Abstract
To study the role of NMDA receptors in dopamine signaling of the striatum, the brain area that receives glutamatergic inputs from various cortical areas and most dopaminergic inputs, we generated striatum-specific NMDA receptor-deficient mice. The mutant pups showed reduced food intake and retarded growth starting at the second postnatal week and died on approximately postnatal day 20 (P20). The time course of postnatal lethality is similar to that of compound mutant, double knockout of dopamine D1/D2 receptors, or genetically engineered dopamine-deficient mouse. In vivo electrophysiological recordings in the mutant pups showed that frequencies in the range of gamma oscillation were reduced in the striatal circuits. Moreover, the number of functional dopamine receptors in the striatum as measured by D1- and D2-binding experiments was greatly diminished in the mutants as compared with control animals. A consequence of diminished dopamine binding in the striatum manifested in an increase of locomotor activity. The administration of D1/D2 agonists paradoxically reduced the hyperactivity of the mutant mice as compared with an increase in locomotor activity in control mice. These results demonstrate that the NMDA receptor plays an essential role in the integration of dopamine signaling in the striatum and that is required in behavioral function.
Keywords: dopamine, knockout, NR1, striatum, locomotion
The striatum receives glutamatergic projections from virtually all areas of the cerebral cortex and dense dopaminergic projections from the substantia nigra and ventral tegmental area (1, 2). The principal neurons of the striatum, medium spiny neurons (MSNs), are GABAergic projection neurons. These neurons are classified into two groups: the MSNs that express D1 dopamine receptors responsible for a direct pathway and the MSNs that express D2 dopamine receptors responsible for an indirect pathway (3). These MSNs constitute 90–95% of the striatal neuronal population (4) and contain high levels of NMDA receptors (5, 6).
During development, dopamine receptor expressions (both D1 and D2) are already abundant by the late embryonic stage, however functional dopamine receptor binding is low at birth and progressively increases to reach adult levels between postnatal day (P)14 and P21 (7). This delayed appearance of functional dopamine receptors in the MSNs of the striatum suggests that maturation of functional dopamine receptors is regulated by an unknown mechanism, other than the mere availability of the dopamine receptor mRNAs.
During postnatal weeks, it has been shown that the NMDA receptor-mediated currents develop later in the MSNs compared with other brain regions such as the hippocampus. There is a ≈2-fold increase from the first to the third postnatal week (8). Recently, molecular interactions between NMDA receptors and dopamine receptors have been reported. For example, NMDA receptors trap diffusible D1 receptors by direct physical interaction (9–11). The interaction between NR2B, a subunit of the NMDA receptor, and the D2 receptor can also disrupt the association of Ca2+/calmodulin-dependent protein kinase II (CaMKII) with NR2B and inhibit NMDA receptor-mediated currents in MSNs (12). These findings have led to an emerging view that the NMDA receptor plays a central role in the integration of these two neurotransmitter systems, and disruption of this integration can lead to various behavioral malfunctions.
Conditional gene knockout methodology provides the ability to study functions of a gene of interest in specific cell types or specific brain regions and is particularly important if general gene knockout limits the extent of analyses due to embryonic death. The conventional knockout of NR1, the essential subunit of the NMDA receptor, causes neonatal death (13, 14), whereas a partial knockout of NR1 in the striatum causes motor learning deficits.
Results
Generation of Striatum-Specific NR1-Deficient Mice.
We first assessed the region specificity of the Dlx5/6-Cre line (15) using the reporter mouse, fLacZ line (as detailed in Materials and Methods) and found that the Cre/loxP deletion is almost exclusively restricted to the striatum including the dorsal striatum (caudate putamen) and ventral striatum (nucleus accumbens) (Fig. 1A). Other brain regions such as the cortex, hippocampus, and thalamus seemed to be absent of the LacZ staining or have only sporadic staining (Fig. 1A). The minor population of LacZ-positive cells seen in the cortex, hippocampus, and thalamus is possibly composed of interneurons. This Cre mouse line was crossed with fNR1 mice (16) to generate striatum-specific NR1-deficient mice (STR-KO). The production of conditional NR1 knockout mice was verified by PCR (Fig. 1B). The loss of NR1 protein in the striatum of the conditional knockout mice was further confirmed by Western blot analysis (Fig. 1C). In situ hybridization using a probe specific to the floxed NR1 exon region shows the complete absence of the NR1 mRNA in the striatum of the conditional mutant pups (P16) (Fig. 1D).
Fig. 1.
Generation of striatum-specific NR1-deficient mouse. (A) Dlx5/6-Cre-mediated lox-P recombination pattern was tested in the flacZ reporter line. Dlx5/6-Cre and fLacZ double-positive mice were subjected to X-Gal staining (Left). Magnified sections from the olfactory bulb (OB), striatum (STR), hippocampus (HP), cortex (CTX), thalamus (TH), and cerebellum (CBM) are shown (Right). Nuclei of recombination positive cells are shown in blue. (B) PCR genotyping pattern of each genotype produced. PCR product of Cre, fNR1, and wild type (WT) NR1 are 248, 398, and 400 bp, respectively. (C) Synaptic NR1 (110 kDa) expression in cortex (CTX), striatum (STR), and hippocampus (HP). Complete absence of NR1 protein expression in the mutant striatum. The same membrane filters were reblotted by using anti-β-actin antibody as a loading control (42 kDa). (D) In situ analysis of NR1 mRNA expression of the control and conditional NR1 mutant mouse brain (P16).
Retarded Growth and Postnatal Lethality of STR-KO.
The conditional mutant pups exhibited normal growth for the initial 12 days after birth. At birth, mutant pups were indistinguishable from their control littermates, suggesting that mutants were normal in terms of their feeding behavior. However, their growth lagged compared with that of their control littermates from P13 [F(5,77) = 5.57, P = 0.0002] (Fig. 2A) and became more significant by P16 (Fig. 2 A and B). The diminished growth in the conditional knockout mice is highly specific to the genotype, because all other control genotypes exhibited normal body growth. In addition, the measurement of the amount of semiliquid diet they consumed reveals that the mutant mice ingested ≈20% of the control level (Fig. 2C). Other than smaller body size, our visual observation of the pups in the home cage did not reveal any obvious abnormal voluntary movements, such as axial and limbic abnormality, in the mutant pups up to P18. However, on P19, abnormal gait became noticeable in the mutant mice, with their hind legs often sprawled [supporting information (SI) Movie S1]. Importantly, most mutant pups died on average P20 ± 2.8 (Fig. 2 B and D). At this stage, these mutant pups were mostly abandoned by their mothers and their control littermates, and they also developed stooped posture, bradykinesia, hypokinesia, and slight tremor. Such postnatal developmental growth retardation and lethality are strikingly reminiscent of the phenotypes of a series of dopamine knockout mice carrying D1 and D2 receptor double-knockout (D1−/−, D2−/−), compound mutant (D1−/−, D2+/−), or dopamine-deficient mice (17, 18). Unlike those dopamine-related mutants, a semiliquid diet or dopamine receptor agonist mixture injection did not improve the growth of our conditional mutants.
Fig. 2.
Retarded growth and postnatal lethality of striatum-specific NR1-deficient mice. (A) Smaller body size of the P16 knockout mice (fNR1/fNR1, Cre/+) placed above a P16 control animal. (Scale bar, 10 mm.) (B) Postnatal developmental growth curve of each genotype. Data are expressed as mean +/− SD. Reduced body weight of mutant pups (fNR1/fNR1, Cre/+) became significant from P13 against all other genotypes (*, P < 0.05 and **, P < 0.01 after P14, post hoc analyses). (C) Reduced food intake by the mutant mice (P16) compared with their control littermates (n = 6). (D) Percentage distribution of mortality in the conditional knockout mice (n = 21). Average lifespan = 19.97 (days) +/− 2.87 (SD). The distribution was leptokurtic (Kurtosis = 4.37).
Synaptic Transmission and Local Field Potential (LFP) in Striatal Circuits.
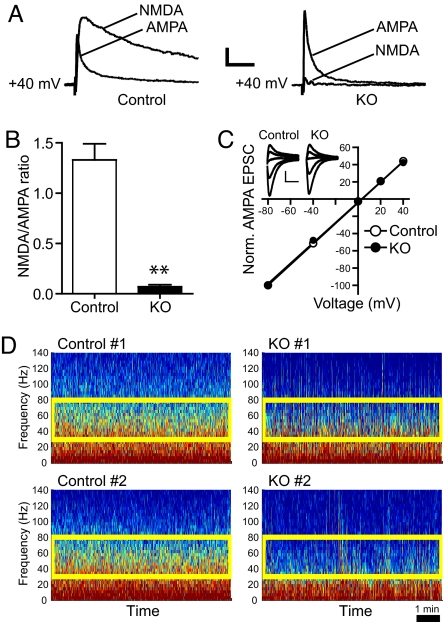
To assess whether loss of the NMDA receptor alters AMPA receptor function in P16 mutant mice, we prepared acute slices of ventral striatal tissue and made whole-cell recordings from the principal cells of this region, i.e., MSNs (19). Although stimulation of excitatory afferents produced robust AMPA receptor- and NMDA receptor-mediated excitatory postsynaptic currents (EPSC) in control neurons, mutant neurons exhibited normal AMPA currents. No functional synaptic NMDA receptor currents were observed (Fig. 3 A and B). Additionally, there was no obvious difference in the magnitude of afferent stimulation necessary to produce an equivalent AMPA receptor-mediated EPSC in mutant neurons (data not shown). Interestingly, mutant and control neurons displayed similar synaptic I–V curves for AMPA receptor EPSCs (Fig. 3C). Because a shift in the shape of this curve can reflect the inclusion or exclusion of synaptic AMPA receptors that lack the GluR2 subunit (20), this result suggests that no such changes have occurred in mutant neurons.
Fig. 3.
Electrophysiological characterizations of striatum properties in knockout and control mice. (A) Measurements of NMDA and AMPA currents in striatal slices from control (Left) and mutant mice (Right). NR1 mutants lack functional synaptic NMDA receptors in striatal neurons. Examples of EPSCs from neurons in control and NR1 mutant mice. (Scale bars, 100 ms and 20 pA.) (B) Mean NMDA receptor/AMPA receptor ratios from control and NR1 mutant groups (n = 6 from three animals in each group). Error bars represent SEM (**, P < 0.0001, Student's t test). (C) Current–voltage (I–V) relationships of AMPA receptor-mediated EPSCs in control mice and mutant mice. (Inset) Examples of evoked AMPA receptor EPSCs at membrane potentials from −80 to +40 mV. (Scale bar, 50 pA and 20 ms; n = 6 from three animals per group; r = 0.99 for each group.) (D) Local field potentials measured in P16 pups. Data from two representative mice of the control and mutant groups are shown. The population oscillatory frequencies at the gamma range (30–80 Hz) are greatly diminished in conditional knockout mice (n = 6; n = 4 in control mice).
To examine whether there is a change in in vivo striatal neuron population properties in freely behaving knockout mice, we have used an extracellular recording method (21) to monitor the LFP, which in the dorsal striatum can reflect a good degree of coherent neuron population firing (22, 23). We succeeded in recording the LFP in the striatum of P16 pups in an awake resting state and found that mutants differ from that of control mice (Fig. 3D). Although there was no obvious difference in the lower frequency range (<30 Hz), there was a striking reduction in the neuronal frequency distribution at the 30- to 80-Hz gamma oscillation range, in which fundamental frequencies of striatum have been identified (24). This indicates that loss of the NMDA current has greatly diminished the firing of those striatal principal cells in freely behaving mutant pups. The involvement of the NMDA receptor in regulating striatal network oscillatory function at this 30- to 80-Hz range was further supported by our observation that injection of the NMDA receptor antagonist, ketamine, into control mice can specifically and significantly reduce gamma signals (data not shown).
Dopamine Receptor Binding.
To investigate whether loss of the NMDA receptor actually alters dopamine receptor functions, we performed a dopamine receptor-binding assay using membrane proteins prepared from the striatum tissues of P16 control and mutant mice. We used 3H-SCH23390 for D1 receptor binding and 3H-spiperone for D2 receptor binding. The competition binding experiment revealed that the IC50 of the mutant striatal membranes was unaltered (Fig. 4), suggesting no alteration in ligand-binding affinity. Interestingly, the Bmax of both the D1 and D2 receptors in the knockout animals was greatly reduced by 53% and 71%, respectively (Fig. 4). The reduction in Bmax strongly indicates there is a significant decrease in the total number of functional D1 and D2 receptors in the striatum of the mutant mice.
Fig. 4.
Alteration in functional dopamine D1 and D2 receptors in the mutant striatum. D1- and D2-like dopamine receptor bindings were measured by using the striatal membrane of P16 mice. The data are expressed as percentage displacement of specific binding. 3H-SCH23390 was competed with cold SCH23390 for D1-like dopamine receptors (Upper). 3H-Spiperone was also competed with cold spiperone for D2-like dopamine receptors (Lower).
Locomotor Activity.
Although P9 mutant mice exhibit indistinguishable locomotor behavior (Fig. 5A), P16 mutants were hyperactive once the pups were separated from their mothers. Their ambulatory activities were increased by an average of 450% [F(5,83) = 38.67, P < 0.0001] (Fig. 5B Left), and their fine movements were also increased by an average of 191% from other genotypes [F(5,83) = 18.65, P < 0.0001] (Fig. 5B Right). Again, this hyperlocomotion induced by the novel environment is highly specific to the conditional knockout, because all other genotypes of the various control groups exhibited normal locomotor activities. This hyperactive phenotype in P16 but not in P9 mutants also correlates nicely with the time course of postnatal development of striatum-mediated locomotor behavior.
Fig. 5.
Hyperactivity of P16 mutants in novelty-induced locomotor activity test. (A) Indistinguishable locomotor actvitity in P9 mutants compared with that of control mice in the novelty-induced locomotion test (mean +/− SEM, n = 11, 15, 13, 14, 15, and 14, in the same order of genotype shown in the graph). (B) Hyperactivity of P16 mutant (mean +/− SEM, n = 13 11, 15, 16, 19, and 13, in the order shown in the graph). Mutant pups were significantly hyperactive in both ambulatory activity and fine movements compared with all other genotypes (post hoc test, **, P < 0.01).
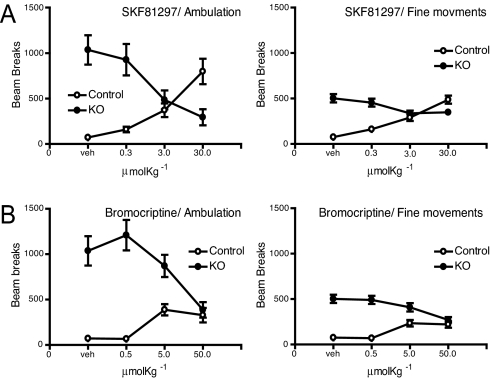
Acute Locomotor Induction by Dopamine Receptor Agonists.
To understand how the lack of NR1 and reduced functional dopamine receptor binding in the striatum affect animals' behavior, acute effects of D1- and D2-like agonists in locomotor activity were measured. The altered D1 and D2 receptor responses in locomotion were further evident from pharmacological experiments. Injections of the D1-like receptor agonist, SKF81297, caused a dose-dependent reduction in locomotor activity in P16 mutant pups, as measured by both ambulation and fine movements, whereas SKF81297 caused a dose-dependent increase in locomotion in control mice, as expected (Fig. 6A). Similarly, an injection of the D2-like receptor agonist bromocriptine produced a dose-dependent suppression of locomotor activity in the mutant mice rather than an increase in locomotor activity that was seen in control mice (Fig. 6B). These experiments collectively suggest significant biochemical and functional disruptions in D1 and D2 receptor signaling properties in the knockout mice.
Fig. 6.
Dopamine receptor-mediated locomotor activity. (A) Acute effect on locomotor activity by the D1 receptor agonist SKF81297. The agonist was i.p.-injected at various doses (n = 8 for each point). (B) Acute effect on locomotor activity by the D2 receptor agonist Bromocriptine. The agonist was i.p.-injected at various doses (n = 8 for each point).
Discussion
Our evidence demonstrates that the NMDA receptor plays an essential role in integration of dopamine signaling in the striatum. We demonstrate here that failure in integration of dopamine signaling by the NMDA receptor in the striatum leads to behavioral consequences.
Partial NR1 knockouts in which only 40–60% of the striatum neurons had NMDA receptor deletion or functional knockdown (e.g., in CaMKII, dopamine D1 receptor, or RGS9 promoter active cells that are presumably D2-positive striatal neurons) have been reported (25–27). These partial knockout mice are viable and exhibit normal locomotion in open field and basal motor function. These results suggest that the NMDA receptors in remaining neuronal population compensate for the functional loss. In contrast, we generated NMDA receptor-deficient mice that lack NR1 in both dopamine D1 and D2 receptor-positive MSNs of the striatum, which surprisingly resulted in premature death.
The reduction in food consumption, diminished body growth during the postnatal weeks, and subsequent postnatal lethality of our striatum-specific complete NR1 knockout mice bear a strong resemblance to that of genetically engineered dopamine-deficient mice. D1 and D2 receptor double-knockout and double-mutant mice carrying homozygous D1 and heterozygous D2 null mutations (17, 18) exhibit a strikingly similar phenotype. The lifespan of these mutants is also closely matched to those of dopamine-deficient mice. The reduced food intake in the conditional mutants is consistent with the idea that striatal-hypothalamic circuits play an important role in the control of food intake (28). The ventral tegmental area that provides dopaminergic inputs back to the ventral striatum is downstream of the ventral striatum, and lateral hypothalamus. GABAergic projections from ventral striatum (nucleus accumbens shell) into lateral hypothalamus are not responsible for lateral hypothalamus-mediated feeding, because infusion of GABA receptor blockers into the lateral hypothalamus does not significantly affect food intake (29). Our mutants could reach and suckle their mothers' nipples, suggesting that a lack of NMDA receptors in the striatum did not reduce appetitive motivation or olfactory function. Lesions of dopaminergic terminals induced by 6-hydroxydopamine in the ventrolateral striatum, a site governing oral motor control, depressed feeding transiently (30, 31). Consistent with these data, we observed dysfunction of oral motor control. When pups were suckling from their mothers' nipples, and the mother was gently lifted, mutant pups hung onto the mother by biting nipples for >10 sec (see Movie S2). This suggests that reduction of food consumption and growth retardation is due to dysfunction of oral motor control.
Our in vivo recording suggests that the NMDA receptors in the striatal circuits play an essential role in controlling and regulating basic circuit properties such as network oscillations. This is quite surprising, given the knowledge that the hippocampal and cortical NMDA receptors are known to be mostly involved in the control of synaptic plasticity and high forms of cognitive process (25). It is conceivable that the diminished gamma oscillation in our striatum-specific NR1 knockout mice, which reflects the reduced coordinated firing in the principal neurons in the striatal circuits, can have devastating consequences on striatal output.
It is important to point out that hyperactivity of P16 mutants was not observed in the early postnatal period (e.g., P9). The onset of this developmental phenotype is not due to the time course of the striatal NMDA receptor knockout, because the NMDA receptors in the striatum have been deleted before P9 (data not shown). It is particularly interesting that dopamine receptor-binding activity in the striatum is low at birth and progressively increases to reach adult levels between P14 and P21 (7). This similar developmental time course correlates well with the dramatic increase in the NMDA currents, which increases ≈2-fold from the first to the third postnatal week (8). The progressive increases in functional D1 and D2 receptors together with NMDA receptor currents in the striatum during the second and third postnatal weeks have long been noticed but poorly understood (7, 8, 32).
The paradoxical effect of dopamine agonists in locomotor activity that reduced hyperlocomotor activity in mutants and increased it in control mice (Fig. 6) was unexpected, because we originally hypothesized dopamine agonists do not have effects in mutants, and hyperactivity is already saturated. One possible explanation is that another ionotropic glutamate receptor, the AMPA receptor-mediated current, is still intact (Fig. 3 A and C). Although the D1 and D2 receptors modulate the AMPA receptor in the striatum negatively and positively, respectively, in contrast, the D1 and D2 receptors modulate the NMDA receptor in the reverse manner (33). Although the number of functional dopamine receptors was decreased, lack of NMDA receptors may have manifested dopaminergic modulation of AMPA receptor function. It is also possible that other brain areas expressing dopamine receptors, such as the substantia nigra and the subthalamic nucleus (STN), may be the key components to invert the effects of dopamine agonists in the induction of locomotor activity. The STN is particularly interesting in the following ways: (i) the STN interacts with the indirect pathway of the basal ganglia, (ii) it also directly receives cortical inputs, and (iii) its firing pattern is modulated by dopamine (34). To understand how striatal NMDA receptors ablation altered effects of dopamine in induction of locomotor activity, technical difficulties need to be resolved to make precise in vivo recording possible from each cell type and multiple areas of basal ganglia simultaneously.
Our observation that the complete deletion of the striatal NMDA receptor results in a drastic reduction of functional D1 and D2 receptor in the striatum leads us to suggest that the NMDA receptor plays an essential role in the regulation of the expression of functional dopamine receptors in the striatum. The data presented in this article, taken together, strongly support the interpretation that NMDA receptors mediate the dopamine signaling that is obligatory for the normal function of the striatum.
Materials and Methods
Generation of Knockout Mice.
Striatum-restricted NR1 mutant mice were generated by using Cre/loxP recombination. Cre transgenic mice in which Cre gene is driven by an intergenic region of Dlx 5/6 (15) were generated by Marc Ekker (University of Ottawa). A line of this transgenic mouse was provided in FVB genetic background, and we backcrossed to C57BL/6 and CBA-mixed genetic background for >8 generations. To generate striatum-specific NR1 mutants, this Dlx5/6-Cre line was crossed with floxed NR1 mouse (fNR1) that is the essential subunit of NMDA receptor (16). After several crossings, offspring carrying fNR1/+, Cre (fNR1/+; Cre/+) were crossed with the fNR1/+ (fNR1/+; +/+) mice to produce wild type (+/+;+/+), fNR1/+ (fNR1/+; +/+), Cre (+/+; Cre/+), fNR1/fNR1 (fNR1/fNR1; +/+), fNR1/+, Cre (fNR1/+; Cre/+), and fNR1/fNR1, Cre (fNR1/fNR1; Cre/+ or simply knockout). Mice carrying fNR1/fNR1 used as a control. No gender bias was found in the production of the Dlx 5/6 Cre line, and the distribution of genotype was close to Mendelian ratio. Genotype was checked by PCR by using primers 5′-TGCTGTTTCACTGGTTATGC-3′ and 5′-TAACATTCTCCCACCGTCAG-3′ for Cre; 5′-CTGATGCCGCCGTGTTCC-3′ and 5′-CCCCTGTGCTCTTCGTC-3′ for fNR1; 5′-CCCCTCCCTTTTCCAGACAG-3′ and 5′-CTTCCTACAACCCTCACACC-3′ for wild-type NR1; 5′-GGTCGCTACCATTACAGTTG-3′ and 5′-ACG CAGGAGTTTTGATGGAC-3′ for fLacZ. All procedures relating to animal care and treatment conformed to institutional and National Institutes of Health guidelines.
Body Weight and Food Consumption Measurement.
Pups were weighed daily. For measuring food intake, P16 animals were separated and isolated into each cage and fed with semiliquid diet. Five grams of rodent diet (Harlan Teklad 2018) was ground into powder and suspended in 50 ml of water. This semiliquid food was served in a small dish (2-cm diameter, 7-mm depth) that was placed in the middle of another plate (12 × 8 cm) to minimize the false positive from spill. Pups were free to access to the liquid food for 8 h between 12:00 and 8:00 p.m. Water evaporation from liquid food was also measured in the same preparation and subtracted from experimental data. Data are given as mean +/− SEM (n = 6 for control and knockout, respectively).
In Situ Hybridization for NR1 mRNA.
The procedures are similar to those have been published (35). Both sagittal and coronal cryosections were prepared at a thickness of 25 μm. These cryostat sections were directly mounted onto silicane-coated slide glasses. For NR1, 5′-TCTACCACTCTTTCTATCCTGCAGGTTCTTCCTCCACACGTT-3′ or alternative splicing insensitive probe, 5′-TGCCTCCAGCCACCAGCATGAAGACCCCTGCCATGTTCTCAAAAG-3′ were used, and both gave the same result. These synthetic nucleotides were labeled with [α-33P]dATP (Perkin–Elmer) by terminal transferase (Roche). Before hybridization, the sections were fixed with ice-cold 4% paraformaldehyde (PFA) in PBS for 5 min, washed for 5 min in PBS, and then subjected to dehydration by incubating for 5 min in 70% and 95% ethanol. The brain sections were hybridized with the oligonucleotide probe (1.0–2.0 × 105 cpm per slide) at 42°C for 18 h. Sections were then rinsed in 2× SSC once and washed in 2× SSC at 60°C for 30 min twice. Then, sections were subjected to dehydration by incubating in 70%, 80%, 90%, and 100% ethanol for 3 min each. Kodak Biomax XAR films were used for autoradiography.
X-Gal Staining.
The Dlx 5/6-Cre line was also crossed with a reporter line that has LacZ below the β-actin promoter and stop cassette with two loxP sequences (36) to monitor recombination pattern. The procedure was carried out as described (36) (see also SI Text).
Western Blot Analysis.
For NR1 immunoblotting, a crude synaptosome fraction was used. P16 mice pups were decapitated, and brains were placed on an ice-cold glass plate. Then the striatum, hippocampus, and cortex were dissected. Tissue was homogenized in 0.32 M sucrose, 5 mM Tris·HCl, pH 7.4, and centrifuged at 800×g for 10 min. The resulting supernatant was further centrifuged at 10,000×g for 20 min. The pellet was suspended in 10 volumes of the same buffer and centrifuged again. Protein samples were separated on 4% SDS/PAGE gel. An anti-NR1 C-terminal region rabbit polyclonal antibody (provided by M. Watanabe of Hokkaido University, Sapporo, Japan) was used. Then the blot was subjected to a secondary antibody reaction with anti-rabbit IgG-HRP from Upstate at a dilution of 1:50,000. After detection using ECL plus from Amersham, all antibodies were stripped in Reblot Plus Mild Antibody Stripping Solution from Chemicon, then incubated with anti-β-actin-HRP monoclonal antibody from Sigma and developed as above.
In Vitro Brain-Slice Recording.
Slice recordings were performed as described (37) (see also SI Text). Briefly, sagittal slices of the ventral striatum (240 μm) were prepared from P16 mice, then cells were visualized by using IR-differential interference contrast microscopy optics, and finally MSNs were identified by their morphology and high resting membrane potential (−75 to −85 mV).
In Vivo Local Field Potential Recording.
The surgery and construction of multielectrodes in stereotrode format are similar to those described (21) (see also SI Text). In these experiments, the headstages, which had a total weight of 0.3 g, were modified to minimize the weight. P16 pups were anesthetized with an i.p. injection of 37.5 mg/kg of ketamine (Bedford Laboratories) and 0.5 mg/kg of dormitor (Pfizer Animal Health). The positions for the two bundles were 1.8 mm lateral and 0.6 mm rostral to the bregma on both the right and left sides based on the adult animal and scaled accordingly. Recovery was validated by the ambulatory activities of the animals, and the survival rate for the surgery was 100%.
Dopamine Receptor-Binding Assay.
Membrane proteins were prepared from striatum by homogenizing in 10 volumes in ice-cold 0.32 M sucrose in a Dounce homogenizer for 10 strokes, then centrifuged at 1,000×g at 4°C for 10 min. The pellet was then homogenized again and centrifuged as above. The resulting two supernatants were combined and centrifuged at 48,000×g for 20 min at 4°C. This pellet was washed twice by using binding assay buffer. For D1 binding, 0.25 nM 3H-SCH23390 (specific activity 85 Ci/mmol) was incubated with 25 μg of membrane protein in 50 mM Tris·Cl, pH 7.4; 150 mM NaCl; 0.025% ascorbic acid; 0.001% BSA at 4°C for 2 h. Cold SCH23390 was added at the same time as 3H-SCH23390 for competition. For D2 binding, 3H-spiperone (s.a. 15 Ci/mmol) was incubated with 20 μg of membrane 40 mM Tris·Cl, pH 7.7; 96 mM NaCl; 4 mM KCl; 1.6 mM CaCl2; 0.8 mM MgCl2; 0.1% ascorbic acid; 1% BSA; and 0.001% polyethyleneimine for 2 h at 4°C. Cold spiperone was used as a competitor. Obtained data from 3H count were normalized upon analysis. Then datasets were fit to the equation Y = AX/(X + B) by using the program Kaleidagraph (Synergy), where Y = the relative amount of binding observed at a given concentration of nonradioactive SCH23390 or nonradioactive spiperone, X = concentration of either nonradioactive SCH23390 or nonradioactive spiperone (mol/liter), A = Bmax or total amount of binding in the normal animal in the absence of nonradioactive SCH23390 or nonradioactive spiperone (expressed as a percentage), B = IC50 or the concentration of cold inhibitor required to inhibit the maximal binding for each set by 50%. The data from the SCH23390 arm and the spiperone arm were not pooled. Therefore, the maximal specific binding based on normal animals was calculated separately for each arm of the study.
Locomotor Activity and Dopamine Receptor Stimulation.
P9 and P16 pups were separated from the mother and put into a new cage environment for 10 min before infrared beam breaks were counted by Photobeam Activity System, Ver. 2 (San Diego Instruments) for 1 h during the daytime, 3:00–5:00 p.m. Sequential beam breaks were counted as ambulatory activity. Repetitive breaks of a same beam were counted as fine movements. For dopamine receptor agonist stimulation experiments, P16 mice were i.p.-injected with the D1 receptor agonist SKF81297 or the D2 receptor agonist bromocriptine 10 min before locomotor activity measurement. Independent naïve animals were used for each drug and dose group (n = 8 mice for each point). The fNR1/fNR1 genotype mice were used as the control group.
Statistics.
Summary data are reported as mean ± SEM, except the growth curve in Fig. 2B. Statistical significance was evaluated by one-way ANOVA after validating the equal variances by Bartlett's test and then followed by posthoc test for multiple comparison (Tukey Kramer test), except the data of Figs. 2C and 3B. For the data of food consumption in Fig. 2C and the NMDA/AMPA ratio in Fig. 3B, statistical significance was assessed by two-tailed Student's t tests.
Supplementary Material
Acknowledgments.
We thank Dr. M. Watanabe for providing the anti-NR1 antibody and Dr. J. J. Soghomonian for helpful discussion. This work was supported by funds from the National Institute of Mental Health, the National Institute on Aging, and the W. M. Keck Foundations (all to J.Z.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806180105/DCSupplemental.
References
- 1.McGeer PL, McGeer EG, Scherer U, Singh K. A glutamatergic corticostriatal path? Brain Res. 1977;128:369–373. doi: 10.1016/0006-8993(77)91003-4. [DOI] [PubMed] [Google Scholar]
- 2.Fonnum F, Storm-Mathisen J, Divac I. Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibres in rat brain. Neuroscience. 1981;6:863–873. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- 3.Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 4.Kemp JM, Powell TP. The structure of the caudate nucleus of the cat: Light and electron microscopy. Philos Trans R Soc Lond Ser B. 1971;262:383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- 5.Albin RL, et al. Excitatory amino acid binding sites in the basal ganglia of the rat: A quantitative autoradiographic study. Neuroscience. 1992;46:35–48. doi: 10.1016/0306-4522(92)90006-n. [DOI] [PubMed] [Google Scholar]
- 6.Standaert DG, Friberg IK, Landwehrmeyer GB, Young AB, Penney JB., Jr Expression of NMDA glutamate receptor subunit mRNAs in neurochemically identified projection and interneurons in the striatum of the rat. Brain Res Mol Brain Res. 1999;64:11–23. doi: 10.1016/s0169-328x(98)00293-9. [DOI] [PubMed] [Google Scholar]
- 7.Schambra UB, et al. Ontogeny of D1A and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience. 1994;62:65–85. doi: 10.1016/0306-4522(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 8.Hurst RS, Cepeda C, Shumate LW, Levine MS. Delayed postnatal development of NMDA receptor function in medium-sized neurons of the rat striatum. Dev Neurosci. 2001;23:122–134. doi: 10.1159/000048704. [DOI] [PubMed] [Google Scholar]
- 9.Scott L, et al. Selective up-regulation of dopamine D1 receptors in dendritic spines by NMDA receptor activation. Proc Natl Acad Sci USA. 2002;99:1661–1664. doi: 10.1073/pnas.032654599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott L, et al. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Natl Acad Sci USA. 2006;103:762–767. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pei L, Lee FJ, Moszczynska A, Vukusic B, Liu F. Regulation of dopamine D1 receptor function by physical interaction with the NMDA receptors. J Neurosci. 2004;24:1149–1158. doi: 10.1523/JNEUROSCI.3922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu XY, et al. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 14.Forrest D, et al. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 15.Ghanem N, et al. Regulatory roles of conserved intergenic domains in vertebrate Dlx bigene clusters. Genome Res. 2003;13:533–543. doi: 10.1101/gr.716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, et al. Simultaneous absence of dopamine D1 and D2 receptor-mediated signaling is lethal in mice. Proc Natl Acad Sci USA. 2004;101:11465–11470. doi: 10.1073/pnas.0402028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 19.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 20.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: Synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Lin L, et al. Large-scale neural ensemble recording in the brains of freely behaving mice. J Neurosci Methods. 2006;155:28–38. doi: 10.1016/j.jneumeth.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Misgeld U, Okada Y, Hassler R. Locally evoked potentials in slices of rat neostriatum: A tool for the investigation of intrinsic excitatory processes. Exp Brain Res. 1979;34:575–590. doi: 10.1007/BF00239150. [DOI] [PubMed] [Google Scholar]
- 23.Ryan LJ, Tepper JM, Young SJ, Groves PM. Frontal cortex stimulation evoked neostriatal potentials in rats: Intracellular and extracellular analysis. Brain Res Bull. 1986;17:751–758. doi: 10.1016/0361-9230(86)90086-9. [DOI] [PubMed] [Google Scholar]
- 24.Masimore B, Schmitzer-Torbert NC, Kakalios J, Redish AD. Transient striatal gamma local field potentials signal movement initiation in rats. NeuroReport. 2005;16:2021–2024. doi: 10.1097/00001756-200512190-00010. [DOI] [PubMed] [Google Scholar]
- 25.Cui Z, et al. Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron. 2004;41:781–793. doi: 10.1016/s0896-6273(04)00072-8. [DOI] [PubMed] [Google Scholar]
- 26.Dang MT, et al. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci USA. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heusner CL, Palmiter RD. Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevents cocaine sensitization and decreases cocaine preference. J Neurosci. 2005;25:6651–6657. doi: 10.1523/JNEUROSCI.1474-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley AE. Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jicha GA, Salamone JD. Vacuous jaw movements and feeding deficits in rats with ventrolateral striatal dopamine depletion: Possible relation to parkinsonian symptoms. J Neurosci. 1991;11:3822–3829. doi: 10.1523/JNEUROSCI.11-12-03822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993;44:605–610. doi: 10.1016/0091-3057(93)90174-r. [DOI] [PubMed] [Google Scholar]
- 32.Jung AB, Bennett JP., Jr Development of striatal dopaminergic function. I. Pre- and postnatal development of mRNAs and binding sites for striatal D1 (D1a) and D2 (D2a) receptors. Brain Res Dev Brain Res. 1996;94:109–120. doi: 10.1016/0165-3806(96)00033-8. [DOI] [PubMed] [Google Scholar]
- 33.David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Bevan MD, Atherton JF, Baufreton J. Cellular principles underlying normal and pathological activity in the subthalamic nucleus. Curr Opin Neurobiol. 2006;16:621–628. doi: 10.1016/j.conb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Rampon C, et al. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 36.Tsien JZ, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 37.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: A neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.