Abstract
TRPV1, a cation channel on sensory nerves sensitive to heat and capsaicin, plays an important role in the transduction of noxious stimuli to the spinal cord. It is expressed by neurons in dorsal root ganglia (DRG) that may also express neuropeptides, which are important for the development of inflammation. Mice with genetic deletion of TRPV1 have been used to study the involvement of this receptor in the mediation of pain and inflammation in animal models of arthritis. However, the expression of TRPV1 in the mouse articular afferents has not been studied. We here provide numerical data on expression of TRPV1 in an identified population of sensory afferents to the mouse L3–L5 DRG that innervate joints, in comparison with that from bladder and skin. A combination of tracing and immunocytochemistry revealed that TRPV1-positive fibers innervate the mouse knee and ankle. At the level of DRG, ~40% of articular afferents from these joints express TRPV1 and the majority of them are peptidergic, as revealed by simultaneous immunostaining for the neuropeptide calcitonin gene-related peptide. These findings are consistent with the idea that activation of TRPV1 in peripheral axons of joint afferents may mediate the synovial release of neuropeptides in arthritis.
Keywords: Articular afferent, Vanilloid receptor TRPV1, Calcitonin gene-related peptide, Dorsal root ganglion, Mouse
1. Introduction
An important step for our understanding of nociception was the cloning of the receptor for capsaicin (the pungent ingredient in hot peppers), the vanilloid receptor TRPV1. TRPV1 is a non-selective cation channel expressed by many neurons in dorsal root ganglia (DRG, Caterina et al., 1997) and in their peripheral processes in skin and viscera (Tominaga et al., 1998) and central processes in the spinal and trigeminal dorsal horn (Guo et al., 1999).
TRPV1 in the skin is important for thermal nociception and inflammatory hyperalgesia and allodynia, and has also been implicated in neuropathic pain (Hudson et al., 2001). In vitro, TRPV1-expressing cells can be activated by protons (pH<6) or heat (>43°C) as well as by vanilloids, like capsaicin. Mice lacking the TRPV1 gene do not respond to vanilloids and have reduced sensitivity to noxious heat and low pH, indicating that TRPV1 contributes to heat and proton sensitivity in vivo (Caterina et al., 2000; Karai et al., 2004). In mice, knocking out the TRPV1 gene prevented thermal hyperalgesia in an experimental model of hindpaw inflammation (Caterina et al., 2000; Davis et al., 2000), and capsazepine (a selective TRPV1 antagonist) prevented the development of mechanical hyperalgesia in experimental inflammation (Walker et al., 2003).
Besides the skin, TRPV1-positive sensory afferents innervate many deep structures of the body (Robinson et al., 2004; Ward et al., 2003); moreover, the fraction of DRG neurons that express TRPV1 is much higher for visceral and muscle afferents than for cutaneous afferents (Christianson et al., 2006a; Hwang et al., 2005a; Zhong et al., 2008). The presence of TRPV1 in joints was proposed based on the articular effects of capsaicin, to which it is the only known receptor (Holzer, 1991). More recently, the expression of TRPV1 in articular afferents has been suggested by immunostaining of nerve fibers within the rat temporomandibular joint (Ichikawa et al., 2004; Ioi et al., 2006) and rat and human knee joints (Fernihough et al., 2005).
TRPV1-KO mice provide an excellent negative control for studies of the involvement of capsaicin-sensitive sensory afferents in several models of painful inflammatory conditions, including arthritis (Bolcskei et al., 2005). The severity of adjuvant-induced arthritis of the knee (Barton et al., 2006; Keeble et al., 2005) and ankle (Szabo et al., 2005) was found to be significantly reduced in TRPV1-KO mice. However, the expression of TRPV1 in mouse articular afferents has not been studied. It is also unclear what is the relationship between the articular afferents that express TRPV1 and those that could release the neuropeptide calcitonin gene-related peptide (CGRP), an important mediator of pain and inflammation in arthritis (Schaible et al., 2002). CGRP has been shown in rat articular afferents at the level of DRG (O'Brien et al., 1989) and the knee joint (Schwab et al., 1997) and the release of CGRP into the joint was found to be increased in experimental arthritis in rats (Ahmed et al., 1995). CGRP-KO mice have reduced behavioral pain responses in experimental arthritis, consistent with a pro-nociceptive role for CGRP in joints (Zhang et al., 2001). The reported increase in expression of both TRPV1 and CGRP in a rat model of arthritis has been controversial (Fernihough et al., 2005; Staton et al., 2007) and data on colocalization of the two markers in mouse articular afferents is lacking.
Thus, further anatomical characterization of the TRPV1-positive afferents from joints is needed to better understand the organization of articular nociceptive pathways. The goal of the present study was to ascertain whether TRPV1 is expressed by articular afferents in the mouse, to provide basic numerical data on the fraction of TRPV1-positive articular afferents, in comparison with afferents of cutaneous and visceral origin to the same DRG, and to determine what fraction of them is peptidergic. For this, we use a combination of tracing and immunocytochemistry and show that TRPV1-positive afferents innervate knee and ankle joints in the mouse and that the majority of them co-express CGRP.
2. Results
In sections of joints, many nerve fibers were strongly immunoreactive for TRPV1 in the periosteum of periarticular bone and within the articular capsule; most TRPV1-positive fibers were also stained for CGRP (Fig. 1). TRPV1-positive fibers were frequently associated with the vasculature of the articular capsule and were often within the vessel wall of larger vessels; virtually all fibers innervating larger blood vessels were double-stained (Fig. 1C). Few fibers were single-stained for PGP9.5, a generic marker for all peripheral nerve fibers, suggesting that the majority of nerve fibers innervating joint tissues are TRPV1-positive (Fig. 1D,E). Specifically, numerous nerve fibers had varicose appearance with immunostaining for TRPV1 concentrating in beads along the fiber. However, some small bundles or individual thin fibers on the surface of the femur and tibia, apparently innervating the periosteum, were immunostained for CGRP but not for TRPV1.
Fig. 1.
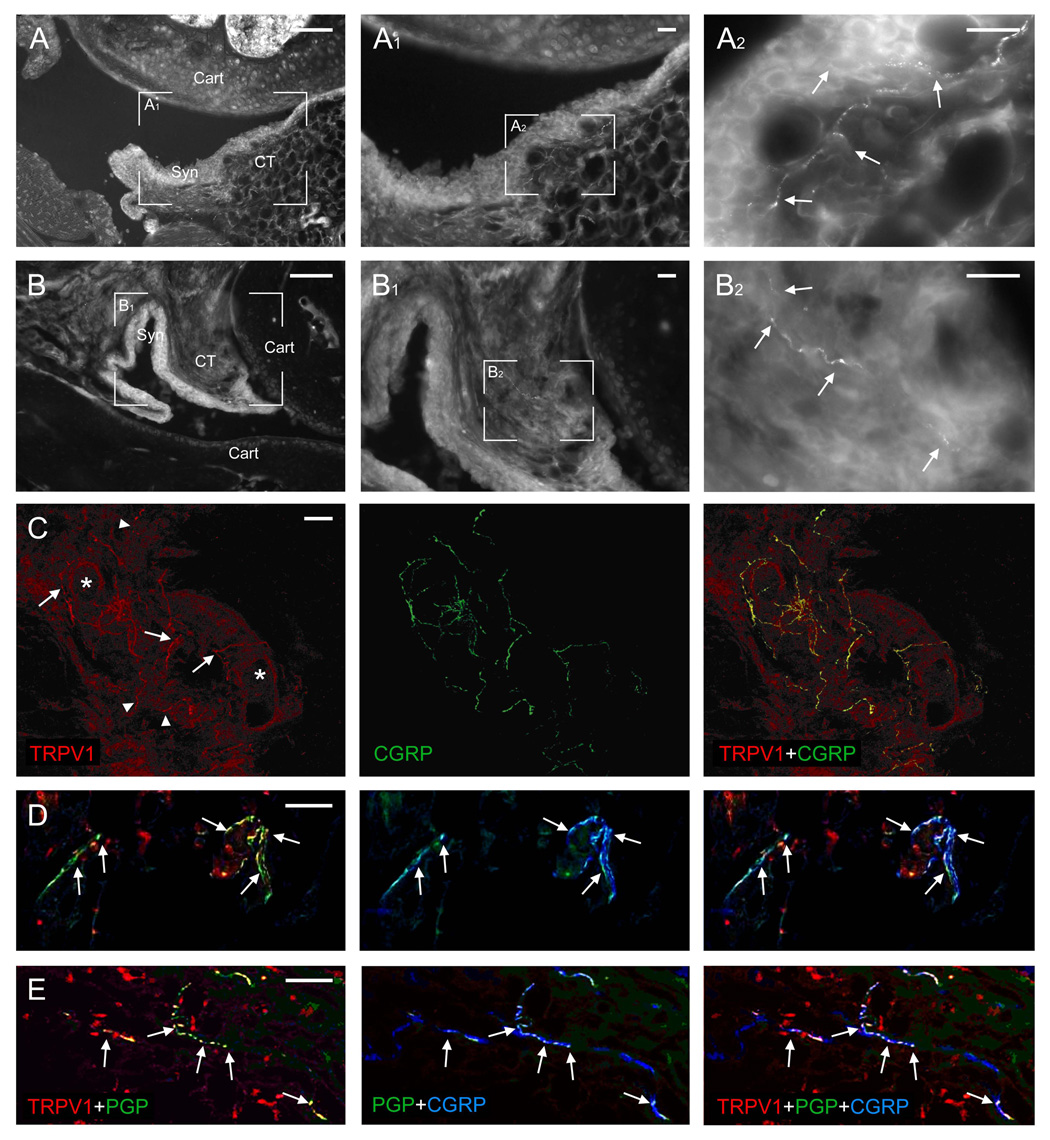
A, B: Fluorescent microscopy of sections through the mouse knee (A) and ankle (B) immunostained for TRPV1; A1, A2 and B1, B2 are enlargements of the respective boxed areas in A and B. Various joint elements are labeled to help orientation: Cart, articular cartilage of the distal epiphysis of the femur (A) and the tibia and talus (B); Syn, synovial layer of the articular capsule; CT, connective tissue layer of the articular capsule. TRPV1-immunopositive nerve fivers in the subsynovial tissues are pointed to with arrows. C–E: Confocal microscopy of triple immunofluorescent staining for TRPV1, CGRP, and the marker for peripheral nerve fibers PGP9.5 in sections through mouse ankle (C, D) and knee (E) joints. C: Some fibers that stain simultaneously for TRPV1 and CGRP (arrows, the staining for PGP9.5 is not shown for clarity) are apparently associated with small blood vessels (asterisks), while others appear to terminate as free nerve fibers (arrowheads). D, E: Colocalization of TRPV1 with CGRP is apparent in varicosities (beads) along the nerve fibers (arrows). Scale bars = 20 µm, except for A, B=100 µm.
Three days after the tracer injections, multiple DRG neurons were labeled with FB on the side of the injection; bladder injections produced bilateral labeling. Most labeled neurons were in L3–L4 DRG after knee injections, in L4–L5 DRG after ankle injections, and in L5 DRG after bladder and skin injections. Double immunofluorescence revealed that many neurons retrogradely-labeled with FB express TRPV1, CGRP, or both (Fig. 2). Cell counts revealed that while in naïve mice ~37% of all DRG neurons expressed TRPV1 and ~ 23% expressed CGRP, the percentages in afferents of identified sources varied according to the type, and for joint afferents were ~44% and ~39% for TRPV1 and ~44% and ~53% for CGRP for the ankle and the knee afferents, respectively (Table 1). In comparison, fractions for skin afferents in the same ganglia were generally lower and for bladder afferents were generally higher: of all identified skin and bladder afferents respectively, ~ 33% and ~58% stained for TRPV1 and ~ 35% and ~69% stained for CGRP. Counts of double- and triple-labeled neurons further revealed that 29–31% of the identified articular afferents expressed both TRPV1 and CGRP and that 70–77% of the TRPV1-positive articular afferents expressed CGRP. These fractions were also lower for the skin afferents (~21% and ~65%) and higher for the blabber afferents (~54% and ~92%).
Fig. 2.
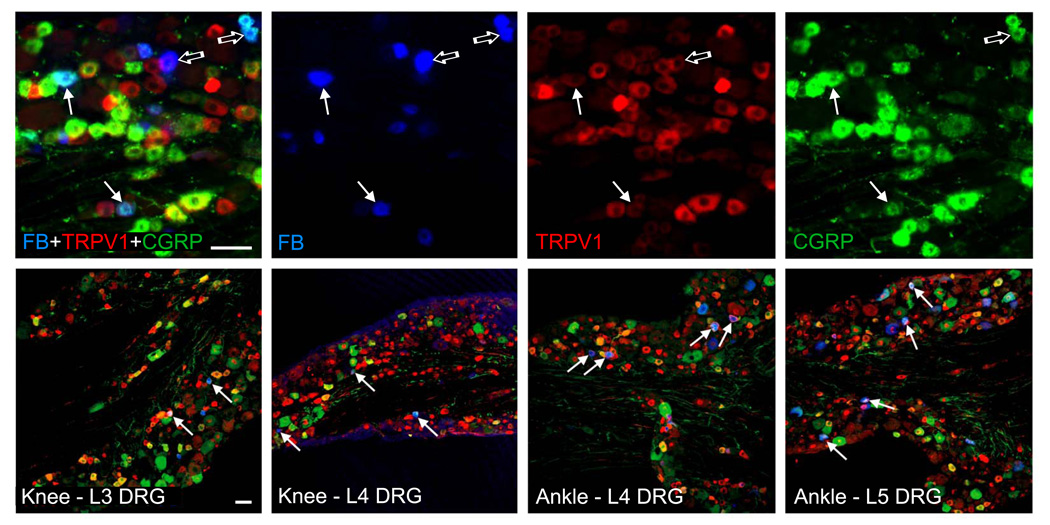
Triple immunofluorescence for the axonal tracer FB (blue), TRPV1 (red), and CGRP (green) in sections of mouse lumbar DRG (tracer injection sites and DRG segments are indicated on the bottom). Intraarticular injections of FB label afferent neurons that innervate joints, many of which co-immunostain for TRPV1 and CGRP (solid arrows; examples of articular afferents that immunostain for either TRPV1 or CGRP alone are pointed to with open arrows). Scale bars = 50 µm.
Table 1.
Expression of TRPV1 in identified sensory afferents and colocalization with CGRP
| Afferents | TRPV1 | CGRP | Double | TRPV1 also CGRP |
|---|---|---|---|---|
| Ankle (228) | 43.5±4.9 | 44.3±4.0 | 30.7±5.1 | 70.1±6.0 |
| Knee (140) | 39.0±2.8 | 52.6±3.7 | 28.5±3.0 | 77.1±11.2 |
| Skin (911) | 32.5±1.5 | 35.3±1.4 | 21.1±1.5 | 64.8±4.4 |
| Bladder (604) | 58.2±1.9 | 68.7±3.4 | 53.7±1.3 | 92.3±1.5 |
| Naïve (1379) | 36.7±1.8 | 23.3±2.0 | 28.3±0.9 | 64.4±1.2 |
Counts of DRG neuronal profiles, labeled after injections of the tracer FB in the ankle, knee, skin or bladder that express TRPV1, CGRP, or both (Double, “TRPV1 also CGRP” is the fraction of TRPV1 neurons that also express CGRP); the total number of profiles counted for each type of afferents are given in parentheses. Values are percentages (mean±SEM) of total FB-labeled profiles, or of all profiles in naïve mice. Counts were not significantly different between animals within a group and were pooled from 4–8 sections in each of L3–L5 DRG per animal in 3 mice for each type of afferents, double-stained for TRPV1 and CGRP as in Fig. 2.
3. Discussion
We here report for the first time that i) TRPV1-positive fibers innervate the mouse knee and ankle, ii) at the level of DRG, ~40% of articular afferents from these joints express TRPV1, and iii) the majority of TRPV1-expressing articular afferents are peptidergic.
The expression of TRPV1 in articular afferents has been suggested by the involvement of capsaicin-sensitive afferents in experimental arthritis (Helyes et al., 2004; Tang et al., 2004) and supported by immunostaining of nerve fibers within the rat temporomandibular joint (Ichikawa et al., 2004; Ioi et al., 2006) and rat and human knee joints (Fernihough et al., 2005). Many of the TRPV1-positive fibers innervating the articular capsule in the knee and ankle, and the posterior aspect of the patellar ligament, appeared to terminate in the sub-synovial connective tissue as free axonal endings and displayed varicosities characteristic of the sensory afferents described in the cat’s knee (Schaible, 1996). The CGRP-positive fiber bundles in the periosteum, some of which were also TRPV1-positive, likely correspond to those described as providing sensory and sympathetic innervation of the mouse femur (Mach et al., 2002). The TRPV1-negative fibers were also likely to be of sympathetic origin (Ioi et al., 2006). The close association of TRPV1-positive fibers with blood vessels and colocalization with CGRP was analogous to that reported in the rat temporomandibular joint (Ioi et al., 2006; Ishikawa et al., 2005). TRPV1 has also been demonstrated in synovial fibroblasts from patients with arthritis, suggesting that it may play a role also in non-neuronal mechanisms of nociception in joints (Engler et al., 2007; Kochukov et al., 2006); we were unable to obtain convincing immunostaining of synovial cells in the mouse. TRPV1 has also been reported to colocalize with the marker for non-peptidergic afferents IB4 in nerve fibers in the rat temporomandibular joint (Ioi et al., 2006), but not knee (Ivanavicius et al., 2004), consistent with the idea that the distinction between peptidergic and non-peptidergic TRPV1-expressing afferents based on co-labeling for IB4 may be unreliable in the rat (Hwang et al., 2005b; Price and Flores, 2007; Woodbury et al., 2004).
We did not attempt to quantify the immunostaining for TRPV1 and CGRP in peripheral nerve fibers in joint sections. However, while the majority of (PGP9.5-positive) nerve fibers in sections of knees and ankles were immunolabeled for TRPV1, a much lower fraction (~40%) of DRG neurons back-labeled from these two joints expressed TRPV1. This apparent discrepancy may be explained if, compared to the cell body in DRG, peripheral axons of articular afferents had higher levels of TRPV1, favoring their detection with immunocytochemistry (Hwang and Valtschanoff, 2003). Alternatively, leakage of the tracer during injection may have caused FB-labeled DRG cells to include neurons from unintended sources, i.e. proprioceptive, muscle, and skin afferents in articular injections, peritoneal afferents in bladder injections, etc. To minimize the influence of this difficult to control variable, we injected a relatively small volume of more highly concentrated tracer (than the standard 1%) through the thinnest available needle and included in the analysis only mice that did not exhibit retrogradely-labeled motor neurons in the lumbar spinal cord. The tracer may further skew the results from cell counts by favoring fibers of a particular class or from a particular source. For example, the tracers wheat-germ agglutinin (WGA) and cholera toxin B (CTB) may label preferentially unmyelinated and myelinated afferents, respectively; however, while this selectivity may apply to afferents of somatic origin (labeled with tracer injections into the sciatic nerve, Valtschanoff et al., 1994), the opposite may be true for visceral afferents (Wang et al., 1998). We here chose FB, which does not have a known bias for a particular class or source of afferents, to provide counts for articular, cutaneous and visceral afferents to the same ganglion, retrogradely-labeled with the same tracer, as a basis for comparison with previously-published data.
Our counts of identified articular afferents that immunostain for TRPV1 and the fraction that also stain for CGRP agree with previously reported data in mice (Salo et al., 2002) and rats (Fernihough et al., 2005; Ichikawa et al., 2004; Ochiai et al., 2007). They were higher that that in cutaneous afferents and lower than that in visceral afferents, also consistent with previous reports. Thus, using the same experimental strategy as the present study, Christianson et al. (2006a) found that mouse colonic afferents (labeled with CTB) contained the highest percentage of both TRPV1- and CGRP-positive neurons, followed by muscle afferents (labeled with WGA) and cutaneous afferents (labeled with WGA and IB4), further supported by Zhong et al. (2008), who reported that of identified gastric neurons in the mouse (labeled with CTB), 77% expressed TRPV1 and 82% expressed CGRP, of muscle neurons, 40% expressed TRPV1 and 39% expressed CGRP, and of skin neurons, 26% expressed TRPV1 and 18% expressed CGRP. These data are also consistent with the previously published percentages for mouse colonic afferents labeled with FB (82% stained for TRPV1 and 81% stained for CGRP, BALB/c mice, Robinson et al., 2004). However, as suggested above, the value of comparing counts of immunopositive primary afferent neurons and/or peripheral fibers across different studies may be limited by multiple difficult to control variables, including the choice of tracer, the strain, age, and gender of the animal, the neuronal segment level, the immunohistochemical protocol, the specific criteria for counting, etc. For example, thoraco-lumbar DRG contained significantly higher percentage of TRPV1-and CGRP-positive colonic afferents than lumbo-sacral DRG in mice but not in rats (Christianson et al., 2006b), the degree of colocalization of TRPV1 and CGRP in peripheral afferent axons varied with the particular viscus within the gastrointestinal tract (Ward et al., 2003), and even our counts in naïve mice differed significantly from those of Zwick et al. (2002), who reported that out of all afferents in the mouse lumbar DRG, 22% expressed TRPV1 and 30% expressed CGRP and those of Robinson et al. (2004), who reported that 42% of all DRG afferents immunostained for TRPV1 and 27% immunostained for CGRP. Yet, the vagaries of the method notwithstanding, our results establish baseline values that can be useful in interpreting the results of experiments based on mouse models of arthritis.
Discussion of the functional importance for TRPV1 has so far been primarily focused on its thermoreceptive role in the skin. That TRPV1 may also play a role in joints and viscera is suggested by its ubiquitous expression in nerve fibers in various deep structures of the body and by the effects of capsaicin on internal organs of experimental animals and humans (Ballet et al., 2003). Since sensory nerves in joints and viscera are not normally exposed to high heat or capsaicin, however, the functional role of TRPV1 in non-cutaneous afferents remains elusive. TRPV1 in articular afferents may be a target for endogenously-released ligands (Di Marzo et al., 2002) or function as a proton detector (Christianson et al., 2006a). It may also contribute directly to the pathogenesis of arthritis by mediating the release of neuropeptides from nerve fibers in joints. In support of such mechanism, studies suggest that activation of TRPV1 may trigger peripheral release of neuropeptides (McVey and Vigna, 2001; Nathan et al., 2001) and that peptidergic afferents in joints may release neuropeptides into the synovial fluid in the course of arthritis (Lotz et al., 1987; Saito and Koshino, 2000). That TRPV1 is expressed by peptidergic nerve fibers in joints is consistent with the possibility that synovial release of neuropeptides during inflammation is triggered by depolarization of these fibers via TRPV1. The issue could be addressed by a functional study using knockout mice or antagonists of TRPV1.
4. Experimental procedures
Twelve male C57/BL6J mice (25–35g; Jackson Laboratories, Bar Harbor, ME) were used in this study. All experimental procedures involving mice were carried out in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, according to a protocol approved by the Institutional Animal Care and Use Committee at UNC.
To label articular afferents and to compare the fraction that are TRPV1 and/or CGRP-positive to that of identified cutaneous and visceral afferents to the same DRG, mice anesthetized with a mixture of ketamine and xylazine (90 mg/kg and 10 mg/kg, i.p.) were injected with 0.5 µl of the axonal tracer Fast Blue (FB, 2% aqueous solution containing 5% DMSO; EMS-Chemie GmbH), using a 30-gauge needle attached to a Hamilton syringe. Mice received a single injection into the knee (n=3) or ankle (n=3) joint on one side and the skin on the posterior side of the thigh on the other side (four sites ~ 1 mm apart), or the wall of urinary bladder (four sites ~ 1 mm apart, n=3), exposed through a lower median laparotomy under asepsis. DRGs of mice that did not receive any injections (n=3) were used to study expression of TRPV1 and CGRP in naïve mice.
Three days after tracer injections, the mice were re-anaesthetized with sodium pentobarbital (80 mg/kg, i.p.) and perfused intracardially with 30 ml heparinized saline, followed by 100 ml freshly prepared solution of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB). Tissues, including the L3–L5 DRG bilaterally, and joint preparations of ankles and knees that were not injected with FB, were dissected out, postfixed in 4% paraformaldehyde for 4 hrs, and stored in PB. Joint preparations were decalcified in Rapid Cal Immuno (BBC Biochemical through Fisher Scientific, Pittsburgh, PA) for 3 days. Tissue blocks were further cryoprotected in 30% sucrose in PB for 24–48 hrs and sectioned on a cryostat at 20 µm, and sections were collected on slides and stored at −20°C.
All incubations for fluorescent immunohistochemistry were carried out on a shaker at room temperature. Sections were permeabilized with 50% ethanol in phosphate-buffered saline (PBS; 0.01 M, pH 7.4) for 30 min, blocked with 10% normal donkey serum (NDS; Jackson Immunoresearch, West Grove, PA) in PBS, and incubated overnight with a mixture of two or three primary antibodies in PBS: goat anti-TRPV1 (Cat# SC12498, Santa Cruz Biotechnology, Santa Cruz, CA; 1:1,000), rabbit anti-CGRP (Cat# AB5920, Chemicon, Temecula, CA; 1:2,000), and guinea pig anti-PGP9.5 (Cat# 14104, Neuromics, Minneapolis, MN; 1:500). After several rinses with PBS and incubation with 2% NDS for 30 min, sections were incubated with appropriate secondary antibodies (fluorescein isothiocyanate-, Cy3-, or Cy5-conjugated antibodies raised in donkey, 1:200; Jackson Immunoresearch). Finally, sections were rinsed, mounted on slides and coverslipped with Vectashield (Vector, Burlingame, CA).
The antibodies used in this study are well characterized and are in common use in our laboratory. As a matter of routine control, sections were processed according to the above protocols, except that primary or secondary antibodies were omitted, or blocking peptides were added at concentrations recommended by the suppliers. Omission of primary or secondary antibodies or preadsorption with blocking peptides completely abolished specific staining.
Joint sections were examined on a laser confocal microscope (SP2, Leica, Wetzlar, Germany); since laser illumination with a wavelength appropriate for visualization of FB-labeled cells was unavailable on the confocal microscope, images of DRG sections were acquired with a Retiga EX cooled CCD camera (QImaging, Burnaby, Canada) attached to a Leitz DMR fluorescent microscope (Leitz, Wetzlar, Germany) at 10X, and saved as TIFF files using OpenLab software (Improvision, Lexington, MA). Brightness and contrast were adjusted with Photoshop CS2 (Adobe Systems, San Jose, CA); all enhancements were applied to the entire image. Some images of joint sections immunostained for TRPV1 were acquired with a conventional fluorescent microscope (Leitz DMR/Retiga EX) and used for Fig. 1.
To quantify colocalization of FB, TRPV1, and CGRP in DRG neurons, neuronal profiles were counted in 4–8 sections per ganglion at 80 µm intervals in each of L3–L5 DRG in each mouse by an investigator blinded to the source material. In every section, positive neuronal profiles were counted in one gray-scale image for each of the three fluorescent channels. Neuronal profiles were identified by their characteristic cellular morphology. The cut-off brightness level (labeling density threshold) was determined by averaging the integral brightness of three neuronal profiles per image that were judged to be minimally positive using Image J 1.38x software (NIH, Bethesda, MD); all profiles whose mean labeling density exceeded this threshold were counted as positive. To avoid counting the same profile more than once, each positive profile was labeled with a dot, color-coded after the respective fluorescent channel. To determine colocalization, the three gray-scale images containing the colored dots were overimposed and the fraction of profiles in each of the eight categories [blue, green, red, yellow (green+red), purple (blue+red), aqua (blue+green), white (triple-labeled), and black (unlabeled; immuno-negative neurons in naïve mice were visualized with phase-interference contrast)] in each section were normalized to the total number of profiles and expressed as a percentage of total counted profiles (Marvizon et al., 2002). Data were analyzed with one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test, using Kaleidagraph (Synergy Software, Reading, PA); statistical significance was set at p<0.05.
Acknowledgements
This work was supported by NIH grant AR053721. We thank Ms. Helen Willcockson for expert help with immunohistochemistry and Dr. R. Weinberg for careful critique of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmed M, Bjurholm A, Schultzberg M, Theodorsson E, Kreicbergs A. Increased levels of substance P and calcitonin gene-related peptide in rat adjuvant arthritis. A combined immunohistochemical and radioimmunoassay analysis. Arthritis Rheum. 1995;38:699–709. doi: 10.1002/art.1780380519. [DOI] [PubMed] [Google Scholar]
- Ballet S, Conrath M, Fischer J, Kaneko T, Hamon M, Cesselin F. Expression and G-protein coupling of mu-opioid receptors in the spinal cord and dorsal root ganglia of polyarthritic rats. Neuropeptides. 2003;37:211–219. doi: 10.1016/s0143-4179(03)00045-3. [DOI] [PubMed] [Google Scholar]
- Barton NJ, McQueen DS, Thomson D, Gauldie SD, Wilson AW, Salter DM, Chessell IP. Attenuation of experimental arthritis in TRPV1R knockout mice. Exp Mol Pathol. 2006;81:166–170. doi: 10.1016/j.yexmp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Bolcskei K, Helyes Z, Szabo A, Sandor K, Elekes K, Nemeth J, Almasi R, Pinter E, Petho G, Szolcsanyi J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–376. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006a;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006b;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Griffin G, De Petrocellis L, Brandi I, Bisogno T, Williams W, Grier MC, Kulasegram S, Mahadevan A, Razdan RK, Martin BR. A structure/activity relationship study on arvanil, an endocannabinoid and vanilloid hybrid. J Pharmacol Exp Ther. 2002;300:984–991. doi: 10.1124/jpet.300.3.984. [DOI] [PubMed] [Google Scholar]
- Engler A, Aeschlimann A, Simmen BR, Michel BA, Gay RE, Gay S, Sprott H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem Biophys Res Commun. 2007;359:884–888. doi: 10.1016/j.bbrc.2007.05.178. [DOI] [PubMed] [Google Scholar]
- Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett. 2005;388:75–80. doi: 10.1016/j.neulet.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Szabo A, Nemeth J, Jakab B, Pinter E, Banvolgyi A, Kereskai L, Keri G, Szolcsanyi J. Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund's adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 2004;50:1677–1685. doi: 10.1002/art.20184. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Valtschanoff JG. Vanilloid receptor VR1-positive afferents are distributed differently at different levels of the rat lumbar spinal cord. Neurosci Lett. 2003;349:41–44. doi: 10.1016/s0304-3940(03)00750-x. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Oh JM, Valtschanoff JG. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005a;1047:261–266. doi: 10.1016/j.brainres.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Oh JM, Valtschanoff JG. The majority of bladder sensory afferents to the rat lumbosacral spinal cord are both IB4- and CGRP-positive. Brain Res. 2005b;1062:86–91. doi: 10.1016/j.brainres.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Fukunaga T, Jin HW, Fujita M, Takano-Yamamoto T, Sugimoto T. VR1-, VRL-1- and P2X3 receptor-immunoreactive innervation of the rat temporomandibular joint. Brain Res. 2004;1008:131–136. doi: 10.1016/j.brainres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Ioi H, Kido MA, Zhang JQ, Yamaza T, Nakata S, Nakasima A, Tanaka T. Capsaicin receptor expression in the rat temporomandibular joint. Cell Tissue Res. 2006;325:47–54. doi: 10.1007/s00441-006-0183-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Miyagi M, Ohtori S, Aoki Y, Ozawa T, Doya H, Saito T, Moriya H, Takahashi K. Characteristics of sensory DRG neurons innervating the lumbar facet joints in rats. Eur Spine J. 2005;14:559–564. doi: 10.1007/s00586-004-0834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanavicius SP, Blake DR, Chessell IP, Mapp PI. Isolectin B4 binding neurons are not present in the rat knee joint. Neuroscience. 2004;128:555–560. doi: 10.1016/j.neuroscience.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble J, Blades M, Pitzalis C, Castro da Rocha FA, Brain SD. The role of substance P in microvascular responses in murine joint inflammation. Br J Pharmacol. 2005;144:1059–1066. doi: 10.1038/sj.bjp.0706131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochukov MY, McNearney TA, Fu Y, Westlund KN. Thermosensitive TRP ion channels mediate cytosolic calcium response in human synoviocytes. Am J Physiol Cell Physiol. 2006;291:C424–C432. doi: 10.1152/ajpcell.00553.2005. [DOI] [PubMed] [Google Scholar]
- Lotz M, Carson DA, Vaughan JH. Substance P activation of rheumatoid synoviocytes: neural pathway in pathogenesis of arthritis. Science. 1987;235:893–895. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O'Leary P, Mantyh PW. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- McVey DC, Vigna SR. The capsaicin VR1 receptor mediates substance P release in toxin A-induced enteritis in rats. Peptides. 2001;22:1439–1446. doi: 10.1016/s0196-9781(01)00463-6. [DOI] [PubMed] [Google Scholar]
- Nathan JD, Patel AA, McVey DC, Thomas JE, Prpic V, Vigna SR, Liddle RA. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1322–G1328. doi: 10.1152/ajpgi.2001.281.5.G1322. [DOI] [PubMed] [Google Scholar]
- O'Brien C, Woolf CJ, Fitzgerald M, Lindsay RM, Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience. 1989;32:493–502. doi: 10.1016/0306-4522(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Ochiai N, Ohtori S, Sasho T, Nakagawa K, Takahashi K, Takahashi N, Murata R, Moriya H, Wada Y, Saisu T. Extracorporeal shock wave therapy improves motor dysfunction and pain originating from knee osteoarthritis in rats. Osteoarthritis Cartilage. 2007;15:1093–1096. doi: 10.1016/j.joca.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Saito T, Koshino T. Distribution of neuropeptides in synovium of the knee with osteoarthritis. Clin Orthop Relat Res. 2000:172–182. doi: 10.1097/00003086-200007000-00024. [DOI] [PubMed] [Google Scholar]
- Salo PT, Seeratten RA, Erwin WM, Bray RC. Evidence for a neuropathic contribution to the development of spontaneous knee osteoarthrosis in a mouse model. Acta Orthop Scand. 2002;73:77–84. doi: 10.1080/000164702317281459. [DOI] [PubMed] [Google Scholar]
- Schaible H, Schmidt RF. Neurobiology of articular nociceptors. In: Belmonote C CF, editor. Neurobiology of Nociceptors. Vol. New York: Oxford University Press; 1996. [Google Scholar]
- Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann N Y Acad Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- Schwab W, Bilgicyildirim A, Funk RH. Microtopography of the autonomic nerves in the rat knee: a fluorescence microscopic study. Anat Rec. 1997;247:109–118. doi: 10.1002/(SICI)1097-0185(199701)247:1<109::AID-AR13>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Staton PC, Wilson AW, Bountra C, Chessell IP, Day NC. Changes in dorsal root ganglion CGRP expression in a chronic inflammatory model of the rat knee joint: differential modulation by rofecoxib and paracetamol. Eur J Pain. 2007;11:283–289. doi: 10.1016/j.ejpain.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Szabo A, Helyes Z, Sandor K, Bite A, Pinter E, Nemeth J, Banvolgyi A, Bolcskei K, Elekes K, Szolcsanyi J. Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: in vivo study using gene-deficient mice. J Pharmacol Exp Ther. 2005;314:111–119. doi: 10.1124/jpet.104.082487. [DOI] [PubMed] [Google Scholar]
- Tang ML, Haas DA, Hu JW. Capsaicin-induced joint inflammation is not blocked by local anesthesia. Anesth Prog. 2004;51:2–9. [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Phend KD, Bernardi PS, Weinberg RJ, Rustioni A. Amino acid immunocytochemistry of primary afferent terminals in the rat dorsal horn. J Comp Neurol. 1994;346:237–252. doi: 10.1002/cne.903460205. [DOI] [PubMed] [Google Scholar]
- Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- Wang HF, Shortland P, Park MJ, Grant G. Retrograde and transganglionic transport of horseradish peroxidase-conjugated cholera toxin B subunit, wheatgerm agglutinin and isolectin B4 from Griffonia simplicifolia I in primary afferent neurons innervating the rat urinary bladder. Neuroscience. 1998;87:275–288. doi: 10.1016/s0306-4522(98)00061-x. [DOI] [PubMed] [Google Scholar]
- Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain. 2001;89:265–273. doi: 10.1016/s0304-3959(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Zhong F, Christianson JA, Davis BM, Bielefeldt K. Dichotomizing axons in spinal and vagal afferents of the mouse stomach. Dig Dis Sci. 2008;53:194–203. doi: 10.1007/s10620-007-9843-z. [DOI] [PubMed] [Google Scholar]


