Abstract
The sense of body ownership represents a fundamental aspect of our self-awareness, but is disrupted in many neurological, psychiatric, and psychological conditions that are also characterized by disruption of skin temperature regulation, sometimes in a single limb. We hypothesized that skin temperature in a specific limb could be disrupted by psychologically disrupting the sense of ownership of that limb. In six separate experiments, and by using an established protocol to induce the rubber hand illusion, we demonstrate that skin temperature of the real hand decreases when we take ownership of an artificial counterpart. The decrease in skin temperature is limb-specific: it does not occur in the unstimulated hand, nor in the ipsilateral foot. The effect is not evoked by tactile or visual input per se, nor by simultaneous tactile and visual input per se, nor by a shift in attention toward the experimental side or limb. In fact, taking ownership of an artificial hand slows tactile processing of information from the real hand, which is also observed in patients who demonstrate body disownership after stroke. These findings of psychologically induced limb-specific disruption of temperature regulation provide the first evidence that: taking ownership of an artificial body part has consequences for the real body part; that the awareness of our physical self and the physiological regulation of self are closely linked in a top-down manner; and that cognitive processes that disrupt the sense of body ownership may in turn disrupt temperature regulation in numerous states characterized by both.
Keywords: body image, consciousness, crossmodal integration, homeostasis
Body ownership refers to the feeling that your body belongs to you and is constantly there (1, 2)—it constitutes a fundamental aspect of self-awareness. That our body is ours is often taken for granted, but disruption of this sense of body ownership is characteristic of numerous pathological conditions, for example stroke, schizophrenia, autism, epilepsy, neuropathic pain, anorexia nervosa, and bulimia. Many of these pathological conditions are also characterized by disruption of temperature regulation, which is attributed in a broad sense to disruption of or damage to structures subserving autonomic control, in the brain or the periphery (3–16) [supporting information (SI) Table S1]. That the effects are often confined to one side of the body or even to a single limb has only been explained in terms of peripheral or somatotopic-specific damage. No one has proposed that disruption of temperature regulation might be linked to disruption of body ownership. We hypothesized that, in healthy adults, temperature regulation in a specific limb could be disrupted by psychologically disrupting the sense of body ownership.
That body ownership can be disrupted psychologically in healthy volunteers has been demonstrated by out-of-body (17–19) and rubber hand (20) illusions. These sorts of illusions exploit the brain's predilection for integrating congruent tactile, visual, and proprioceptive inputs. The most-studied of these illusions, the rubber hand illusion (RHI), is typically induced by brushing a person's hand (hidden from view) while synchronously brushing a visible rubber hand. Many people perceive the touch as if it were actually coming from the rubber hand and a measurable shift in the perceived location of the experimental limb (toward the rubber one) is observed (20). In the RHI, it is as although the artificial body part is in some sense treated by participants as being part of their own body. Brain imaging studies corroborate this observation: When one “takes ownership” of the rubber hand, threatening the rubber hand evokes cortical responses (that are commensurate in magnitude with the reported strength of the illusion and are consistent with the withdrawal of the hand from threat), in parietal, premotor, and insula areas (21, 22). Notably, these areas are also important in sympathetic control and temperature regulation (23)—the insula cortex has been labeled the “interoceptive cortex” (24).
Until now, no one has investigated the consequences, for the real body part, of taking ownership of an artificial counterpart. In six separate experiments, with six independent groups of healthy volunteers, we demonstrate that the RHI evokes a limb-specific decrease in the temperature of the participant's own hand and a decrease in the weight given to tactile information from that hand. The magnitude of both effects correlates with the strength of the illusion (for a summary of experiments, see Table S2).
Results
The RHI was elicited by using the standard experimental protocol (20). In Experiment 1, we compared skin temperature of the experimental hand during the RHI to that when the rubber hand was removed and the participant's own unseen hand was no longer stimulated. The mean ± SEM skin temperature of the experimental hand was 0.27 ± 0.11°C lower during the RHI trials than during the control trials [t(10) = −2.34, P = 0.041] (Fig. S1). Experiment 2 investigated whether the decrease in skin temperature might reflect a body-wide sympathetic response, for example, because of increased general arousal. The unstimulated hand was now hidden from view, and the experimental hand was brushed, during both the RHI and control trials. Skin temperature recordings from the experimental hand corroborated the results of the previous study (mean ± SEM decrease = 0.25 ± 0.09°C), but skin temperature recordings from the unstimulated hand revealed no difference between the RHI and control trials (0.01 ± 0.09°C; ANOVA revealed a Hand × Condition interaction [F(1, 10) = 41.11, P < 0.001; Fig. S2], demonstrating that this illusion-induced change in skin temperature is not a body-wide response. By keeping the unstimulated hand hidden during the experiment, we excluded the sight of the unstimulated hand as a confounding influence on the localized nature of this effect. By stroking the experimental hand in an identical manner during the control and RHI trials, we excluded stroking itself, which could feasibly reduce skin temperature via activation of c-fibers that project to insula cortex (25), as the cause of the temperature decrease.
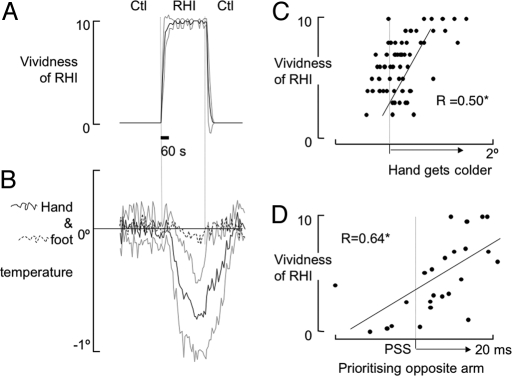
Experiment 3 was identical to Experiment 2 except that the control condition involved the asynchronous stroking of the rubber hand and the participants' experimental hand (asynchronous stroking reduces the vividness of the RHI, although it does not necessarily eliminate the effect (26)). Skin temperature was again lower during RHI trials than during asynchronous stroking trials (mean ± SEM decrease = 0.24 ± 0.13°C for the experimental hand; +0.03 ± 0.15°C for the unstimulated hand; ANOVA revealed a Hand × Condition interaction [F(1, 9) = 7.98, P = 0.020; Fig. S3]. After each RHI trial, the participants rated the vividness of the RHI. Pooling the data from Experiments 1–3 revealed that the vividness of the illusion was positively correlated with the magnitude of the decrease in skin temperature on the experimental hand (r = 0.50, P < 0.001; Fig. 1C). In other words, the greater a participant's sense of ownership of the rubber hand, the greater the side-specific drop in skin temperature.
Fig. 1.
Skin temperature, tactile processing, and vividness of the illusion. (A) Mean (bold line) and standard deviation (gray line) vividness of the rubber hand illusion (RHI). (B) Mean (bold line) and standard deviation (gray line) hand skin temperature, and mean foot skin temperature (broken line) during control (Ctl) and RHI conditions. (C) Vividness of the RHI and decrease in hand skin temperature for all participants across RHI and asynchronous stroking. Note: When the RHI is more vivid, so too is the decrease in skin temperature on the real hand. (D) Vividness of the RHI and point of subjective simultaneity (PSS). PSS to the right means that the brain is prioritiszing tactile input from the opposite side over identical tactile input from the experimental hand. *, significant difference at P < 0.02.
Experiment 4 aimed to determine whether the drop in skin temperature was specific to side or specific to the limb involved in the illusion and to quantify the time course of the response. We assessed skin temperature on the experimental hand and on the ipsilateral ankle, during two control conditions interspersed with a RHI condition. Only participants who reported a very vivid RHI were included. Skin temperature was lower during the RHI than during the control conditions at the hand (mean ± SEM decrease = 0.82 ± 0.21°C; Fig. 1C) but not at the foot (0.08 ± 0.12°C; F(5, 35) = 14.94, P = 0.001; Fig. 1B). The RHI (Fig. 1A) preceded the decrease in skin temperature of the experimental hand (Fig. 1B), which shows that the RHI does not result from the drop in skin temperature, which might have been expected according to the somatic marker hypothesis (27).
Experiment 5 assessed skin temperature while participants watched one of their hands being stroked while their other hand was hidden behind an occluding screen. This experiment did not involve the RHI (the rubber hand was removed from the table), and was different from the asynchronous stroking condition of Experiment 3, because in that condition, participants saw a hand being stroked and felt their own hand being stroked, although the timing of the strokes was not matched. No differences between conditions, nor hands, nor any interaction, were observed, which shows that the decrease in skin temperature is not evoked simply by simultaneously seeing and feeling one's own hand being stroked. That is, it is not simply a result of synchronous visual and tactile input, but depends on a simultaneous induction of the illusion of ownership over an artificial counterpart.
Perhaps the decrease in skin temperature results from a shift in attention toward the limb concerned. The final experiment used an established protocol to investigate this issue, by interrogating the processing of tactile information during the RHI. Participants made temporal order judgments (TOJs) concerning pairs of tactile stimuli, one applied to the index finger of either hand, at a range of interstimulus intervals. The outcome of interest of the TOJ task is the point of subjective simultaneity (PSS), which provides a measure of the relative weighting given by the brain to tactile input from either limb. The TOJ task was undertaken during three conditions: RHI, asynchronous stroking and control (the rubber hand was on the table to the right of the participant, but was not stroked). During the RHI trials, the tactile stimulus had to be applied to the experimental hand before an identical stimulus was applied to the other hand, in order for the two stimuli to be perceived as simultaneous (PSS = 11.0 ± 1.2 ms), which means less weight was given to processing tactile information from the experimental hand. The PSS was greater during the RHI trials than during the control trials (−1.6 ± 1.9 ms) and during asynchronous stroking of the rubber hand and the real hand (2.3 ± 2.2 ms) (ANOVA main effect [F(2, 28) = 10.22, P < 0.005; post hoc P < 0.01 for both; Fig. 2). The vividness of the RHI was positively related with the PSS (r = 0.64, P < 0.001; Fig. 1D).
Fig. 2.
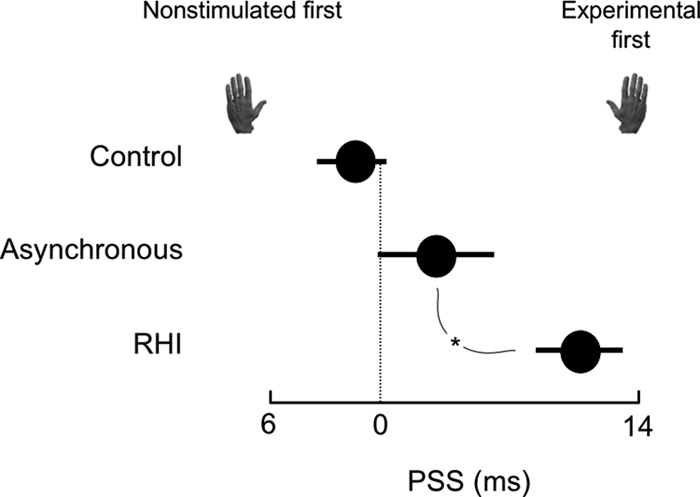
RHI is associated with slowed tactile processing. Mean ± SD point of subjective simultaneity (PSS) for temporal order judgments (TOJs) made during control trials, during asynchronous stroking of the rubber and the experimental hand, and during the rubber hand illusion (RHI). Positive PSS values indicate that the tactile stimulus had to be presented to the experimental hand before the tactile stimulus was presented to the unstimulated hand in order for them to be perceived as occurring at the same time. *, significant difference at P < 0.01.
Discussion
These six experiments yield important new findings. First, they uphold our hypothesis that temperature regulation can be disrupted in healthy volunteers by psychologically disrupting the sense of body ownership. This is the first empirical evidence that the taking ownership of a rubber hand is accompanied by a significant drop in skin temperature for the real hand. Second, and crucially, the decrease in skin temperature was limb-specific: That is, the effect does not occur in the opposite, unstimulated hand, nor in the ipsilateral foot. The effect is not evoked by tactile or visual input per se, nor by simultaneous tactile and visual input per se, nor by a shift in attention toward the experimental side or limb. That the illusion-induced drop in skin temperature was confined to a single limb also provides the first evidence of cortically mediated local changes in homeostatic control.
Disruption of both body ownership and temperature regulation are characteristic of numerous clinical states (Table S2). Notably, the disruption of body ownership and temperature regulation can be confined to one side of the body or to a single limb (6, 8). The magnitude of the effect reported here was similar to that observed in some states, for example schizophrenia, but smaller than that reported in neuropathic pain states such as complex regional pain syndrome (1–2°C). Notably, when only participants with a particularly vivid RHI were evaluated (Experiment 4), the magnitude of the temperature drop approached that (0.8 ± 0.2°C). One might expect a smaller effect in healthy volunteers undergoing a perceptual illusion than in patients with neurological dysfunction, because there is a fundamental difference in the change in body ownership involved: For the RHI, participants invariably know the rubber hand is not actually theirs, even though it feels like it. Patients however, can have the absolute conviction that the limb they see protruding from their trunk is not their own limb but that of an “imposter.”
Our findings build on a range of neuroimaging and psychophysiological studies that have shown that the taking ownership of an artificial body part engages homeostatic processes similar to those engaged by real body parts (28, 29). However, the current results provide the first evidence in healthy human participants of limb-specific changes in temperature regulation and the first evidence that such a localized effect on body temperature can be evoked via a cognitive illusion. Moreover, the psychological induction of an illusory body part decreases the weighting given to tactile information from the real limb.
The vividness of the RHI was positively related with the PSS. Remarkably, this type of shift in PSS, away from the concerned limb, has been demonstrated in neurological disorders associated with limb disownership, for example unilateral spatial neglect after stroke. In such conditions, the shift in PSS is thought to reflect defective deployment of spatial attention (30) or damage to the neural systems that subserve higher order representation of body and space (31). In other words, the shift in PSS away from the experimental limb implies a functional disownership of that limb. Although decreased skin temperature can reduce receptor sensitivity and nerve conduction velocity (32), the magnitude of the drop in temperature that occurred during the RHI is far too small to explain the PSS effect (33).
These findings have implications for our understanding of self-awareness. First, these findings show that the conscious sense of our physical self, and the physiological regulation of our physical self, are linked. In fact, our results suggest that the conscious sense of our physical self may actually contribute to its homeostatic regulation. This finding is particularly important because temperature dysregulation in pathological conditions has only ever been attributed to damage or dysfunction of autonomic networks in the central nervous system before. The current results suggest that higher order cognitive processing associated with the representation of our physical body in space, may also contribute. Second, perhaps the RHI induces a form of experimental autotomy by in some way “replacing” the limb with an artificial counterpart. Relevant to that possibility are two reports of skin temperature abnormalities at the site of repetitive self-inflicted injury in severely intellectually disabled individuals (34) and in patients with neuropathic pain (35). Both papers suggest that the repetitive self-injury may result from skin temperature changes and the latter describes the behavior as a human equivalent of animal autotomy. Perhaps however, both skin temperature dysregulation and self-injury are epiphenomena of a psychologically mediated disownership of the body part. The final implication of the current work is that the current assumption that comorbid disruption of body ownership and disruption of temperature regulation that we see in many pathological states are unrelated phenomena, is wrong, and that cognitive mechanisms underpinning the former may cause the latter.
Materials and Methods
All experiments involved separate groups of participants.
Experiment 1.
Participants and methods.
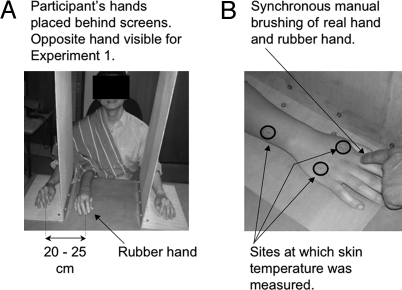
Eleven (five female) right-handed volunteers (mean ± SD age = 25 ± 4 years) participated in the study, and sat with their forearms resting on a table. The participants could see the rubber hand but a screen was used to prevent them from seeing their own stimulated hand during the RHI condition (Fig. 3). A towel was placed over the participant's shoulder and the proximal end of the rubber hand to hide both from the participant's view. The RHI was evoked by the synchronous stroking of the rubber hand and the participant's own hand (the location of stroking on the two hands was carefully matched). The participants were instructed to report any changes in perception relating to the rubber hand. Data collection began after five minutes of stroking, or sooner, whether the participant indicated, according to the questions typically used to evaluate the illusion (20), that they were already experiencing the RHI (for example, “It feels like I am actually being touched on the rubber hand.”). Brushing continued throughout the trial. Each trial lasted seven-eight minutes. There were four trials, two RHI trials and two control trials, which were undertaken in random order. In the control condition, the rubber hand was removed. The participants were instructed to look toward a fixation spot placed in the same location as the rubber hand was placed during the RHI.
Fig. 3.
Experimental setup to induce the rubber hand illusion (RHI). (A) The RHI is typically induced by brushing a participant's unseen hand while synchronously brushing a rubber hand in full view of the participant. The orientation of the rubber hand was aligned with that of the real hand. (B) Sites at which skin temperature was measured on the experimental hand (Experiment 1) and on both the experimental (i.e., stimulated) and unstimulated hands (Experiments 2–4).
Skin temperature was measured by means of a hand-held AutoPro laser thermometer (Raytek), every 30 s (interval between assessments was randomized between 25–35 s). Five readings of skin temperature were taken from each of three sites (Fig. 3B), which gave a total of 15 temperature readings for each trial. The order in which the three skin sites were measured during each trial was randomized and counterbalanced across participants. The duration of each trial was 7–8 min.
Analysis.
The hypothesis that skin temperature on the stimulated limb would be lower during the RHI trials than during the control trials was evaluated by using a paired samples t test on the mean skin temperature readings.
Experiment 2.
Participants and methods.
Eleven (six female) right-handed volunteers (mean ± SD age = 26 ± 4 years) participated. None had participated in Experiment 1. The protocol was identical to that used in Experiment 1, with the following exceptions: The participants' skin temperature was now recorded from identical sites on both hands, which were hidden from the participant's view during both conditions. The experimental hand was once again stroked in synchrony with the rubber hand in both conditions.
Analysis.
A repeated-measures ANOVA with first factor, condition (RHI, control); second factor, hand (experimental, opposite). There was no main effect of Condition [F(1, 10) = 2.215, P = 0.168] or hand [F(1, 10) = 2.229, P = 0.166].
Experiment 3.
Participants and methods.
Ten (seven female) right-handed volunteers (mean ± SD age = 28 ± 6 years) participated. None had participated in Experiments 1 or 2. The protocol used to induce the RHI was identical to that reported in Experiment 1. However, the control condition in Experiment 3 now involved the asynchronous brushing of the rubber hand and the real hand.
Analysis.
A repeated-measures ANOVA with first factor, condition (RHI, control); second factor, hand (experimental, opposite). There was no main effect of condition [F(1, 9) = 1.237, P = 0.295] or of hand [F (1, 9) = 1.826, P = 0.210].
Relating the vividness of the RHI to the magnitude of limb-specific temperature change.
The skin temperature during each RHI trial was subtracted from the mean skin temperature during both control trials to provide a measure of the temperature change for each trial, for each participant. This variable was calculated for Experiments 1–3. Participants rated the vividness of the RHI on a 10-point numerical rating scale (from zero, “not at all vivid,” to 10, “completely vivid”). This variable was calculated for Experiments 1–3. The data were entered into a linear regression to relate the vividness rating of the RHI to the magnitude of the change in skin temperature of the participant's experimental hand. The vividness score was the independent variable and temperature change the dependent variable.
Experiment 4.
Participants and methods.
Eight (four female) right-handed volunteers (mean ± SD age = 27 ± 6 years) participated. Four participants had participated in Experiment 2, but none had participated in Experiment 1 or 3. Participants were excluded if they reported vividness of the RHI as <9 on the 0–10 scale used to rate the strength of the illusion. There were two control periods (5 min), in which the participant sat with their right hand behind the screen and the rubber hand was not present, separated by a RHI condition (5 min). The vividness of the RHI was rated every 10 s on the 0–10 scale. Skin temperature was also measured on the back of the right hand and on the anterior right ankle every 10 s. The order of the vividness rating and the two skin temperature measures was different for each participant.
Skin temperature readings during each of six epochs of 60 s were used for analysis. The epochs were from 90 to 150 s and from 240 to 300 s in each condition.
Analysis.
Average skin temperature during each epoch was analyzed by using a repeated measures ANOVA with first factor, time (6 epochs); second factor, site (hand, foot). This analysis revealed a significant main effect of Site [F(1, 7) = 32.599, P = 0.001] and a main effect of time [F(5, 35) = 25.872, P < 0.001], driven by the interaction between these two factors [F(5, 35) = 14.942, P = 0.001].
Experiment 5.
Participants and methods.
Ten (five female) right-handed volunteers (mean ± SD age = 26 ± 5 years) participated. None had participated in Experiments 1, 2, 3, or 4. This experiment did not involve the RHI. The experimental condition involved the participant watching one of their hands being stroked while their other hand remained out of sight. The left and right hands were tested in a random order.
Analysis.
A repeated measures ANOVA with first factor, condition (control, looking toward the stroked hand, and looking away from the stroked hand); second factor, hand (left, right). There was no main effect of condition [F(2, 8) = 1.145, P = 0.365] nor hand [F(1, 9) = 5.029, P = 0.052], nor any interaction between these two factors [F(2, 8) = 2.035, P = 0.193].
Experiment 6.
Participants and methods.
Fifteen (7 female) right-handed volunteers (mean ± SD age = 26 ± 6 years) participated. None had participated in Experiments 1, 2, 3, 4, or 5. A pair of identical vibrotactile stimuli were presented to the index fingers of both hands (i.e., one stimulus to each hand), at a range of interstimulus intervals (5, 15, 30, 60, or 120 ms; the interstimulus interval and the order of presentation of the stimuli (left vs. right first) were randomized] (Fig. 4). Participants responded to each pair of stimuli by lifting their toes off a footpedal under their right foot if they thought the first stimulus had been presented on the right, and by lifting their toes off a footpedal under their left foot if they thought the first stimulus had been presented on the left. There were 200 pairs of stimuli in each block of TOJ trials.
Fig. 4.
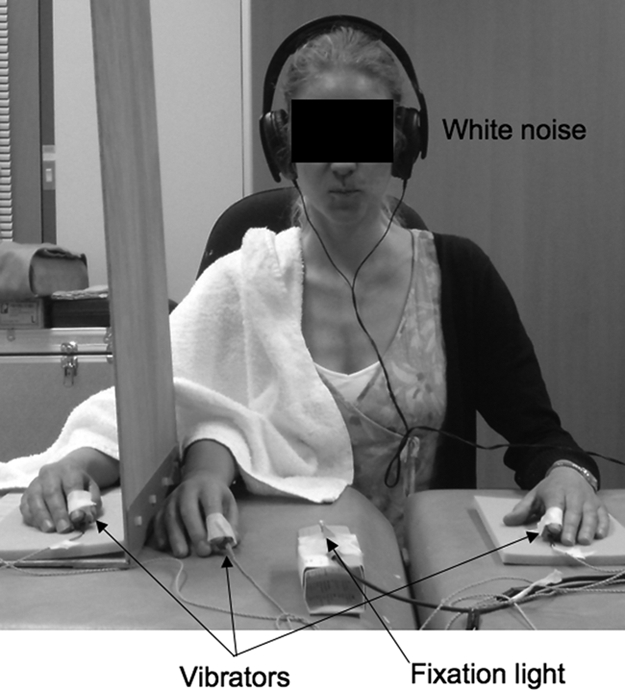
Experiment setup to evaluate tactile processing (Experiment 6). Temporal order judgment (TOJ) task. Pairs of tactile stimuli were delivered at various interstimulus intervals (one to each index finger). The onset of a visual cue at fixation informed the participant that a pair of stimuli was imminent. The participants responded by lifting their toes off of a footpedal under their left or right foot to indicate the perceived side of the first stimulus. Note that a sham vibrator was fixed to the rubber hand. White noise was delivered through the headphones to conceal any noise made by the vibrators.
One block of TOJ trials was completed during each of three conditions, the order of which was randomized: control, asynchronous stroking, and synchronous stroking (RHI). In the control condition, a rubber hand was present on the right of the participant, but was not stroked. A tactile vibrator identical to that attached to each index finger was also attached to the index finger of the rubber hand.
Analysis.
The point of subjective simultaneity (PSS) and the just noticeable difference (JND) were calculated for each participant and were the primary outcome variables [see Shore et al. (36) for methods used to psychometrically fit data to extract the PSS and JND measures]. Participants rated the vividness of the RHI after each block of TOJ trials.
PSS data were analyzed by using repeated measures ANOVA with factor condition (RHI, asynchronous, control). JND data were analyzed with a second identical ANOVA. JND was greater during the RHI and asynchronous stroking conditions than during the control condition [repeated measures ANOVA: F(2,28)= 9.75, P < 0.001; Duncan post hoc P < 0.004 for both; RHI vs. asynchronous stroking n.s.]. This result shows that the participants required a larger interstimulus interval to correctly determine the order in which the two stimuli had been presented, during the RHI and asynchronous stroking, than they did during the control condition. The vividness of the RHI was related to PSS by linear regression.
Did Participants Notice the Change in Their Skin Temperature?
When the data collection was completed, each participant was asked whether they had noticed any change in the temperature of either arm, or throughout the rest of their body, during the course of the experiment. Across Experiments 1–6 (excluding Experiment 5), 17% of the participants responded in the affirmative. They all reported that their experimental arm felt cooler during the RHI, but subsequent analysis revealed that the mean change in their skin temperature was no different from that of the remainder of the participants (n.s.).
Supplementary Material
Acknowledgments.
We thank David Hall for assistance with data collection. G.L.M. was supported by a Nuffield Medical Research Fellowship. N.O., A.V., M.W., and S.D. were supported by the Erasmus Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803768105/DCSupplemental.
References
- 1.Gallagher S. Philosophical conceptions of the self: Implications for cognitive science. Trends Cognit Sci. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- 2.James W. Principles of Psychology. New York: Henry Holt; 1890. [Google Scholar]
- 3.Priebe S, Rohricht F. Specific body image pathology in acute schizophrenia. Psychiatr Res. 2001;101:289–301. doi: 10.1016/s0165-1781(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 4.Chong TWH, Castle DJ. Layer upon layer: Thermoregulation in schizophrenia. Schizophrenia Res. 2004;69:149–157. doi: 10.1016/s0920-9964(03)00222-6. [DOI] [PubMed] [Google Scholar]
- 5.Moseley GL. Distorted body image in complex regional pain syndrome. Neurology. 2005;65:773–773. doi: 10.1212/01.wnl.0000174515.07205.11. [DOI] [PubMed] [Google Scholar]
- 6.Janig W, Baron R. Complex regional pain syndrome: Mystery explained? Lancet Neurol. 2003;2:687–697. doi: 10.1016/s1474-4422(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 7.Halligan PW, Marshall JC, Wade DT. Three arms: a case study of supernumerary phantom limb after right hemisphere stroke. J Neurol Neurosurg Psychiatry. 1993;56:159–166. doi: 10.1136/jnnp.56.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riedl B, Beckmann T, Neundorfer B, Handwerker HO, Birklein F. Autonomic failure after stroke–is it indicative for pathophysiology of complex regional pain syndrome? Acta Neurol Scand. 2001;103:27–34. doi: 10.1034/j.1600-0404.2001.00139.x. [DOI] [PubMed] [Google Scholar]
- 9.Bruch H. Perceptual and conceptual disturbances in anorexia nervosa. Psychosom Med. 1962;24:187–195. doi: 10.1097/00006842-196203000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Lautenbacher S, Pauls AM, Strian F, Pirke K-M, Krieg J-C. Pain sensitivity in anorexia nervosa and bulimia nervosa. Biol Psychiatry. 1991;29:1073–1078. doi: 10.1016/0006-3223(91)90249-l. [DOI] [PubMed] [Google Scholar]
- 11.Slade P. A review of body-image studies in anorexia nervosa and bulimia nervosa. J Psychiatr Res. 1985;19:255–265. doi: 10.1016/0022-3956(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 12.Papezova H, Yamamotova A, Uher R. Elevated pain threshold in eating disorders: Physiological and psychological factors. J Psychiatr Res. 2005;39:431–438. doi: 10.1016/j.jpsychires.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Boesebeck F, Ebner A. Paroxysmal alien limb phenomena due to epileptic seizures and electrical cortical stimulation. Neurology. 2004;63:1725–1727. doi: 10.1212/01.wnl.0000143064.81746.e9. [DOI] [PubMed] [Google Scholar]
- 14.Holtkamp M, Schmitt FC, Buchheim K, Meierkord H. Temperature regulation is compromised in experimental limbic status epilepticus. Brain Res. 2007;1127:76–79. doi: 10.1016/j.brainres.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Rogers SJ, Ozonoff S. Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatr. 2005;46:1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- 16.Satoshi T. A thermographic study on the alteration of facial skin temperature in autistic patients by exercise loading. J Kyorin Med Soc. 2000;31:357–364. [Google Scholar]
- 17.Altschuler EL, Ramachandran VS. Last but not least—A simple method to stand outside oneself. Perception. 2007;36:632–634. doi: 10.1068/p5730. [DOI] [PubMed] [Google Scholar]
- 18.Ehrsson HH. The experimental induction of out-of-body experiences. Science. 2007;317:1048. doi: 10.1126/science.1142175. [DOI] [PubMed] [Google Scholar]
- 19.Lenggenhager B, Tadi T, Metzinger T, Blanke O. Video Ergo Sum: Manipulating bodily self-consciousness. Science. 2007;317:1096–1099. doi: 10.1126/science.1143439. [DOI] [PubMed] [Google Scholar]
- 20.Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd D, Morrison I, Roberts N. Role for human posterior parietal cortex in visual processing of aversive objects in peripersonal space. J Neurophysiol. 2006;95:205–214. doi: 10.1152/jn.00614.2005. [DOI] [PubMed] [Google Scholar]
- 22.Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: A sensory network for bodily self-consciousness. Cereb Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- 23.Hilz MJ, et al. Right ventromedial prefrontal lesions result in paradoxical cardiovascular activation with emotional stimuli. Brain. 2006;129:3343–3355. doi: 10.1093/brain/awl299. [DOI] [PubMed] [Google Scholar]
- 24.Craig A. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 25.Kramer HH, et al. Activation of the cortical pain network by soft tactile stimulation after injection of sumatriptan. Pain. 2007;133:72–78. doi: 10.1016/j.pain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Ehrsson HH, Spence C, Passingham RE. That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- 27.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc London Ser B. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 28.Armel KC, Ramachandran VS. Projecting sensations to external objects: Evidence from skin conductance response. Proc R Soc London Ser B. 2003;270:1499–1506. doi: 10.1098/rspb.2003.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrsson HH, Wiech K, Weiskopf N, Dolan RJ, Passingham RE. Threatening a rubber hand that you feel is yours elicits a cortical anxiety response. Proc Natl Acad Sci USA. 2007;104:9828–9833. doi: 10.1073/pnas.0610011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heilman KM, Bowers D, Coslett HB, Whelan H, Watson RT. Directional Hypokinesia - Prolonged Reaction-Times for Leftward Movements in Patients with Right Hemisphere Lesions and Neglect. Neurology. 1985;35:855–859. doi: 10.1212/wnl.35.6.855. [DOI] [PubMed] [Google Scholar]
- 31.Bisiach E, Luzzatti C, Perani D. Unilateral neglect, representational schema and consciousness. Brain. 1979;102:609–618. doi: 10.1093/brain/102.3.609. [DOI] [PubMed] [Google Scholar]
- 32.Dioszeghy P, Stalberg E. Changes in motor and sensory nerve-conduction parameters with temperature in normal and diseased nerve. Electroencephal Clin Neurophysiol. 1992;85:229–235. doi: 10.1016/0168-5597(92)90110-w. [DOI] [PubMed] [Google Scholar]
- 33.von Bekesy G. Interaction of paired sensory stimuli and conduction in peripheral nerves. J App Physiol. 1963;18:1276–1284. doi: 10.1152/jappl.1963.18.6.1276. [DOI] [PubMed] [Google Scholar]
- 34.Symons FJ, Sutton KA, Bodfish JW. Preliminary study of altered skin temperature at body sites associated with self-injurious behavior in adults who have developmental disabilities. Am J Ment Retard. 2001;106:336–343. doi: 10.1352/0895-8017(2001)106<0336:PSOAST>2.0.CO;2. and erratum (2001) 106:469. [DOI] [PubMed] [Google Scholar]
- 35.Mailis A. Compulsive targeted self-injurious behaviour in humans with neuropathic pain: A counterpart of animal autotomy? Four case reports and literature review. Pain. 1996;64:569–578. doi: 10.1016/0304-3959(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 36.Shore DI, Spry E, Spence C. Confusing the mind by crossing the hands. Cognit Brain Res. 2002;14:153–163. doi: 10.1016/s0926-6410(02)00070-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.