Abstract
Primary neuron cultures are widely used in research due to the ease and usefulness of observing individual cells. Therefore, it is vital to understand how variations in culture conditions may affect neuron physiology. One potential variation for cultured neurons is a change in intracellular transport. As transport is necessary for the normal delivery of organelles, proteins, nucleic acids, and lipids, it is a logical indicator of a cell’s physiology. We test the hypothesis that organelle transport may change with varying in vitro population densities, thus indicating a change in cellular physiology. Using a novel background subtraction imaging method we show that, at 5 days in vitro (DIV), transport of vesicular organelles in embryonic rat spinal cord neurons is positively correlated with cell density. When density increased 6.5 fold, the number of transported organelles increased 2.2 ± 0.3 fold. Intriguingly, this effect was not observable at 3–4 DIV. These results show a significant change in cellular physiology with a relatively small change in plating procedure; this indicates that cells appearing to be morphologically similar, and at the same DIV, may still suffer from a great degree of variability.
Keywords: organelle, transport, MEDIC, population density, in vitro, neuron, spinal cord
Introduction
Active transport is the primary mechanism by which organelles, proteins, nucleic acids, and lipids are delivered to relatively distant regions of a growing neuron [6]. Intracellular transport is accomplished by many molecular motors that carry this essential cargo along microtubules and actin filaments [9]. Indeed, the proper transport and delivery of such organelles and molecules may be critical to neuronal survival during cytotoxic insult; impairment of that delivery may result in neurons that are more sensitive to injury and disease.
Previous reports have found a correlation between reduced axonal transport and Huntington’s disease, Alzheimer’s disease, Amyotrophic Lateral Sclerosis (ALS), Spinal and Bulbar Muscular Atrophy (SBMA), and other neurodegenerative diseases [5, 13]. Decreases in cell density and synaptic connections have also been shown to accompany Alzheimer’s disease, suggesting that cell density, number of synaptic connections, and intracellular transport may all play significant roles in neural pathology in vivo [14]. Given this relationship, we were curious if alterations in cell density in vitro could recapitulate the altered intracellular transport observed in some neurodegenerative diseases.
Primary cultures of neurons are widely used in neuroscience research because many questions can only be examined under the simpler and easily manipulated environment of cell culture. At the same time, it is important for in vitro physiological conditions to mimic those found in vivo as closely as possible. This requirement requires tailoring culture conditions to those that maintain neurons in a “healthy” state, i.e. operating as they would in vivo. However, there is little objective data on what constitutes such a healthy state in vitro. Interestingly, neuron culture density is known to play a role in cell survival [2], phenotypic expression of neurotransmitters [1], and the rate of neurite growth and synapse formation [15]. Furthermore, studies have also found that in high density cultures, neurons exhibit greater excitability [16]. Thus, although this may not prove that higher density cell cultures are healthier per se, it does suggest that they mature faster and are more robust.
In our study, we demonstrated that increased cell density resulted in increased transport of vesicular organelles through use of Motion Enhanced Differential Interference Contrast (MEDIC) microscopy, an improved version of video-enhanced DIC [8]. MEDIC is a unique program which allowed us to observe the transport of intracellular organelles with diameters larger than 100nm, suggesting that the moving organelles we observed include the following: mitochondria (up to 800nm) [7], lysosomes (100–500nm) [3], peroxisomes (150–300nm) [12], and endosomes (<500nm) [11]. Identifying which organelles, in particular, were being observed was not within the scope of this study; instead, we were concerned with the fact that transport, in general, was changing and that this transport indicates an important physiological change within the neurons.
Our data show that at 5 days in vitro (DIV), transport of vesicular organelles in embryonic rat spinal cord neurons increases with increasing population density: as the density increases 6.5 fold, the number of transported organelles increases by 2.2 ± 0.3 fold. Our data indicate that there are obvious physiological differences between low and high density cultures with respect to organelle transport, and these differences could result in the change of other physiological markers.
It is worth noting that population density and length of time in vitro, combine to play an important role in synaptogenesis. Synaptic formation in vitro is both dependent upon target distance (i.e. population density) and DIV; in particular, after 5 DIV, active synapses are found to be prevalent in vitro [15]. In light of this study, the change in transport observed in our experiments at 5 DIV, but not at 3–4 DIV, shows the importance of time in vitro, as well as plating density, in regard to at least two physiological markers – organelle transport and number of synaptic connections.
Material and Methods
Cell Culture
All rats were handled according to procedures approved by the Animal Care and Use Committee of Wake Forest University. E18 embryos were surgically removed from a pregnant Sprague-Dawley rat anesthetized with 1% isoflurane (Butler – Dublin, Ohio). Seven of the embryos were placed in cold phosphate buffered saline (PBS). While in PBS, the spinal cord was dissected from each and then placed into cold Hibernate-E supplemented with B27 (45mL+1mL respectively; Brain Bits LLC, Springfield, IL and Invitrogen, Carlsbad, CA). After all spinal cords were collected in 2 mL Hibernate-E + B27, trypsin (Invitrogen) and DNase (Sigma Aldrich, St. Louis, MO) were added to final concentrations of 0.1% and 50µg/mL, respectively. After five minutes of incubation at 37°C, the tissue was gently triturated; the resulting cell suspension was collected, leaving the debris that settled to the bottom of the tube. After centrifuging the suspension at 60 × g for five minutes, the cell pellet was resuspended in 4mL of plating medium to create the cell suspension stock. The plating medium consisted of 250mL Neurobasal Medium to which was added 625µL of 200mM L-glutamine, 4mL B27 (both from Invitrogen), 62.5µL of 16.9mg/ml glutamate (Sigma), and 0.5mL of an antibiotic/antimycotic solution (10mg/mL streptomycin, 25µg/mL amphotericin, and 10,000units/mL penicillin [Sigma]).
Round, No. 1.5 coverslips (12mm diameter, Fisher Scientific – Pittsburg, PA) were washed by sequential cleaning with a 1% Alconox solution, 70% ethanol, glacial acetic acid, and deionized water before being autoclaved. They were then coated overnight with >70KDa poly-D-lysine (40µg/mL, Sigma), and placed into the central well of a 35mm glass-bottomed culture dish (MatTek Corp., Ashland, MA). The suspensions of neurons were added (400µL) to cover the coverslip. Three suspension densities were used, low, medium and high, consisting of 20–25, 38–53 and 67–127 cells/mm2, respectively. Cultures were kept in a 5% CO2 incubator at 37°C, fed the day after plating (bringing the total volume to 1.4mL), and allowed to grow for 3–5 days before observation. The feeding medium was the same as the plating medium, except for the exclusion of glutamate. The B27 present in the plating and feed media prevented the proliferation of glia [4]. For the observations reported here, a total of five cultures consisting of 15–20 coverslips at each of the three densities were analyzed. Five cells per coverslip were selected for analysis of transport at randomly generated X,Y coordinates. If there was not a neuron at the randomly generated X,Y coordinate, we chose the next closest neuron. These were then viewed using MEDIC and analyzed as described below.
Microscopy
The MEDIC microscopy set-up consisted of a Nikon E600 FN microscope equipped with a Nikon 60X water-immersion objective (NA 1.0), condenser (NA 0.9), and DIC prisms. Images were collected using a 12-bit, Hamamatsu C4742-95 ORCA ER cooled CCD camera at a frame rate of 8.3 frames per second. This system was located in a separate building from the culture facility. To transport the cultures, the growth medium was replaced with Hibernate-E+B27 (a pH-independent medium) and maintained at 37°C. Before observation, the Hibernate-E was replaced via perfusion with artificial cerebrospinal fluid (aCSF), consisting of (in mM) 137 NaCl, 5 KCl, 10 NaHCO3, 20 HEPES, 5.5 glucose, 0.6 KH2PO4, 0.6 Na2HPO4, 1.4 CaCl2, and 0.9 MgSO4. The pH of the solution was adjusted with NaOH to 7.4. Perfusion with aCSF was performed for approximately 10–15 minutes at a rate of 1mL/. The perfusion was stopped five minutes prior to viewing. The total volume of aCSF covering the cells at any given time was approximately 5mL. The cells were kept at 37°C during observation. A single dish was never analyzed for more than 45 minutes, although previous studies performed with our setup on the same cell type had shown that the cells maintain consistent levels of transport for at least four hours. Unfortunately, sterility could not be maintained for day-to-day observations of the same cells, so each day (3, 4, or 5) new cells from the same culture were observed.
MEDIC
This real-time image processing addition to the microscope is described in detail elsewhere [8]. As shown in Figure 1, MEDIC can take a DIC image (A) and provide a background subtracted image (B) where organelle movement can be clearly seen (inset) within the neurite or soma (see also Movie 1, Supplemental Information). These MEDIC images allowed counts to be made for all the organelles moving within the region of interest for a given neuron.
1. Example of the use of MEDIC for imaging organelle transport.
MEDIC takes a DIC image and continually subtracts the background to make vesicles or organelles more visible when moving. The image on the left (A) is a raw DIC image; Panel B is a background-subtracted image that shows organelles undergoing transport within the neuron’s soma (lower left arrow) and processes (upper and lower right arrows). Inset: Arrow in the digital enlargement indicates the same vesicular organelle indicated by upper right arrow. Panel C shows the manner in which areas were determined - the background (red) was deleted, allowing the neuronal ROI to be measured. The scale bar represents 10µm.
Data Analysis
Each of the two digital video recordings, per neuron, of organelle movement was analyzed by separate individuals to control for any observer bias. An organelle was defined as moving if it was transported a distance more than 3x its diameter. The neuronal region of interest (ROI) did not include the entirety of a neuron’s area, but instead, the area visible within the field of view, which included the soma and a portion of the processes extending outwards from the cell body (Figure 1). The counts of moving organelles from two videos of the same neuronal ROI were averaged. Then, all of the single-cell averages for a given density were compiled in order to obtain the average number of moving organelles per neuronal ROI during a 24-second recording (the length of a MEDIC video).
Fixation, Density Determination, and Area Determination
After observation, the neurons were fixed in 4% PFA (paraformaldehyde; Electron Microscopy Sciences, Washington, PA] for 15–30 minutes then replaced with PBS and stored at 4°C until mounted on slides. Density counts of nuclei were done on neurons stained with DAPI-containing Vectashield HardSet Mounting Media (Vector Labs, Burlingame, CA). Two diagonally adjacent 4x images per coverslip were captured from two coverslips at each density for each of the five cultures analyzed. The nuclei visible in these images were counted and the counts from the four images (two images for each of the two coverslips) were averaged to arrive at the density for each of the categories.
The area of each neuronal ROI was determined by tracing the cell perimeter by hand using Adobe Photoshop CS (Figure 1C); after which, ImageJ [rsb.info.nih.gov/ij] was used to find the area of the tracing. Area determinations were done for the first two neurons in each density category resulting in a total of 102 neuronal ROIs being measured.
Statistical Analyses
After compiling the number of moving organelles in each of the neurons, the averaged population densities and transport values were plotted on an X,Y graph. A linear regression of the data provided a p-value that confirmed a positive linear relationship between organelle movement and cell density and the r2-value was calculated to show the degree to which these two measures were correlated. A one-way ANOVA was used to confirm that there were no significant differences in the areas of the neurons analyzed in each cell density group. This same test was used to confirm the consistency of the population densities.
Results
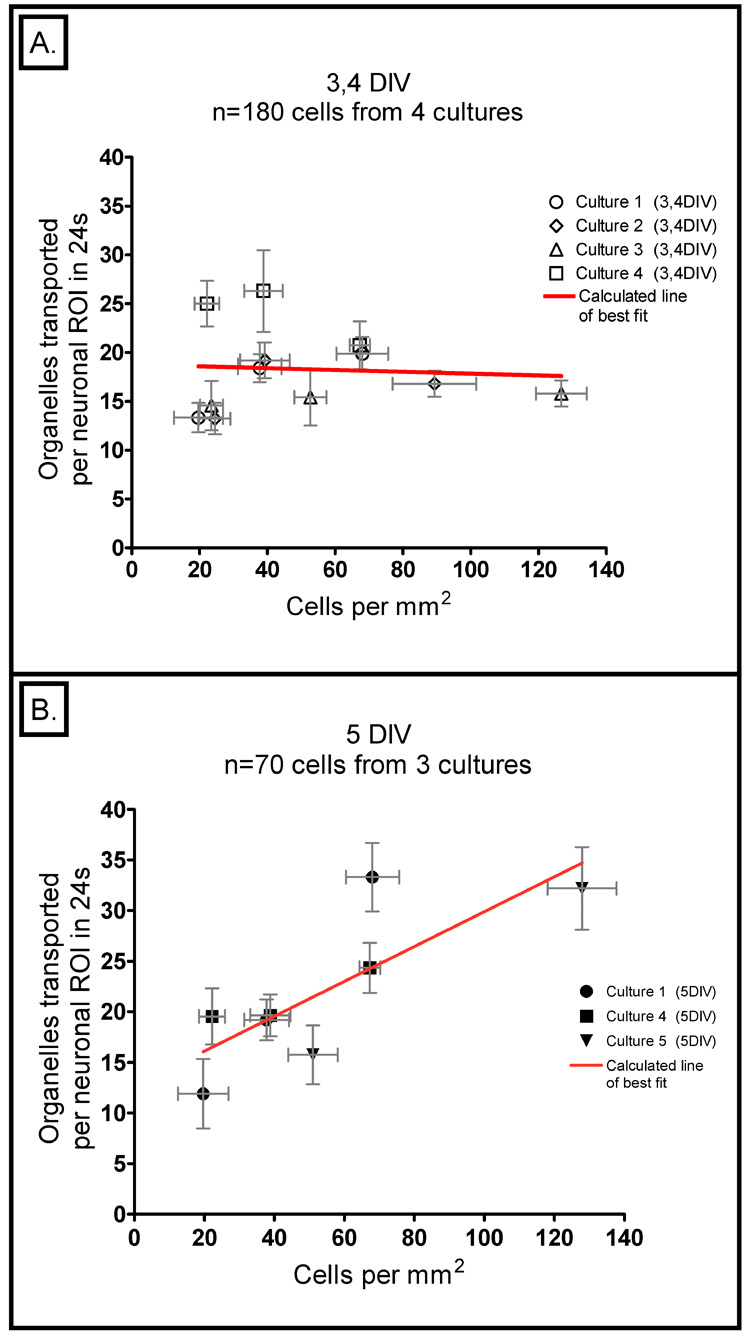
Figure 1A is an example of the typical neuron in which organelle transport was tracked; Figure 1B illustrates how the MEDIC technique enhances the detection of moving structures within the stationary features of the cell. When we analyzed how many moving organelles were present in neurons at 3–4 DIV, we found there was considerable variation from one culture to the next, but it appeared to be random and unrelated to the cell density (Fig. 2A). The p-value provided by a linear regression of these points was 0.83, showing that the slope of the line formed by these data is not statistically different from zero. Also an r2-value of 0.00 for this regression further indicates completely random scatter. Repeating the same analysis on another set of neurons cultured for just one more day (5 DIV), revealed a striking change. A clear positive relationship was observed between cell density and the number of transported organelles: the neurons cultured at high densities showed significantly more transport compared to those at low densities (Figure 2b). This data provided a slope that is statistically different from zero with a p-value of 0.018 and an r2-fit of 0.635. The line fit to this data, over the 6.5 fold change in density, resulted in a 2.2 ± 0.3 fold increase in transport.
2. Plot of the number of transported organelles versus cell density.
A) At 3 – 4 DIV, the data fall on a line with a slope that is not statistically different from zero (p-value = 0.83) and there is an extremely poor fit to for this regression (r2=0.00). This graph represents a total of 180 neurons from 3 and 4 DIV; these data were gathered from four different cultures. In both panels of this figure, each data point represents the average of all the neurons from a single density category (Low, Medium, or High) from a single culture; the horizontal error bars reflect the standard error of the mean (SEM) of cell density and the vertical ones reflect the SEM of the number of transported organelles observed. B) The same analysis at 5 DIV provides data that fall on a line with a slope that is significantly different from zero (p-value = 0.018) with a much higher correlation (r2=0.635). This graph represents a total of 70 neurons from three different cultures.
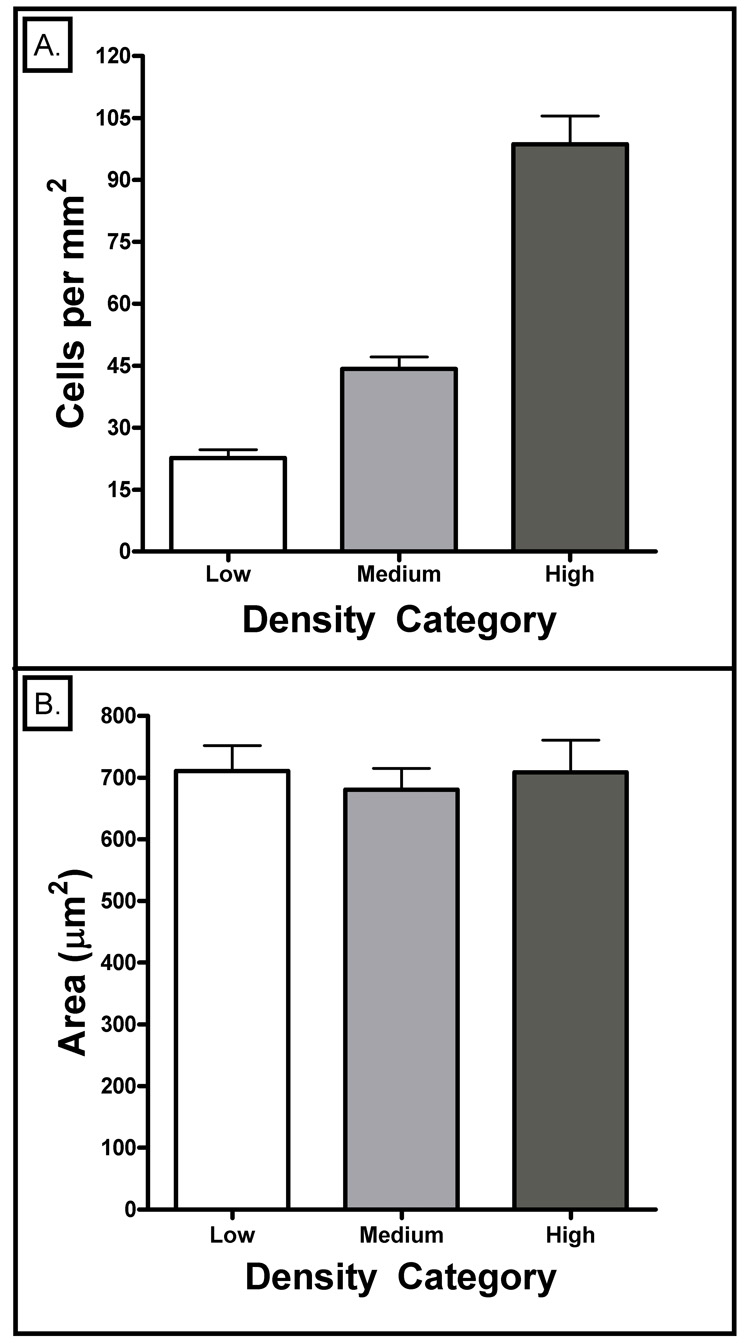
To confirm that the cell densities in each group really were different, we measured them. Figure 3A shows that the low, medium and high density groups had averages of 23, 44 and 99 cells/mm2, respectively. An ANOVA statistical analysis with a Bonferroni post-hoc test provided p-values <<0.05 indicating that the density categories were significantly different. We also wanted to make sure that the cell densities did not have a systematic effect on the sizes of the cells, as that would likely alter the number of moving organelles being observed. Figure 3B shows that all the analyzed neuronal ROIs covered similar areas of the culture dish. This was verified through use of a one-way ANOVA analysis with a Bonferroni post-hoc test which provided p-values for all column comparison that were greater than 0.05 (Figure 3b).
3. Analysis of neuronal density and area (3–5 DIV).
A) Cell density counts were done on two coverslips, per category (Low, Medium, High), for each of the five cultures. Error bars represent the SEM. All three densities were statistically different according to a Bonferroni post-hoc ANOVA analysis (all column comparisons had p-values << 0.05).
B) Mean areas were analyzed for the first two neurons on each coverslip, for a total of 34 neurons per category and 102 neurons total. Error bars represent the SEM. All three densities had statistically similar areas according to a Bonferroni post-hoc ANOVA analysis (all column comparisons had p-values > 0.05).
Discussion
Primary neuron cultures are frequently used in neuroscience research. It is generally assumed that if the neurons look similar from one culture to the next, and are at the same DIV, then their physiological states are similar as well. Therefore, they should be expected to respond similarly to whatever experimental manipulation is being performed. However, our observations showing dramatic differences in organelle transport in neurons that appear morphologically similar (Fig. 2B), bring this assumption into question. We found that there is a systematic effect of a small difference in cell density on the number of organelles being transported (Fig. 2B). Since a reduction in axonal transport is interpreted as an impaired neuronal function [5, 13], one could possibly infer that lower density cultures are not as optimal as higher density cultures at 5 DIV. Even though MEDIC is not capable of observing in vivo transport, our observations of 5 DIV cultures also show that in vitro cells can not consistently be accurate representations of in vivo physiology - specifically, in regard to intracellular transport of organelles; even if one of these culture densities had similar levels of transport to what might be seen in vivo, the other cultures would consequently not be reliable indicators of normal in vivo physiology.
Another important point to glean from our results is the fact that organelle transport increases with population density and is not merely an artifact of observing more or less neuronal area within the ROI (Figure 3b). Moreover, this density dependence of transport is also time dependent, as it is only observed at 5 DIV but not at 3–4 DIV (Figure 2). There is some precedence for changes in intracellular transport with DIV. For example, one study using the MEDIC technique showed that velocities of organelle transport in chick motor neurons slowed with increasing time in vitro [10].
Figure 2A also shows that even at 3–4 DIV, the pattern of increasing transport with increasing density begins to vaguely emerge as can be seen in the overlapping points seen at 20, 40, and 70 cells per mm2. However, the pattern is not consistent as can be seen by the very high p-value and very low r2-value for the data from 3–4 DIV
The questions that arise from our study are: (1) What neuronal density is necessary for optimal cell physiology; (2) What is the time in culture during which this optimal state is maintained; and (3) Does axonal transport in vitro ever approximate what occurs in vivo? In future studies using the MEDIC technique, we will examine the relationship between numbers of transported organelles and neuronal viability. For example, at what point does a decrease in axonal transport predict cell death? Additionally, we plan to devise ways to track the transport of specific organelles, like mitochondria, which will allow us to understand better the functional importance of the overall transport changes we have described.
These results reveal a method for quantifying subtle changes in neuronal physiology in vitro that will likely be important to many different studies of neuronal function and survival. However, the significance of our current results lies primarily in the form of a cautionary note. Labs that use cell culture work in an attempt to mimic in vivo physiology, or those that have seen inconsistency with in vitro cultures, may be dealing with variations caused by changes in the population densities of their cultures.
Consequently, it is important to very strictly control plating densities and time in vitro for optimal reproducibility.
Supplementary Material
Acknowledgements
A special thanks to Dr. George Holzwarth (Wake Forest University), Dr. Mac Robinson (Wake Forest University Medical School), and Dr. Susan E. Fahrbach (Wake Forest University) for the thoughts, suggestions, reviews, and supplies that they provided. Funding for these experiments was provided by Wake Forest University’s Faculty Start-up Grant to JCM and an NIH grant (R01 NS051632) to CPT. Experiments and data analysis were performed by the first four authors; manuscript writing was done by the first and fourth authors; intellectual and material contributions were made by all authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler JE, Black IB. Sympathetic neuron density differentially regulates transmitter phenotypic expression in culture. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:4296–4300. doi: 10.1073/pnas.82.12.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12:243–260. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 3.Bednarski E, Ribak CE, Lynch G. Suppression of cathepsins B and L causes a proliferation of lysosomes and the formation of meganeurites in hippocampus. J Neurosci. 1997;17:4006–4021. doi: 10.1523/JNEUROSCI.17-11-04006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. Journal of neuroscience research. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 5.Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochimica et biophysica acta. 2006;1762:1094–1108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 7.Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap AP, Wieloch T. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J Neurosci. 1998;18:5151–5159. doi: 10.1523/JNEUROSCI.18-14-05151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill DB, Macosko JM, Holzwarth G. Motion-enhanced, differential interference contrast (MEDIC) microscopy of moving vesicles in live cells: VE-DIC updated. Accepted for publication in Journal of Microscopy. 2008 doi: 10.1111/j.1365-2818.2008.02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nature reviews. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- 10.Macosko JC, Newbern JM, Rockford J, Chisena EN, Brown CM, Holzwarth GM, Milligan CE. Fewer active motors per vesicle may explain slowed vesicle transport in chick motoneurons after three days in vitro. Brain Research. doi: 10.1016/j.brainres.2008.03.014. In Press, Accepted Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin EJ, Kim M, Velier J, Sapp E, Lee HS, Laforet G, Won L, Chase K, Bhide PG, Heller A, Aronin N, Difiglia M. Analysis of Huntingtin-associated protein 1 in mouse brain and immortalized striatal neurons. The Journal of comparative neurology. 1999;403:421–430. [PubMed] [Google Scholar]
- 12.Moreno S, Nardacci R, Ceru MP. Regional and ultrastructural immunolocalization of copper-zinc superoxide dismutase in rat central nervous system. J Histochem Cytochem. 1997;45:1611–1622. doi: 10.1177/002215549704501204. [DOI] [PubMed] [Google Scholar]
- 13.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science (New York, N.Y. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 14.Terry RD. Cell death or synaptic loss in Alzheimer disease. Journal of neuropathology and experimental neurology. 2000;59:1118–1119. doi: 10.1093/jnen/59.12.1118. [DOI] [PubMed] [Google Scholar]
- 15.van den Pol AN, Obrietan K, Belousov AB, Yang Y, Heller HC. Early synaptogenesis in vitro: role of axon target distance. The Journal of comparative neurology. 1998;399:541–560. doi: 10.1002/(sici)1096-9861(19981005)399:4<541::aid-cne7>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Wyart C, Ybert C, Douarche C, Herr C, Chatenay D, Bourdieu L. A New Technique to Control the Architecture of Neuronal Networks in vitro. New York: Karger, Basel; 2005. pp. 23–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.