expression. Furthermore, we found that the typical BE(2)-C cell morphology of a flat and aggregated appearance (shCON) changed to a round and more polarized shape with GRP-R silencing (Fig. 2A). Thus, in addition to its mitogenic properties, GRP/GRP-R might also function as a morphogen for human neuroblastoma cells and thereby, regulate their invasive properties. As an initial in vitro measure of GRP-R-mediated invasiveness, we performed a wound-healing assay and found that GRP-R siRNA (siGRP-R) significantly prevented wound closure in BE(2)-C cells (Fig. S2), adding credence to our hypothesis.
Fig. 2.
GRP-R silencing induces changes in cell morphology, reduces cell size and decreases p-p70S6K and S6 expression. (A) The morphology of BE(2)-C cells transfected with either shGRP-R or shCON were revealed by phase contrast microscopy (magnification 100×). (B) The size of cells expressing shGRP-R and shCON was determined as described in Materials and Methods. The average size is represented as the mean FSC-H ± SD of the counted cells (*, P < 0.0001 vs. shCON). (C) Western blot analysis of p-p70S6K, p-S6, and S6.
Silencing GRP-R or GRP Decreases Anchorage Independence in Vitro.
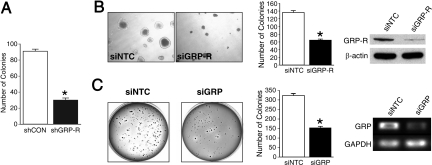
Anchorage-independent growth in soft agar is another well-established property of in vitro tumorigenicity, reflecting the malignant potential of cells in vivo (16). To evaluate whether GRP-R is critical for anchorage-independent neuroblastoma growth, we assessed the ability of BE(2)-C cells to grow in soft agar. Cells with stable expression of shGRP-R developed 60% fewer soft agar colonies than control cells (Fig. 3A). To confirm, we additionally decreased the expression of GRP-R in BE(2)-C cells by transient transfection with siRNA. Both the size and number of colonies were significantly decreased with siGRP-R (47% of control; Fig. 3B Left and Center). In addition, colonies also formed more slowly than the control colonies transfected with a nontargeting control (siNTC). The efficiency of transfection was confirmed by Western blotting (Fig. 3B Right). To determine whether these effects were mediated by the binding of GRP ligand to GRP-R, and not just a result of receptor manipulation, we transfected BE(2)-C cells with GRP siRNA (siGRP), before soft agar analysis. Transfection with siGRP blocked the colony growth of BE(2)-C cells by 54% when compared to cells transfected with siNTC (Fig. 3C Left and Center). Effective GRP knockdown was confirmed by RT-PCR (Fig. 3C Right). Therefore, GRP binding to its cell surface receptor is an important step in the process of GRP-R-induced colony formation. Of note, Akt silencing produced similar inhibitory effects on colony growth as GRP-R knockdown (Fig. S3).
Fig. 3.
Knockdown of GRP-R or GRP inhibits soft agar colony formation. (A) BE(2)-C cells expressing either shGRP-R or shCON were plated in soft agar (2.5 × 103 cells per well) for 3 weeks, and colony formation was quantitatively assessed (*, P < 0.0001 vs. shCON). (B) BE(2)-C cells were transfected with siGRP-R or siNTC for 48 h and then plated in soft agar (2.5 × 103 cells/well) for 3 weeks (live cells, magnification 40×; Left). Bar graph represents the quantitative assessment (*, P < 0.05 vs. siNTC; Center). Western blotting confirmed inhibition of GRP-R protein levels by siRNA (Right). (C) BE(2)-C cells were transfected with siGRP or siNTC for 48 h, incubated in soft agar at 2.5 × 103 cells/well for 3 weeks, and then photographed after staining (Left). Bar graph represents the quantitative assessment of colony growth (*, P < 0.05 vs. siNTC; Center). The knockdown of GRP mRNA by siRNA was confirmed with RT-PCR (Right).
GRP-R Expression Level Correlates with Malignant Potential in Human Neuroblastoma Cells.
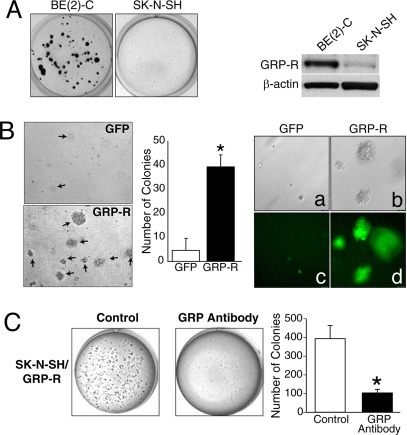
To examine whether endogenous GRP-R expression correlates with anchorage independence in neuroblastoma cells and to eliminate cell-line specific effects, we compared the colony formation of BE(2)-C to another human neuroblastoma cell line, SK-N-SH. We found that BE(2)-C cells, which behave aggressively in an in vivo xenograft model (data not shown), exhibited significantly increased malignant potential by formation of soft agar colonies when compared to SK-N-SH cells (Fig. 4A Left). Furthermore, BE(2)-C cells expressed higher levels of GRP-R than SK-N-SH cells by Western blotting (Fig. 4A Right). This finding is consistent with our previous studies, in which more abundant GRP-R expression was noted in poorly differentiated, more aggressive neuroblastomas (3).
Fig. 4.
Constitutive expressions of GRP-R correlate to anchorage-independent growth in human neuroblastoma cells. (A) BE(2)-C and SK-N-SH cells were incubated in soft agar at 2.5 × 103 cells/well in a six-well plate for 3 weeks and 5 × 103 cells per well for 4 weeks, respectively, and then photographed after staining (Left). Western blot analysis of endogenous levels of GRP-R in BE(2)-C and SK-N-SH cells (Right). (B) Soft agar analysis of SK-N-SH cells stably transfected to overexpress GFP or GFP-tagged GRP-R; arrows indicate colonies (live cells, magnification 40×; Left). Quantitative analysis of soft agar assay (*, P < 0.05 vs. GFP control cells; Center). GFP and GFP-tagged GRP-R were expressed in SK-N-SH cells stably transfected with pEGFP and pEGFP-GRP-R plasmids (Right). (C) GRP-R stably transfected SK-N-SH cells (5 × 103 cells per well) were incubated in soft agar for 4 weeks. GRP-neutralizing antibody (1 ng/ml) was applied to the top of the soft agar and colonies were photographed after staining (Left). Quantitative analysis of soft agar assay (*, P < 0.05 vs. without antibody; Right).
To further examine the role of GRP-R on neuroblastoma malignant potential, we stably transfected SK-N-SH cells with either pEGFP or pEGFP-GRP-R plasmid, and then incubated the cells in soft agar for 4 weeks. GRP-R overexpressing SK-N-SH neuroblastoma cells demonstrated a significantly increased number of colonies in soft agar, indicating stimulation of anchorage-independent cell growth by increased GRP-R expression (Fig. 4B Left). In contrast, cells transfected with control vector, pEGFP, showed only a few smaller, isolated soft agar colonies. The number of colonies induced by GRP-R overexpression was markedly increased by nearly 8-fold (Fig. 4B Center). The expression of GFP and GFP-tagged GRP-R was confirmed by fluorescent microscopy, where intense green fluorescence was noted in GRP-R overexpressing SK-N-SH cells (Fig. 4B Right). Similar results were obtained by transient transfection of SK-N-SH cells with a pEGFP-GRP-R plasmid (data not shown). Our findings suggest that the cell surface receptor, GRP-R, is tumorigenic in human neuroblastoma SK-N-SH cells. To further confirm the role of the ligand GRP in neuroblastoma colony formation, we added a specific GRP-neutralizing antibody to the culture media. The presence of the GRP-specific antibody significantly inhibited colony formation in SK-N-SH cells overexpressing GRP-R (Fig. 4C Left). The number of colonies was significantly decreased to 25% of control (Fig. 4C Right). These results further demonstrate that GRP, secreted by neuroblastoma cells, binds to GRP-R to act as a growth factor in soft agar assay, suggesting that the mitogenic actions of GRP may be an important mechanism in enhancing anchorage-independent growth.
Decreased Expression of GRP-R Inhibits Tumor Growth and Metastasis in Vivo.
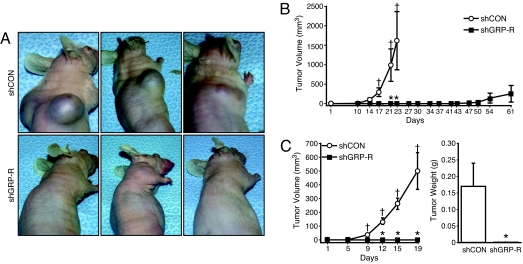
Based on the above in vitro findings, both GRP and GRP-R appear to be important in the anchorage-independent growth of neuroblastoma cells. To investigate whether the effects of GRP-R knockdown on neuroblastoma growth inhibition are sustained in vivo, we next established shGRP-R stably transfected BE(2)-C xenografts in athymic nude mice. Strikingly, as depicted in Fig. 5A, mice injected with shGRP-R cells failed to establish any noticeable tumors, whereas the control mice were significantly affected by tumor burden by day 23. In fact, the nude mice xenografts with shGRP-R expression did not develop tumors up to 43 days after subcutaneous (s.c.) injection, whereas control mice developed tumors within 10 days (Fig. 5B). In another experiment, for the purpose of same-day comparison, tumor volumes and weights were assessed 19 days after injection. The tumor volumes of mice bearing shCON xenografts were significant by day 9, in comparison to day 1 (Fig. 5C Left). As expected, because of the minute size of shGRP-R xenografts, it was not possible to measure the tumor volumes; however, there were constant GFP signals observed with the bioluminescent imaging system throughout the duration of the experiment. Correspondingly, the tumor weights (Fig. 5C Right) of mice injected with shGRP-R cells were drastically lower than mice bearing shCON xenografts (n = 5 per group).
Fig. 5.
GRP-R silencing blocks tumorigenesis of BE(2)-C cells in vivo. (A) BE(2)-C cells stably transduced with shGRP-R or shCON were grown in subscapular region of athymic nude mice. Pictures were taken at 23 and 61 days postinjection for shCON group (Upper) and shGRP-R group (Lower), respectively. (B) Tumor volumes of transduced BE(2)-C xenografts of mice killed on day 23 (shCON) or 61 (shGRP-R) postinjection (n = 3 per group (*, P < 0.05 vs. shCON; †, P < 0.05 vs. shCON, day 1). (C) Tumor volumes (Left) of transduced xenografts of mice killed on day 19 (n = 5 per group; *, P < 0.05 vs. shCON; †, P < 0.05 vs. shCON, day 1). Tumor weights (Right) of mice killed on day 19 (n = 5 per group; *, P < 0.05 vs. shCON).
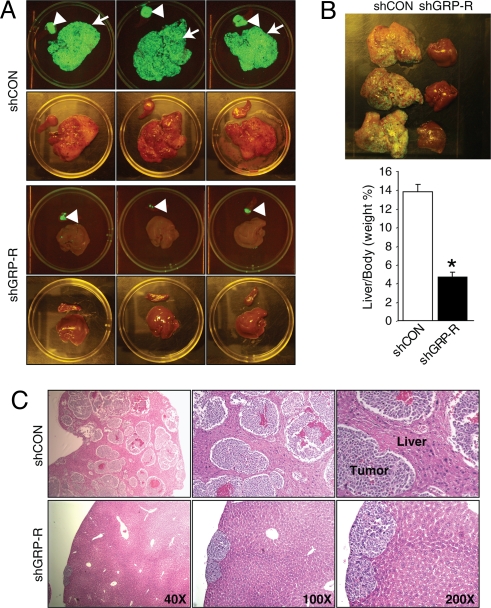
Metastatic disease is common in neuroblastoma, and because we observed anchorage-independence in vitro, we next examined the effect of GRP-R silencing on tumor metastasis in vivo. We used a spleen-liver metastasis model using BE(2)-C cells transfected with pEGFP/shGRP-R or pEGFP/shCON. Before death, strong green fluorescence signals were detected in the abdominal region of control mice, whereas fluorescence was barely detectable in the shGRP-R group. However after death, we observed that shGRP-R cells formed primary tumors with demonstrable GFP signal in the spleen (Fig. 6A, arrowheads), in contrast to control tumors which formed not only at primary sites, but also aggressively metastasized to the livers (Fig. 6A, arrows). The livers of mice with shGRP-R tumors appeared fairly normal, whereas the control mice livers had multiple metastatic lesions (Fig. 6 A and B). In addition, the average liver weight (relative to mouse body weight) of the shGRP-R group was 34% of the control group (Fig. 6B). Furthermore, H&E staining of the livers demonstrated numerous, densely packed neuroblastoma metastases in the shCON group, whereas a relatively low number of micrometastases were noted on the periphery of the livers in the shGRP-R group (Fig. 6C). Taken together, these results demonstrate that GRP-R knockdown is an advantageous strategy for in vivo neuroblastoma growth inhibition and metastasis suppression.
Fig. 6.
Knockdown of GRP-R inhibits tumor cell metastasis in vivo. (A) Representative GFP and gross images of primary tumor (arrowheads) in spleen and secondary liver metastases (arrows) from mice injected with BE(2)-C/GFP/shCON (Upper) and BE(2)-C/GFP/shGRP-R (Lower) cells. (B) Representative images of livers from mice injected with BE(2)-C/GFP/shGRP-R and BE(2)-C/GFP/shCON cells (Upper). Quantitative analysis of liver weight relative to body weight (*, P < 0.05 vs. shCON; Lower). (C) Representative H&E-stained sections of livers from mice injected with BE(2)-C/GFP/shCON (Upper) and BE(2)-C/GFP/shGRP-R (Lower) cells.
Discussion
We previously demonstrated the growth-stimulatory function of GRP in neuroblastoma cells and its correlation to PI3K/Akt pathway activation (3, 6, 7). In the present study, we show that GRP-R overexpression led to anchorage-independent growth in soft agar, which is considered a criterion for cell transformation and malignant potential in vivo. Conversely, GRP-R knockdown with siRNA and shRNA inhibited colony growth in soft agar. Furthermore, GRP-R silencing resulted in the inhibition of tumor growth and loss of cell metastatic potential in vivo. Previously uncharacterized, these results show that GRP/GRP-R plays a role in the oncogenic process of anchorage-independence and is critical for tumor growth and metastasis in human neuroblastoma. Our findings further corroborate the multiple mitogenic functions of GRP, which are known to be associated with various malignant and aggressive human tumors (4, 5, 17, 18).
GRP and its equivalent bombesin act as autocrine and/or paracrine growth factors to promote cell proliferation in cancer cells (19, 20). In accordance with this, overexpression of the bombesin peptide receptor has been shown to promote cell proliferation in Rat-1 fibroblasts (21). Similarly, we have previously shown that GRP-R overexpression in SK-N-SH neuroblastoma cells increased the proliferative capacity of the cells (6). In addition, we have found that exogenous bombesin promotes neuroblastoma growth and that its antagonist suppresses tumor progression in vivo (8). We now report that GRP-R overexpression induces anchorage-independent growth that requires the GRP ligand, because a neutralizing antibody reversed the effects. We also determined that cell proliferation, DNA synthesis, and cell cycle progression are intricately related to GRP-R expression, as silencing GRP-R inhibited each process. GRP has also been thought to be a morphogen, because it is capable of altering cell morphology in colon cancer cells (22). In this study, GRP-R silencing induced a round, polarized shape in BE(2)-C cells, which normally exist in flatter, densely packed formations. Dynamic cytoskeletal modifications are a function of cell motility and, thus, characteristic of invasive cells (23). This is consistent with our results because GRP-R knockdown inhibited metastatic potential in vitro and in vivo, correlating to the loss of the anchorage-independent phenotype. Consequently, xenograft development was significantly delayed in mice injected with shGRP-R cells. Moreover, GRP-R-silenced neuroblastoma xenografts in the spleen lacked the metastatic aggressiveness of control tumors, suggesting that in addition to proliferation, GRP-R signaling is also an effective regulator of malignant transformation in neuroblastoma cells.
PI3K/Akt pathway activation is frequently observed in human cancers; specifically in neuroblastoma, activation of Akt predicts poor outcome (9). Akt contributes to diverse cellular roles, which include cell survival, growth, proliferation, angiogenesis, metabolism, and migration (24). One of the best-conserved functions of Akt is its role in promoting cell growth and increasing cellular mass by regulating nutrient uptake and metabolism (25). The Akt-regulated mediators of this process include mTOR and its target S6K (14, 15, 26). In this study, we found that GRP-R silencing down-regulated the phosphorylation of Akt and its downstream effectors p70S6K and S6, in addition to reducing cell size. Interestingly, we did not see significant changes in mTOR (data not shown), suggesting that Akt may additionally regulate p70S6K and S6 independent of mTOR. This is consistent with many studies in which Akt functions in both mTOR-dependent and -independent pathways (24). Akt also plays a role in the regulation of cell cycle G2/M phase transition (27) and correspondingly, our results demonstrated that GRP-R knockdown resulted in a decrease in activated Akt and further caused a decrease in DNA synthesis and cell cycle arrest at G2/M phase. GRP-R knockdown also appears to indirectly inhibit Akt activation through PTEN, the endogenous inhibitor of PI3K/Akt, whose expression was increased with shGRP-R. Therefore, down-regulation of Akt activity is most likely an important factor coordinating tumor growth inhibition subsequent to GRP-R silencing.
In conclusion, our study demonstrates that GRP/GRP-R is a crucial regulator of neuroblastoma cell growth, transformation, and metastasis and that Akt may be an important downstream effector of GRP/GRP-R-mediated oncogenic properties. These in vitro and in vivo results are consistent with previous histological findings in which GRP and GRP-R expression were increased in poorly differentiated, aggressive human tumor samples (3). In a recent review (10), the biological importance of GRP and GRP-R in relation to various cancers, was discussed. However, it also emphasized the need for further investigation in regards to cancer-specific strategies. This study clearly demonstrates the roles of GRP-R in neuroblastoma tumorigenesis and secondary metastasis formation. These findings are clinically relevant because advanced-stage neuroblastomas are refractory to current treatment modalities; hence, understanding GRP/GRP-R regulation of tumor metastatic potential could provide a novel therapeutic adjunct for aggressive, undifferentiated neuroblastomas.
Materials and Methods
Antibodies and Reagents.
Primary antibodies used include GRP-R (Abcam), p-ERK1/2 (Promega), and PTEN, ERK, p-Akt (Ser-473), Akt, p-p70S6K (Thr-389), p-S6 (Ser 235/236 and Ser-240/244), and S6 from Cell Signaling. Rabbit anti-GRP IgG (GRP-neutralizing antibody) was from Bachem. Agarose was from Cambrex Bio Science. β-actin antibody and all other reagents were from Sigma-Aldrich.
Cell Culture and Transfections.
SK-N-SH and BE(2)-C cells were transfected with plasmids or siRNA pool (Dharmacon) as described (6, 7). All stably transfected cells were selected with G418 (Cellgro) at 300 μg/ml and/or zeocin at 50 μg/ml for 2 weeks. For GRP-R overexpression and silencing, pEGFP-GRP-R and pENTR/H1/TO (Invitrogen) were used, respectively. The sequence targeting GRP-R (NM_005314) is underlined in the following shRNA sequence: 5′-CACCGTAACGTGTGCTCCAGTGGACGAATCCACTGGAGCACACGTTA-3′, the nonspecific control shCON was: 5′-CACCGGGCGCGCTTTGTAGGATTCGCCGAAGCGAATCCTACAAAGCGCGCC-3′.
RT-PCR and Western Blot Analyses.
Total RNA was isolated using RNAqueous kit (Ambion) according to the manufacturer's instructions. Isolated RNA was used to synthesize cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Primers designed to amplify a 151-bp GRP fragment (NM_002091): forward primer 5′-GCTGGGTCTCATAGAAGCAAAG-3′; reverse primer 5′-TGGAGCAGAGAGTCTACCAAC-3′. GRP-R and GAPDH-specific oligonucleotide primers were the same as described (28). Amplification was performed for 40 cycles (30 cycles for GAPDH) of 2 min at 94°C, 30 sec at 55°C, and 40 sec at 72°C. GAPDH was used as a control. Western blot analysis was performed using β-actin as a loading control as described (6).
Cell Proliferation and DNA Synthesis Analyses.
Cells were seeded onto 96-well plates at a density of 1 × 104 cells per well in RPMI culture medium with 10% FBS and cell number was assessed using Cell Counting Kit-8 (Dojindo Molecular Technologies) for cell proliferation. For DNA synthesis, BrdU was added to the cell culture for 4 h before detection by Cell Proliferation ELISA (Roche) according to the manufacturer's instructions.
Flow Cytometry Analyses.
Cell cycle analyses were assessed as described (7). For cell size determination, the average cell size in 100,000-cell samples was assessed using CellQuest v3.3 software (BD), according to a previous report (29). The resulting parameter, mean forward scatter height (FSC-H), is a measure of relative cell size.
Soft Agar Colony Formation Assay.
Cells were trypsinized and resuspended in RPMI medium 1640 containing 0.4% agarose and 7.5% FBS. SK-N-SH and BE(2)-C cells were overlaid onto a bottom layer of solidified 0.8% agarose in RPMI medium 1640 containing 5% FBS, at cell concentrations of 5 × 103 cells per well and 2.5 × 103 cells per well of a six-well plate, and incubated for 4 and 3 weeks, respectively. Colonies were stained with 0.05% crystal violet, photographed, and quantified.
Xenograft Tumor Growth.
Male athymic nude mice (4–6 weeks old) were maintained as described (8). All studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and were conducted in accordance with guidelines issued by the National Institutes of Health. BE(2)-C cells transfected with shGRP-R or shCON alone or cotransfected with pEGFP and xenografts were established as previously described (8). Briefly, 1 × 106 cells/100 μl of HBSS were injected s.c. into the bilateral flanks using a 26-gauge needle (n = 3–5 per group). Tumor growth was assessed biweekly by measuring the two greatest perpendicular tumor dimensions with vernier calipers (Mitutoyo), and body weights were recorded weekly. At 23 days postinjection, tumors were excised, weighed, and fixed in formalin.
Liver Metastasis Model.
BE(2)-C cells were stably transfected with pEGFP(N3) vector (Clontech) and shGRP-R or shCON. Mice were anesthetized with isofluorane, and a small left flank incision was made to isolate and exteriorize the spleen; viable pEGFP/shCON or pEGFP/shGRP-R cells (4 × 106 cells/100 μl of HBSS) were injected into the splenic capsule using a 27-gauge needle. The spleen was returned, and the abdominal wall was closed with metal wound clips. Tumor growth was observed biweekly with Illumatool TLS (Lightools Research). At death, livers were excised, weighed and fixed in formalin for further assessment.
Statistical Analysis.
For in vitro experiments, conditions were compared by using Student's paired t test. One-way ANOVA on the ranks for repeated measures was performed for multiple comparisons. In vivo experiments were analyzed as described previously (8). Body weight was analyzed using ANOVA for a two-factor experiment with repeated measures on time. The two factors were treatment group (control and target knockdown) and day. For all experiments, P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
We thank Karen Martin for manuscript preparation and Tatsuo Uchida for statistical analysis. This work was supported by Grants RO1 DK61470, RO1 DK48498, RO1 CA104748, PO1 DK35608, and F31 DK079422 from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711861105/DCSupplemental.
References
- 1.Kushner BH, et al. Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J Clin Oncol. 2004;22:4888–4892. doi: 10.1200/JCO.2004.02.101. [DOI] [PubMed] [Google Scholar]
- 2.Gustafson WC, De Berry BB, Evers BM, Chung DH. Role of gastrointestinal hormones in neuroblastoma. World J Surg. 2005;29:281–286. doi: 10.1007/s00268-004-7815-4. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, et al. Gastrin-releasing peptide is a growth factor for human neuroblastomas. Ann Surg. 2002;235:621–629. doi: 10.1097/00000658-200205000-00003. discussion 629–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moody TW, Carney DN, Cuttitta F, Quattrocchi K, Minna JD. High affinity receptors for bombesin/GRP-like peptides on human small cell lung cancer. Life Sci. 1985;37:105–113. doi: 10.1016/0024-3205(85)90413-8. [DOI] [PubMed] [Google Scholar]
- 5.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999;59:1152–1159. [PubMed] [Google Scholar]
- 6.Qiao J, Kang J, Cree J, Evers BM, Chung DH. Gastrin-releasing peptide-induced down-regulation of tumor suppressor protein PTEN (phosphatase and tensin homolog deleted on chromosome ten) in neuroblastomas. Ann Surg. 2005;241:684–691. doi: 10.1097/01.sla.0000161173.47717.71. discussion 691–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishola TA, Kang J, Qiao J, Evers BM, Chung DH. Phosphatidylinositol 3-kinase regulation of gastrin-releasing peptide-induced cell cycle progression in neuroblastoma cells. Biochim Biophys Acta. 2007;1770:927–932. doi: 10.1016/j.bbagen.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang J, et al. Bombesin induces angiogenesis and neuroblastoma growth. Cancer Lett. 2007;253:273–281. doi: 10.1016/j.canlet.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opel D, Poremba C, Simon T, Debatin KM, Fulda S. Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res. 2007;67:735–745. doi: 10.1158/0008-5472.CAN-06-2201. [DOI] [PubMed] [Google Scholar]
- 10.Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide and cancer. Biochim Biophys Acta. 2006;1766:23–41. doi: 10.1016/j.bbcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Paszek MJ, Weaver VM. The tension mounts: Mechanics meets morphogenesis and malignancy. J Mamm Gland Biol Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 12.van den Brink GR, Offerhaus GJ. The morphogenetic code and colon cancer development. Cancer Cell. 2007;11:109–117. doi: 10.1016/j.ccr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen P, Tyers M. How cells coordinate growth and division. Curr Biol. 2004;14:R1014–R1027. doi: 10.1016/j.cub.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Fingar DC, Blenis J. Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 15.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponten J. Spontaneous and virus induced transformation in cell culture. In: Herausgegeben V, Gard S, Hallauer C, Meyer KF, editors. Virology Monographs. vol 8. New York: Springer; 1971. pp. 4–19. [DOI] [PubMed] [Google Scholar]
- 17.Ferris HA, Carroll RE, Rasenick MM, Benya RV. Constitutive activation of the gastrin-releasing peptide receptor expressed by the nonmalignant human colon epithelial cell line NCM460. J Clin Invest. 1997;100:2530–2537. doi: 10.1172/JCI119795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lui VW, et al. Mitogenic effects of gastrin-releasing peptide in head and neck squamous cancer cells are mediated by activation of the epidermal growth factor receptor. Oncogene. 2003;22:6183–6193. doi: 10.1038/sj.onc.1206720. [DOI] [PubMed] [Google Scholar]
- 19.Alexander RW, et al. Effects of bombesin on growth of human small cell lung carcinoma in vivo. Cancer Res. 1988;48:1439–1441. [PubMed] [Google Scholar]
- 20.Rozengurt E, Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci USA. 1983;80:2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlesworth A, Broad S, Rozengurt E. The bombesin/GRP receptor transfected into Rat-1 fibroblasts couples to phospholipase C activation, tyrosine phosphorylation of p125FAK and paxillin and cell proliferation. Oncogene. 1996;12:1337–1345. [PubMed] [Google Scholar]
- 22.Carroll RE, Matkowskyj KA, Tretiakova MS, Battey JF, Benya RV. Gastrin-releasing peptide is a mitogen and a morphogen in murine colon cancer. Cell Growth Differ. 2000;11:385–393. [PubMed] [Google Scholar]
- 23.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlob K, et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohanna M, et al. Atrophy of S6K1(-/-) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol. 2005;7:286–294. doi: 10.1038/ncb1231. [DOI] [PubMed] [Google Scholar]
- 27.Kandel ES, et al. Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Mol Cell Biol. 2002;22:7831–7841. doi: 10.1128/MCB.22.22.7831-7841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatzistamou I, Schally AV, Sun B, Armatis P, Szepeshazi K. Inhibition of growth of OV-1063 human epithelial ovarian cancers and c-jun and c-fos oncogene expression by bombesin antagonists. Br J Cancer. 2000;83:906–913. doi: 10.1054/bjoc.2000.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruvinsky I, et al. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.