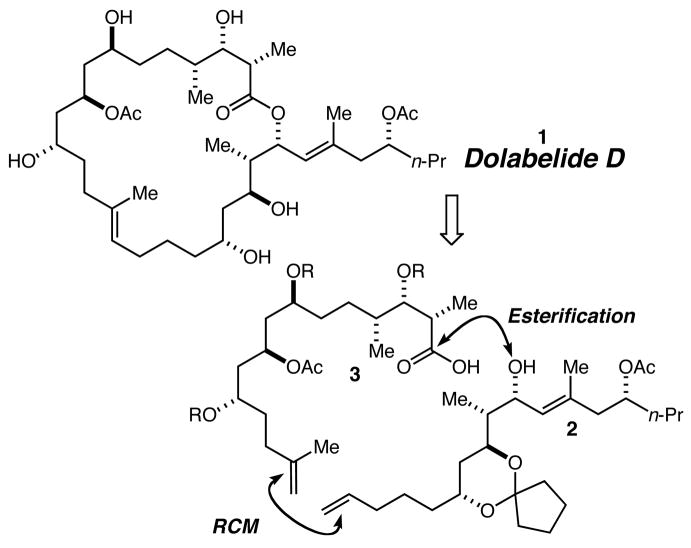
In 1995 researchers reported the isolation and structural characterization of two new 22-membered macrolides they termed dolabelides A and B from Japanese specimens of the sea hare Dolabella auricularia.1 These compounds exhibited cytotoxic activity against HeLa-S3 cells with IC50s of 6.3 and 1.3 μg/mL, respectively. Two years later, two new members of this class of natural products, dolabelides C and D were reported.2 These 24-membered macrolides are also cytotoxic against HeLa-S3 cells with IC50s of 1.9 and 1.5 μg/mL, respectively. This biological activity and the interesting stereostructure of these natural products have combined to elicit attention from synthetic chemists3 including our own group.4 Herein we describe our investigations that have led to the first total synthesis of dolabelide D, by way of the synthesis and coupling of fragments 2 and 3 by esterification and ring-closing metathesis (Scheme 1).
Scheme 1.
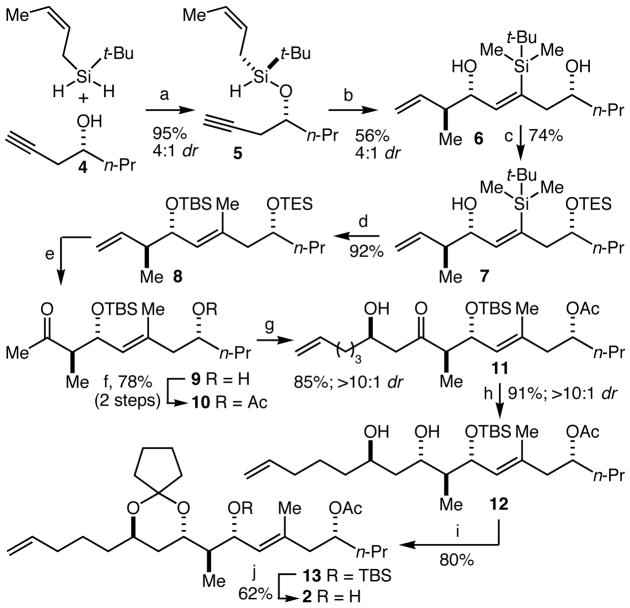
The synthesis of fragment 24 commenced with an application of our recently developed catalytic asymmetric silane alcoholysis5 with alcohol 4 and t-butyl-cis-crotylsilane to provide 5 as the major component of a 4:1 mixture of diastereomers in 95% yield (Scheme 2). Rhodium-catalyzed tandem silylformylation-crotylsilylation6 proceeded stereospecifically to provide, after quenching with methyllithium, a 4:1 mixture of diastereomers favoring 1,5-syn-diol 6 in 56% yield. Selective protection of the less-hindered alcohol as its triethylsilyl (TES) ether led, after separation of the diastereomers, to the isolation of 7 in 74% yield. Treatment of alcohol 7 with n-BuLi and then CuBr•SMe2 and DMPU initiated a Brook-like 1,4 carbon (sp2) to oxygen silane migration,7 and the resulting vinylcopper species was then alkylated with MeI to provide 8 in 92% yield. This sequence illustrates the power of the tandem silylformylation chemistry to provide access to different functionalities and substitution patterns in the 1,5-diol products. In addition, it is noteworthy that the t-butylsilane serves multiple purposes before being morphed into the desired t-butyldimethylsilyl (TBS) ether. A Wacker oxidation was optimized for concurrent removal of the TES ether, and the resulting alcohol 9 was acetylated to provide 10 in 78% overall yield (2 steps). Asymmetric aldol coupling8 with 5-hexenal then gave aldol 11 in 85% yield and with >10:1 diastereoselectivity. Anti-diastereoselective (>10:1 dr) β-hydroxyketone reduction9 then gave 12 in 91% yield. Protection of the diol as a cyclopentylidene ketal10 gave 13 and TBS removal provided fragment 2 in 50% yield (2 steps from 12). The synthesis of 2 was thus achieved in 10 steps and 11% overall yield from 4.
Scheme 2.
(a) 4 mol% CuCl, 4 mol% NaO-t-Bu, 4 mol% (R, R)-BDPP, PhH. (b) i. 2 mol% [Rh(acetone)2-(P(OPh)3)2]BF4, CO, PhH, 60 °C; ii. MeLi, Et2O −78 to 23 °C. (c) TESCl, Et3N, CH2Cl2, −20 °C. (d) n-BuLi, THF, −78 °C; CuBr•Me2S, DMPU, 23 °C; MeI, −78 to 23 °C. (e) 25 mol% PdCl2, CuCl, DMF, THF, H2O, O2. (f) Ac2O, pyridine, DMAP, CH2Cl2. (g) (+)-(ipc)2BCl, Et3N, 5-hexenal, Et2O −78 to 23 °C. (h) Me4NBH(OAc)3, AcOH, CH3CN, THF, −40 to −20 °C. (i) 1,1-dimethoxycyclopentane, PPTS, CH2Cl2. (j) n-Bu4NF, THF.
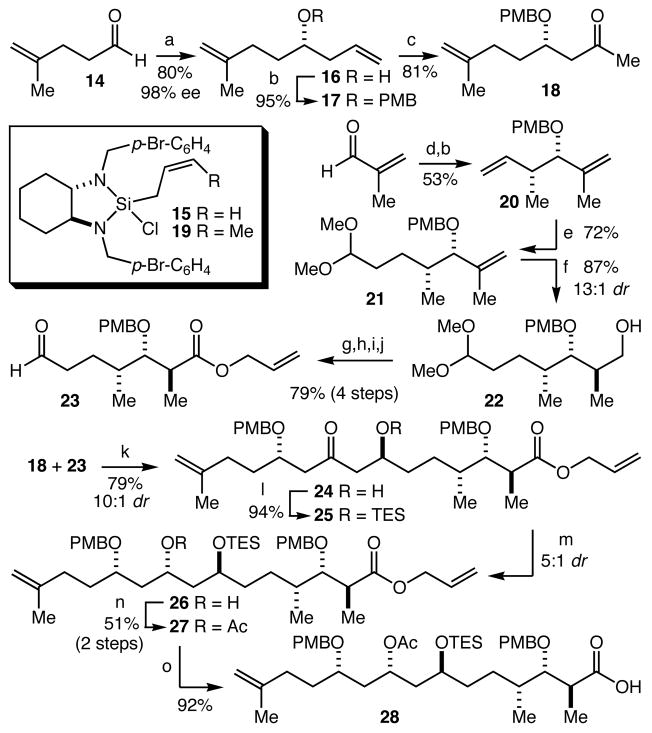
Allylation of aldehyde 14 with our recently developed reagent 1511 proceeded smoothly to provide 16 in 80% yield and 98% ee (Scheme 3). Protection of the alcohol as its p-methoxybenzyl (PMB) ether gave 17 in 95% yield, and was followed by a Wacker oxidation to give ketone 18 in 81% yield. Crotylation of methacrolein with crotylsilane ent-1912, followed by protection of the resultant alcohol as its PMB ether produced 20 in 53% yield (based on ent-19, 2 steps) and 88% ee. Hydroformylation in the presence of 2,2-dimethoxypropane proceeded smoothly and selectively to give acetal 21 in 72% yield. Still-Barrish hydroboration13 gave alcohol 22 with 13:1 dr. A 4 step oxidation-oxidation-protection-deprotection sequence then provided aldehyde 23 in 79% overall yield. 1,5-Anti selective aldol coupling14 of ketone 18 and aldehyde 23 proceeded smoothly to give aldol 24 in 79% yield as a 10:1 mixture of diastereomers. Protection of the alcohol as a triethylsilyl (TES) ether gave 25 in 94% yield and was followed by a diastereoselective (~5:1) ketone reduction with L-Selectride to give 26. Following acetylation, the diastereomers were separated and 27 was isolated in 51% yield. Finally, deprotection of the allyl ester gave the target acid 28 in 92% yield. The synthesis of 28 was carried out with a longest linear sequence of 13 steps from methacrolein in 9% overall yield.
Scheme 3.
(a) 15, CH2Cl2, −20 °C. (b) NaH, PMBBr, THF, reflux, (c) 25 mol% PdCl2, CuCl, DMF, H2O, O2. (d) ent-19, CH2Cl2. (e) 2 mol% Rh(acac)(CO)2, 10 mol% PPh3, H2/CO, 2,2-dimethoxypropane, PPTS, 60 °C. (f) 9-BBN, THF, −78 to 23 °C; H2O2, NaOH. (g) (COCl)2, DMSO, Et3N, CH2Cl2, −78 °C. (h) NaClO2, NaH2PO4,2-methyl-2-butene, t-BuOH, H2O. (i) K2CO3, CH2=CHCH2Br, acetone, reflux, (j) PPTS, acetone, H2O, reflux, (k) n-Bu2BOTf, i-Pr2NEt, Et2O, −110 °C. (1) TESCl, imidazole, CH2Cl2. (m) L-Selectride, CH2Cl2, −78 °C. (n) Ac2O, pyridine, DMAP, CH2Cl2. (o) 10 mol% Pd(PPh3)4, morpholine, THF.
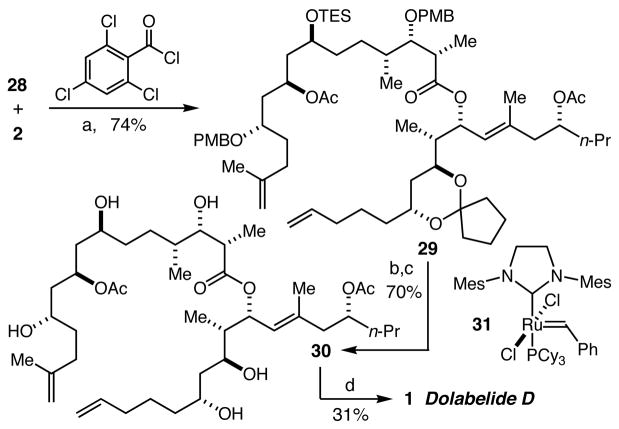
Esterification of alcohol 2 with acid 28 proceeded smoothly to give 29 in 74% yield (Scheme 4). Methanolysis of the TES ether and cyclopentylidene ketal protecting groups was followed by oxidative cleavage of the PMB ether groups to provide pentaol 30 in 70% overall yield (2 steps). Initial attempts at macrocyclization by ring closing metathesis with the “second-generation” Grubbs catalyst 31 were plagued not only by (not unexpected) low stereoselectivity (~1.3:1 E:Z), but also by significant amounts of byproducts presumably derived from olefin isomerization pathways.15 Despite these setbacks, dolabelide D could be isolated in 31% yield. Although a sample of the natural product was unavailable, comparison (1H and 13C NMR, IR, HRMS, [α]D) to published data confirmed the identity of our synthetic material.
Scheme 4.
(a) Et3N, DMAP, toluene, −78 to 0 °C. (b) PPTS, MeOH. (c) DDQ, CH2Cl2, pH 7 buffer, (d) 25 mol% 31, CH2Cl2, reflux.
The first synthesis of dolabelide D (and of any of the dolabelides) has been achieved. Methodologically, the four step sequence that converts alcohol 4 into protected diol fragment 8 is especially noteworthy and serves as a demonstration of the power of the catalytic asymmetric silane alcoholysis and tandem silytformylation-crotylsilylation methods. That the pathway from alcohol 4 to dolabelide D comprises just 14 linear steps (the longest linear sequence is from methacrolein to dolabelide D in 17 steps) is testament to the efficiency of these methods.
Supplementary Material
Experimental procedures, characterization data, and stereochemical proofs. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgments
The NIH (NIGMS GM58133) is acknowledged for their generous support of this work, and for a postdoctoral fellowship to D.R.S. P.K.P. is supported by the NIH Medical Scientist Training Program. We thank Bristol-Myers Squibb for a graduate fellowship to S.J.O.
References
- 1.Ojika M, Nagoya T, Yamada K. Tetrahedron Lett. 1995;36:7491. [Google Scholar]
- 2.Suenaga K, Nagoya T, Shibata T, Kigoshi H, Yamada K. J Nat Prod. 1997;60:155. [Google Scholar]
- 3.(a) Grimaud L, de Mesmay R, Prunet J. Org Lett. 2002;4:419. doi: 10.1021/ol017122w. [DOI] [PubMed] [Google Scholar]; (b) Desroy N, Le Roux R, Phansavath P, Chiummiento L, Bonini C, Genêt JP. Tetrahedron Lett. 2003;44:1763. [Google Scholar]; (c) Le Roux R, Desroy N, Phansavath P, Genêt JP. Synlett. 2005:429. [Google Scholar]; (d) Keck GE, McLaws MD. Tetrahedron Lett. 2005;46:4911. doi: 10.1016/j.tetlet.2005.04.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt DR, Park PK, Leighton JL. Org Lett. 2003;5:3535. doi: 10.1021/ol035431b. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt DR, O’Malley SJ, Leighton JL. J Am Chem Soc. 2003;125:1190. doi: 10.1021/ja0283201. [DOI] [PubMed] [Google Scholar]
- 6.(a) Zacuto MJ, O’Malley SJ, Leighton JL. J Am Chem Soc. 2002;124:7890. doi: 10.1021/ja026511y. [DOI] [PubMed] [Google Scholar]; (b) Zacuto MJ, O’Malley SJ, Leighton JL. Tetrahedron. 2003;59:8889. [Google Scholar]
- 7.Taguchi H, Ghoroku K, Tadaki M, Tsubouchi A, Takeda T. Org Lett. 2001;3:3811. doi: 10.1021/ol016837w. [DOI] [PubMed] [Google Scholar]
- 8.(a) Paterson I, Goodman JM, Lister MA, Schumann RC, McClure CK, Norcross RD. Tetrahedron. 1990;46:4663. [Google Scholar]; (b) Paterson I, Florence GJ, Gerlach K, Scott JP, Sereinig N. J Am Chem Soc. 2001;123:9535. doi: 10.1021/ja011211m. [DOI] [PubMed] [Google Scholar]
- 9.Evans DA, Chapman KT, Carreira EM. J Am Chem Soc. 1988;110:3560. [Google Scholar]
- 10.For a discussion of the advantages of this diol protecting group, see: Evans DA, Connell BT. J Am Chem Soc. 2003;125:10899. doi: 10.1021/ja027638q.
- 11.Kubota K, Leighton JL. Angew Chem Int Ed. 2003;42:946. doi: 10.1002/anie.200390252. [DOI] [PubMed] [Google Scholar]
- 12.Hackman BM, Lombardi PJ, Leighton JL. Org Lett. 2004;6:4375. doi: 10.1021/ol0480731. [DOI] [PubMed] [Google Scholar]
- 13.Still WC, Barrish JC. J Am Chem Soc. 1983;105:2487. [Google Scholar]
- 14.(a) Paterson I, Gibson KR, Oballa RM. Tetrahedron Lett. 1996;37:8585. [Google Scholar]; (b) Evans DA, Coleman PJ, Côté B. J Org Chem. 1997;62:788. [Google Scholar]
- 15.The recently reported method to suppress such pathways did not provide significant improvement in this case. See: Hong SH, Sanders DP, Lee CW, Grubbs RH. J Am Chem Soc. 2005;127:17160. doi: 10.1021/ja052939w.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, characterization data, and stereochemical proofs. This material is available free of charge via the internet at http://pubs.acs.org.