Abstract
Objective
Calculate first and second trimester reference ranges and within-woman correlations for TSH, free T4, and thyroid antibodies.
Study Design
Measure TSH, free T4, and thyroid antibodies in paired sera from 9,562 women in the FaSTER trial of Down syndrome screening.
Results
Median first trimester TSH (1.05 mIU/L) is lower than second (1.23 mIU/L); and 98th centile is higher (4.15 vs. 3.77 mIU/L). Within-woman paired TSH correlations are moderately strong (r2=0.64). Among women with first trimester TSH values above the 98th centile, second trimester values are over the 95th centile in 68%. Median first trimester free T4 values (1.10 ng/dL) are higher than second (1.01 ng/dL). Paired free T4 measurements correlate weakly (r2=0.23). Among women with first trimester free T4 values below the 2nd centile, second trimester values are below the 5th centile in 32%. Antibody measurements correlate strongly between trimesters (thyro-peroxidase r2=0.79, thyroglobulin r2=0.83).
Conclusions
TSH and free T4 measurements require gestation-specific reference ranges.
Keywords: TSH, T4, Antibodies, Gestational Ranges
Introduction
In January 2004, a workshop was held in Atlanta, Georgia entitled, “The Impact of Maternal Thyroid Diseases on the Developing Fetus: Implications for Diagnosis, Treatment, and Screening”. One conclusion of that meeting was that more data were needed on reference ranges for T4 (free or total) and TSH by month of pregnancy, especially during the first trimester.1
Two subsequent publications addressed this recommendation by providing data on TSH.2, 3 The first study obtained samples from each of 1,126 woman in both first and second trimesters. Reference ranges were determined for TSH and thyroid antibodies during gestational weeks eight through 21, and within-person variability was analyzed. The second study was larger (a cohort of 13,599 singleton and 132 twin pregnancies) and reported TSH values for single samples obtained from individual women between six gestational weeks and term. Differences in 50th centile values between the two studies, while not great, indicated that any laboratory offering this type of testing must establish its own reference range for pregnant women, prior to reporting interpretive results for clinical purposes. Authors of the second study suggested that conversion of TSH measurements to multiples of the median (MoM) might take into account differing assay standards and thereby improve the ability of laboratories to compare results. Free T4 reference ranges have also been reported recently from the second cohort.3,4 Median values were higher in the first than the second trimester, while the 2.5th centile ranged between 0.77 ng/dL and 0.92 ng/dL, with an average of 0.86 ng/dL.
The current study analyzes paired first and second trimester serum samples from a large cohort of women to provide further reference data on TSH levels in pregnancy and, in addition, to examine levels of free T4 and two thyroid antibodies (thyroperoxidase (TPO) and thyroglobulin (TG)), between 11 and 18 completed weeks’ gestation.
Materials and Methods
Women were participants in the multicenter FaSTER (First And Second Trimester Risk of aneuploidy) trial, as previously described.5 Only women with singleton pregnancies were enrolled. At five of the 15 recruitment centers, participants were asked to give supplementary consent to allow their residual sample to be used for additional research studies. Samples from those centers (Montefiore Medical Center, Bronx, NY; Swedish Medical Center, Seattle WA; LDS Hospital, Salt Lake City, UT; Utah Valley Regional Medical Center, Provo, UT; and McKay Dee Hospital, Ogden, UT) were eligible for inclusion in the present study. Women who did not consent and those whose pregnancies were affected by Down syndrome were excluded.
Inclusion criteria required documentation that thyroid-related measurements were available from both first and second trimester samples and that gestational dates were established by ultrasound. From the 10,329 women who met those inclusion criteria, 111 with initial gestational dates less than 11 weeks were excluded, because the numbers were judged insufficient to establish reliable reference ranges. Also excluded were 389 women with known hypothyroidism. Finally, 267 women were excluded because data regarding known hypothyroidism were missing; 9,562 women remained. Samples were collected between 1999 and 2002, stored at −80°C, and tested between July 2004 and May 2005 (stored for three to six years). Levels of TSH, free T4, and anti-TPO and anti-TG antibodies were measured using the Immulite 2000 methodology (Los Angeles, CA). Women were considered positive if the anti-TPO antibodies were greater than 35 IU/mL or if anti-TG antibodies were greater than 40 IU/mL. Samples were thawed overnight before assay, and first and second trimester samples from each woman were assayed within 24 hours of each other. Long-term coefficients of variation were 5.3%, 6.9% and 3.8% at TSH concentrations of 0.53, 4.5 and 21.9 mIU/L; 8.1%, 6.5% and 7.9% at free T4 concentrations of 0.9, 1.8 and 3.2ng/dL; 2.5%, 6.6% and 4.7% at anti-TG concentrations of 20, 35 and 556 IU/mL; and 4.9%, 7.5% and 5% at anti-TPO concentrations of 30, 39 and 546 IU/mL.
Analyses for all analytes were performed after logarithmic transformation. Differences in group means were compared using the t-test, and differences in variances were compared using the F-test. Significance was 2-tailed at the 0.05 level. Correlation analyses and graphics were done in GraphPad Prism version 4.03 (San Diego, CA). Remaining analyses were performed using SAS version 9.1 (Cary, NC).
Results
Table 1 lists selected centiles of first and second trimester TSH values in all 9,562 women. Table 1 also shows values in antibody-negative women and in subgroups of women with elevations of one or more antibodies. In the first trimester (top half of Table 1), the median TSH level is significantly higher (1.64 mIU/L) among the 1,211 women with elevated antibody levels than among the remaining 8,351 women (1.00 mIU/L), (p<0.0001). Among antibody positive women, the median TSH levels are least elevated in comparison to antibody negative women for those with thyroglobulin (TG) antibodies (1.43 mIU/L), higher with thyroid peroxidase (TPO) antibodies (1.75 mIU/L) and highest with both antibodies (1.94 mU/L) (p<0.0001). Similar relationships between antibody status and TSH levels are found in the second trimester (bottom half of Table 1).
For the entire cohort, the median TSH level is lower in the first trimester than in the second (1.05 vs 1.23 mIU/L, p<0.0001), and the 5th, 25th, and 75th centiles are also lower in the first trimester (0.13, 0.63, and 1.66 mIU/L) than in the second (0.36, 0.82, and 1.78 mIU/L). In contrast, the 98th centile is 4.15 mIU/L in the first trimester, and 3.77 mIU/L in the second trimester, indicating that the spread (variance) of measurements is tighter in the second trimester than the first. Figure 1 shows the centiles of TSH at each gestational week between the 11th and 18th weeks of gestation. When TSH measurements are converted to MoM, the 98th centile is 3.95 MoM in the first, and 3.06 MoM in the second, once again demonstrating the increased spread of values in the first trimester. A similar effect is found for subgroups of the cohort (e.g., antibody negative).
Figure 1. Selected TSH centiles for 9,562 women by gestational week in the first and second trimesters.
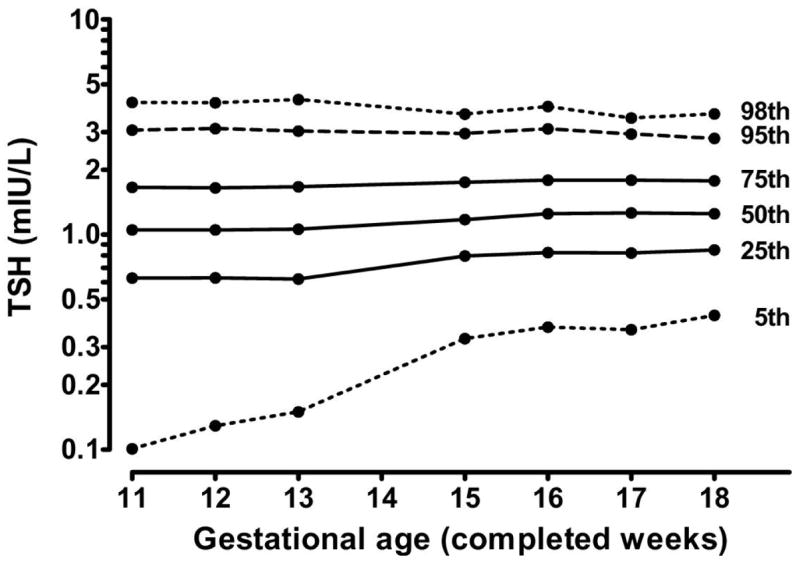
The variance (spread) of TSH values is greater at weeks 10 through 13 than at weeks 15 through 18, at both the lower and upper extremes of the distribution.
Figure 2, a scatter plot on logarithmic scales, shows within-woman measurements of TSH in the first and second trimesters. The overall correlation is high (r2=0.64), even with inclusion of the more extreme first trimester TSH values below 0.1 mIU/L. The extent of between-trimester correlation is of special interest for TSH values in the upper range, because a value above the 98th centile is one indication of thyroid deficiency. Figure 3 shows TSH values from 191 women whose values fall above the 98th centile during the first trimester. When those women are re-tested in the second trimester, 130 (68%) of the values remain above the 95th centile, and only four are below the median. Measurements from 7 women are above 10 mIU/L in both trimesters. Among the 191 women, seven (3.7%) were positive for TG antibodies only, 57 (29.8%) were positive for TPO antibodies only, and 61 (31.9%) were positive for both antibodies.
Figure 2. TSH measurements in 9,562 women who provided a sample in the first trimester (horizontal axis) and in the second trimester (vertical axis).
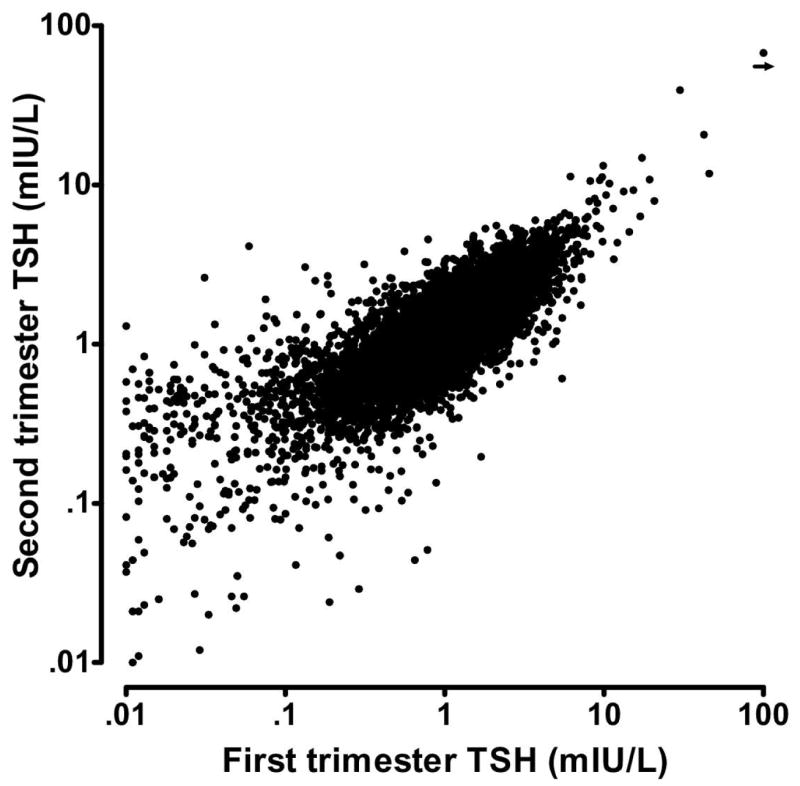
In this Figure, 75 paired samples were excluded, because at least one of the TSH measurements was below 0.01 mIU/L. The shown measurements are highly correlated (r2 = 0.64).
Figure 3. TSH measurements in 191 women with TSH values greater than the 98th centile in the first trimester.
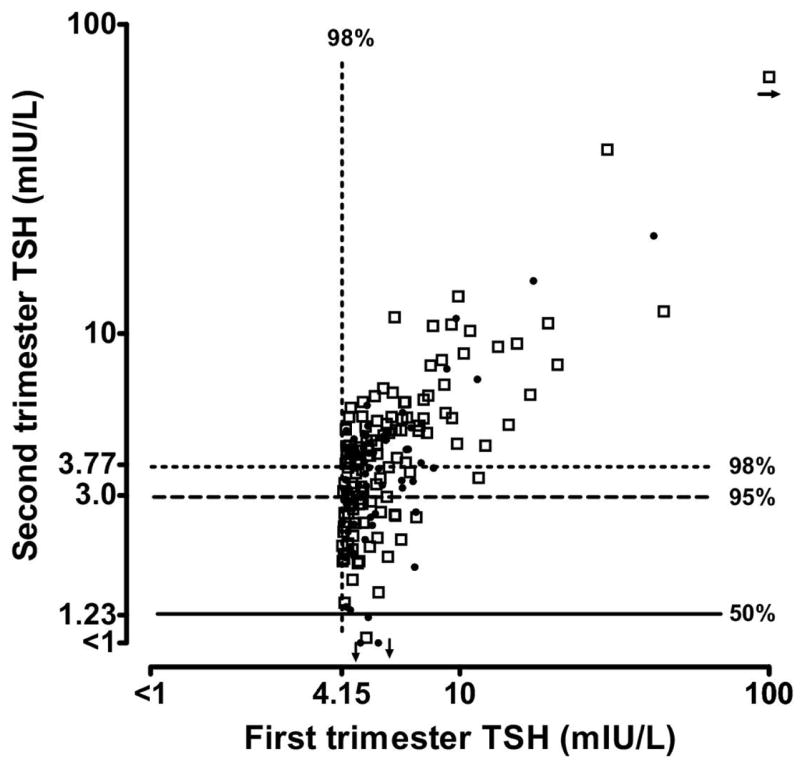
Overall, 130 (68%) of the values remain above the 95th centile, and only four are below the 50th centile, in the second trimester. Open squares indicate TSH values associated with positive antibodies.
Table 2 lists selected centiles of first and second trimester free T4 values for the entire cohort of 9,562 women and for the subgroups classified by antibody status. Free T4 values are higher in the first trimester than in the second at all centiles shown (p<0.0001). Figure 4 is a scatter plot on a linear scale that shows the correlation between first and second trimester free T4 values in these same women. The overall correlation is relatively weak (r2=0.23), even with the two outlying values removed. An initial free T4 value below the second centile is an indication of thyroid deficiency, and the extent of between-trimester correlation is again of special interest for values in this range. Figure 5, also on a linear scale, shows that free T4 measurements from 177 of the women are below the second centile during the first trimester. When these women are re-tested in the second trimester, 56 of the values (32%) remain below the 5th centile.
Figure 4. Free T4 measurements in 9,562 women who provided samples in both the first (horizontal axis) and second (vertical axis) trimesters.
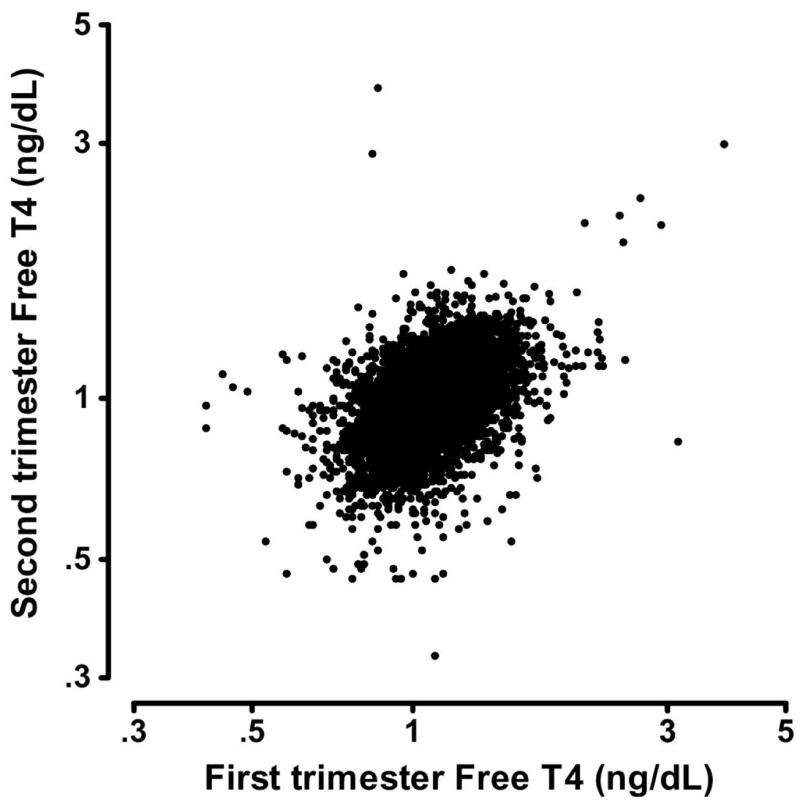
In this Figure, two paired samples were excluded because at least one of the free T4 measurements was below 0.3 or greater than 5 ng/dL. The shown measurements are less highly correlated (r2 = 0.23) than TSH.
Figure 5. Free T4 measurements in 177 women with free T4 values less than the 2nd centile in the first trimester.
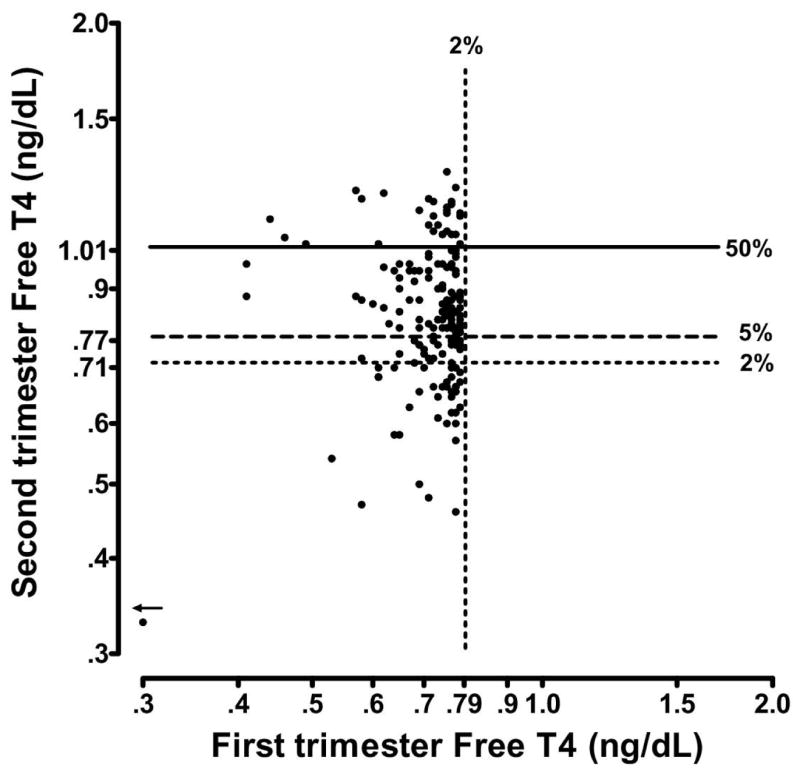
Overall, 56 of the values (32%) remain below the 5th centile, in the second trimester. In addition, 28 of the values (16%) fall above the median in the second trimester.
Figure 6 shows TPO antibody measurements in 933 women with elevations (>35 IU/mL) in either, or both, serum samples. In 14 women, the level is below the limit of sensitivity (<5 IU/mL) in one trimester, but elevated in the other. With these samples removed, the correlation is high (r2=0.79), but values in the second trimester are systematically lower (most observations fall below the line of identity). Results are similar (data not shown) for women with elevated TG antibody in one or both samples (r2=0.83 after trimming 32 observations). In both the first and second trimester, there is a statistically significant association (p < 0.0001) between elevated levels of TPO and TG antibody in individual women (r2=0.25 and r2=0.23, respectively) (data not shown).
Figure 6. Thyro-peroxidase (TPO) antibody measurements in women having at least one elevated result in the first or second trimester of pregnancy.
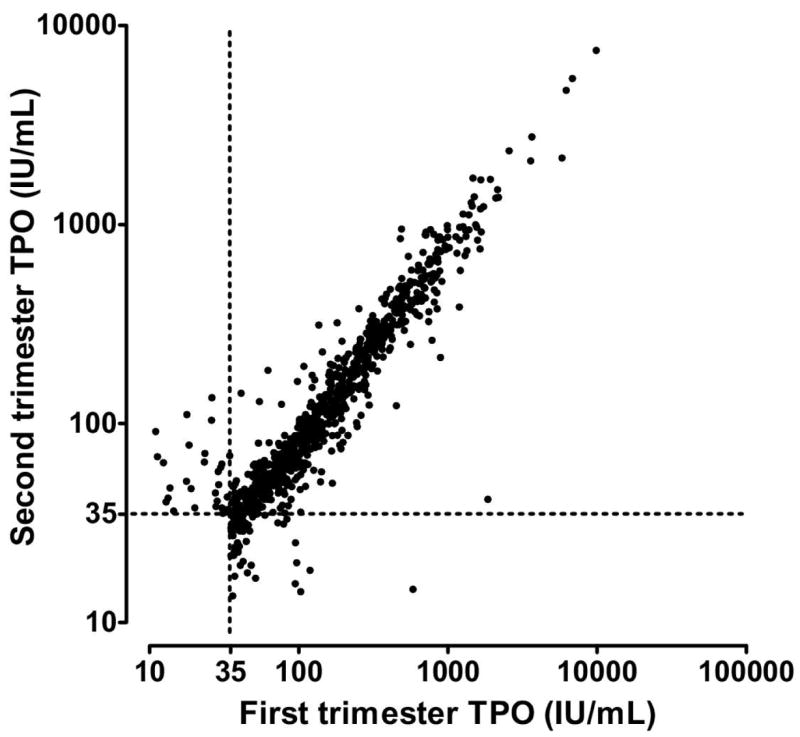
An elevated TPO antibody level (defined as 35 IU/mL or greater) in the first trimester was found in 933 women in the cohort (9.8%). The two TPO antibody measurements in this subgroup are highly correlated (r2=0.79, after logarithmic transformation and trimming of fourteen women having one measurement under 10 IU/mL).
Comment
The present study provides further documentation that TSH levels average lower and show greater variability in the first trimester than in the second. Within-woman correlations of TSH measurements between the two trimesters are moderately strong, however. Measurements obtained in the first trimester can, therefore, be interpreted meaningfully for clinical purposes, especially when elevated. Our data also support existing observations that free T4 levels average higher in the first trimester than in the second. Within-woman correlations for free T4 between the two trimesters are considerably less strong than for TSH. First and second trimester within-woman correlations of the two antibodies (TPO and TG) are very strong, with a downward trend in concentration. Once again, this supports previously published data.2
The main strengths of the present population-based study are its size (9,562 women), the availability of paired first and second trimester sera from all of the women and documentation of clinically diagnosed hypothyroidism (allowing those women to be removed from the reference population). A weakness is that observations are limited to the late first, and early second, trimesters. TSH and other thyroid-related testing were performed after completion of pregnancy, meaning that no clinical action could be taken during pregnancy on the basis of our measurements. However, it is not known how many women with hypothyroidism were diagnosed and treated as part of standard medical practice between the first and second trimesters. Figure 3 shows that all but one of the 16 women with first trimester TSH values above 10 mIU/L remain above the 98th centile in the second representing either undiagnosed or under-treated cases, and seven (44%) remain above 10 mIU/L.
TSH is the most reliable test for diagnosing primary hypothyroidism, but pregnancy presents a challenge for establishing reliable reference ranges and cut-offs.2, 3 One well known example is the temporary downward shift in TSH values in the first trimester, due to the surge in human chorionic gonadotropin (hCG) which exhibits a weak TSH-like effect.6 Normative data for six through 40 weeks’ gestation are catalogued for TSH in a large population-based study from Texas.3 In that study, cut-offs were successfully established for defining subclinical hypothyroidism, and the association between this condition and pregnancy outcome reported. This demonstrated that reliable clinical interpretations can be achieved during pregnancy, with appropriate attention to gestational age-specific normative data. It is especially important to have reference data available for the first trimester, because one objective of TSH testing is to identify and treat hypothyroidism as early in pregnancy as possible, preferably beginning with the first prenatal visit.
While gestational age trends in TSH medians are similar in the Texas study to those reported in a cohort of Maine pregnancies2, the actual values are systematically lower, even though the same commercially available assay kit was used. Such between-laboratory differences are not uncommon and often cannot be explained. Women in the Texas study were mostly Hispanic, while Maine women were nearly all non-Hispanic Caucasian. The present cohort, which is largely non-Hispanic Caucasian, yields measurements that are closer to Maine’s. The NHANES III study, however, shows little difference in median TSH values between these population groups among non-pregnant women.7 Based on these data, any laboratory providing services to pregnant women should establish and monitor TSH reference ranges to assure reliability.
Given that median TSH values vary somewhat between laboratories and that within-laboratory reference ranges differ with gestational age, expressing results in a way that is readily interpretable for clinical purposes is not straightforward. Dashe et al have proposed that TSH measurements be converted to MoM, as a way to unify expression of test results between laboratories.3 This approach is universally used by laboratories that provide prenatal screening services for Down syndrome and neural tube defects.8 Clinicians, therefore, are familiar with the terminology. One drawback to applying this method to TSH is that MoM cut-offs would be different in first versus second trimesters. Dashe et al find the 97.5th centile at 4.0 MoM in the first, and 2.5 MoM in the second.3 The current study finds the 98th centile at 3.95 MoM in the first trimester and 3.06 MoM in the second. Alternative approaches might be to express results either in centiles, or simply in mU/L, with trimester-specific, or even week-specific, cutoff levels specified.
The most common cause of hypothyroidism in the United States is autoimmune thyroiditis, a disorder that can be detected by measuring thyroid antibodies.9 The present study demonstrates that thyro-peroxidase (TPO) antibodies appear to have a more powerful effect than thyroglobulin (TG) antibodies, based on median TSH values in pregnant women with elevated levels of one antibody. When both antibody levels are elevated, the effect is greater than with either, alone. The recognized effect of pregnancy on lowering antibodies is also seen in comparing the number of antibody-positive women in the first trimester (1,211) with the number in the second (1,147). The current data support our earlier finding that TPO antibody measurements are highly correlated between trimesters2, but extend the finding to TG antibody measurements.
Free T4 values average higher in the first trimester than in the second, consistent with other published data.4,10 The within-person variability of free T4 between the first and second trimesters, however, has not previously been reported. For the entire cohort of 9,562 women in this portion of the FaSTER trial, the between-trimester correlation of free T4 is relatively weak (r2=0.23). Free T4 values at the low extreme of the population distribution are of clinical interest, because of their association with hypothyroidism, and the present study examines between-trimester variability for that subset, as well. Among women with free T4 values in the lowest two centiles in the first trimester, only 32% remain below the fifth centile in the second trimester. This contrasts with TSH values, where the between-trimester correlation is much higher (r2=0.64), and 68% of the values in the uppermost two centiles of the first trimester population distribution (also associated with hypothyroidism) remain above the 95th centile in the second trimester.
Just under one women per 600 in the present cohort (16 in 9,562) had first trimester TSH values of 10 mIU/L, or higher. Values in that range are consistent with overt hypothyroidism and, when identified, call for prompt treatment, aimed at normalizing the TSH level. Two earlier population-based cohorts also documented a similar frequency of TSH values in that range.11, 12 In view of recent documentation of limitations in the case-finding approach to identifying hypothyroidism during pregnancy, it appears timely to re-emphasize the need for establishing reliable reference ranges for thyroid-related tests to diagnose thyroid disease in pregnancy..13–16
Acknowledgments
Supported by Grant Number RO1 HD 38652 from the National Institutes of Health and the National Institute of Child Health and Human Development.
The authors acknowledge the work of the members of the FaSTER Research Consortium: K. Welch, MS, R. Denchy, MS (Columbia University, New York, NY) R. Ball, MD, M. Belfort, MD, B. Oshiro, MD, L. Cannon, BS, K. Nelson, BSN, C. Loucks, RNC, A. Yoshimura (University of Utah, and IHC Perinatal Centers, Salt Lake City, Provo, and Ogden, UT), D. Luthy, MD, S. Coe, MS (Swedish Medical Center, Seattle, WA) C. Comstock, MD, J. Esler, BS (William Beaumont Medical Center, Royal Oak, MI) R. Bukowski, MD, G. Hankins, MD, G. Saade, MD, J. Lee MS, (UTMB Galveston, TX) R. Berkowitz, MD, K. Eddleman, MD, Y. Kharbutli MS (Mount Sinai Medical Center, New York, NY) I. Merkatz, MD, S. Carter, MS (Montefiore Medical Center, Bronx, NY) L. Dugoff, MD, J. Hobbins, MD, L. Schultz, RN (University of Colorado Health Science Center, Denver, CO) I.. Timor-Tritsch, MD, M. Paidas, MD, J. Borsuk, MS (NYU Medical Center, New York, NY) S. Craigo, MD, D. Bianchi, MD, B. Isquith, MS, B. Berlin, MS (Tufts University, Boston, MA) S. Carr, MD, C. Duquette, RDMS (Brown University, Providence, RI) H. Wolfe, MD, R. Baughman, MS (University of North Carolina, Chapel Hill, NC) J. Hanson, MD, F. de la Cruz, MD (National Institute of Child Health and Human Development) K. Dukes, PhD, T. Tripp, MA, D. Emig, MPH, L. Sullivan, PhD (DM-STAT, Inc, Medford, MA).
Footnotes
Condensation: TSH, free T4, and antibody reference ranges differ between 1st and 2nd trimesters. Between-trimester correlations are strong for antibodies and moderate for TSH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smallridge RC, Glinoer D, Hollowell JG, Brent G. Thyroid function inside and outside of pregnancy: what do we know and what don’t we know? Thyroid. 2005;15:54–9. doi: 10.1089/thy.2005.15.54. [DOI] [PubMed] [Google Scholar]
- 2.Haddow JE, Knight GJ, Palomaki GE, McClain MR, Pulkkinen AJ. The reference range and within-person variability of thyroid stimulating hormone during the first and second trimesters of pregnancy. J Med Screen. 2004;11:170–4. doi: 10.1258/0969141042467340. [DOI] [PubMed] [Google Scholar]
- 3.Dashe JS, Casey BM, Wells CE, McIntire DD, Byrd EW, Leveno KJ, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. 2005;106:753–7. doi: 10.1097/01.AOG.0000175836.41390.73. [DOI] [PubMed] [Google Scholar]
- 4.Casey BM, Dashe JS, Spong CY, McIntire DD, Leveno KJ, Cunningham GF. Perinatal significance of isolated maternal hypothyroxinemia identified in the first half of pregnancy. Obstet Gynecol. 2007;109:1129–35. doi: 10.1097/01.AOG.0000262054.03531.24. [DOI] [PubMed] [Google Scholar]
- 5.Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N Engl J Med. 2005;353:2001–11. doi: 10.1056/NEJMoa043693. [DOI] [PubMed] [Google Scholar]
- 6.Glinoer D. The systematic screening and management of hypothyroidism and hyperthyroidism during pregnancy. TEM. 1998;9:403–411. doi: 10.1016/s1043-2760(98)00095-2. [DOI] [PubMed] [Google Scholar]
- 7.Haddow JE, McClain MR, Palomaki GE, Hollowell JG. Urine iodine measurements, creatinine adjustment, and thyroid deficiency in an adult United States population. J Clin Endocrinol Metab. 2007;92:1019–22. doi: 10.1210/jc.2006-2156. [DOI] [PubMed] [Google Scholar]
- 8.Wald NJ, Cuckle H, Brock JH, Peto R, Polani PE, Woodford FP. Maternal serum-alpha-fetoprotein measurement in antenatal screening for anencephaly and spina bifida in early pregnancy. Report of U.K. collaborative study on alpha-fetoprotein in relation to neural-tube defects. Lancet. 1977;1:1323–32. [PubMed] [Google Scholar]
- 9.Poppe K, Glinoer D. Thyroid autoimmunity and hypothyroidism before and during pregnancy. Hum Reprod Update. 2003;9:149–61. doi: 10.1093/humupd/dmg012. [DOI] [PubMed] [Google Scholar]
- 10.Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–90. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7:127–30. doi: 10.1136/jms.7.3.127. [DOI] [PubMed] [Google Scholar]
- 12.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya B, Bilous M, Hutchinson RS, Connolly V, Jones S, Kelly WF, et al. Screening for thyroid disease in pregnancy: an audit. Clin Med. 2002;2:599–600. doi: 10.7861/clinmedicine.2-6-599a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaidya B, Anthony S, Bilous M, Shields B, Drury J, Hutchison S, et al. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007;92:203–7. doi: 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- 15.Brent GA. Diagnosing thyroid dysfunction in pregnant women: Is case finding enough? J Clin Endocrinol Metab. 2007;92:39–41. doi: 10.1210/jc.2006-2461. [DOI] [PubMed] [Google Scholar]
- 16.Stagnaro-Green A. Can a high-risk case-finding approach identify all women with thyroid dysfunction during pregnancy? Nat Clin Pract Endocrinol Metab. 2007;3:216–7. doi: 10.1038/ncpendmet0421. [DOI] [PubMed] [Google Scholar]


