Abstract
Introduction
The transformation of smooth muscle cells (VSMCs) in the vessel wall to osteoblast like cells is known to precede arterial calcification which may cause bleeding complications. The vitamin K-dependent protein MGP has been identified as an inhibitor of this process by binding BMP-2, a growth factor known to trigger the transformation. In this study, we determined if the vitamin K-dependent Gla region in MGP by itself can inhibit the growth factor activity of BMP-2 and if menaquinone-4 (MK4) regulates gene expression in VSMCs.
Materials and Methods
A synthetic γ-carboxyglutamic acid (Gla) containing peptide covering the Gla region in human MGP was used to test its ability to inhibit BMP-2 induced transformation of mouse pro-myoblast C2C12 cells into osteoblasts. MK4 was tested by microarray analysis as a gene regulatory molecule in VSMCs.
Results and Conclusions
The results show that the Gla- but not the Glu-peptide inhibited the transformation which provide evidence that the Gla region in MGP is directly involved in the BMP-2/MGP interaction and emphasizes the importance of the vitamin K-dependent modification of MGP. From the data obtained from the microarray analysis, we focused on two quantitatively altered cDNAs representing proteins known to be associated with vessel wall calcification. DT-diaphorase of the vitamin K-cycle, showed increased gene expression with a 4.8-fold higher specific activity in MK4 treated cells. Osteoprotegrin gene expression was down regulated and osteoprotegrin protein secretion from the MK4 treated cells was lowered 1.8-fold. These findings suggest that MK4 acts as an anti-calcification component in the vessel wall.
Keywords: Vitamin K, Arterial calcification, Matrix Gla Protein (MGP), MK4 is an abbreviation for vitamin K4 of the vitamin K2 compounds
Monkeberg’s sclerosis is a non-inflammatory induced form of vascular calcification seen in the tunica media of the arterial vessel. The tunica media is built by layers of vascular smooth muscle cells (VSMCs) separated by elastic internal laminas (1). This medial calcification pathology is commonly observed in aging people and patients with diabetes and end-stage renal disease (ESRD) (2) and may lead to letal thrombotic events (3). Extensive research on the cellular and molecular mechanisms leading to arterial calcification has provided strong support for the idea that the mechanisms are similar to the mechanisms underlying bone formation (4). Several proteins suspected to be involved have been identified (1–5). However the findings that 1) deletion of the gene for the vitamin K-dependent protein matrix Gla protein (MGP) (6) and 2) prevention of vitamin K to work as a cofactor for production of Gla containing proteins in the vessel wall (7) caused massive calcification of the arterial system in rodents led to massive thrombosis and death (6), suggested, for the first time, that vitamin K is an important factor in prevention of arterial calcification.
As a vitamin K-dependent protein, four glutamic acid (Glu) residues in newly synthesized precursors of MGP are post translationally converted to γ-carboxyglutamic acid (Gla), Ca++ binding residues by the γ-carboxylation system in the endoplasmic reticulum (ER). The system requires reduced vitamin K as cofactor (8).
In early experiments aimed at understanding the mechanism by which MGP works as a calcification inhibitor, our laboratory (9) identified by ligand blotting, and Zebboudj et al. (10) by transfection experiments, that MGP binds BMP-2, a growth factor known for osteoblastic differentiation. Indication that such transformations could take place in the aortic wall has been provided by Watson et al. (11) who showed that a subpopulation of cultured VSMCs were transformed to calcifying vascular cells (CVCs) capable of forming a mineralized matrix. We have shown (12) that BMP-2 and MGP are both synthesized by VSMCs and Murshed et al. (13) have shown that MGP synthesized in the vessel wall and not blood born MGP is the in vivo pool of the protein that is active as the vascular calcification inhibitor. These findings emphasize the importance to understand the MGP-BMP-2 interaction in inhibition of vascular calcification.
Since vessel wall synthesized MGP is a vitamin K-dependent protein, knowledge about vitamin K metabolism in VSMCs is important. It is now confirmed by different research groups that the essential nutritional form of vitamin K, vitamin K1, derived from plants is, to different extents, converted to menaquinone-4 (MK4) in various extrahepatic tissues (14–16). In the brain the conversion is almost 100% (17) and Spronk et al. (16) have shown a significant conversion in the aortic wall. In feeding studies Spronk et al. (16) showed that MK4 but not vitamin K1 could prevent arterial calcification in rats when given in combination with warfarin. Certain combinations of vitamin K and warfarin given to rodents have been shown to inhibit vitamin K-dependent γ-carboxylation in extrahepatic tissues but not in the liver which maintains a normal coagulation system (18). As reduced vitamin K1 and reduced MK4 are both cofactors in the γ-carboxylation system, this finding has raised questions about additional effects of MK4 in the arterial wall which may enhance inhibition of arterial calcification.
MK4 has been shown to effect gene expression of osteoblastic bone markers by binding to the steroid and xenobiotic receptor (SXR) [17]. This observation triggered our curiosity to find out if MK4 could affect gene expression in VSMCs which could result in altered concentrations of known calcification inhibitory proteins in the vessel wall to levels that would enhance inhibition of calcification. In this paper, we present data which strengthen this hypothesis. We also present data which show that the Gla region in MGP is active in neutralization of the growth factor activity of BMP-2.
MATERIALS AND METHODS
Materials
The synthetic peptides VQGlaRIRGlaRSKPVHGlaL (Gla-peptide) and VQERIRERSKPVHEL (Glu-peptide) covering the 35–49 amino acid sequence of human MGP were reagents provided by the Cardiovascular Research Institute of Maastricht, Department of Biochemistry, University of Maastricht, The Netherlands. Gla is the symbol used for gamma-carboxyglutamic acid. Human recombinant bone morphogenetic protein-2 (BMP-2) and human recombinant noggin were from R&D Systems, Minneapolis, MN. Osteoprotegrin polyclonal antibodies were from Santa Cruz Biotechnology, Santa Cruz, CA. The mass spectrometry compatible silver stain kit, DODECA Silver Stain, and CRITERION 8–16% SDS-PAGE gels were from BIO-RAD, Richmond, Ca. Vitamin K2 (menaquinone-4; MK4) was from Sigma, St. Louis, MO. Cell medium containing charcoal treated fetal bovine serum (CDMEM) were prepared at the Cell culture core facility by Wake Forest University School of Medicine, Winston-Salem, NC.
Cell culture assay on BMP-2’s osteoinductive growth factor activity
Mouse pro-myoblast C2C12 cells (ATCC, Manassas, VA.) were grown in DMEM medium with 10% fetal bovine serum (FBS). Cells were split and plated, in complete medium, in 96 well containing plates at a density of 3 × 104 cells per well. After 24 hours the cells were washed three times with DPBS to remove any loosely and dead cells. Medium with 10% charcoal treated FBS (CDMEM) were added to adherent cells in the wells and stimulated with either 5 nM of BMP-2 or with 5nM of BMP-2 containing either 500 µM or 750 µM of the Gla- or Glu-peptide respectively. In some of the wells, adherent cells were incubated with 5 nM of BMP-2 + 43 nM of noggin. After these additions, cells in each well were grown for an additional 48 hours in 200 µl of CDMEM. At this point in time the medium was removed and the cells were washed 3 times with DPBS before they were lysed in 100 µl of 0.1 M of glycine buffer, pH 9.6 containing 1% NP-40, 1 mM MgCl2 and 1 mM ZnCl2. After lysis, 100 µl of a 1 mg/ml solution of the alkaline phosphatase (ALP) substrate p-nitrophenylphosphate was added to each well and the plates incubated for 15 minutes at 37°C. The developed color intensity was measured at 405 nm in a plate reader. One unit of ALP activity was defined as 1.0 optical density (OD) change measured per min at 405 nm per 3 × 104 cells as reported by Kirsch et al. (19).
Cell culture of rat aortic vascular smooth muscle cells
Vascular smooth muscle cells (VSMCs) were isolated from the thoracic aorta by the procedure of explant culture described by Freeman and al. (20). The cells were grown in DMEM containing 10% FBS.
Treatment of VSMCs with menaquinone-4 (MK4) and isolation of mRNA for Affimatrix gene chip array analysis
Rat aortic VSMCs were grown in DMEM with 10 % FBS. At 70% confluency, cells were washed with DPBS and continued to be cultured in CDMEM. Washed cells in CDMEM were treated either with 25 µM MK4 or ethanol (control; the solvent for stock MK4). After 24 hours, the MK4 and ethanol containing media were replaced with identical fresh CDMEM and the cells were allowed to grow for another 24 hours. At this time the cells were washed with DPBS and total RNA isolated using the QIAGEN maxiprep RNA kit according to the instructions provided by the company. Total RNA was resuspended in RNA free water, and precipitated overnight at −20 °C in a 1:2 part mixture of 3M Na acetate and 100% ethanol. The RNA precipitate was centrifuged at 1,500 × g for 30 min at 4 °C. Pellets were washed 3 times with 70% ethanol, dried in the hood and resuspended in RNAse free water. RNA purity was checked by electrophoresis in 1% TBE agarose gels and by capillary chromatography. Differential gene expression was carried out by the Wake Forest University Affimatrix Core Facility on The Rat Genome 230 2.0 Array Gene Chips purchased from AFFIMATRIX, Santa Clara, Ca.
Treatment of VSMCs with menaquinone-4 (MK4) and isolation of the cellular proteome for2D-SDS-PAGE and mass spectrometry analysis
Rat aortic VSMCs were grown in CDMEM and treated with MK4 and ethanol as described above for preparation of RNA samples for Affimatrix gene array analysis. At the end of the incubation period, cells were harvested and washed in DPBS. MK4 treated and ethanol treated (control) cells were lysed in RIPA buffer and solubilized proteins prepared for isoelectric focusing (IEF) by acetone, TCA and ether/ethanol washes before IEF as described by our laboratory (12). The second dimension 2D-SDS-PAGE for IEF and one-dimentional SDS-PAGE were carried out as described (12) using 8–16% gradient CRITERION gels. Gels were silver stained with the DODECA Silver Stain kit and proteins spots selected for MS/MS analyses by their differential staining intensity in the two gels excised from the gel and analyzed by the Proteomic Core Laboratory at Virginia Bioinformatics Institute, Blacksburg, VA.
Treatment of VSMCs with menaquinone-4 (MK4) and isolation of secreted proteins
Rat aortic VSMCs were grown in CDMEM and treated with MK4 and ethanol as described above for 24 hours with 10% of the charcoal treated serum containing medium. The cells were washed and continued growing for an additional 16 hours in serum free media containing 25 µM MK4 and ethanol (control) respectively. Medium from each culture was collected and concentrated to equal volumes in a Vivaspin concentrator with a 3 kDa cut off. Aliquots representing total protein present in each concentrate were subjected to SDS-PAGE and Western blotting (12).
Quantitative imaging of Westen blots
Digitized images of immunoreactive protein bands on FUJI Medical X-Ray Film SuperRX (Fisher Scientific, Pittsburg, PA) were analyzed with Kodak 1D software (Eastman Kodak, Rochester, NY) to determine the integrated areas representing the protein bands. The density of the images were in the linear range of plots of protein concentration versus integrated image density.
DT-diaphorase activity
DT-diaphorase activity was measured by the standard assay using dichlorophenol indophenol (DCPIP) as the electron acceptor. The activity that could be inhibited with 100 µM dicumarol was used to represent the activity.
RESULTS
In previous studies the intact BMP-2 and MGP proteins have been used to demonstrate a binding interaction between the two proteins (10). To specifically investigate the role the Gla region in MGP plays in neutralizing the BMP-2 growth factor activity, we studied the effect our synthetic Gla-peptide, had on BMP-2 induced differentiation of the myoblast C2C12 cells into osteoblast like cells (21). Fig.1 shows the morphological changes the C2C12 cells underwent, when they were exposed to BMP-2. Expression of the osteoblastic ALP marker activity by the transformed cells is also shown. We used this BMP-2 transformation to determine if the Gla-peptide would interfere with the ability of BMP-2 to trigger the transformation. Fig.2, shows the results when C2C12 cells were incubated with BMP-2 in the presence of the Gla-peptide and the corresponding Glu-peptide respectively. We also included as a positive control incubations with noggin, a known potent inhibitor of BMP-2 (22). Because of it poor solubility, fully γ-carboxylated MGP could not be used as a positive control. As shown, C2C12 cells exposed to 5 nM of BMP-2 acquired significant ALP activity (open bar). The presence of 500 µM of the Glu peptide did not affect BMP-2’s growth factor activity as determined by the ALP activity expressed by the transformed cells (black bar), but 500 µM of the Gla-peptide significantly inhibited the growth factor activity triggered with 5 nM BMP-2 (crosshatched bar) and this inhibition was increased when 750 µM of the Gla-peptide was added (Diagonal lines bar)which indicated a dose response effect. Noggin, at 43nM, had a significant neutralizing effect on the growth factor activity of BMP-2 at 5 nM (vertical line shaped bar). These results show that the Gla region in MGP is involved in neutralization of the growth factor activity of BMP-2.
Figure 1.
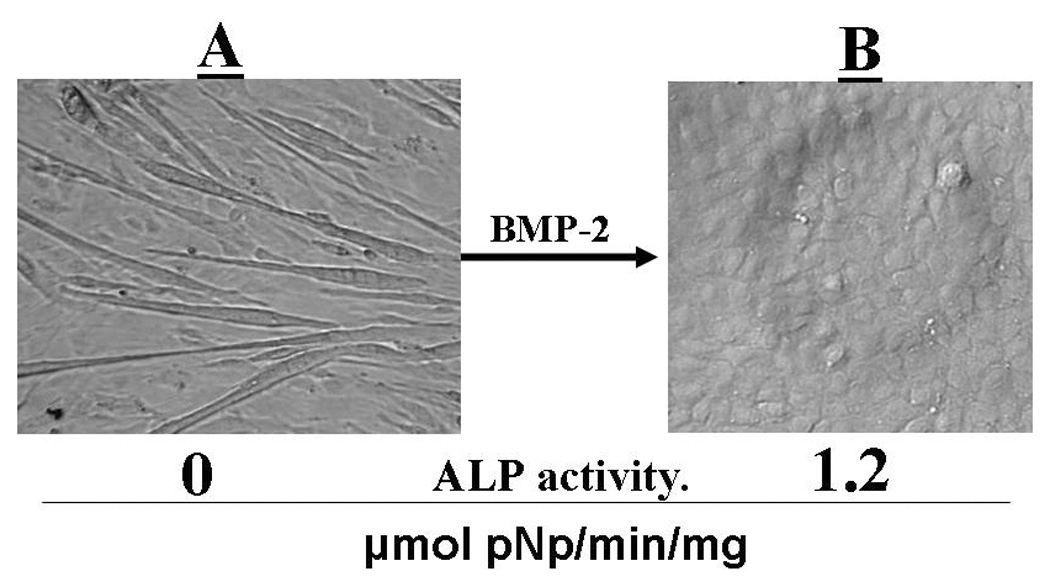
BMP-2 transformation of pro-myoblast C2C12 cells into osteoblasts. C2C12 cells were cultured for 48 hours in CDMEM medium (see MATERIALS AND METHODS) containing 5 nM of recombinant BMP-2. Panels A and B show images of adherent cells at times 0 and 48 hours respectively. Cells were tested for ALP activity as described in MATERIALS AND METHODS
Figure 2.
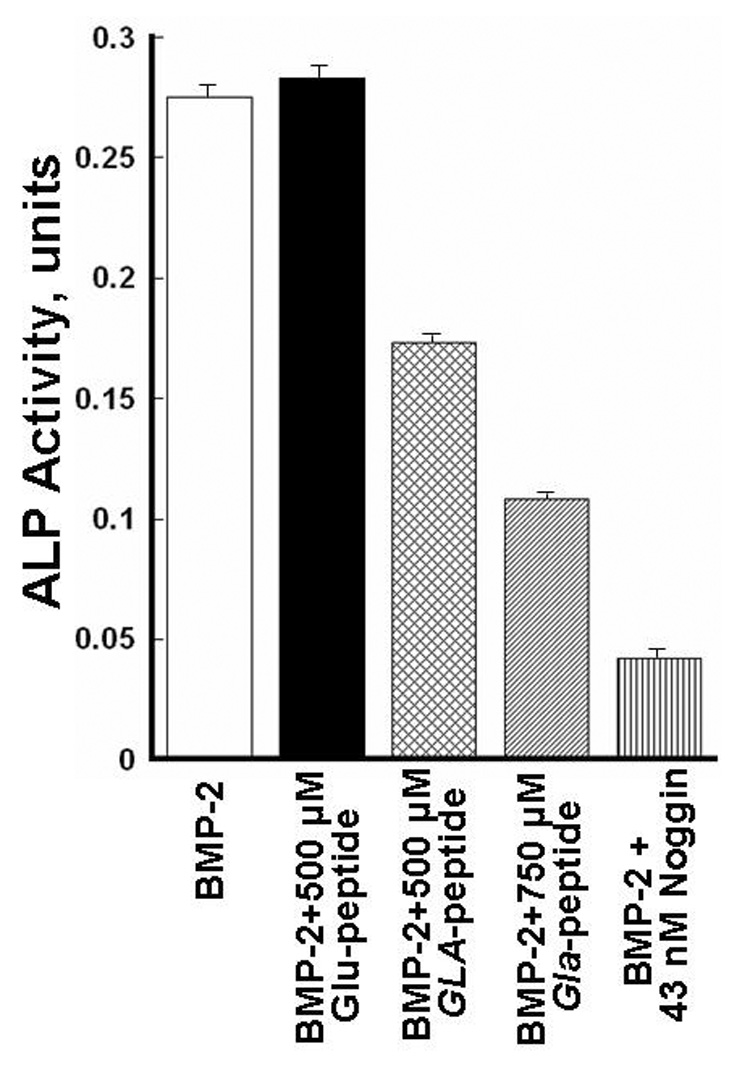
Gla-peptide inhibition of BMP-2 induced transformation of pro-myoblast C2C12 cells into osteoblasts. Cells were plated in 96 well containing plates and cultured in CDMEM as described in MATERIALS AND METHODS for 48 hours in the presence of 5 nM of BMP-2 (open bar), 5 nM of BMP-2 + 0.5 mM Glu-peptide (black bar), 5 nM of BMP-2 + 0.5 mM Gla-peptide (cross hatched bar), 5 nM BMP-2 + 750 µM Gla-peptide (diagonal lines bar) and 5 nM BMP-2 + 43 nM of noggin respectively. ALP activities in the various wells measured as units (see MATERIALS AND METHODS) are shown for the various 48 hours cultured cells. Standard deviations are indicated as lines on the top of each bar in the graph. No ALP activity could be measured in pro-myoblasts and a P-value could not be given for the transformed cells.
The next set of experiments focused on MK4 and its potential as a regulator of gene expression in cultured VSMCs. Messenger-RNA from control cells and cells incubated with MK4 for 48 hours were isolated and exposed to microarray analysis on Rat Genome 230 2.0 Array Gene Chips representing approximately 29,700 well substantiated rat genes. From the extensive set of data of unknown and known genes obtained, we found gene expression of two proteins known to be involved in calcification biology and biosynthesis of vitamin K-dependent proteins to be altered by MK4. These genes were osteoprotegrin and DT-diaphorase. Since our objective was to search for known proteins involved in arterial calcification we focused on the effects osteoprotegrin and DT-diaphorase could have on arterial calcification. MK4 treatment decreased osteoprotegrin gene expression >2-fold and increased DT-diaphorase gene expression >2-fold. Increased expression of DT-diaphorase would result in increased vitamin K reducing capacity of the vitamin K-cycle (22) which indicate increased synthesis of functional γ-carboxylated MGP. Since osteoprotegrin is a decoy receptor protein for RANKL (1,24), decreased expression of osteoprotegrin could result in increased osteoclastic activity in the vessel wall.
To confirm the microarray analysis data on DT-diaphorase and osteoprotegrin gene expression at the proteome level, we measured cellular DT-diaphorase activity and carried out Western blotting of osteoprotegrin secreted from the MK4 treated and control VSMCs. As shown in Fig. 3, panel A, specific DT-diaphorase activity had increased 4.2-fold in MK4 treated VSMCs. Western blotting showed 1.8-fold less osteoprotegrin secreted from the vitamin MK4 treated cells (panel B). Thus, the microarray analysis data were consistent with the data obtained at the proteome level. To image the proteomes of VSMCs treated and not treated with MK4 we carried out 2-D-SDS-PAGE of proteins harvested from the two cell populations by RIPA buffer extraction. The images of the two captured proteomes are shown in Fig.4 as silver stained 2-D-SDS-PAGE gels. Visual inspection of the two gels clearly identified differences in protein concentration of some of the proteins present in the two gels. The proteins spots labeled by arrows were significantly more stained in the MK4 gel than the same spots seen in the control gel. By visual inspection, differences in staining intensity between most of the other spots seen on the two gels were difficult to identify. We did not have to our disposition a computerized 2-D-SDS-PAGE analysis system which however was not needed to accomplish the objectives with this work. However, the most differently stained protein spot seen on the two gels (broken arrow) was subjected to MS/MS and conclusively identified as tropomyosin 4. Thus the MS/MS analysis identified increased synthesis of tropomyosin 4 when the cultured VSMCs were treated with MK4. A difference in tropomyosin 4 gene expression was not reported by the microarray analysis.
Figure 3.
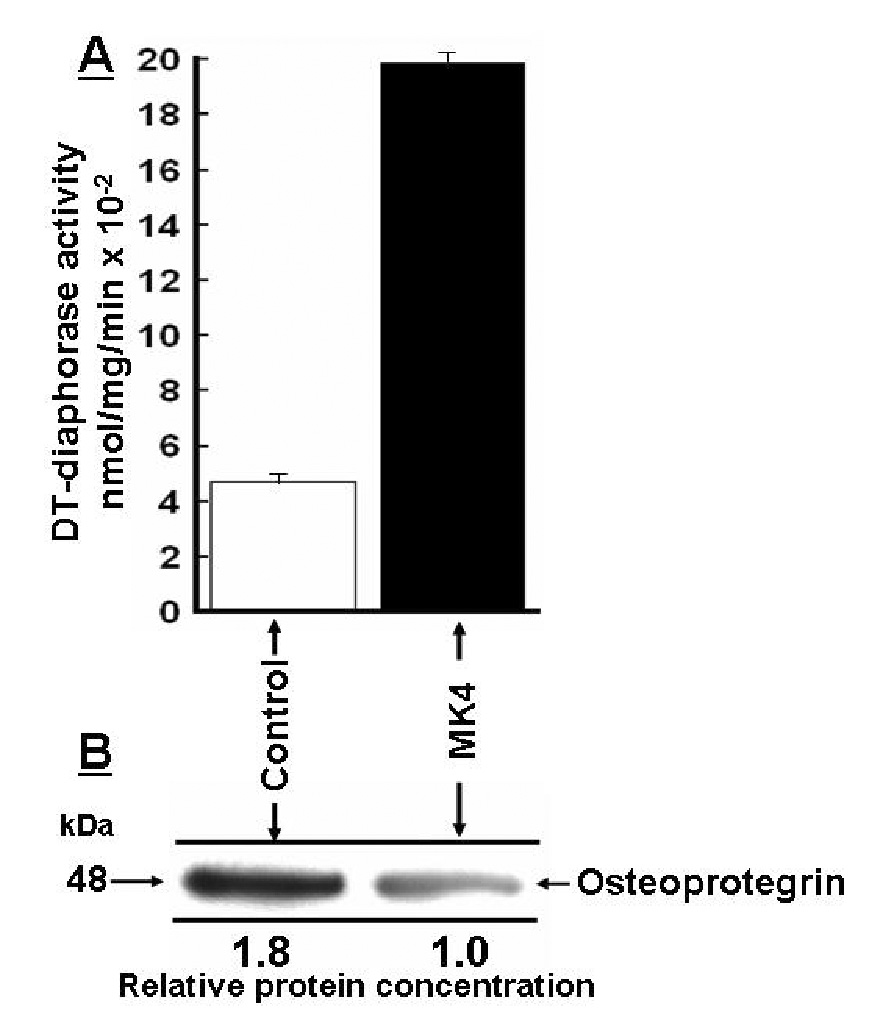
Menaquinone-4 (MK4) altered gene expression of DT-diaphorase and osteoprotegrin detected at the proteome level. Rat aortic vascular smooth muscle cells (VSMCs) were cultured as described in MATERIALS AND METHODS for 24 hours in medium containing 1) 10% CDMEM + 25 µM MK4 (MK4) or 2) 10% CDMEM + no additions (control). After 24 hours the cells were continued growing for 6 hours in serum free medium containing 1) 25 µM MK4 and 2) no additions (control). DT-diaphorase activity was measured in the adherent cells as dicumarol sensitive DCPIP reducing activity (panel A). Osteoprotegrin secreted from control and MK4 treated cells are shown in panel B as Western blots which also represent total secreted osteoprotegrin (see MATERIALS AND METHODS). Standard deviations estimated for the measured DT-diaphorase activities are indicated on the top of the bars (n=3). The P-value was P<0.0001
Figure 4.
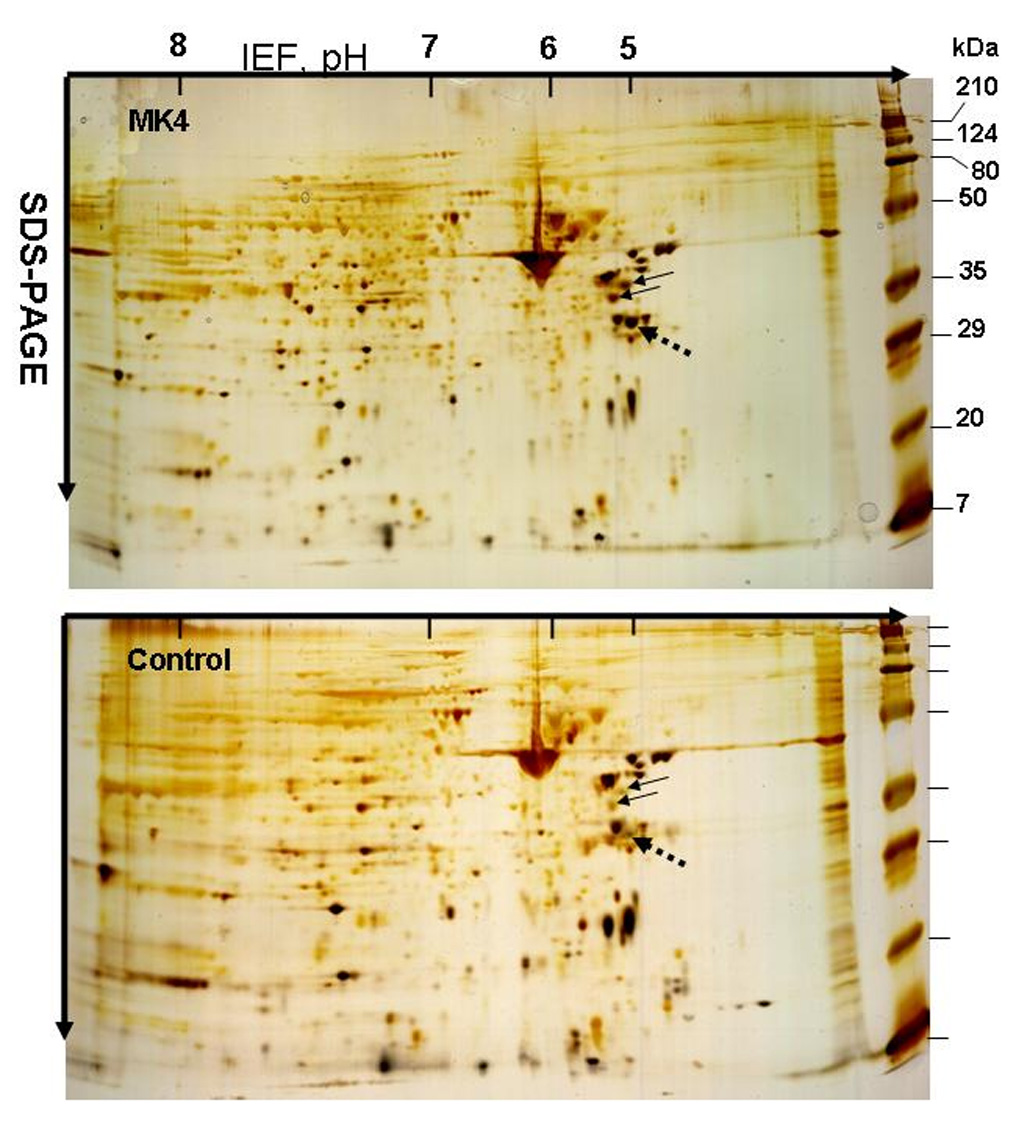
Display by 2D-SDS-PAGE of the proteomes of rat aortic smooth muscle cells (VSMCs) treated and not treated with MK4. Rat aortic VSMCs were cultured for 48 hours as described in MATERIALS AND METHODS in medium containing 1) CDMEM + 25 µM MK4 (MK4) or 2) CDMEM + no additions (control). MK4 and control cells were extracted with RIPA buffer and solubilized cell proteins prepared for 2D-SDS-PAGE as described in MATERIALS AND METHODS. The MK4 and Control gels were silver stained. Proteins labels by arrows were identified to be significantly more stained in the gel containing proteins from the MK4 treated cells. The spot labeled by the broken arrow in the MK4 gel was excised and subjected to MS/MS analysis. The protein was identified as tropomyosin 4.
DISCUSSION
This work provide data which support the growing notion that vitamin K and vitamin K-dependent proteins are important in prevention of arterial calcification (5). The data also support the widely accepted hypothesis that VSMCs in the vessel wall play a dominating role in vessel wall calcification pathology and prevention (1). In the studies reported in this work, VSMCs isolated from explants of the aorta were used. Previously we have shown that these proliferating cells maintain their phenotype as VSMCs (25).
The formation in the vessel wall of osteoblast and chondrocyte like cells have been well documented (26). MGP, as a Gla modified functional protein, must play an important role as a regulatory protein in transformation of the cells because deletion of the MGP gene or γ-carboxylation of the protein cause extensive calcification of arteries and cartilage (6). In order to strengthen the hypothesis that the Gla region in MGP indeed is involved in BMP-2 binding and inhibition of the growth factor activity of BMP-2 we have in this work investigated the ability of the Gla region in MGP to inhibit the transforming growth factor activity of BMP-2. The synthetic Gla-peptide used to represent the Gla region has been shown previously to undedrgo the Ca++ induced conformational change characteristic of fully γ-carboxylated vitamin K-dependent proteins (9) which justifies its use in our assays.
As all previously published data on the ability of MGP to inhibit the growth factor activity of BMP-2 has been obtained from experiments where the genes for BMP-2 and MGP have been overexpressed by transfection (10), the γ-carboxylation status of the synthesized MGP protein pool was not known. We (27) and others (28) have shown that overexpression of vitamin K-dependent proteins exceeds the capacity of a cell’s vitamin K-dependent γ-carboxylation system to fully γ-carboxylate the total pool of precursors of recombinant vitamin K-dependent proteins in the ER. Thus all previous experiments with the recombinant proteins have investigated BMP-2 binding to a mixture of differently γ-carboxylated forms of MGP. Our experiments with the Gla-peptide eliminates the uncertainty of the presence of none- and under-γ-carboxylated forms of MGP in the experiments. The results conclusively demonstrate that the Glu peptide had no noticeable effect on BMP-2’s growth factor activity. On the other hand, the Gla-peptide clearly had an inhibitory effect on BMP-2’s growth factor activity. These data support the hypothesis that the Gla region in MGP indeed is involved in BMP-2 binding and regulation of the ostergenic growth factor activity of BMP-2. The data also add support to our earlier finding that overexpression of non γ-carboxylated MGP in calcified lesions in the aorta of aging rats results in unopposed BMP-2 activity which may contribute to osteoblastic differenation of cells laying down the observed calcified matrix (29). BMP-2 at 5 nM was used in all experiments and significantly higher concentrations of the Gla- and the Glu-MGP peptides were needed to measure significant inhibitory effects. The data on the involvement of the Gla- peptide residues in BMP-2 binding and inhibition are conclusive but can not exclude the possibility that the intact MGP protein may be involved in enhancing BMP-2 growth factor inhibition. This could mimic the strong inhibitory effect noggin has on BMP-2/BMP-4 as reported by Zimmerman et al. (30) Exposing cultured VSMCs to MK4 clearly had an effect on expression of genes representing a variety of proteins. Among the MK4 affected genes representing proteins known to affect arterial calcification [1] we found the genes of DT-diaphorase and osteoprotegrin to be up and down regulated respectively. The altered expression of these two proteins could contribute to inhibition of arterial calcification. The increase in cellular activity of the warfarin insensitive, vitamin K reducing enzyme DT-diaphorase adds support to the observation by Spronk et al.(16) that MK4 and warfarin, but not vitamin K1 and warfarin, prevented calcification of the arterial wall. An additional contributing factor could be that the γ-carboxylase cofactor MK4 is a better substrate for DT-diaphorase than vitamin K1. It is well known that the smallest form of the vitamin K family ,menadione, is an excellent substrate for DT-diaphorase (31).
The finding that MK4 reduced VSMC synthesis of osteoprotegrin, the RANKL decoy receptor protein, indicate generation of an additional mechanism involved in inhibition of arterial wall calcification. Reduced osteoprotegrin production in the vessel wall would increase the free RANKL concentration and potentially increase osteoclastic activity. Osteoclast like cells have been identified in calcified human aortic plaques (32) and Tintut et al. (33) have demonstrated osteoclast formation by arterial wall produced cell differentiation factors. Together, our findings suggest that MK4 could inhibit vessel wall calcification by increasing DT-diaphorase activity and enhancing osteoclastic activity resulting from reduced osteoprotegrin synthesis. Osteoprotegrin (−/−) mice have been shown to have increased osteoporosis and increased arterial calcification (34). In these mice the extreme onset of calcium phosphate metabolism may override the capacity of the vessel wall to prevent calcification.
Our data from the MK4 gene expression studies are supportive of the current use of MK4 in medicine. MK4 has been shown conclusively to reduce osteoporosis and has been used for this purpose in Japan for several years (35). MK4 intake has also been reported in a population study by Geleijnse et al. (36) to reduce aortic calcification. In support of these clinical data is the demonstration by Spronk et al. (16) of MK4 being an effective inhibitor of arterial calcification in the rat.
As shown by our proteomic 2D-SDS-PAGE analyses (Fig.4) of MK4 and control treated VSMCs, a protein spot, significantly more stained in the MK4 treated cells, was identified as tropomyosin 4 by MS/MS. Tropomyosin 4 has been shown to be a marker for the transformation of contractive VSMCs to a synthetic phenotype (37). Our finding with cultured VSMCs indicate that MK4 accelerates this transformation. The physiological consequences of MK4 induced acceleration of this transformation are currently unknown. The mass spectrometry data were conclusive but the change in gene expression of tropomyosin 4 was not detected by the Affimatrix gene chip analysis. Based on company information, we confirmed that the thropomyosin 4 cDNA was present among the 29,7000 rat genes. The difference in the results may indicate that gene array analyses may miss detection of genes or the result could reflect a difference in the concentration of tropomyosin 4 at the proteome level which did not correlate with gene expression data. Inconsistency between measured mRNA levels and cellular protein levels is not an uncommon finding.
ACKNOWLEDGEMENTS
This work was supported was supported by grant RO1HL69331 to Reidar Wallin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abedin M, Tintut Y, Demer LL. Vascular Calcification: Mechanisms and Clinical Ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 2.Giachelly CM, Speer MY, Li X, Rajachar RM, Yang H. Regulation of Vascular Calcification. Circ Res. 2005;96:717–722. doi: 10.1161/01.RES.0000161997.24797.c0. [DOI] [PubMed] [Google Scholar]
- 3.Taylor AJ, Burke AP, O’Malley PG, Farb A, Malcom GT, Smialek J, Virmani R. A Comparison of the Framingham Risk Index, Coronary Artery Calcification, and Culpit Plaque Morphology in Sudden Cardiac Death. Circulation. 2000;101:1234–1248. doi: 10.1161/01.cir.101.11.1243. [DOI] [PubMed] [Google Scholar]
- 4.Wallin R, Wajih N, Greenwood GT, Sane DC. Arterial Calcification: A Review of Mechanisms, Animal Models, and the Prospects for Therapy. Med Res Rev. 2001;21:274–301. doi: 10.1002/med.1010. [DOI] [PubMed] [Google Scholar]
- 5.Shanahan M, Proudfoot D, Farzaneh-Far A, Weisberg PL. The Role of Gla Proteins in Vascular Calcification. Crit Rev Eukar Gene Exp. 1998;8:357–375. doi: 10.1615/critreveukargeneexpr.v8.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 6.Luo G, Ducy P, McKee MD, Pinero GJ, Behringer RR, Karsenty G. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 7.Wallin R, Hutson SM. Warfarin and the vitamin K-dependent gamma-carboxylation system. Trends Mol Med. 2004;10:299–302. doi: 10.1016/j.molmed.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–1407. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 9.Wallin R, Cain D, Hutson SM, Loeser R. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2) Thromb Haemostas. 2000;84:1039–1044. [PubMed] [Google Scholar]
- 10.Zebboudj AF, Imura M, Bostrom K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem. 2002;277:4388–4394. doi: 10.1074/jbc.M109683200. [DOI] [PubMed] [Google Scholar]
- 11.Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-ß 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wajih N, Borras T, Xue W, Hutson SM, Wallin R. Processing and transport of matrix gamma-carboxyglutamic acid protein and bone morphogenetic protein-2 in cultured human vascular smooth muscle cells: evidence for an uptake mechanism for serum fetuin. J Biol Chem. 2004;279:43052–43060. doi: 10.1074/jbc.M407180200. [DOI] [PubMed] [Google Scholar]
- 13.Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thijssen HHW, Drittij-Reijnders MJ. Vitamin K distribution in rat tissues: tissue specific accumulation of phylloquinone and menaquinone-4. Br J Nutr. 1994;72:415–425. doi: 10.1079/bjn19940043. [DOI] [PubMed] [Google Scholar]
- 15.Davidson RT, Foley Al, Engelke JA, Suttie JW. Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent upon bacteria. J Nutr. 1998;128:220–223. doi: 10.1093/jn/128.2.220. [DOI] [PubMed] [Google Scholar]
- 16.Spronk HMH, Soute BAM, Schurgers LJ, Thjissen HHW, De Mey JGR, Vermeer C. Tissue-Specific Utilization of Menaquinone-4 Results in the Prevention of Arterial Calcification in Warfarin-Treated Rats. J Vasc Res. 2003;40:531–537. doi: 10.1159/000075344. [DOI] [PubMed] [Google Scholar]
- 17.Carrie I, Portoukalian J, Vicaretti R, Rochford J, Potvin S, Ferland G. Menaquinone-4 Concentration is Correlated with Sphingolipid Concentrations in Rat Brain. J Nutr. 2004;134:167–172. doi: 10.1093/jn/134.1.167. [DOI] [PubMed] [Google Scholar]
- 18.Tabb MM, Sun A, Zhou C, Grün F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B. Vitamin K2 Regulation of Bone Homeostasis Is Mediated by the Steroid and Xenobiotic Receptor SXR. J Biol Chem. 2003;278:43919–43927. doi: 10.1074/jbc.M303136200. [DOI] [PubMed] [Google Scholar]
- 19.Kirsch T, Nickel J, Sebald W. BMP-2 antagonists emerge from alterations in the low-affinity binding epitope for receptor BMPR-II. EMBO J. 2000;19:3314–3324. doi: 10.1093/emboj/19.13.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman EJ, Chisholm GM, Ferrario CM, Tallant EA. Angiotensin-1-7 inhibits vascular smooth muscle cell growth. Hypertension. 1996;28:104–108. doi: 10.1161/01.hyp.28.1.104. [DOI] [PubMed] [Google Scholar]
- 21.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosem V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone Morphogenetic Protein-2 Converts the Differenation Pathway of C2C12 Myoblasts into the Osteoblast Linage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fakhry A, Ratosoontorn C, Vedhachalam C, Salhab I, Koyama E, Leboy P, Pacifici M, Kirschner RE, Nah HD. Effects of FGF-2/-9 in calvarial bone cell cultures: differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone. 2005;36:254–266. doi: 10.1016/j.bone.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Wallin R, Martin LF. Warfarin poisoning and vitamin K antagonism in rat and human liver. Design of a system in vitro that mimics the situation in vivo. Biochem J. 1987;241:389–396. doi: 10.1042/bj2410389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan M, Shi X, Feng X, Cao X. Transcriptional mechanisms of bone morphogenetic protein-induced osteoprotegrin gene expression. J Biol Chem. 2001;267:10119–10125. doi: 10.1074/jbc.M006918200. [DOI] [PubMed] [Google Scholar]
- 25.Wallin R, Cain D, Sane DC. Matrix Gla protein synthesis and gamma-carboxylation in the aortic vessel wall and proliferating vascular smooth muscle cells—a cell system which resembles the system in bone cells. Thromb Haemost. 1999;82:1764–1767. [PubMed] [Google Scholar]
- 26.Tyson KL, Reynolds JL, McNair R, Zang Q, Weissberg PL, Shanahan CM. Osteo/Chondrocytic Transcription Factors and Their Target Genes Exhibit Distinct Patterns of Expression in Human Arterial Calcificaton. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 27.Wajih N, Sane DC, Hutson SM, Wallin R. Engineering of a recombinant vitamin K-dependent gamma-carboxylation system with enhanced gamma-carboxyglutamic acid forming capacity: evidence for a functional CXXC redox center in the system. J Biol Chem. 2005;280:10540–105. doi: 10.1074/jbc.M413982200. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman RJ, Wasley LC, Furie BC, Furie B, Shoemaker CB. Expression, purification, and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cells. J Biol Chem. 1986;261:9622–9628. [PubMed] [Google Scholar]
- 29.Sweatt A, Sane SC, Hutson SM, Wallin R. Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats. J Thromb Haemost. 2003;1:178–185. doi: 10.1046/j.1538-7836.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman LB, De Jesus-Escobar JM, Hartland M. The Spemann Organizer Signal noggin Binds and Inactivates Bone Morphogenetic Protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 31.Lind C, Segura-Aguilar JE. Isolation and Characterization of DT Diaphorase Isozymes from Rat Liver. Chemica Scripta. 1987;27A:37–41. [Google Scholar]
- 32.Hofbauer LC, Shui C, Riggs BL, Dunstan CR, Spelsberg TC, O’Brien T, Khosla S. Effects of immunosupperssants on receptor activator of NF-kappaB ligand and osteoprotegrin production by human osteoblastic and coronary artery smooth muscle cells. Biochem Biophys Res Commun. 2001;280:334–339. doi: 10.1006/bbrc.2000.4130. [DOI] [PubMed] [Google Scholar]
- 33.Tintut Y, Abedin M, Cho J, Choe A, Lim J, Demer LL. Regulation of RANKL-induced osteoclastic differentiation by vascular cells. J Mol Cell Cardiol. 2005;39:389–393. doi: 10.1016/j.yjmcc.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Cappareli, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegrin-deficient mice develop early onset osteoporosis and arterial calcification. Genes and Develop. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15:515–521. doi: 10.1359/jbmr.2000.15.3.515. [DOI] [PubMed] [Google Scholar]
- 36.Geleijnse JM, Vermeer C, Schurgers LJ, Grobbee DE, Pols HAP, Witteman JCM. Inverse association of dietary vitamin K-2 intake with cardiac events and aortic atherosclerosis: The Rotterdam Study. Thromb Haemostas. 2001 Suppl July:P473. [Google Scholar]
- 37.Abouhamed M, Reichenberg S, Robenek H, Plenz G. Tropomyosin 4 expression is enhanced in dedifferentiating smooth muscle cells in vitro and during atherogenesis. Eur J Cell Biol. 2003;82:473–482. doi: 10.1078/0171-9335-00333. [DOI] [PubMed] [Google Scholar]


