Abstract
There are few objective staging systems to assess severity of Dupuytren’s disease (DD). Previous methods to assess severity of DD were based primarily on the degree of contracture of an affected digit measured using a goniometer. Nonetheless, this method of assessment alone may be incomplete, and other factors should be considered. White (n = 92) patients diagnosed with DD from northwest of England were assessed for DD. Objective criteria for evaluating severity incorporated quantified variables. The revised severity stage was correlated to a known staging system of DD (Tubiana’s staging system) which measures total flexion deformity for a single affected digit. Total revised severity staging scores ranged between 4 and 53 (mean = 18.7) and revealed significant positive correlation to Tubiana’s original staging system (r2 = 0.8, p < 0.001). There was significant difference between severity staging scores in those with a positive family history compared to those without (p < 0.01). In current practice, often, the degree of contracture in an affected digit is used solely as a measure of disease severity. Additional objective clinical information may provide useful prognostic indices for disease progression as well as postoperative outcome.
Keywords: Dupuytren’s disease, Contracture, Dupuytren diathesis, Ectopic dupuytren disease, Garrod’s pads, Disease prognosis, Risk factor, Family history, Severity
Introduction
Dupuytren’s disease (DD) is a benign condition that can present with varying severity. DD is a progressive fibroproliferative disorder resulting in abnormal “scar-like” tissue in the palmar fascia [6] leading to irreversible, permanent, and progressive contracture of the involved digits. DD is commonly bilateral, and “Dupuytren-like” fibrotic tissue can occur on the dorsum of the hand over the knuckles (Garrod’s pads), feet (Lederhose’s disease), and penis (Peyronies disease) [28]. DD is not only physically and psychologically disabling [12], but can also be aesthetically displeasing.
The decision to carry out surgical correction is often dependent upon the surgeon’s evaluation of clinical severity of the disease. Severity is often based upon measurement of flexion deformity using a goniometer. Another common assessment of severity of disease is the table top test; unfortunately, these methods of assessment may not be sufficient in planning surgical management, as factors which may affect recurrence of disease may not have been considered. Severity of DD and outcome after surgery based upon the measurement of contracture of an affected digit [10] (Table 3) [30] was a system introduced by Tubiana and has been used by clinicians in aiding surgical management. This method does not objectively associate other relevant risk factors with disease severity, which may alter treatment plans.
Table 3.
Assessing risk factors in disease severity.
| Risk factor | Frequency | Mean severity score in presence of factor | Mean severity score in absence of factor | Significance of risk factor on severity (p value) |
|---|---|---|---|---|
| Positive family history | 35/92 (38%) | 23.1 | 15.9 | 0.006 |
| Substantial alcohol history | 20/92 (22%) | 24.6 | 17.0 | 0.04 |
| Manual labor | 58/92 (63%) | 19.8 | 16.7 | 0.20 |
| Diabetes mellitus | 13/92 (14%) | 22.1 | 18.1 | 0.31 |
| Smoker | 45/92 (49%) | 19.8 | 17.6 | 0.34 |
| Carpal tunnel syndrome | 4/92 (4%) | 23.5 | 18.5 | 0.39 |
| Age at onset <55 years | 34/92 (37%) | 19.4 | 18.2 | 0.62 |
| Male gender | 80/92 (87%) | 18.5 | 19.3 | 0.71 |
| Frozen shoulder | 18/92 (20%) | 19.3 | 18.5 | 0.80 |
| Rheumatoid arthritis | 3/92 (3%) | 19.3 | 18.6 | 0.95 |
| Hand injury | 9/92 (9%) | 18.6 | 18.6 | 0.99 |
List of known associated risk factors and their significance in Dupuytren’s disease severity
Hueston introduced the concept of DD diathesis and proposed that presence of diathesis indicated degree of disease severity and may predict postoperative outcome. DD diathesis described four characteristics of the disease that included bilateral disease (described as bilateral palmar lesions), family history of DD, ectopic lesions (DD found outside the palmar surface), and ethnicity [14].
The four diathesis factors dictated an aggressive course of the disease and greater tendency for recurrence after surgical treatment. Recurrent disease also had a tendency to be more severe in these patients who presented with DD diathesis. A predictive measure of various features of DD diathesis was recently introduced and evaluated [14].
A combined method to assess disease severity utilizing Tubianas original method of measuring degrees of digital contracture combined with other relevant risk factors is introduced here. This may provide a means of monitoring disease progression as well as prognosis after surgery, which can be used for surgical audit, clinical, and research studies assessing new trials or treatments.
Materials and Methods
Study Population
A hospital-based retrospective study design was used to recruit patients with a diagnosis of DD. We identified 300 successive white patients with a diagnosis of DD who had surgery between January 2003 and December 2004 from surgical records at a hospital in the northwest of England. Of these 300 patients, 92 respondents agreed to take part in the study and were subsequently examined. Eighty men [age range 37–88 years; mean age 65.6 years SD = 8.3)] and 12 women [age range 58–81 years; mean age 68.8 years (SD = 8.1)] participated in the study after completing an ethically approved consent form. All patients were enrolled after surgical management for DD ensuring that the diagnosis of DD was accurate. The presence of DD nodules and cords in the palmar/digital or plantar fascia with or without contraction of affected digits/toes on examination was used to confirm the diagnosis. The local research ethics committee granted full ethical approval for this study.
Assessment of Severity
A detailed medical history and clinical examination were performed by the first author based on a standard methodology agreed on by all authors. Each patient was assessed between 1 and 4 years postoperatively at one time point.
Data on associated risk factors were collected, including history of diabetes (insulin or non-insulin-dependent) [4, 5], epilepsy, carpal tunnel syndrome [7], frozen shoulder, rheumatoid arthritis [3], smoking and alcohol history [8], history of manual labor [9], and history of injury to the hand [19]. Clinical examination evaluated for true recurrence of DD.
No specific exclusion criteria were considered to differentiate true and false recurrence; however, clinical examination aimed to differentiate extension, true, and false recurrent DD. False recurrence can include scar and joint contracture, whereas true recurrence is the development of new DD tissue within the same area of previous surgery. Extension describes the development of new DD tissue away from the area of surgery.
Detailed clinical evaluation made it possible to produce a revised severity staging system for assessing disease severity in each patient. This revised staging system incorporated a known staging system developed by Tubiana [30] which is based upon measurement of the total flexion deformity of each affected digit (Table 1). Measurements of digit contractures were made using a goniometer. As per Tubiana’s original method, measurements were made of the degree of flexion contracture at the metacarpophalangeal, proximal, and distal interphalangeal joints.
Table 1.
Staging of Dupuytren’s disease.
| Stage | Deformity |
|---|---|
| 0 | No lesion |
| N | Palmar nodule without presence of contracture |
| 1 | TFD between 0° and 45° |
| 2 | TFD between 45° and 90° |
| 3 | TFD between 90° and 135° |
| 4 | TFD greater than 135° |
Original staging of DD introduced by Tubiana [30]. Total flexion deformity (TFD) is measured with a goniometer at the metacarpalphalangeal, proximal, and distal interphalangeal joints.
To assess severity of DD, additional variables were also considered (Table 2). The total number of surgical procedures for treatment of DD were deemed important, as this identified recurrence of DD requiring repeat surgical intervention. The total number of digits affected with DD (i.e., the presence of a digit contracture) is a measure of severity and also a measure of hand function. The presence of true recurrence after surgical management for each digit identifies more severe disease, as it suggests that the patient has a strong DD diathesis. The presence of nodules, palmar pits, Garrod’s pads, Lederhose’s or Peyronie’s disease; the presence of unilateral or bilateral disease; the presence of a positive family history with focus on first- and second-degree relatives; and a young age at onset are all aspects of the DD diathesis [14]. Mean onset of disease is in the fifth decade of life [11, 21]. A strong diathesis suggesting an increase risk of disease recurrence and more severe disease [18].
Table 2.
Assessing severity in Dupuytren’s disease. (Revised Tubiana Staging System)
| Criteria | Score | |
|---|---|---|
| 1 | Surgical procedures | Total no of operations for left and right hand |
| 2 | Recurrence | Total no of recurrences for left and right hand |
| 3 | Number of digits affected | Total for left and right hand |
| 4 | Number of nodules | Total for left and right hand (palmar/digital) |
| 5 | Number of pits | Total for left and right hand |
| 6 | Garrod’s pads | 1 |
| 7 | Lederhoses’ disease | 1 |
| 8 | Peyronies’ disease | 1 |
| 9 | Bilateral/unilateral DD | 1 for unilateral; 2 for bilateral |
| 10 | Stage 1a = total flexion deformity (TFD) of each digit | 1 |
| 11 | Stage 2 = TFD | 2 |
| 12 | Stage 3 = TFD | 3 |
| 13 | Stage 4 = TFD | 4 |
| Total severity score |
The revised Tubiana staging system incorporates the original total flexion deformity (TDF) measurements and 9 other clinical objective criteria.
aA stage and score are given for each digit and scores summated for all digits.
Data Analysis
All data were transcribed categorically using binary variables. All known associated risk factors and relevant aspects of clinical examination were analyzed using Student’s t test to assess which factors (including known associated risk factors and modified Hueston’s diathesis factors) should be used to incorporate into the revised severity staging system. The revised staging was correlated to the original staging system [27] introduced by Tubiana to evaluate whether or not additional factors should be considered when assessing severity and planning operative management for DD. Statistical analyses were calculated using the Microsoft Excel and SPSS software packages.
Results
Observations
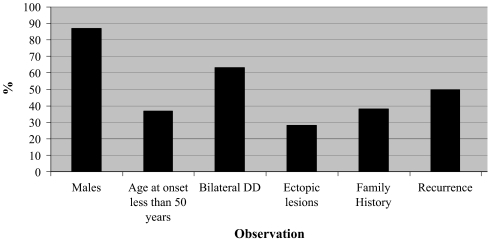
Of the 80 men and 12 women included, observations included 34 (37%) with an age at onset less than 50 years, 58 (63%) with bilateral DD, 26 (28%) with ectopic lesions, 35 (38%) with a positive family history of DD, the number of surgical procedures for DD (median = 2, range = 1–10), the number of digits affected (median = 2, range = 1–10), and combined staged TFD (median = 3, range = 0–20; Fig. 1). Mean severity staging score for the study population was 18.7 (median = 16, range = 4–53).
Figure 1.
Observational findings of 80 men and 12 women with DD.
Correlating Tubiana’s Original and Revised Staging Systems
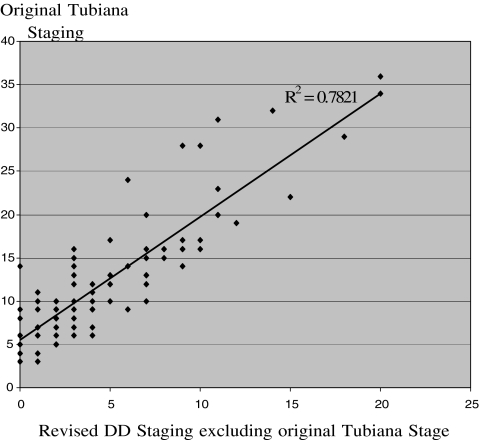
The staging system introduced by Tubiana correlated significantly with the revised staging (r2 = 0.78; p < 0.001; Fig. 2). The revised staging included the after objective factors: age at onset, presence of ectopic lesions, presence of bilateral disease, presence of true recurrent disease, presence of nodules and pits in the palms of the hands, the total number of digits affected, and the total number of surgical procedures.
Figure 2.
Correlation of original DD staging system introduced by Tubiana [30] with the revised staging system, for analysis the original stage that is incorporated in the revised staging system is executed.
Correlating the Revised Staging System and Frequency of Surgery
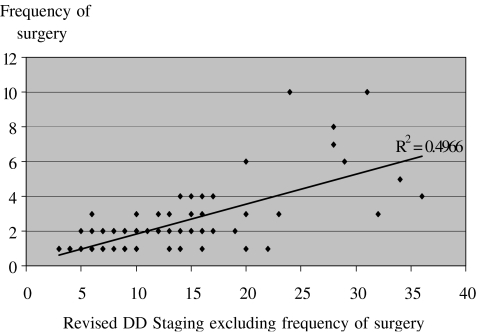
The revised severity stage was correlated to the total number of surgical procedures for each patient. This revealed a significant positive correlation (r2 = 0.50; p < 0.001), suggesting that the greater the number of surgical procedures the greater the severity of disease (Fig. 3).
Figure 3.
Correlation of frequency of number of surgical procedures to the revised DD Tubiana staging system, for analysis the frequency of surgical procedures is excluded from the revised DD stage.
Associated Etiological Factors and Their Influence on Disease Severity
Mean severity staging scores in the presence or absence of known associated risk factors were compared and the significance of each factor upon severity of disease calculated (Table 3). Family history is a significant factor influencing disease severity. There was a significant difference (p = 0.006) between the mean revised severity staging system score for those with a positive family history equalling 23.1 (range = 6–53, median = 21) compared to a score of 15.5 (range = 4–38, median = 16) for those with a negative family history of DD. Clinical examination of patients also indicated that the severity of DD was greater in those with a positive family history than those without. Patients with a positive family history demonstrated a trend towards a greater number of digits being affected with more severe contractures, nodules, cords, and pits. Patients who consumed considerable amounts of alcohol (more than 21 units in men, more than 14 units in women in 1 week) also demonstrated a significant increase in disease severity (p = 0.04). The mean revised severity staging score for those with considerable alcohol consumption equalled 24.6 (range = 4–53, median = 17.5) compared to 17.0 (range = 4–45, median = 16) in those patients who did not. Other associated etiological factors including tobacco smoking did not appear to have a significant effect upon disease severity.
Discussion
A clinically objective tool is presented for assessment of the degree of severity in DD which may be used either pre- or postoperatively. Preoperative quantification of disease severity may aid in accurate and reliable prediction of the postoperative outcome. A quantifiable severity assessment may also help both the surgeon and patient alike to understand the degree of disease progression and implications of recurrence after surgical treatment.
One of the earliest attempts to assess DD involved measuring the degree of disability in the patient’s hand [20]. Tubiana introduced a grading system based upon the total flexion deformity of the affected digit [30]. A not too dissimilar grading system was introduced by Tropet et al. [29], introducing the grading system based upon the degree of pre- and postoperative retraction [29]. Adaptations of Tubiana’s staging system, focusing upon the particular joint contracture of each digit and subsequently grading the disease for a hand based upon the worst affected finger [2], was considered a useful tool in deciding operative management.
A clinical grading system incorporating predisposing risk factors, risk of recurrence, and sympathetic tone in individual patients has also been introduced [32]. The outcomes in this study were based upon timing of individual surgical procedures and experience of operators. This particular staging system may have been adapted as a clinical tool; nevertheless, aspects such as sympathetic tone are difficult to measure and extremely variable particularly when assessed by more than one clinician.
The revised severity staging system introduced here provides a simple and objective method to evaluate the severity of a patient’s disease incorporating the known staging system introduced by Tubiana [30]. It is evident from the outcomes of this study that in addition to measuring digit contracture, other clinical features are a reflection of disease severity and should be taken into account.
There are other well-validated and useful tools to assess hand function such as the Disabilities of the Arm, Shoulder and Hand Questionnaire (DASH) and the Michigan Hand Outcomes Questionnaire (MHQ). However, these tools are not specific enough for assessment of severity in DD. For example, DD does not affect the arm or the shoulder, and hence, the DASH is too broad and can become confusing when assessing severity of a hand-specific condition such as DD. We feel that the DASH would be more suited to assess severity in a condition which is likely to affect the entire upper limb such as rheumatoid arthritis. The DASH has been used to assess specific upper limb conditions such as carpal tunnel syndrome [16]; however, it has been suggested that the MHQ is more reliable when assessing function in patients with carpal tunnel syndrome and can be used for trauma patients with wrist fractures [17]. The MHQ again is a very useful tool to assess hand function; however, we feel there is a need for a more specific staging system for a complex condition like DD. One aspect of the MHQ which has been shown to be useful in patients with burns [31] and with rheumatoid arthritis [22] is the assessment of pain. Patients with DD rarely complain of pain, an aspect of the MHQ which is not required in assessing severity of DD. The staging criteria devised from this study were aimed to aid the clinician in appropriate planning and timing of surgery in DD cases. Timing of surgery in DD patients can be of paramount importance depending upon the risk of recurrence and severity of disease. To clarify the benefit of the DASH or MHQ in assessing severity of DD, it would be useful to compare the validity and reliability of this revised staging system to either the DASH or MHQ.
We have shown that there is a positive correlation between Tubiana’s staging system and our revised clinical assessment. This study further supports an objective severity staging system with its significant positive correlation with frequency of surgical procedures for DD. Nevertheless, it must be emphasized that the proposed revised staging system has only presented preliminary data with interesting findings of potential clinical relevance. We are aware of its limitations and would recommend that the potential superiority of this revised system in providing additional information to Tubiana’s original staging system should be replicated using a larger cohort of patients in a well-designed prospective study.
The etiopathogenesis of DD remains unknown; however, a genetic basis is implicated, as the disease is strongly familial. Thirty-eight percent of patients reported a positive family history; this is consistent with a previous figure [25]. We have found that in the presence of a positive family, a patient is more likely to have more severe disease based upon objective assessment. The presence of DD in an individual significantly increases the risk of a sibling developing DD [13]; therefore, presence of a positive family history of DD should be considered when assessing severity [14].
This study has demonstrated that considerable (in excess 21 units for men and 14 units for women of alcohol consumption per week) alcohol consumption also increases the possibility of patients presenting with more severe DD. Although mean disease severity was greater in smokers, the result was not statistically significant. Data regarding the role of smoking and alcohol are not consistent [23, 26]; however, alcohol consumption may be considered when assessing severity of disease and when educating patients about the risk of disease severity in the case of recurrent DD after surgical management.
In assessment of the other associated risk factors, although they have a known association with DD, they did not seem to have a statistically significant effect on the severity of disease. Therefore, when assessing severity of DD, gender, a history of carpal tunnel syndrome, rheumatoid arthritis, frozen shoulder, hyperlipidemia, epilepsy, diabetes mellitus, hand injury, or a history of manual labor do not appear to be of significant importance in this cohort of patients.
In our assessment, we did not find a significant association between severity and a young age at onset. This may be related to a smaller sample-sized study, unlike contrary findings in previous studies with larger cohorts of patients [14]. Nonetheless, Hueston noted that recurrence is more common in the younger age group [15], so younger patients who may present with a low clinical severity preoperatively may indeed be predisposed to developing higher recurrence rates and more severe disease postoperatively.
A severity staging system alone, although a useful clinical tool, can be made more precise when used with known prognostic indicators [1]. To manage DD effectively, clinical assessment should incorporate clinical severity and indicators predicting disease recurrence. Other factors which can influence recurrence include surgical extent of the disease and particular surgical techniques used to treat the disease (fasciectomy vs dermofasciectomy). We propose that the revised severity staging system introduced here may provide a new entity in evaluating disease severity in the future by the introduction of a prognostic index. Prognostic indices are derived following models of differential analysis which use logistic regression to identify factors that are significantly associated with outcome. A novel prognostic indicator will also aid in identifying and classifying patients in prospective clinical or scientific studies. To validate such a prognostic index in clinical practice, a further larger prospective study is required.
It is evident from this study that other factors should also be considered when grading severity that may influence postoperative results. Factors such as assessing the nature of DD nodules, pits, and cords may be valuable in surgical time management for individual patients with particular risks or a diathesis [24]. We feel that the introduction of this revised staging system may be a useful tool in providing guidance to the patient and surgeon, as it may provide a more objective, accurate, and precise method of clinical assessment. This may provide added benefit of predicting surgical outcome. It may be necessary to validate this revised staging system in a larger cohort of patients before it is being used in clinical practice.
Acknowledgments
The authors would like to thank Mrs. Sarah Rhodes of Research and Development Department, Pennine Acute Hospitals NHS Trust for her statistical expertise.
References
- 1.Abe Y, Rokkaku S, Ofuchi S, Tokunaga S, Takahashi K, Moriya H. An objective method to evaluate the risk of recurrence and extension of Dupuytren’s disease. J Hand Surg (Br) 2004;29-B(5):427–30. [DOI] [PubMed]
- 2.Abe Y, Rokkaku S, Ofuchi S, Tokunaga S, Takahashi K, Moriya H. Surgery for Dupuytren’s disease in Japanese patients and a new preoperative classification. J Hand Surg (Br) 2004;29-B(3):233–7. [DOI] [PubMed]
- 3.Arafa M, Steingold RF, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg (Br) 1984;9-B:165–6. [PubMed]
- 4.Arkkila PE, Kantola IM, Viikari JS, Ronnemaa T, Vahatalo MA. Dupuytren’s disease in type I diabetic patients: a five year prospective study. Clin Exp Rheumatol 1996;14:59–65. [PubMed]
- 5.Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol 1997;24:153–9. [PubMed]
- 6.Bayat A, McGrouther DA. Management of Dupuytren’s disease—clear advice for an elusive condition. Ann R Coll Surg Engl 2006;88(1):3–8. [DOI] [PMC free article] [PubMed]
- 7.Bonnici AV, Birjandi F, Spencer JD, Fox SP, Berry AC. Chromosomal abnormalities in Dupuytren’s contracture and carpal tunnel syndrome. J Hand Surg (Br) 1992;17(3):349–55. [DOI] [PubMed]
- 8.Burge P, Hoy G, Regan P, Milne R. Smoking, alcohol and the risk of Dupuytren’s contracture. J Bone Jt Surg 1997;79-B(2):206–10. [DOI] [PubMed]
- 9.de la Caffiniere JY, Wagner R, Etscheid J, Metzger F. Manual labor and Dupuytren’s disease. The results of a computerized survey in the field of iron metallurgy. Ann Chir Main 1983;2(1):66–72. [DOI] [PubMed]
- 10.Draviaraj KP, Chakrabarti I. Functional outcome after surgery for Dupuytren’s contracture: a prospective study. J Hand Surg (Am) 2004;29-A(5):804–8. [DOI] [PubMed]
- 11.Early PF. Population studies in Dupuytren’s contracture. J Bone Jt Surg 1962;44-B(3):602–13.
- 12.Hayton MJ, Gray ICM. Dupuytren’s contracture: a review. Curr Orthop 2003;17:1–7. [DOI]
- 13.Hindocha S, John S, Stanley JK, Watson SJ, Bayat A. The heritability of Dupuytren’s disease: familial aggregation and its clinical significance. J Hand Surg (Am) 2006;31(2):204–10. [DOI] [PubMed]
- 14.Hindocha S, Stanley JK, Watson SJ, Bayat A. Dupuytren’s diathesis—evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg (Am) 2006;31(10):204–10. [DOI] [PubMed]
- 15.Hueston JT. Dupuytren’s contracture. Edinburgh & London: E&S Livingstone; 1963: p. 51–120.
- 16.Kotsis SV, Chung KC. Responsiveness of the Michigan Hand Outcomes Questionnaire and the Disabilities if the Arm, Shoulder and Hand Questionnaire in carpal tunnel surgery. J Hand Surg (Am) 2005;30(1):81–6. [DOI] [PubMed]
- 17.Kotsis SV, Lau FH, Chung KC. Responsiveness of the Michigan Hand Outcomes Questionnaire and physical measurements in outcome studies of distal radius fracture treatment. J Hand Surg (Am) 2007;32(1):84–90. [DOI] [PubMed]
- 18.Lettin AW. Dupuytren’s diathesis. J Bone Jt Surg (Br) 1964;46-B(2):220–4. [PubMed]
- 19.McFarlane RM. Dupuytren’s disease: relation to work and injury. J Hand Surg (Am) 1991;16:775–9. [DOI] [PubMed]
- 20.Meyerding HW. Dupuytren’s contracture. Arch Surg 1936;32:320–33.
- 21.Mikkelsen OA. Dupuytren’s disease—initial symptoms, age of onset and spontaneous course. Hand 1977;9(1):11–15. [DOI] [PubMed]
- 22.Mussy-Westropp N, Krishnan J, Ahern M. Comparing the AUSCAN osteoarthritis hand index, Michigan Hand Outcomes Questionnaire and sequential occupational dexterity assessment for patients with rheumatoid arthritis. J Rheumatol 2004;31(10):1996–2001. [PubMed]
- 23.Noble J, Arafa M, Royle SG, McGeorgeG, Crank S. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg (Br) 1992;17-B:71–4. [DOI] [PubMed]
- 24.Reilly RM, Stern PJ, Goldfarb CA. A retrospective review of the management of Dupuytren’s nodules. J Hand Surg (Am) 2005;30-A(5):1014–1018. [DOI] [PubMed]
- 25.Skoog T. Dupuytren’s contracture with special reference to aetiology and improved surgical treatment, its occurrence in epileptics, note on Knuckle pads. Acta Chir Scand 1948;96(Suppl 39):25–175.
- 26.Smith AC. Diagnosis and indications for surgical treatment in Dupuytren’s contracture. Hand Clin 1991;7(4):635–41. [PubMed]
- 27.Smith SP, Devaraj VS, Bunker TD.The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg 2001;10(2):149–51. [DOI] [PubMed]
- 28.Thurston AJ. Dupuytren's disease. J Bone Jt Surg (Br) 2003;85-B(4):469–77. [DOI] [PubMed]
- 29.Tropet Y, Deck D, Vichard P. Lesions of the little finger in Dupuytren’s disease. Ann Chir Main Memb Super 1994;13(2):101–6. [DOI] [PubMed]
- 30.Tubiana R. Dupuytren’s disease of the radial side of the hand. Hand Clin 1999;15(1):149–59 (Feb). [PubMed]
- 31.Umraw N, Chan Y, Gomez M, Cartotto RC, Fish JS. Effective hand function assessment after burn injuries. J Burn Care Rehabil 2004;25(1):134–9. [DOI] [PubMed]
- 32.Woodruff MJ, Waldram MA. A clinical grading system for Dupuytren’s contracture. J Hand Surg (Br) 1998;23(3):303–305. [DOI] [PubMed]