Abstract
Partitioning of the mammalian Golgi apparatus during cell division involves disassembly at M-phase. Despite the importance of the disassembly/reassembly pathway in Golgi biogenesis, it remains unclear whether mitotic Golgi breakdown in vivo proceeds by direct vesiculation or involves fusion with the endoplasmic reticulum (ER). To test whether mitotic Golgi is fused with the ER, we compared the distribution of ER and Golgi proteins in interphase and mitotic HeLa cells by immunofluorescence microscopy, velocity gradient fractionation, and density gradient fractionation. While mitotic ER appeared to be a fine reticulum excluded from the region containing the spindle-pole body, mitotic Golgi appeared to be dispersed small vesicles that penetrated the area containing spindle microtubules. After cell disruption, M-phase Golgi was recovered in two size classes. The major breakdown product, accounting for at least 75% of the Golgi, was a population of 60-nm vesicles that were completely separated from the ER using velocity gradient separation. The minor breakdown product was a larger, more heterogenously sized, membrane population. Double-label fluorescence analysis of these membranes indicated that this portion of mitotic Golgi also lacked detectable ER marker proteins. Therefore we conclude that the ER and Golgi remain distinct at M-phase in HeLa cells. To test whether the 60-nm vesicles might form from the ER at M-phase as the result of a two-step vesiculation pathway involving ER–Golgi fusion followed by Golgi vesicle budding, mitotic cells were generated with fused ER and Golgi by brefeldin A treatment. Upon brefeldin A removal, Golgi vesicles did not emerge from the ER. In contrast, the Golgi readily reformed from similarly treated interphase cells. We conclude that Golgi-derived vesicles remain distinct from the ER in mitotic HeLa cells, and that mitotic cells lack the capacity of interphase cells for Golgi reemergence from the ER. These experiments suggest that mitotic Golgi breakdown proceeds by direct vesiculation independent of the ER.
INTRODUCTION
Membrane-bound organelles are generally thought to be acquired by inheritance rather than de novo synthesis (Nunnari and Walter, 1996). Segregation of the inherited organelles into daughter cells can either be directed or passive, depending on the copy number and distribution of the organelle components at the time of cytokinesis (Warren and Wickner, 1996). Segregation of the mammalian endoplasmic reticulum (ER) and Golgi is thought to be passive despite the fact that these are single-copy organelles during interphase. This is because the ER and Golgi components are present in abundant dispersed membranous structures at M-phase (Maul and Brinkley, 1970; Moskalweski et al., 1977; Zeligs and Wollman, 1979; Lucocq and Warren, 1987; Lucocq et al., 1987, 1989). Although segregation of the disassembled ER and Golgi may not require specific molecular machinery, their disassembly/reassembly reactions, which ensure accurate partitioning, must involve specific cell cycle-regulated activities. While in vitro systems have been used to successfully identify components required for Golgi disassembly and reassembly (Misteli and Warren, 1994; Acharya et al., 1995a, 1995b; Rabouille et al., 1995a, 1995b), the pathway of Golgi breakdown and reassembly in vivo remains controversial. Specifically, it is not clear whether the Golgi breaks down directly and is inherited as discrete Golgi fragments, or whether it is fused with the ER at M-phase and then reemerges after cytokinesis (Thyberg and Moskalewski, 1992a; Cole et al., 1996). The latter model is distinct from de novo synthesis in that the components of the Golgi are not lost, but it requires Golgi assembly without the inheritance of a preexisting organelle.
The mammalian interphase ER is a large interconnected tubular membrane network that includes the nuclear membrane. The interphase Golgi is a smaller interconnected membrane network consisting of flattened membrane stacks situated in a perinuclear position near the microtubule-organizing center. In mitotic cells, ER and Golgi membrane proteins are found in numerous dispersed cisternae and/or vesicles (Lucocq et al., 1987, 1989; Misteli and Warren, 1995a). Whether these structures represent independent ER and Golgi membranes or the product of ER–Golgi fusion, is the crucial distinction between the two models of ER and Golgi disassembly. Examination of mitotic cells has revealed significant relocalization of mannosidase II, a medial Golgi marker, to structures identified as fragmented ER cisternae (Thyberg and Moskalewski, 1992a, 1992b), suggesting that the cell cycle-driven Golgi disassembly/reassembly reaction might involve reversible fusion with the ER. That mechanisms exist that allow reversible ER-Golgi fusion is clear from experiments with the drug brefeldin A, which reversibly induces ER-Golgi fusion (Doms et al., 1989; Lippincott-Schwartz et al., 1989; Sandvig et al., 1991). Furthermore, Golgi dispersal upon drug-induced microtubule depolymerization has been suggested to involve fusion with the ER followed by budding of Golgi proteins at peripheral ER sites (Cole et al., 1996). Therefore, microtubule reorganization at mitosis may lead to ER-Golgi fusion, and Golgi reemergence from the ER after cytokinesis may account for its positioning near ER export sites (Lucocq and Warren, 1987). On the other hand, galactosyltransferase, a trans-Golgi/trans-Golgi network marker, is localized in mitotic cells to clusters of vesicular and tubulovesicular membranes that lack ribosomes, a marker of the rough ER (Lucocq et al., 1987). Furthermore, in vitro Golgi disassembly triggered by mitotic cytosol takes place with isolated Golgi (Misteli and Warren, 1994) suggesting that the ER is not required for mitotic Golgi vesiculation.
To determine the pathway of Golgi disassembly during mitosis, we used a combination of morphological and subcellular fractionation assays to test for fusion of ER and Golgi. Our results indicated that the Golgi and ER are not fused in mitotic cells and that mitotic cells lack the capacity for fusion or budding of ER and Golgi. These experiments suggest that the Golgi directly vesiculates and disperses to allow equal partitioning at cytokinesis.
MATERIALS AND METHODS
Cell Culture
Monolayer cultures of HeLa cells were grown in minimal essential medium (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum and 100 IU/ml penicillin-streptomycin (Life Technologies). Where indicated, the growth medium was supplemented with 0.5 μg/ml nocadazole (Sigma, St. Louis, MO) and/or 10 μg/ml brefeldin A (Sigma). Confluent interphase (nonsynchronized) cultures were harvested using a cell scraper. Mitotic cells were isolated by shake-off from subconfluent cultures incubated with nocadazole for 24 h (Knehr et al., 1995). For brefeldin A washout, the media was carefully removed and any detached cells were collected. Both detached and attached cells were washed three times with Dulbecco’s phosphate-buffered saline (PBS) (Life Technologies, Inc.), recultured in Eagle’s minimal essential medium supplemented with nocodazole for 3 h, and then subjected to the shake-off procedure.
Immunofluorescence
Both cells and membrane fractions were analyzed by immunofluorescence. For analysis of cells, HeLa or COS-7 were grown on 22-mm glass coverslips, fixed with 3% paraformaldehyde (in PBS) for 30 min, washed three times with PBS, and then permeabilized for 30 min with block solution (PBS containing 0.1% Triton X-100, 2.5% fetal bovine serum, 0.2 M glycine, and 0.02% sodium azide). Primary antibody incubation was in 0.04 ml block solution containing mouse anti-p63 (Schweizer et al., 1993) and rabbit anti-giantin (Linstedt et al., 1995) for 30 min. After five washes with PBS, 0.04 ml block solution containing fluorescein-labeled goat anti-mouse (dilution 1:100; Cappel, West Chester, PA) and rhodamine-labeled goat anti-rabbit (dilution 1:100; Cappel) was added for 30 min. After five final washes with PBS, the coverslips were attached to glass slides over mounting medium containing the DNA stain (glycerol with 0.1 mg/ml phenylenediamine and Hoechst 33258), and viewed as previously described (Linstedt et al., 1997). For analysis of membrane fractions, 0.02 ml of each membrane fraction was placed between two glass coverslips and submerged in liquid nitrogen. After the sample had been removed, the coverslips were rapidly separated and submerged in methanol at −20°C for at least 10 min. Analysis of the coverslip-attached, fixed, and permeabilized membrane fraction was performed exactly as for cells, except that all incubations and washes were in PBS containing 0.2% gelatin. The resulting acquired digital images were false colored to green or red and then merged to form a composite using Photoshop (Adobe Systems, Mountain View, CA).
Gradient Fractionation
To prepare mitotic postnuclear supernatants, nocodazole-blocked HeLa cells from three 15-cm plates were collected by shake-off and centrifugation (2,000 rpm in a SA-600 rotor (Sorvall, Newtown, CT) for 5 min, washed once in PBS (containing 1 mM EDTA), washed once in sucrose buffer (250 mM sucrose, 10 mM triethylamine pH 7.4, 1 mM EDTA, with protease inhibitors: 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml pepstatin A), homogenized by 20 passages through a 25-gauge needle in 0.5 ml buffer (50 mM NaCl, 10 mM triethylamine, pH 7.4, 1 mM EDTA, with protease inhibitors), and centrifuged at 1,000 × g for 2 min. The postnuclear supernatant was then fractionated on either velocity gradients or flotation gradients. In the standard velocity gradient procedure the postnuclear supernatant (0.4 ml) was layered on linear 5–25% (vol/vol) glycerol gradients (5 ml in 10 mM triethylamine, pH 7.4, and 1 mM EDTA on a 0.5 ml 80% sucrose cushion) and centrifuged at 150,000 × g for 30 min in a SW50.1 rotor (Beckman, Fullerton, CA), and 0.4 ml fractions were collected from the top. The sedimentation coefficient was calculated as described using a proportionality constant value of 3.38 cm (Fritsch, 1973). For flotation gradient analysis, the postnuclear supernatant was adjusted to 1.6 M sucrose (1.1 ml), layered on 2 M sucrose (0.5 ml), covered with three sucrose layers (1.1 ml each: 1.4 M, 1.2 M, 0.8 M), and centrifuged at 105,000 × g for 105 min in a SW50.1 rotor (Beckman), and 0.4-ml fractions were collected from the top (Nagata-Kuno et al., 1990). All steps were carried out at 0–4°C. Immunoblotting was used to assay each fraction for the presence of GPP130 (Linstedt et al., 1997), giantin (Linstedt and Hauri, 1993), galactosyltransferase (Berger et al., 1986), p63 (Schweizer et al., 1993), and calnexin (StressGen Biotechniologies, Victoria, British Columbia, Canada) as previously described (Linstedt and Hauri, 1993). In some cases the membrane proteins in each fraction were concentrated by centrifugation or trichloroacetic acid precipitation before SDS-PAGE. Detection was by enhanced chemiluminescence (Pierce, Rockford, IL) followed by densitometric scanning. Previously described enzymatic assays were used to determine galactosyltransferase (Aoki et al., 1990) and glucose-6-phosphatase activities in each fraction (Arosond and Touster, 1974).
Electron Microscopy
For electron microscopic analysis of vesicles in the 115 S Golgi peak, velocity gradient fractions containing the vesicles were pooled, adjusted to 1.6 M sucrose, and subjected to flotation gradient analysis as indicated above. The 1.4 M–1.6 M sucrose interface was collected, concentrated by centrifugation, and resuspended in 250 mM sucrose, 10 mM triethylamine, pH 7.4, and 1 mM EDTA by trituration. Samples were stained with 1% uranyl acetate and viewed as previously described (Linstedt et al., 1997). Vesicle diameter was measured with NIH Image software after calibration with a catalase crystal. For mitotic Golgi vesicle immunoisolation from the 115 S Golgi peak, affinity-purified goat anti-mouse IgG (Cappel) secondary antibody was covalently coupled to Dynabeads M-500 (Dynal, Great Neck, NY) magnetic beads (7.5 μg/107 beads) according to manufacturer’s instructions. An anti-giantin monoclonal antibody (Linstedt and Hauri, 1993) was incubated with coupled beads at a twofold M excess over secondary antibody and washed four times with PBS + 0.1% bovine serum albumin (BSA). Anti-giantin and control (no primary antibody) beads were incubated with velocity gradient fractions containing the vesicles for 12 h at 4°C with constant rotation in PBS containing 0.5% BSA. The beads were then washed three times in PBS containing 0.5% BSA using a magnet, transferred to a fresh tube, and prepared for electron microscopy as previously described (Saucan and Palade, 1994).
RESULTS
The two models for Golgi disassembly, direct vesiculation and ER fusion, make distinct predictions regarding the fractionation of Golgi and ER membrane marker proteins. If the Golgi vesiculates independently of the ER, then ER and Golgi membranes should be separable. Alternatively, if the ER and Golgi fuse, then the markers will always cofractionate. To test these models, we compared the distribution of ER and Golgi proteins in interphase and mitotic HeLa cells. This analysis was carried out using immunofluorescence microscopy, velocity gradient fractionation, and density gradient fractionation.
Mitotic ER and Golgi Have Distinct Cellular Distributions and Morphologies
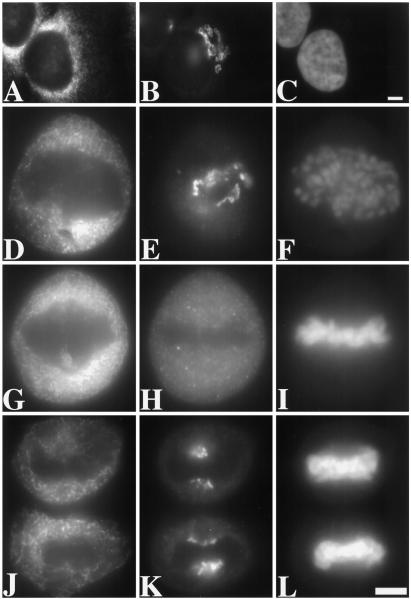
For immunofluorescence microscopy, paraformaldehyde-fixed HeLa cells were triply stained. Cell-cycle state was determined by DNA staining with Hoechst 33258, ER morphology was determined using a monoclonal anti-p63 antibody (Schweizer et al., 1995), and Golgi morphology was determined using a polyclonal anti-giantin antibody (Linstedt et al., 1995). As expected, the Golgi staining at interphase was restricted to perinuclear membranes with a characteristic ribbon-like appearance, whereas the ER staining at interphase was distributed in a fine reticulum filling the cytoplasm with concentration near the nuclear membrane (Figure 1, A–C). Importantly, at no stage of mitosis was a correspondence between Golgi and ER staining discerned. At prometaphase, Golgi staining was restricted to the area containing the chromosomes and lacking the ER marker (Figure 1, D–F). At metaphase, the Golgi partially penetrated the area containing the spindle apparatus, whereas the ER was excluded from this area (Figure 1, G-I). In contrast to the ER, the Golgi exhibited a clear vesicular, rather than reticular, pattern. By late anaphase, the Golgi was localized at either end of the chromosomes, while the ER remained distributed throughout the cytoplasm (Figure 1, J–L). Thus, it was clear from a simple immunofluorescence analysis that ER and Golgi markers were not coincident in mitotic cells, suggesting that the two compartments remain independent at M-phase. Similar results were obtained after analysis of COS-7 cells, a different mammalian cell line, with the same markers. Similar results were also obtained after analysis of methanol- rather than paraformaldehyde-fixed cells. Also, the giantin pattern was exactly coincident with that of another Golgi protein, GPP130 (Linstedt et al., 1997), at all stages of the cell cycle. Although these observations did not exclude the possibility of continuities between mitotic ER and Golgi membranes, they suggested that the ER and Golgi markers are segregated from one another in distinct compartments in mitotic cells, the ER membranes being larger than the Golgi membranes.
Figure 1.
Examination of triply stained interphase and mitotic HeLa cells by fluorescence microscopy. The staining pattern of the ER marker protein p63 (A, D, G, and J), the Golgi marker protein giantin (B, E, H, and K), and the DNA stain Hoechst 33258 (C, F, I, and L) is shown for cells at interphase (A–C), prometaphase (D–F), metaphase (G–I), and late anaphase (J–L). To allow direct comparison, each label was viewed at the same focal plane. Compared with the Golgi staining, the ER staining was significantly more reticular and was excluded from a larger area near the condensed chromosomes. The pictures shown are representative of hundreds of different cells examined at the different stages of division. Bars, 25 μm.
The Major Golgi Breakdown Product at M-Phase Is 60-nm Vesicles That Lack Detectable ER Marker Proteins
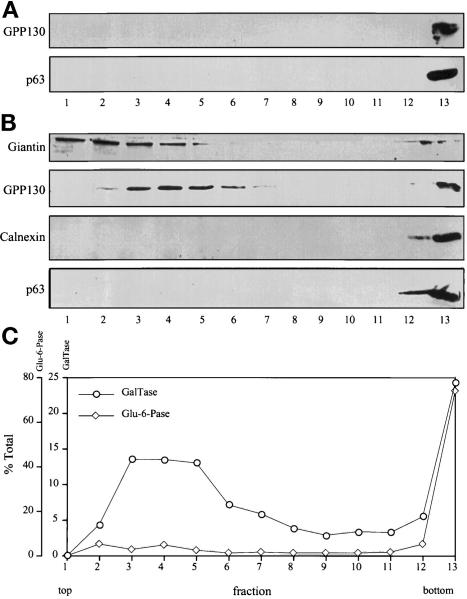
If ER and Golgi membranes are not fused and differ in size, it should be possible to resolve mitotic ER and Golgi vesicles on gradients. Therefore, we analyzed the distribution of ER and Golgi markers after fractionation of postnuclear supernatants from interphase (nonsynchronized) or nocodazole-blocked mitotic HeLa cells on velocity gradients. After fractionation of interphase cells, the Golgi protein GPP130 (Linstedt et al., 1997) and the ER protein p63 (Schweizer et al., 1995) were recovered at the bottom of the gradient (Figure 2A). Presumably, this reflects the fact that interphase Golgi and ER exist as relatively large membranes, not as small vesicles. In contrast, fractionation of mitotic cells revealed that, at M-phase, most of the Golgi marker GPP130 was recovered in a slowly sedimenting population, with relatively minor amounts remaining in larger structures (Figure 2B). Two other Golgi-localized integral membrane proteins, galactosyltransferase (Nilsson et al., 1993) and giantin, were also recovered in slowly sedimenting membranes in mitotic cells (Figure 2, B and C). Clearly, the slowly sedimenting Golgi membranes represented a major breakdown product of Golgi at M-phase. Importantly, three ER-localized integral membrane proteins, p63, calnexin (Anhquyen et al., 1994), and glucose-6-phosphatase (DePierre and Dallner, 1976), were not detectable in the fractions containing the slowly sedimenting mitotic Golgi (Figure 2, B and C). The separation of mitotic Golgi vesicles and ER was highly reproducible. In more than 50 separate velocity gradient fractionations under a variety of conditions, at least 75% of the mitotic Golgi was recovered as a membrane peak that lacked detectable ER markers. The fact that the major breakdown product of the Golgi at M-phase lacks ER marker proteins is incompatible with the view that the end point of mitotic Golgi disassembly is fusion with the ER.
Figure 2.
Distribution of ER and Golgi markers after velocity gradient sedimentation. (A) Interphase (nonsynchronized) HeLa cell postnuclear supernatants were fractionated on glycerol gradients and immunoblotted with antibodies against the Golgi marker protein GPP130 and the ER marker protein p63. Both Golgi and ER marker proteins were recovered on a sucrose cushion at the bottom of the gradient. (B) Mitotic HeLa cell postnuclear supernatants were fractionated and immunoblotted with antibodies against Golgi marker proteins (GPP130 and giantin) and ER marker proteins (calnexin and p63). The cells were blocked at mitosis with 0.5 μg/ml nocodazole and collected by shake-off. In contrast to interphase, a major portion of the mitotic Golgi was recovered in a slowly sedimenting position near the top of the gradient. These fractions lacked detectable ER. (C) Fractions resulting from separation of mitotic postnuclear supernatants were also assayed by enzymatic activity for the Golgi marker galactosyltransferase and the ER marker glucose-6-phophatase. By activity assay also, mitotic Golgi, but not mitotic ER, was recovered in a slowly sedimenting position. Centrifugation in these experiments was for 30 min at 150,000 × g; similar results were obtained under a variety of centrifugation conditions.
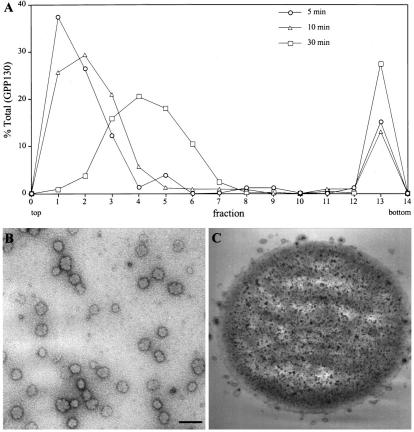
Further analysis of the slowly sedimenting mitotic Golgi suggested that it represented a relatively uniform population of small vesicles. The migration of the GPP130-containing vesicles increased linearly with time of sedimentation (Figure 3A). The calculated sedimentation coefficient for these vesicles was 115 S. These mitotic Golgi vesicles were pooled, further purified on a density gradient, and then examined by electron microscopy after negative staining (Figure 3B). The resulting vesicle fraction exhibited a uniform size distribution with an average diameter of 60 nm (57 ± 7 nm, n = 81). Therefore, the average vesicle size in the partially purified fraction containing the 115 S vesicles was identical to the 57-nm diameter previously reported for Golgi vesicles in mitotic HeLa cells (Lucocq et al., 1989), and these lack detectable ER markers. To confirm the presence of giantin and GPP130 in the 60-nm diameter vesicles, we performed vesicle immunoisolation experiments using magnetic beads either coated with an anti-giantin antibody or a control antibody. As expected, 60-nm vesicles were specifically immunoisolated by beads coated with anti-giantin antibodies (Figure 3C). Immunoblotting confirmed that both giantin and GPP130 were recovered with the anti-giantin, but not the control, immunobeads.
Figure 3.
Analysis of the major mitotic Golgi breakdown product. (A) Mitotic postnuclear supernatants were fractionated on glycerol velocity gradients by centrifugation at 75,000 × g for 5 or 10 min or 150,000 × g for 30 min. The recovery of GPP130 in each fraction was determined by densitometry of immunoblots. The slowly sedimenting mitotic Golgi was recovered as a uniform peak of 115 S. (B) The 115 S mitotic Golgi peak was collected on a sucrose cushion and analyzed by electron microscopy after negative staining with uranyl acetate. A 60-nm diameter population of vesicles was present. To exclude potential artifacts, the vesicles were also treated with protease or detergent before analysis. Similar results were obtained for samples treated with protease, and no structures were visualized for samples treated with detergent. Bar, 150 nm. (C) Mitotic Golgi vesicles immunoisolated from 115 S vesicle peak fractions on anti-giantin–coated magnetic beads are shown after analysis by electron microscopy. No vesicles were observed when control magnetic beads were incubated with identical velocity gradient fractions.
Separation of ER and Golgi in mitotic cells was also examined using a flotation gradient. Interphase Golgi was mostly recovered near the top of the gradient, whereas interphase ER was recovered near the bottom of the gradient (Figure 4A). Surprisingly, in mitotic cells, the relative density of ER and Golgi was reversed; mitotic Golgi was recovered near the bottom of the gradient, whereas mitotic ER was now recovered near the top of the gradient (Figure 4B). Clearly, the ER and Golgi compartments underwent dramatic and distinct density shifts during mitosis, presumably due to cell cycle-regulated alterations in their protein/lipid ratios. The distinct density distributions of mitotic ER and Golgi membranes were consistent with our finding from velocity gradient separation that the major Golgi breakdown product was not fused with the ER at M-phase.
Figure 4.
Fractionation of interphase and mitotic ER and Golgi on flotation gradients. (A) The relative distribution of GPP130 and p63 flotation on a sucrose step gradient is shown for a postnuclear supernatant from a culture of nonsynchronized HeLa cells. (B) Identical analysis for cells blocked at mitosis with 0.5 μg/ml nocodazole and collected by shake-off. Note that the distribution of mitotic ER significantly differed from that of mitotic Golgi. (C) Identical analysis for cells in which brefeldin A was used to induce ER–Golgi fusion during progression to M-phase. Subconfluent cultures of HeLa cells were treated with 0.5 μg/ml nocodazole and 10 μg/ml brefeldin A for 24 h. Mitotic cells were obtained by shake-off, and the postnuclear supernatant was fractionated and analyzed as above. Unlike nontreated mitotic cells, cells that progressed to M-phase during brefeldin A treatment yielded equivalent ER and Golgi distributions.
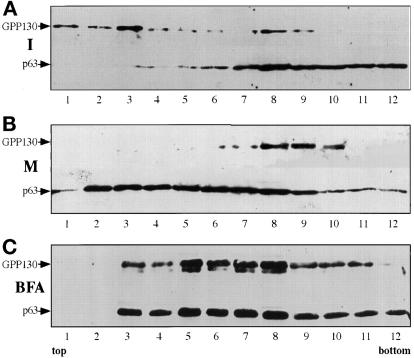
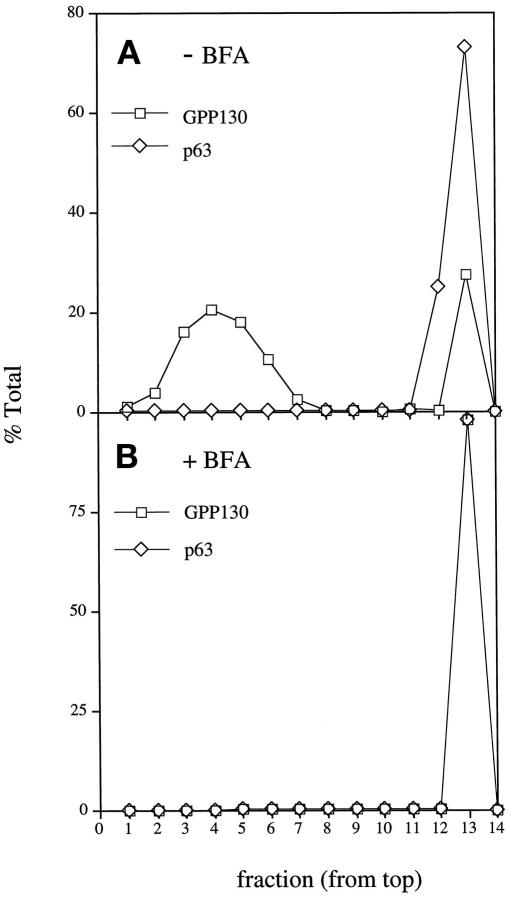
As a control for these experiments, and those presented below, we simultaneously examined mitotic cells in which fusion of ER and Golgi was experimentally induced using brefeldin A treatment. To generate a fused ER and Golgi at M-phase, HeLa cells were exposed to brefeldin A and nocodazole continuously as they progressed from interphase to the M-phase block. The cells were removed from plates by shake-off and fractionated on density and velocity gradients. Consistent with fusion of the ER and Golgi in these mitotic cells, the ER and Golgi cosedimented to a density intermediate to mitotic ER and Golgi (Figure 4C). Furthermore, the 60-nm Golgi vesicles were no longer recovered in these mitotic cells. Instead the mitotic Golgi cosedimented with the ER at the bottom of the velocity gradient (Figure 5). The difference in the behavior of mitotic Golgi in untreated and continuous brefeldin A-treated cells underscores the significance of the difference between the physiological situation and the fused state.
Figure 5.
Fractionation of mitotic ER and Golgi after treatment with brefeldin A during progression to M-phase. Subconfluent cultures of HeLa cells were treated with 0.5 μg/ml nocodazole in the absence (A) or presence (B) of 10 μg/ml brefeldin A for 24 h. Mitotic cells were obtained by shake-off, and the postnuclear supernatants were fractionated on glycerol velocity gradients as indicated above. The recovery, in each fraction, of the Golgi marker GPP130 and the ER marker p63 was determined by densitometry of immunoblots. Unlike nontreated mitotic cells, cells that progressed to M-phase during brefeldin A treatment lacked the 115 S major mitotic Golgi breakdown product. Instead, the mitotic Golgi was completely recovered at the bottom of the gradient together with the ER.
The Rapidly Sedimenting Mitotic Golgi Membranes Also Lack Detectable ER Marker Proteins
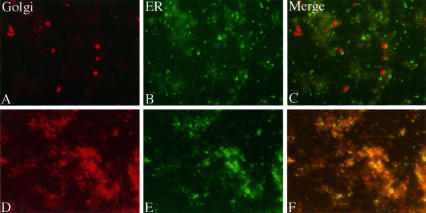
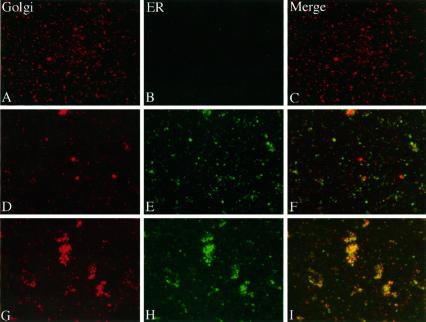
The velocity gradient separations presented above indicated that mitotic Golgi was recovered mostly as a population of 60-nm diameter vesicles, with the remainder being in larger heterogeneously sized membranes. Despite numerous fractionation attempts, we could never achieve a complete separation of the rapidly sedimenting Golgi breakdown product from the ER membranes. Therefore, on the basis of sedimentation behavior, we could not exclude the possibility that this subfraction of the Golgi (<25%) had fused with the ER. Alternatively, the rapidly sedimenting mitotic Golgi may have represented aggregated clusters of the 60-nm vesicles, interphase Golgi contaminating our preparations, ER containing newly synthesized or mistargeted Golgi proteins, or a distinct Golgi breakdown product. To address this issue, we devised a novel assay to determine whether the rapidly sedimenting mitotic Golgi membranes contain ER marker proteins. We reasoned that vesicle populations that represent distinct ER and Golgi breakdown products should yield noncoincident staining patterns after immunofluorescence microscopy, whereas membranes derived from a fused ER and Golgi should produce coincident patterns. To test this notion, we first analyzed membranes in homogenates from nontreated and brefeldin A-treated interphase HeLa cells for coincidence of ER and Golgi marker proteins. The ER and Golgi are known to be distinct in nontreated interphase cells, whereas they are fused in brefeldin A-treated interphase cells (Lippincott-Schwartz et al., 1990). The membranes were fixed to coverslips as described in MATERIALS AND METHODS and stained using anti-giantin and anti-p63 antibodies. As expected, ER and Golgi staining was distinct in membranes from nontreated cells (Figure 6, A–C), whereas ER and Golgi staining was coincident in membranes from brefeldin A-treated cells (Figure 6, D–F). Controls in which either or both primary antibodies were omitted confirmed the specificity of the staining. Based on these results, the assay appeared capable of discriminating between fused and non-fused ER and Golgi. We next analyzed membranes in the 60-nm vesicle peak from a velocity gradient. Consistent with the immunoblot data, this fraction exhibited strong Golgi staining but lacked ER immunoreactivity (Figure 7, A–C). Finally, we examined the membranes in the rapidly sedimenting fraction from the velocity gradient. We noted a striking noncoincidence of Golgi and ER marker proteins in this membrane fraction, indicating that the membranes recovered at the bottom of the velocity gradients representing approximately 25% of the mitotic Golgi were not fused to a significant extent with the ER (Figure 7, D–F). As a further control, we also examined the rapidly sedimenting fraction from cells blocked in M-phase in the continuous presence of brefeldin A to generate a situation in which the ER and Golgi are fused. As expected, in this membrane fraction a striking coincidence of ER and Golgi markers was observed (Figure 7, G–I). Therefore, we conclude that at M-phase there is no significant fusion of either the major or minor Golgi breakdown products with the ER.
Figure 6.
Examination of double-stained control and brefeldin A-treated cell extracts. Membrane vesicles present in a control HeLa cell homogenate were double-stained for the Golgi marker protein giantin (A), and the ER marker protein p63 (B). The Golgi and ER images are also shown after merging (C). Identical analysis of homogenates from brefeldin A-treated cells is also shown (D–F). Note that Golgi and ER staining patterns were coincident in the extracts from brefeldin A-treated, but not nontreated cells.
Figure 7.
Examination of double-stained mitotic membrane fractions. Membrane vesicles present in the 115 S mitotic Golgi velocity gradient peak were double-stained for the Golgi marker protein giantin (A), and the ER marker protein p63 (B). The Golgi and ER images are also shown after merging (C). Identical analysis of membranes in the rapidly sedimenting mitotic Golgi fraction at the bottom of the velocity gradient is also shown (D–F). In addition, an identical analysis of membranes recovered at the bottom of the velocity gradient from cells treated with brefeldin A during progression to M-phase is shown (G–I). Note that Golgi and ER membrane-staining patterns were coincident in the rapidly sedimenting membrane fraction from brefeldin A-treated, but not nontreated, mitotic cells.
Mitotic Cells Lack the Capacity for Reversible ER–Golgi Fusion
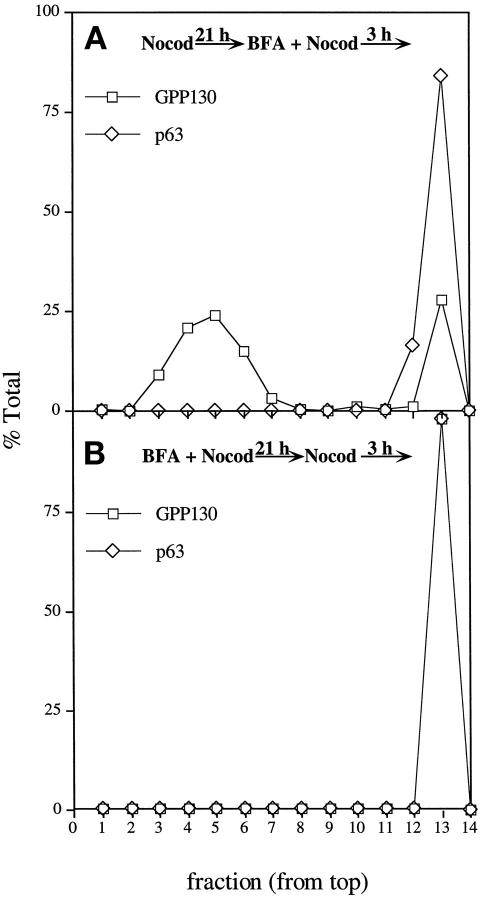
While the experiments presented above demonstrated that the mitotic Golgi breakdown products are not fused with mitotic ER, it remained possible that the ER serves as an intermediate in the Golgi breakdown pathway. That is, the Golgi may first fuse with the ER and then reemerge from the ER, as postulated for Golgi redistribution after nocodazole treatment (Cole et al., 1996). A prediction of this model is that mitotic cells retain the capacity for reversible ER-Golgi fusion. To test this possibility, we again used the drug brefeldin A, which causes reversible ER-Golgi fusion in interphase cells. To test whether brefeldin A could induce fusion of the 60-nm mitotic Golgi vesicles with the ER, we treated nocodazole-blocked M-phase cells with brefeldin A for 3 h. The M-phase cells were collected by shake-off and fractionated on velocity gradients. The 115 S mitotic Golgi vesicles were recovered in the same position as for nonbrefeldin A-treated cells and well separated from the ER (Figure 8A). Clearly, brefeldin A treatment of mitotic cells did not cause ER–Golgi fusion. Nocodazole treatment itself did not block brefeldin A-induced fusion because the Golgi collapsed into the ER in the fraction of nocodazole-treated cells that had not reached M-phase at the time of brefeldin A addition (these remained attached to the plate during the shake-off). Presumably, nocodazole slows, but does not prevent, ER–Golgi fusion in interphase cells (Lippincott-Schwartz et al., 1990). Next, to examine whether mechanisms exist to reform the Golgi from the ER during M-phase, we generated a fused ER and Golgi at M-phase by continuous brefeldin A and nocodazole treatment. As shown above, these cells lacked the 115 S mitotic Golgi vesicles (Figure 5). The brefeldin A was then removed, and the cells were recultured for 3 h in nocodazole to prevent cell cycle progression. Under these conditions, the Golgi and ER were recovered in the same fractions at the bottom of the velocity gradient, indicating that mitotic cells lack the capacity to form the 115 S mitotic Golgi vesicles from the ER (Figure 8B). As a control for the reversibility of the brefeldin A treatment, we examined, by immunofluorescence, identically treated cells that had not reached the M-phase block (these remained attached to the plate during the shake-off). In these interphase cells, the brefeldin A removal was followed by Golgi reemergence from the ER, indicating that ER–Golgi fusion induced by continuous brefeldin A treatment is reversible in interphase but not mitotic cells. Because mitotic Golgi neither fused with, nor budded from the ER, we concluded that the ER is unlikely to be involved as an intermediate in Golgi breakdown at M-phase.
Figure 8.
Fractionation of mitotic ER and Golgi after continuous brefeldin A treatment. (A) Subconfluent cultures of HeLa cells were incubated with 0.5 μg/ml nocodazole for 21 h followed by an additional incubation with 10 μg/ml brefeldin A and 0.5 μg/ml nocodazole for 3 h. Mitotic cells were collected by shake-off, and the postnuclear supernatant was fractionated on a 5–25% glycerol velocity gradient. The relative distribution of GPP130 and p63 was determined by immunoblotting. (B) Subconfluent cultures of HeLa cells were incubated with 0.5 μg/ml nocodazole and 10 μg/ml brefeldin A for 21 h followed by complete brefeldin A washout. Cultures were allowed to incubate with 0.5 μg/ml nocodazole for an additional 3 h and analyzed as above.
DISCUSSION
As single-copy organelles, the ER and Golgi must be broken down before cytokinesis to allow inheritance. In general, preexisting organelles (copied or fragmented) are inherited by the daughter cell rather than synthesized de novo (Nunnari and Walter, 1996; Warren and Wickner, 1996). These then serve as templates for the growth of the organelle in preparation for the next division. Although it is clear that Golgi components are not lost at cytokinesis, it has been suggested that the Golgi may not be inherited as a discrete entity, but rather in the form of a fused compartment with the ER, the organelle that gives rise to most of the components that constitute the Golgi apparatus (Thyberg and Moskalewski, 1992a; Cole et al., 1996). This view suggests that a type of de novo synthesis underlies Golgi partitioning, challenges a more commonly held belief that the Golgi directly vesiculates at mitosis and then self-reassembles after cytokinesis, and calls into question the studies on Golgi disassembly and reassembly carried out in vitro in the absence of ER.
For these reasons we compared the distribution of ER and Golgi markers in mitotic HeLa cells to determine whether Golgi disassembly at mitosis involves fusion with the ER. Based on immunofluorescence microscopy, mitotic ER appeared to be a fine reticulum excluded from the region containing the spindle-pole body. In contrast, mitotic Golgi appeared to be dispersed small vesicles that penetrated the area containing spindle microtubules. We demonstrated that after cell disruption, M-phase Golgi is recovered in two size classes. The major breakdown product, accounting for at least 75% of the Golgi, is a population of 60-nm vesicles that could be completely separated from the ER using velocity gradient separation. The minor breakdown product is a larger, more heterogenously sized, membrane population. Using a double-label fluorescence assay, we were able to demonstrate that these mitotic Golgi membranes also lacked detectable ER marker proteins. Therefore we conclude that there is no significant fusion of ER and Golgi at M-phase in HeLa cells. Furthermore, mitotic Golgi vesicles did not fuse with the ER upon brefeldin A addition, and mitotic Golgi vesicles did not reemerge from the ER upon brefeldin A removal from cells subjected to continuous brefeldin A treatment during progression to M-phase. These observations indicate that the Golgi remains distinct from the ER during its cell cycle-driven disassembly reaction. Thus, the integrity of the Golgi is maintained during partitioning such that daughter cells inherit preexisting, although disassembled, Golgi.
The observation that interphase cells have the capacity to reform the Golgi apparatus out of the ER upon brefeldin A washout (Lippincott-Schwartz et al., 1989) suggests that ER–Golgi fusion might underlie mitotic Golgi breakdown, resulting in vesicles containing markers for both organelles at M-phase. Although the Golgi readily undergoes reversible fusion with the ER upon brefeldin A treatment of interphase cells, mitotic cells lack this capacity (Figure 8). Therefore, similar to other transport steps in mitotic cells (Hesketh et al., 1984; Collins and Warren, 1992), budding of Golgi proteins from the ER is inhibited and cannot mediate formation of mitotic Golgi vesicles. Because our fractionation experiments were carried out in cells blocked at (or near) metaphase with nocodazole, it remains possible that the Golgi passes rapidly and sequentially through the ER in the short time before the nocodazole block. Because we observed inhibition of Golgi budding from the ER in nocodazole-blocked cells, this model would imply a cell cycle switch activated near metaphase that inhibits budding. However, because ER residents lack Golgi modifications, it can be argued that Golgi enzymes do not pass through the ER in significant amounts at any time in the cell cycle. That Golgi enzymes mislocalized to the ER are capable of modifying ER residents is clear from studies of brefeldin A-induced ER–Golgi fusion (Lippincott-Schwartz et al., 1989; Hsu et al., 1992; Ivessa et al., 1992). Why was mannosidase II staining observed in the ER of mitotic cells or okadaic acid-treated cells (Thyberg and Moskalewski, 1992a, 1992b)? Perhaps the ER was mistakenly identified, as previous workers did not use antibodies against ER marker proteins. Alternatively, a misleading conclusion may have been drawn because the previous microscopic analysis was not quantitative. That is, a small amount of mannosidase II present in the mitotic ER (perhaps newly synthesized) may have yielded a disproportionately large signal.
Our results imply that mechanisms must exist for direct disassembly and reassembly of the Golgi and lend further physiological significance to the in vitro disassembly and reassembly reactions that have been studied using Golgi membrane fractions that lack the ER (Misteli and Warren, 1994, 1995b; Sönnichsen et al., 1996). Golgi disassembly in vitro results in the production of two populations of vesicles. Approximately 65% of the Golgi membrane is recovered as 54-nm vesicles produced by coatomer I (COPI)-mediated budding, and the remainder is recovered as a vesicle population of larger more variable size (100–200 nm) produced by a COPI-independent pathway (Misteli and Warren, 1994). Similar size classes have been identified in mitotic cells by electron microscopy (Lucocq et al., 1989; Misteli and Warren, 1995b), and we identified two size classes (60-nm vesicles and a more rapidly sedimenting membrane population) by fractionation of mitotic cell postnuclear supernatants on velocity gradients. Approximately 75% of each Golgi marker we tested was recovered with the 60-nm size class. If these are indeed derived by COPI-mediated budding, a substantial proportion of mitotic Golgi vesiculation is mediated by a constitutive vesicle transport-budding reaction (Warren, 1985; Warren et al., 1995).
On the other hand, it is unclear whether the 60-nm mitotic Golgi vesicles that we isolated are derived by COPI-mediated budding. COPI vesicles formed in vitro in the presence of either interphase or mitotic cytosol exclude Golgi residents such that the concentration of Golgi resident proteins in COPI vesicles is approximately 20% of their concentration in the Golgi (Sönnichsen et al., 1996). This result implies that no more than 20% of the Golgi residents can be recovered in COPI vesicles. Because we recovered approximately 75% of each of three Golgi residents in the 60-nm vesicle population, the vesicles must have been derived from a budding process with a sorting efficiency of at least 0.75 (i.e., the concentration of a resident in the mitotic vesicle membrane must be at least 75% its concentration in the interphase Golgi membrane). Interestingly, the density of the 60-nm mitotic Golgi vesicles was greater than that of interphase Golgi, indicating that Golgi vesiculation at mitosis produces vesicles with an increased protein to lipid ratio (Figure 5, and our unpublished results). This result is consistent with efficient sorting of Golgi residents into the 60-nm vesicles. We conclude that either the sorting efficiency of COPI budding in vitro differs from that in vivo or that Golgi vesiculation at mitosis is primarily independent of COPI-mediated budding. Further analysis of the vesicle populations recovered from mitotic cells after velocity gradient centrifugation should resolve this issue.
A recent report in which Golgi proteins were localized in mitotic cells by confocal microscopy indicated that the end product of mitotic Golgi disassembly is 130 relatively large vesicle clusters per cell (Shima et al., 1997). If this is the case, the 60-nm vesicles that we recovered after fractionation must be derived from clusters that were disrupted during subcellular fractionation. By this view the larger mitotic Golgi membranes that we recovered may represent partially disrupted or intact vesicle clusters. Nevertheless, in our numerous examinations of Golgi protein distribution in mitotic cells using conventional fluorescence microscopy, we rarely observed staining patterns suggestive of vesicle clusters at M-phase. In our experiments the vast majority of M-phase cells exhibited a completely dispersed staining pattern, as shown in Figure 1H. It is not clear whether the confocal microscopic study failed to detect a large population of isolated 60-nm vesicles, or whether out-of-focus information in our experiments somehow obscured the presence of discrete large vesicle clusters. It appears that further work is necessary to resolve this issue.
In summary, our results strongly support the view that mitotic Golgi breakdown proceeds independently of the ER, producing fragmented Golgi that appear to be randomly distributed at the time of cytokinesis. As mitotic Golgi vesicles must fuse to reform the interphase Golgi, inhibition of vesicle fusion at M-phase must mediate at least part of the Golgi vesiculation process (Warren, 1985; Levine et al., 1996). Upon cytokinesis the random distribution of the vesiculated Golgi most likely ensures accurate partitioning without an active mechanism (Birky, 1983; Lucocq et al., 1989). An important area for future study is the characterization of Golgi-localized proteins that are modified at mitosis to allow complete Golgi vesiculation.
ACKNOWLEDGMENTS
We thank J. Suhan for the negative-staining electron microscopy, Dr. T. Lee, and Dr. B. Glick for their critical reading of the manuscript, and Dr. H-P. Hauri for providing the anti-p63 antibody.
REFERENCES
- Acharya U, McCaffery JM, Jacobs R, Malhotra V. Reconstitution of vesiculated Golgi membranes into stacks of cisternae: requirement of NSF in stack formation. J Cell Biol. 1995a;129:577–589. doi: 10.1083/jcb.129.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya U, Jacobs R, Peters J-M, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995b;82:895–904. doi: 10.1016/0092-8674(95)90269-4. [DOI] [PubMed] [Google Scholar]
- Aoki D, Appert HE, Johnson D, Wong SS, Fukuda MN. Analysis of the substrate binding sites of human galactosyltransferase by protein engineering. EMBO J. 1990;9:3171–3178. doi: 10.1002/j.1460-2075.1990.tb07515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anhquyen L, Steiners JL, Ferrell GA, Shaker JC, Sifers RN. Association between calnexin and a secretion-incompetent variant of human alpha 1-antitrypsin. J Biol Chem. 1994;269:7514–7519. [PubMed] [Google Scholar]
- Arosond NN, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;21:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Berger EG, Aegerter E, Mandel T. Monoclonal antibodies to soluble human milk galactosyltransferase (lactos synthase A protein) Carbohydr Res. 1986;149:23–33. doi: 10.1016/s0008-6215(00)90366-5. [DOI] [PubMed] [Google Scholar]
- Birky CW. The partitioning of cytoplasmic organelles at cell division. Int Rev Cytol. 1983;15:49–89. doi: 10.1016/b978-0-12-364376-6.50009-0. [DOI] [PubMed] [Google Scholar]
- Cole NB, Sciaky N, Marotta A, Song J, Lipponcott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol Biol Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RN, Warren G. Sphingolipid transport in mitotic HeLa cells. J Biol Chem. 1992;267:24906–24911. [PubMed] [Google Scholar]
- DePierre JW, Dallner G. Structural aspects of the membrane of the endoplasmic reticulum. Biochim Biophys Acta. 1976;415:411–475. doi: 10.1016/0304-4157(75)90006-4. [DOI] [PubMed] [Google Scholar]
- Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch F. Properties of rotors used for zone centrifugation. Anal Biochem. 1973;55:57–71. doi: 10.1016/0003-2697(73)90290-x. [DOI] [PubMed] [Google Scholar]
- Hesketh TR, Beaven MA, Burke RJB, Warren G. Stimulated release of histamine by a rat mast cell line is inhibited during mitosis. J Cell Biol. 1984;98:2250–2254. doi: 10.1083/jcb.98.6.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu VW, Shah N, Klausner RD. A brefeldin A-like phenotype is induced by the overexpression of a human ERD-2-like protein, ELP-1. Cell. 1992;69:625–635. doi: 10.1016/0092-8674(92)90226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivessa NE, De Lemos-Chiarandini C, Tsao YS, Takatsuki A, Adesnik M, Sabatini DD, Kreibich G. O-glycosylation of intact and truncated ribophorins in brefeldin A-treated cells: newly synthesized intact ribophorins are only transiently accessible to the relocated glycosyltransferases. J Cell Biol. 1992;117:949–958. doi: 10.1083/jcb.117.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knehr M, Poope M, Enulescu M, Eickelbaum W, Stoehr M, Schroeter D, Paweletz N. A critical appraisal of synchronization methods applied to achieve maximal enrichment of HeLa cells in specific cell cycle states. Exp Cell Res. 1995;217:546–553. doi: 10.1006/excr.1995.1121. [DOI] [PubMed] [Google Scholar]
- Levine TP, Rabouille C, Kieckbusch R, Warren G. Binding of the vesicle docking protein p115 to Golgi membranes is inhibited under mitotic conditions. J Biol Chem. 1996;271:17304–17311. doi: 10.1074/jbc.271.29.17304. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Hauri H-P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Foguet M, Renz M, Seelig HP, Glick BS, Hauri H-P. A C-terminally-anchored Golgi protein is inserted into the endoplasmic reticulum and then transported to the Golgi apparatus. Proc Natl Acad Sci USA. 1995;92:5102–5105. doi: 10.1073/pnas.92.11.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Mehta A, Suhan J, Reggio H, Hauri H-P. Sequence and overexpression of GPP130/GIMPc: evidence for saturable pH-sensitive targeting of a type II early Golgi membrane protein. Mol Biol Cell. 1997;8:1073–1087. doi: 10.1091/mbc.8.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri H-P, Yuan LC, Klausner RD. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with Brefeldin A, evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq JM, Warren G. Fragmentation and partitioning of the Golgi apparatus during mitosis in HeLa cells. EMBO J. 1987;6:3239–3246. doi: 10.1002/j.1460-2075.1987.tb02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq JM, Berger EG, Warren G. Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J Cell Biol. 1989;109:463–474. doi: 10.1083/jcb.109.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq JM, Pryde Jg, Berger EG, Warren G. A mitotic form of the Golgi apparatus in HeLa cells. J Cell Biol. 1987;104:865–874. doi: 10.1083/jcb.104.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul GG, Brinkley BR. The Golgi apparatus during mitosis in human melanoma cells in vivo. Cancer Res. 1970;30:2326–2335. [PubMed] [Google Scholar]
- Misteli T, Warren G. COP-coated vesicles are involved in the mitotic fragmentation of Golgi stacks in a cell-free sytem. J Cell Biol. 1994;125:269–282. doi: 10.1083/jcb.125.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T, Warren G. Mitotic disassembly of the Golgi apparatus in vivo. J Cell Sci. 1995a;108:2715–2727. doi: 10.1242/jcs.108.7.2715. [DOI] [PubMed] [Google Scholar]
- Misteli T, Warren G. A role for tubular networks and a COPI-independent pathway in the mitotic fragmentation of Golgi stacks in a cell-free system. J Cell Biol. 1995b;130:1027–1039. doi: 10.1083/jcb.130.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalewski S, Thyberg J, Hinek A, Friberg U. Fine structure of the Golgi complex during mitosis of cartilaginous cells in vitro. Tissue Cell. 1977;9:185–196. doi: 10.1016/0040-8166(77)90015-5. [DOI] [PubMed] [Google Scholar]
- Nagata-Kuno K, Yukinobu H, Nanri H, Shibata Y, Minakami S. Fragmentation and re-formation of mitotic Golgi apparatus detected by a centrifugal method. Exp Cell Res. 1990;191:273–277. doi: 10.1016/0014-4827(90)90014-2. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Pypaert M, Hoe MH, Slusarewicz P, Berger EG, Warren G. Overlapping distribution of two glycosyltransferases in the Golgi apparatus of HeLa cells. J Cell Biol. 1993;120:5–13. doi: 10.1083/jcb.120.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J, Walter P. Regulation of organelle biogenesis. Cell. 1996;84:389–394. doi: 10.1016/s0092-8674(00)81283-0. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Misteli T, Watson R, Warren G. Reassembly of Golgi stacks from mitotic Golgi fragments in a cell-free system. J Cell Biol. 1995a;129:605–618. doi: 10.1083/jcb.129.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C, Levine TP, Peters J-M, Warren G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell. 1995b;82:905–914. doi: 10.1016/0092-8674(95)90270-8. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Prydz K, Hansen SH, van Deurs B. Ricin transport in brefeldin A-treated cells: correlation between Golgi structure and toxic effect. J Cell Biol. 1991;115:971–981. doi: 10.1083/jcb.115.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucan L, Palade GE. Membrane and secretory proteins are transported from the Golgi complex to the sinusoidal plasmalemma of hepatocytes by distinct vesicle carriers. J Cell Biol. 1994;125:733–741. doi: 10.1083/jcb.125.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Ericsson M, Bachi T, Griffiths G, Hauri H-P. Characterization of a novel 63 kDa membrane protein. J Cell Sci. 1993;104:671–683. doi: 10.1242/jcs.104.3.671. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Rohrer J, Slot JW, Geuze HJ, Kornfeld S. Reassessment of the subcellular localization of p63. J Cell Sci. 1995;108:2477–2485. doi: 10.1242/jcs.108.6.2477. [DOI] [PubMed] [Google Scholar]
- Sönnichsen B, Watson R, Clausen H, Misteli T, Warren G. Sorting by COP I-coated vesicles under interphase and mitotic conditions. J Cell Biol. 1996;134:1411–1425. doi: 10.1083/jcb.134.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima DT, Haldar K, Pepperkok R, Watson R, Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg J, Moskalewski S. Reorganization of the Golgi complex in association with mitosis: redistribution of mannosidase II to the endoplasmic reticulum and effects of brefeldin A. J Submicrosc Cytol Pathol. 1992a;24:495–508. [PubMed] [Google Scholar]
- Thyberg J, Moskalewski S. Disorganization of the Golgi complex and the cytoplasmic microtubule system in CHO cells exposed to okadaic acid. J Cell Biol. 1992b;103:1167–1175. doi: 10.1242/jcs.103.4.1167. [DOI] [PubMed] [Google Scholar]
- Warren G. Mitosis and membranes. Nature. 1985;342:857–858. doi: 10.1038/342857a0. [DOI] [PubMed] [Google Scholar]
- Warren G, Wickner W. Organelle inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- Warren G, Levine T, Misteli T. Mitotic disassembly of the mammalian Golgi apparatus. Trends Cell Biol. 1995;5:413–416. doi: 10.1016/s0962-8924(00)89094-7. [DOI] [PubMed] [Google Scholar]
- Zeligs JD, Wollman SH. Mitosis in rat thyroid epithelial cells in vivo. I. Ultrastructural changes incytoplasmic organelles during the mitotic cycle. J Ultrastruct Res. 1979;66:53–77. doi: 10.1016/s0022-5320(79)80065-9. [DOI] [PubMed] [Google Scholar]