Abstract
Background
The lateral hypothalamic neuropeptide, orexin (or hypocretin), is implicated in drug addiction. While a role for orexin has been shown in reward and dependence, the molecular and neural mechanisms are unclear. Here, we investigated the mechanism and neuroanatomical basis of orexin’s role in morphine withdrawal.
Methods
C57BL/6J mice received chronic morphine followed by naloxone (0 or 1 mg/kg, s.c.) to precipitate withdrawal. Prior to naloxone, mice received SB-334867 (0 or 20 mg/kg, i.p.), an orexin 1 receptor (Ox1r) antagonist. Using immunohistochemistry, c-Fos, a marker of cell activation, was quantified in the nucleus accumbens (Acb), lateral hypothalamus (LH), ventral tegmental area (VTA), and locus coeruleus (LC). Retrograde tracing with fluorogold (FG) was performed to determine whether orexin neurons project directly to the Acb.
Results
SB-334867 prior to naloxone significantly attenuated withdrawal symptoms. Withdrawal was accompanied by an increase in c-Fos expression in the Acb shell (AcbSh), which was reduced by SB-334867, but had no effect on the VTA or the LC. Morphine withdrawal increased c-Fos expression in the dorsomedial (DMH) and perifornical (PFA) regions, but not in the lateral region of the LH (LLH). Orexin neurons do not appear to form direct connections with the Acb neurons.
Conclusions
Altogether, these data demonstrate that orexin, acting via Ox1r, is critical for the expression of morphine withdrawal. AcbSh activation during withdrawal is dependent on Ox1r function and is likely mediated by indirect action of LH orexin neurons.
INTRODUCTION
Many models of drug addiction include both positive and negative reinforcement as key components. Continued drug use is partly a result of positive reinforcement from rewarding effects of drug taking, and negative reinforcement from withdrawal that accompany cessation of drug taking. The mesocorticolimbic dopamine (DA) system, originating in the ventral tegmental area (VTA) and projecting to terminal regions such as the nucleus accumbens (Acb), amygdala, and prefrontal cortex, has been identified as an essential neural network in which drug-induced neuroadaptations occur that lead to both types of reinforcement. The reinforcing effects of drugs of abuse are associated with increased dopaminergic neurotransmission (1,2) in the Acb (3-6). During cocaine self-administration, animals will lever-press to maintain elevated DA levels (7). In contrast, decreased accumbal DA levels have been associated with morphine withdrawal (8-10).
Drug abstinence results in somatic (“physical”) withdrawal as well as motivational (“psychological”) withdrawal. Some evidence suggests that these components of withdrawal are mediated by distinct neural systems. In morphine dependent animals, opiate antagonists in the locus coeruleus (LC) (11-14) and the periaqueductal gray (13,15) precipitate robust somatic withdrawal syndromes whereas infusions into the Acb generates only a few somatic symptoms (13). Administration of opiate antagonists directly into the Acb and amygdala in morphine dependent animals results in motivational withdrawal as indicated by the attenuation of lever pressing for food (16) and conditioned place aversion (17). However, other experimental data suggest some overlap among these neural systems exists. For instance, direct administration of opioid antagonists in the amygdala of morphine-dependent animals is associated with moderate somatic withdrawal (13). Furthermore, systemic DA agonist administration attenuates both conditioned place aversions and somatic withdrawal symptoms in morphine-dependent animals treated with naloxone, while increasing phosphorylation of GluR1 in the Acb (18), indirectly implicating the Acb in both withdrawal components. Altogether, these findings suggest a role for limbic structures in both somatic and motivational withdrawal.
The lateral hypothalamus (LH) has also been shown to be involved in reinforcement and neuroadaptations in response to drugs of abuse. Evidence for the role of the LH in positive reinforcement comes from the work of Olds and Milner (19), which showed that animals will robustly lever-press for LH electrical self-stimulation. Furthermore, animals will self-administer opiates, such as morphine, directly into the LH (20,21) and administration of an opioid antagonist directly into the LH blocks systemic heroin self-administration (22). Similarly, opioid administration directly into the LH results in a conditioned place preference (CPP) effect (23). We have previously presented evidence demonstrating a role for the LH in negative reinforcement by implicating orexin (also called hypocretin), a hypothalamic neuropeptide, in morphine dependence and withdrawal (24).
Orexin-containing neurons are restricted to a few regions of the LH - the lateral region of the lateral hypothalamus (LLH), perifornical area (PFA), and dorsomedial hypothamalus (DMH) (25-27) - and have been shown to project broadly throughout the brain (26,28,29). The orexin ligands, orexin A and orexin B, arise from the precursor peptide prepro-orexin by proteolytic processing (27,29) and activate the two G-protein coupled receptors, orexin 1 receptor (Ox1r) and orexin 2 receptor (Ox2r) (27). Interestingly, the orexin receptors and orexinergic projections from the hypothalamus are localized in regions previously shown to play a role in drug addiction, such as the VTA (26,30-32), Acb, substantia nigra (26), and LC (33,34). Orexin neurons located in the LH innervate several regions along the trajectory of the mesocorticolimbic pathway (26,32). Ox1r mRNA is colocalized with the selective DA neuronal marker tyrosine hydroxylase (TH) in the VTA and in vitro activation of orexin receptors activates dopaminergic VTA neurons leading to excitation (34), whereas activation of orexin receptors in the Acb leads to inhibition (35). In the LC, orexin has been shown to excite neurons primarily via Ox1r activation (36).
Early studies suggest a role of orexin in sleep regulation as orexin knockout mice are narcoleptic and loss of orexin neurons leads to narcolepsy (25,37-39). Further behavioral experiments have expanded beyond sleep regulation to orexin’s role in general arousal, feeding, and metabolism (40,41) and suggest a possible role of orexin in drug and natural reinforcement. For instance, animals that express conditioned place preference (CPP) in response to morphine, cocaine, or food express increased c-Fos activation in orexin neurons of the LH, and blockade of orexin receptors attenuates the CPP effect in response to the above stimuli (42). Systemic administration of orexin A leads to increased reinstatement of extinguished lever-pressing for cocaine (43) and blockade of the Ox1r at the level of the VTA significantly blocked the development, but not the expression, of cocaine sensitization (44). By using orexin knockout mice, our laboratory has previously shown that orexin neurons respond to morphine withdrawal and that orexin mutant mice demonstrate attenuated somatic morphine withdrawal symptoms (24).
Although it is clear that orexin is important in components of drug addiction, little is known about the neural circuits and mechanisms by which orexin mediates drug dependence. In the present study, pharmacological Ox1r blockade, c-Fos analysis, and retrograde tracing techniques are used to establish a role for Ox1r in naloxone-precipitated somatic morphine withdrawal.
MATERIALS AND METHODS
Subjects
Subjects were 72 male C57BL/6J mice, obtained from Jackson Laboratories, between 8-12 weeks of age, prior to the onset of the experiments. Mice were housed in groups of five and maintained on a 12:12 hour light:dark cycle with ad libitum access to food and water except during the morphine withdrawal observation session.
Morphine Treatment and Withdrawal
For chronic morphine treatments, mice (n=36) were injected with escalating morphine doses (20, 40, 60, 80, 100 and 100 mg/kg, i.p.) every 8 hours for 2.5 days. To assess morphine withdrawal, each mouse was injected with naloxone (0 or 1 mg/kg, s.c.) two hours after the last morphine injection. Mice were removed from their home cage and placed in a standard clear plastic mouse home cage without the bedding, which served as the observation chamber. Withdrawal sessions took place in the early phase of the light, or inactive, cycle; naloxone injections were administrated four hours into the light cycle. Prior to naloxone treatments, the presence of Straub tail was noted to confirm morphine dependence (45). The occurrence of each withdrawal symptom (jumping, paw tremors, gnawing, head swoops, tremors, wet dog shakes, ptosis, and backward walking) was counted by an investigator blind to treatment conditions for 20 minutes following naloxone treatment. To evaluate the severity of withdrawal, a global withdrawal score was computed by multiplying the sum total obtained for each sign by a constant and adding the scores for each sign.
The constant assigned to each symptom, designed to reflect the severity and occurrence of a particular symptom, was adapted from previously published global score calculations for both rats (46) and mice (47,48). Jumps and paw tremors were multiplied by 0.1; gnawing, ptosis, and head swoops were multiplied by 0.5; tremors, wet dog shakes, and backward walking were multiplied by 1.0. To assess the effects of orexin antagonism in the expression of morphine withdrawal, mice received an injection of SB-334867 (0 or 20 mg/kg, i.p.), an Ox1r antagonist, 20 minutes prior to naloxone injections and global withdrawal scores were calculated.
Immunohistochemical Studies
Please see Supplemental Methods.
Retrograde Tracing
All surgeries were performed under aseptic conditions, using sodium pentobarbital for anesthesia. Fluorogold (FG) injections (0.05 μl of 2% FG in 0.9% saline) were made bilaterally using a 5 μl Hamilton microsyringe (Hamilton, Reno, NV). The coordinates for Acb injections were: 1.2 mm rostral to bregma, ±0.5 mm from midline, and 3.5 mm below the surface of the dura matter. Mice were perfused seven days post injections and the brains were removed and immunostained for orexin or TH following the same immunohistochemical procedures described above.
Drugs
Morphine sulfate (provided by the National Institute on Drug Abuse) and naloxone (Sigma, St. Louis, MO) were dissolved in saline. SB-334867 (1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea hydrochloride) was synthesized by GlaxoSmithKline (Harlow, UK) and dissolved in 10% (w/v) (2-hyroxypropyl)-β-cyclodextrin in sterile water. The dose of SB-334867 is consistent with other recent studies (42,50).
Data Analysis
To assess the role of orexin antagonism on the expression of morphine withdrawal, the results were expressed as mean ± SEM of global withdrawal scores. To quantify colocalization of orexin with c-Fos in the LH and TH with c-Fos in the VTA and LC, results wereexpressed as mean ± SEM of percentage of c-Fos positive orexin or TH cells. Multiple two-way ANOVAs were conducted with dose of SB-334867 and dose of naloxone as between-subject factors.
RESULTS
Ox1r blockade attenuates the expression of morphine withdrawal
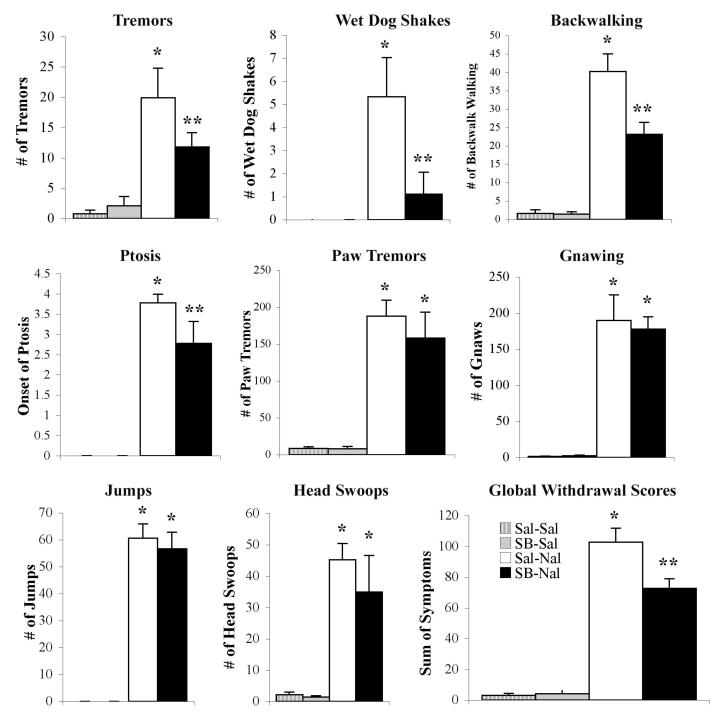
To investigate the role of Ox1r in the expression of morphine withdrawal, mice chronically injected with morphine received naloxone to precipitate withdrawal. Precipitation of withdrawal by naloxone was accompanied by somatic behavioral signs of withdrawal. Animals pretreated with SB-334867 (20 mg/kg, i.p.) prior to naloxone-precipitated withdrawal demonstrated a striking reduction in several withdrawal symptoms, including tremors, wetdog shakes, backward walking, and ptosis and global withdrawal scores were significantly lower than animals pretreated with saline (Fig. 1). A two-way ANOVA withdose of SB-334867 and dose of naloxone as factors revealed a significant main effect of naloxone (F(1, 33) = 485.35, P<0.0001) and a significant SB-334867 by naloxone interaction (F(1, 33) = 16.72, P<0.0001).
Figure 1.
Naloxone precipitated morphine withdrawal in animals chronically treated with morphine is attenuated following pretreatment with an Ox1r antagonist. Animals pretreated with SB-334867 prior to naloxone (black bar, n=9) are compared with those pretreated with saline prior to naloxone (white bar, n=9) and with animals pretreated with SB-334867 (gray bar, n=9) or saline (white striped bar, n=9) prior to a saline control injection for the noted signs or the global withdrawal score. Vertical lines represent the standard error of the mean (SEM). * represents a significant naloxone effect (P<0.05). ** represents a significant naloxone interaction with SB-334867 (P<0.05).
LH activation accompanies naloxone-precipitated withdrawal
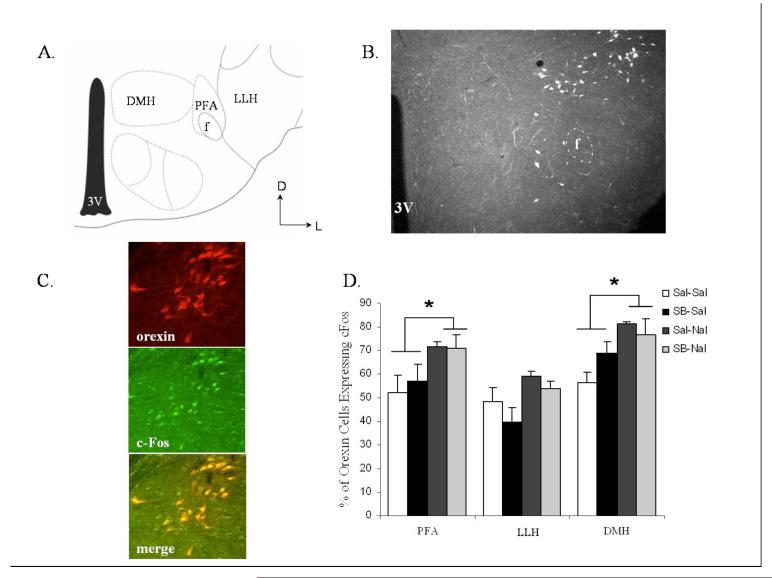
To characterize better the role of orexin neurons in response to morphine withdrawal, c-Fos analysis was completed following naloxone-precipitated morphine withdrawal. The LH was further subdivided into the dorsomedial hypothalamus (DMH), perifornical area (PFA), and lateral area of the lateral hypothalamus (LLH) (Fig. 2A-B), and responses were evaluated following opiate withdrawal with or without treatment with the Ox1r antagonist SB-334867 (0 or 20 mg/kg, i.p.). Precipitation of withdrawal by naloxone was accompanied by increases in c-Fos expression in orexin neurons (Fig. 2C) in the PFA and DMH regions, whereas no significant increase was found in the LLH (Fig. 2D, Table 1A). A mixed-factorial ANOVA with anatomical region as the repeated measures factor and dose of SB-334867 and dose of naloxone as between-subjects factors revealed a significant region main effect (F(2, 24) = 80.42, P<0.0001), a significant naloxone main effect (F(1, 12) = 12.99, P<0.004), but not a significant SB-334867 main effect nor a significant interaction. Tests of simple main effects of naloxone dose at each location revealed a significant naloxone effect in the PFA (F(1, 12) = 5.20, P<0.05) and in the DMH (F(1, 12) = 5.06, P<0.05), but not in the LLH. These results suggest that medial regions of the LH are more responsive to morphine withdrawal and that local Ox1r signaling is not critical for this response.
Figure 2.
Naloxone-induced morphine withdrawal results in activation of orexin cells in the DMH and PFA regions of the LH, but not those in the LLH. SB-334867 failed to affect c-Fos expression. (A) Schematic anatomical representation of LH subdivisions adapted from Paxinos and Franklin’s (49) Stereotaxic Atlas. Labeled areas delineate regions where c-Fos expression was examined. (B) Representative photograph illustrating orexin cell expression in the LH. (C) Microscopy showing double-label immunohistochemistry for c-Fos and orexin in the LH. (D) Regional expression of c-Fos in orexin positive cells. Vertical lines represent the standard error of the mean (SEM). Abbreviations used: LH, lateral hypothalamus; PFA, perifornical area; LLH, lateral region of the lateral hypothalamus; DMH, dorsal medial hypothalamus; 3V, third ventricle; f, fornix. * represents a significant naloxone effect (P<0.05).
Table 1.
Cell counts for immunohistochemical quantification are indicated. (A) The average number of counted orexin-positive cells, c-Fos positive cells, and the percentage of TH-positive cells double-labeled with c-Fos in the LH. (B) The average number of counted TH-positive cells, c-Fos positive cells, and the percentage of TH-positive cells double-labeled with c-Fos in the VTA and LC and the average number of c-Fos positive cells in the Acb. Values in parenthesis represent the standard error of the mean (SEM). Abbreviations used: LH, lateral hypothalamus; PFA, perifornical area; LLH, lateral region of the lateral hypothalamus; DMH, dorsal medial hypothalamus; Orx, orexin; Fos, c-Fos; TH, tyrosine hydroxylase; VTA, ventral tegmental area; LC, locus coeruleus; Acb, nucleus accumbens; AcbC, nucleus accumbens core; AcbSh, nucleus accumbens shell.
A. Orexin and c-Fos double-labeling in the LH
B TH and c-Fos double-labeling in the VTA and LC; c-Fos single-labeling in the NAc
| PFA | LLH | DMH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Fos+ | Orx+ | Percentage+ | Fos+ | Orx+ | Percentage+ | Fos+ | Orx+ | Percentage+ |
| Sal-Sal | 75.8(20.3) | 122.5(19.7) | 52.0(7.7) | 135.5(60.2) | 231.0(82.7) | 48.3(5.8) | 108.0(36.4) | 164.5(46.2) | 56.1(4.5) |
| SB-Sal | 86.3(18.7) | 148.0(18.9) | 56.9(7.3) | 117.0(23.5) | 296.0(45.8) | 39.5(6.4) | 158.0(14.8) | 230.6(16.2) | 68.7(5.1) |
| Sal-Nal | 95.0(11.6) | 132.3(15.7) | 71.7(2.0) | 164.3(21.9) | 277.2(34.6) | 59.2(2.1) | 132.3(11.8) | 163.6(15.5) | 81.1(1.1) |
| SB-Nal | 77.5(22.2) | 111.7(29.8) | 70.6(6.0) | 114.2(31.6) | 209.3(46.7) | 53.6(3.6) | 108.5(9.30) | 145.2(17.3) | 76.6(6.6) |
| VTA | LC | NAc Core | NAc Shell | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Fos+ | TH+ | Percentage+ | Fos+ | TH+ | Percentage+ | Fos+ | Fos+ |
| Sal-Sal | 184.0(86.1) | 686.7(76.7) | 28.9(13.9) | 294.0(42.4) | 357.0(46.9) | 81.7(3.3) | 874.8(136.4) | 840.6(69.5) |
| SB-Sal | 105.0(32.5) | 464.0(196.2) | 33.7(14.2) | 297.0(30.7) | 369.0(8.5) | 80.2(6.6) | 934.6(52.6) | 924.2(59.9) |
| Sal-Nal | 260.0(118.9) | 771.3(58.1) | 31.9(12.3) | 284.7(27.4) | 353.2(30.0) | 80.4(2.3) | 1530.8(193.5) | 2321.8(170.0) |
| SB-Nal | 147.0(81.6) | 598.0(129.5) | 22.69(11.7) | 192.0(32.4) | 277.2(56.2) | 74.5(2.5) | 1677.8(139.6) | 1675.3(93.8) |
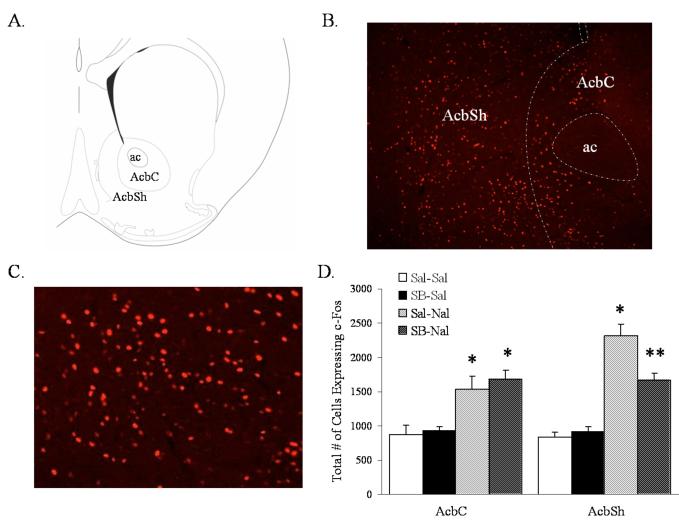
Activation of the AcbSh, but not of the VTA or LC correlates with degree of morphine withdrawal
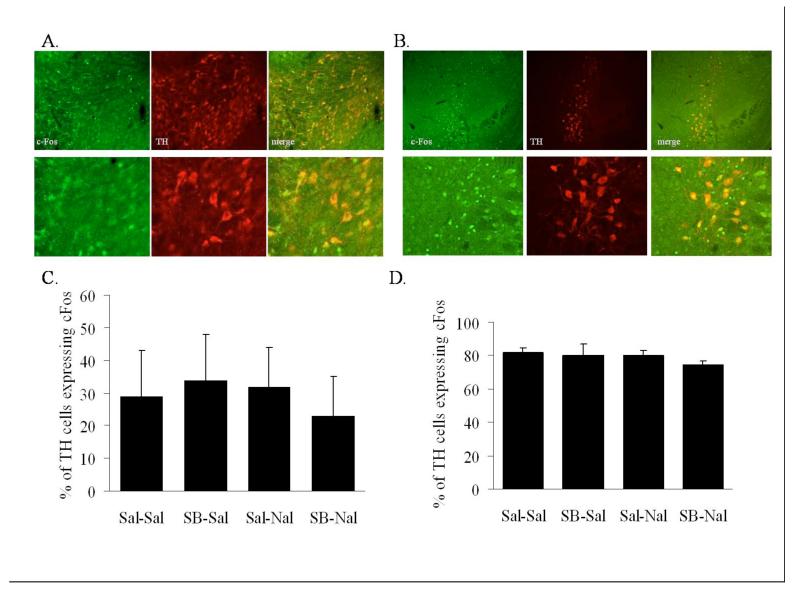
Blockade of Ox1r with SB-334867 resulted in attenuated withdrawal symptoms but did not alter c-Fos expression in the LH. Orexin neurons have extensive extra-LH targets that may mediate the effects of orexin neuron activation in morphine withdrawal. To better understand the neuroanatomical basis of orexin in morphine withdrawal, we evaluated molecular responses to naloxone-precipitated morphine withdrawal in the VTA, Acb, and LC, following the blockade of Ox1r via SB-334867 administration. As a marker of cell activation, the immediate early gene c-Fos was assessed in TH-positive cells in the VTA (Fig. 3A), TH-positive cells in the LC (Fig. 3B) and in cells in the medial core and dorsal shell regions of the Acb (Fig. 4A-B).
Figure 3.
Naloxone-induced morphine withdrawal fails to affect the activity of TH-containing neurons in the VTA and LC. (A) Microscopy showing double-label immunohistochemistry for c-Fos and TH in the VTA. Bottom series of panels are higher magnification from same animal. (B) Microscopy showing double-label immunohistochemistry for c-Fos and TH in the LC. Bottom series of panels are higher magnification from same animal. (C) Quantification of c-Fos expression in TH-positive cells in the VTA. (D) Quantification of c-Fos expression in TH-positive cells in the LC. Vertical lines represent the standard error of the mean (SEM).
Figure 4.
Naloxone-induced morphine withdrawal results in the activation of Acb cells. SB-334867 attenuates naloxone-induced morphine withdrawal induction of c-Fos expression in the AcbSh. (A) Schematic anatomical representation of Acb subdivisions adapted from Paxinos and Franklin’s (49) Stereotaxic Atlas. Labeled areas delineate regions where c-Fos expression was examined. (B) Representative photograph illustrating c-Fos expression in the Acb of an animal pretreated with saline-naloxone. (C) Representative photograph of c-Fos expression in the AcbSh/AcbC boundary in higher magnification from the same animal presented in A. (D) Quantification of cells expressing c-Fos in the AcbC and AcbSh. Vertical lines represent the standard error of the mean (SEM). Abbreviations used: AcbC, nucleus accumbens core; AcbSh, nucleus accumbens shell; ac, anterior commisure. * represents a significant naloxone effect (P<0.05). ** represents a significant naloxone interaction with SB-334867 (P<0.05).
Examination of c-Fos expression in the VTA failed to reveal a response to naloxone-precipitated withdrawal or an effect of Ox1r blockade (Fig. 3C). Similarly, naloxone-precipitated withdrawal and/or Ox1r antagonism had no effect on c-Fos expression in the LC (Fig. 3D). However, precipitation of withdrawal by naloxone was accompanied by an increased number of c-Fos expressing neurons in the Acb (Table 1B). Interestingly, c-Fos expression in the AcbSh, but not the AcbC, was significantly reduced in animals pretreated with SB-334867 (Fig. 4C). c-Fos expression analysis in the AcbC using a two-way ANOVA with dose of SB-334867 and dose of naloxone as factors revealed a significant main effect of naloxone (F(1, 15) = 24.5, P<0.001), but not a significant SB-334867 main effect nor a significant interaction. However, c-Fos expression analysis in the AcbSh using a two-way ANOVA with dose of SB-334867 and dose of naloxone as factors revealed a significant main effect of naloxone (F(1, 15) = 103.166, P<0.0001), a significant SB effect (F(1, 15) = 6.561, P<0.022), and a significant SB-334867 by naloxone interaction (F(1, 15) = 11.038, P<0.005).
Ox1r blockade indirectly alters cellular activation in the Acb
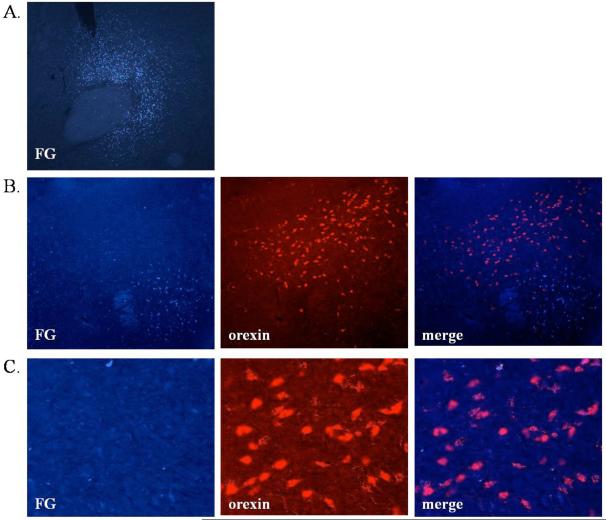
It is possible that orexin neurons directly innervate the Acb and that the SB-334867 effects could be a result of blocking direct orexin action in the Acb. To investigate this, the retrograde tracer FG was infused directly into the medial AcbC/dorsal AcbSh. After seven days, FG labeled neurons were found in the AcbC and/or the dorsal medial AcbSh (Fig. 5A). FG labeled neurons were also found in the LH, but did not co-localize with orexin immunoreactivity (Fig. 5B-C), indicating few if any synaptic contacts arising from the orexin neurons of the LH. In contrast, FG labeled neurons examined in the VTA co-localized with TH immunoreactivity (62.5% of cells expressing FG also expressed TH, data not shown), demonstrating the expected direct dopaminergic projection from the VTA to the Acb.
Figure 5.
Retrograde tracing using fluorogold (FG) suggest that orexin neurons do not form extensive synaptic connections with Acb neurons. (A) Representative photograph illustrating the injection site (FG-labeled neurons are visible in the AcbC and part of the medial/dorsal AcbSh). (B) Microscopy showing single-label immunohistochemistry for orexin in the LH as well as FG. (C) Microscopy showing single-label immunohistochemistry for orexin in the LH as well as FG in higher magnification from the same animal presented in B.
DISCUSSION
We have previously shown that mice lacking the orexin gene demonstrate attenuated somatic symptoms associated with naloxone-precipitated morphine withdrawal (24). Here, we demonstrate that orexin action through Ox1r contributes to the expression of the somatic withdrawal symptoms associated with naloxone-precipitated morphine withdrawal. Moreover, analysis of c-Fos expression suggests that the AcbSh may be a critical region in mediating this effect.
Previous studies investigating morphine withdrawal have used varying paradigms ranging from oral self-administration to pellet implantation. Here, we chose an injection paradigm where animals receive morphine injections of escalating doses (47,48). This allows for better control over morphine dosing, while also reflecting escalating drug intake that is seen in addicts. Naloxone administration to animals chronically treated with escalating doses of morphine leads to robust somatic withdrawal symptoms, including but not limited to tremors, wet dogs shakes, and backwalking. Such symptoms were significantly attenuated in animals that received SB-334867, an Ox1r antagonist, prior to naloxone administration. Ox1r blockade attenuated several, but not all, of the somatic morphine withdrawal symptoms observed. For instance, tremors, wet dog shakes, backward walking, and ptosis were significantly reduced, whereas no effect of SB-334867 was seen on jumping, paw tremors, and gnawing. Although not all symptoms were affected by SB-334867, those affected are the more severe, less frequent symptoms of withdrawal.
Interestingly, some differences in withdrawal symptoms were observed in animals treated with Ox1r antagonist compared with our previous observations on animals genetically lacking the orexin peptide (24). For instance, orexin knock-out mice, but not normal animals treated with SB-334867, displayed significant reductions in jumps. While SB-334867 treated animals displayed significant reductions in wet dog shakes, which were normal in orexin knock-out mice. These disparities may be the result of methodological differences; the orexin knock-out mice received chronic morphine via pellets and withdrawal was precipitated with naltrexone. Another possibility lies in the different orexinergic receptor mechanisms affected. For instance, SB-334867 has a higher binding affinity (pKb = 7.4) and ∼50-fold selectivity for the Ox1r over the Ox2r (pKb = 5.7) (51). Therefore, pharmacological manipulation of orexin action via SB-334867 selectively antagonizes Ox1rs, whereas orexin’s action on both orexin receptors is lacking in orexin knock-out mice. Ox2r may contribute to the morphine withdrawal response and the ongoing development of Ox2r specific reagents or use of Ox2r mutant animals will allow for direct analysis of its role.
By using the immediate early gene c-Fos to assess cellular activation, we observed that orexin neurons in the medial portions of the LH are activated in response to naloxone, and this response is not modified by SB-334867 administration. Withdrawal symptoms were further accompanied by increased c-Fos expression in the Acb. Although SB-334867 administration failed to affect c-Fos expression in the AcbC, Ox1r antagonism significantly attenuated neuronal activation in the AcbSh, implicating the AcbSh as a critical region associated with both somatic withdrawal and orexin action.
Our data demonstrate that attenuation of withdrawal by Ox1r blockade also blocks withdrawal-induced activation of the Acb, a region long postulated to be critical for the aversiveness of opiate withdrawal (16,17). While acute morphine administration results in an increase in accumbal extracellular DA, microdialysis studies have shown that in response to chronic morphine, DA outflow is normal until withdrawal where there is a reduction (8-10). Systemic administration of the DA agonist SKF 82958 reduces somatic signs of morphine withdrawal while increasing phosphorylation of GluR1 in the Acb of morphine dependent rats treated with naloxone (18). Furthermore, in response to morphine withdrawal, there is a reduction in spine density that is specific to the dendrites of medium spiny neurons located in the AcbSh. This reduction correlates with the severity of somatic symptoms of morphine withdrawal (52). Here, we also demonstrate that Acb neurons respond to naloxone-induced morphine withdrawal, a finding consistent with the literature, and suggest a role for orexin in this response. It remains undetermined whether AcbSh activation contributes to somatic withdrawal symptoms or is activated as a consequence of an Ox1r-dependent withdrawal process.
In contrast to the Acb, VTA DA cells fail to respond to naloxone-precipitated withdrawal as assessed by c-Fos. A role for the VTA in mediating orexin action has been previously reported in the context of locomotor activity (32), psychostimulant effects and reinforcement (42,44), and morphine reinforcement (53). Examination of the effects of intra-VTA orexin on DA levels in the Acb has yielded mixed results. While some results suggest a link (53), others report that orexin action in the VTA results in increased prefrontal cortex DA levels with no change in Acb DA levels (54). Moreover, previous evidence suggests that the VTA may not play a role in morphine withdrawal. Although VTA DA neurons increase their firing in response to chronic morphine, they are no longer responsive to acute morphine administration and exhibit similar firing rates as naïve animals following withdrawal (55). Together with our findings, the data suggest that orexin, acting via Ox1r receptors, may be able to activate Acb neurons via VTA-independent mechanisms. This may occur via action on dopamine neuron terminals resulting in dopamine release in the Acb, and/or through dopamine independent mechanisms.
Interestingly, we demonstrate that following chronic morphine treatment, LC norepinephrine neurons display a high basal level of c-Fos activation, which is not altered by naloxone-precipitated withdrawal. The role of the LC in response to states of morphine dependence and withdrawal is well established. Chronic morphine enhances LC levels of cAMP response element-binding protein (CREB) (56), a transcription factor linked with the development of morphine dependence (47,57). Naloxone-precipitated withdrawal is associated with increased activity of LC neurons (11) and intra-LC administration of opiate antagonists in morphine dependent animals results in a robust somatic withdrawal syndrome (13). Here, we demonstrate that in response to chronic morphine, basal c-Fos expression in TH-containing neurons is high (81.72% ±3.3) with no change in c-Fos expression following naloxone-precipitated withdrawal. The failure of naloxone to change c-Fos may be due to the already high basal level seen following chronic morphine, which, in turn, may be due to our use of morphine and saline injections 2-4 hours before sacrifice. It also remains possible that during withdrawal, neurons previously activated by chronic morphine increase their activity which would not be readily detected with c-Fos analysis.
The current results suggest that orexin-containing neurons, particularly those located in the DMH and PFA, are activated by morphine withdrawal, a negative component of drug addiction. It has been recently shown that orexin mRNA is also elevated in the LH in response to spontaneous morphine withdrawal in morphine dependent rats (58). Here, we show that the DMH and PFA demonstrate cellular activation in response to naloxone-precipitated withdrawal, while the LLH does not. This finding may suggest a dissociation between the medial portion and the lateral portion of the LH in response to the positive and negative reinforcement components of drug addiction. This is consistent with previous suggestions based on findings demonstrating that orexin cell activation in the LLH, but not DMH and PFA, is correlated with CPP preference scores for morphine, cocaine, and food (42). In contrast, more medial orexin cells in the DMH and PFA are preferentially activated in response to footshock (42). Additional evidence for the possible role of orexin in negative reinforcement comes from studies in which central administration of the orexin A neuropeptide reinstates extinguished lever pressing for both cocaine and food as well as raising intracranial self-stimulation thresholds (43).
Orexin neurons in the LH demonstrated a high basal level of activity in control animals chronically treated with morphine. In fact, this high basal activity is much higher than that we previously reported in animals treated with morphine pellets (24). This discrepancy may be due to our use of a chronic series of dose-escalating morphine injections, which results in more robust withdrawal and may account for higher basal c-Fos expression. Additionally, repeated injections also serve as stressful stimuli which may elicit a stress response. Orexinergic activity has been previously linked with a stress response (43,59,60) and orexin’s role in morphine withdrawal may indeed be to mediate components of stress that are integral to withdrawal.
The present data suggest that, during morphine withdrawal, orexin bypasses the VTA and acts upon the AcbSh via direct or indirect circuitry. Using retrograde tracing, we show that the AcbSh does not receive direct orexin afferents from the LH, suggesting that the orexin-Ox1r mediated AcbSh activation occurs through an indirect circuit. However, these results may not be surprising since Ox1r levels are very low in the Acb (33). The absence of direct orexin afferents to the Acb suggests that Ox1r activation by morphine withdrawal occurs elsewhere in the brain, perhaps in other regions along the mesocorticolimbic pathway, such as the amygdala or prefrontal cortex, and further investigation into orexin’s neuroanatomical pathway is necessary. A caveat to these experiments is that traditional anatomical tracing techniques may not reveal all functional targets of orexin neurons. In fact, a recent study suggests that only a small proportion of orexin neurons form synaptic contacts in the VTA suggesting that orexin may be released extrasynaptically in the region (61).
Overall, our current data suggest a positive role for orexin function in the expression of somatic morphine withdrawal, expanding our current mechanistic understanding of a clinically relevant component of morphine addiction. These findings, in combination with multiple lines of evidence implicating the orexinergic system in morphine reinforcement and response, suggest that orexin may be a critical substrate underlying different components aspects of opiate addiction. The current findings suggest a role of orexin in the aversive, negatively reinforcing, components of morphine addiction. We demonstrate that in response to morphine withdrawal, orexin achieves its behavioral effects partly via Ox1r-dependent functions. The ability of the Ox1r antagonist to acutely ameliorate withdrawal symptoms may suggest a potential therapeutic use of these agents in treatment of opiate-addicted patients during withdrawal. Although additional studies are necessary to elucidate the pathway by which orexin activates Acb neurons, these data implicate an interaction between the LH and the limbic system in mediating drug withdrawal. Additional studies are now necessary to further assess anatomical pathways and the role of orexin in other, non-somatic withdrawal symptoms as well as opiate self-administration and relapse.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Douglas Guarnieri for technical assistance, Darlene Brunzell for relevant comments about the experimental design, and members of the DiLeone lab for suggestions on the manuscript. This work was supported by a National Institutes of Health grant (DA017676) to R.J.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES: Dr. Sharf, Dr. Sarhan and Dr. DiLeone report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 2.Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152:215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- 3.Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 4.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 5.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 6.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 7.Pettit HO, Justice JBJ. Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- 8.Acquas E, Di Chiara G. Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate abstinence. J Neurochem. 1992;58:1620–1625. doi: 10.1111/j.1471-4159.1992.tb10033.x. [DOI] [PubMed] [Google Scholar]
- 9.Pothos E, Rada P, Mark GP, Hoebel BG. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res. 1991;566:348–350. doi: 10.1016/0006-8993(91)91724-f. [DOI] [PubMed] [Google Scholar]
- 10.Rossetti ZL, Melis F, Carboni S, Gessa GL. Dramatic depletion of mesolimbic extracellular dopamine after withdrawal from morphine, alcohol or cocaine: a common neurochemical substrate for drug dependence. Ann N Y Acad Sci. 1992;654:513–516. doi: 10.1111/j.1749-6632.1992.tb26016.x. [DOI] [PubMed] [Google Scholar]
- 11.Aghajanian GK. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- 12.Esposito E, Kruszewska A, Ossowska G, Samanin R. Noradrenergic and behavioural effects of naloxone injected in the locus coeruleus of morphine-dependent rats and their control by clonidine. Psychopharmacology. 1987;93:393–396. doi: 10.1007/BF00187263. [DOI] [PubMed] [Google Scholar]
- 13.Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- 14.Taylor JR, Elsworth JD, Garcia EJ, Grant SJ, Roth RH, redmond DEJ. Clonidine infusions into the locus coeruleus attenuate behavioral and neurochemical changes associated with naloxone-precipitated withdrawal. Psychopharmacology. 1988;96:121–134. doi: 10.1007/BF02431544. [DOI] [PubMed] [Google Scholar]
- 15.Laschka E, Teschemacher h, Mehraein P, Herz A. Sites of action of morphine involved in the development of physical dependence in rats. II. Morphine withdrawal precipitated by application of morphine antagonists into restricted parts of the ventricular system and by microinjection into various brain areas. Psychopharmacologia. 1976;46:141–147. doi: 10.1007/BF00421383. [DOI] [PubMed] [Google Scholar]
- 16.Koob GF, Wall TL, Bloom FE. Nucleus accumbens as a substrate for the aversive stimulus effects of opiate withdrawal. Psychopharmacology. 1989;98:530–534. doi: 10.1007/BF00441954. [DOI] [PubMed] [Google Scholar]
- 17.Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- 18.Chartoff EH, Mague SD, Barhight MF, Smith AM, Carlezon WAJ. Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone-precipitated morphine withdrawal. J Neurosci. 2006;26:6450–6457. doi: 10.1523/JNEUROSCI.0491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 20.Cazala P, Darracq C, Saint-Marc M. Self-administration of morphine into the lateral hypothalamus in the mouse. Brain Res. 1987;416:283–288. doi: 10.1016/0006-8993(87)90908-5. [DOI] [PubMed] [Google Scholar]
- 21.Olds ME. Hypothalamic substrate for the positive reinforcing properties of morphine in the rat. Brain Res. 1979;168:351–360. doi: 10.1016/0006-8993(79)90175-6. [DOI] [PubMed] [Google Scholar]
- 22.Corrigall WA. Heroin self-administration: effects of antagonist treatment in lateral hypothalamus. Pharmacol Biochem Behav. 1987;27:693–700. doi: 10.1016/0091-3057(87)90196-1. [DOI] [PubMed] [Google Scholar]
- 23.van der Kooy D, Mucha RF, O’Shaughnessy M, Bucenieks P. Reinforcing effects of brain microinjections of morphine revealed by conditioned place preference. Brain Res. 1982;243:107–117. doi: 10.1016/0006-8993(82)91124-6. [DOI] [PubMed] [Google Scholar]
- 24.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 26.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 28.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FSn, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 31.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–187. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- 33.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 35.Martin G, Fabre V, Siggins GR, De Lecea L. Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regul Pept. 2002;104:111–117. doi: 10.1016/s0167-0115(01)00354-8. [DOI] [PubMed] [Google Scholar]
- 36.Soffin EM, Evans ML, Gill CH, Harries MH, Benham CD, Davies CH. SB-334867-A antagonises orexin mediated excitation in the locus coeruleus. Neuropharmacology. 2002;42:127–133. doi: 10.1016/s0028-3908(01)00156-3. [DOI] [PubMed] [Google Scholar]
- 37.Lin L, Faraco J, Li R, Kadotani H, Rogers W, lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 38.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 39.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 40.Sutcliffe JG, De Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 41.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 42.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 43.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, De Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Kameyama T, Nabeshima T, Ukai M, Yamaguchi K. Morphine-induced Straub tail reaction and spinal catecholamine metabolite content: antagonism of naloxone to morphine-induced effects in mice. Chem Pharm Bull. 1978;26:2615–2618. doi: 10.1248/cpb.26.2615. [DOI] [PubMed] [Google Scholar]
- 46.Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–546. [PubMed] [Google Scholar]
- 47.Maldonado R, Blendy JA, Tzavara E, Gass P, Roques BP, Hanoune J, Schutz G. Reduction of morphine abstinence in mice with a mutation in the gene encoding CREB. Science. 1996;273:657–659. doi: 10.1126/science.273.5275.657. [DOI] [PubMed] [Google Scholar]
- 48.Zachariou V, Brunzell DH, Hawes J, Stedman DR, Bartfai T, Steiner RA, Wynick D, Langel U, Picciotto MR. The neuropeptide galanin modulates behavioral and neurochemical signs of opiate withdrawal. Proc Natl Acad Sci U S A. 2003;100:9028–9033. doi: 10.1073/pnas.1533224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. Elsevier Academic Press; San Diego: 2004. [Google Scholar]
- 50.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, Jerman J, Brough S, Smart D, Johns A, Chan W, Porter RA, Upton N. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl) 2001;153:203–209. doi: 10.1007/s002130000550. [DOI] [PubMed] [Google Scholar]
- 52.Spiga S, Puddu MC, Pisano M, Diana M. Morphine withdrawal-induced morphological changes in the nucleus accumbens. Eur J Neurosci. 2005;22:2332–2340. doi: 10.1111/j.1460-9568.2005.04416.x. [DOI] [PubMed] [Google Scholar]
- 53.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake T, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–495. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- 55.Georges F, Le Moine C, Aston-Jones G. No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci. 2006;26:5720–5726. doi: 10.1523/JNEUROSCI.5032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Widnell KL, Russell DS, Nestler EJ. Regulation of expression of cAMP response element-binding protein in the locus coeruleus in vivo and in a locus coeruleus-like cell line in vitro. Proc Natl Acad Sci U S A. 1994;91:10947–10951. doi: 10.1073/pnas.91.23.10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- 59.Feng P, Vurbic D, Wu Z, Strohl KP. Brain orexins and wake regulation in rats exposed to maternal deprivation. Brain Res. 2007;1154:163–172. doi: 10.1016/j.brainres.2007.03.077. [DOI] [PubMed] [Google Scholar]
- 60.Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am J Physiol Regul Integr Comp Physiol. 2007;292:382–387. doi: 10.1152/ajpregu.00496.2006. [DOI] [PubMed] [Google Scholar]
- 61.Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.