Abstract
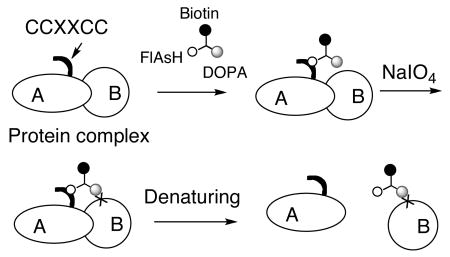
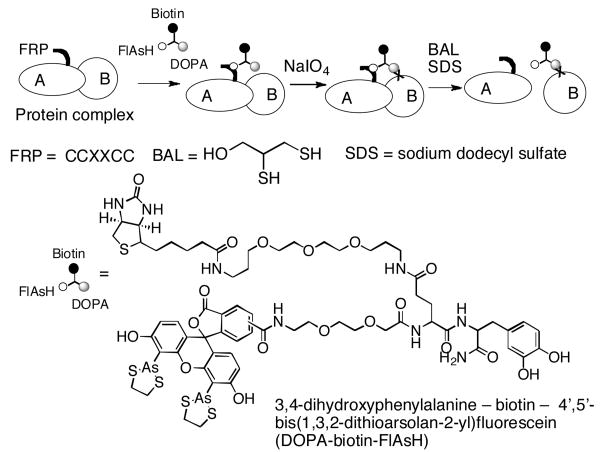
A new label transfer method is presented that overcomes most of the limitations of current systems. A protein of interest is tagged with tetra-cysteine sequence (FlAsH Receptor Peptide (FRP)) that binds tightly and specifically to a chimeric molecule 3,4-dihydroxyphenylalanine – biotin – 4′,5′-bis(1,3,2-dithioarsolan-2-yl)fluorescein (DOPA-biotin-FlAsH). Upon brief periodate oxidation, the DOPA moiety is cross-linked to nearby surface-exposed nucleophiles. Boiling the products in excess dithiol dissolves the FlAsH-FRP interaction, resulting in transfer of the biotin tag to the partner proteins, allowing them to be identified by standard methods.
Most methods to characterize protein-protein interactions require the protein of interest to be displayed as an artificial fusion outside of its native environment. To overcome such limitations, a biochemical method called label transfer has been developed, in which a latent cross-linking agent and some easily detectable tag such as a biotin or a radiolabel are connected to the protein of interest via a cleavable linker. Once the modified protein is incorporated into its native complex, the cross-linking moiety is activated and the linker arm is then cleaved, resulting in transfer of the tag to the partner protein1. While this approach has been employed in the study of some protein complexes,2,3 the utility of current label transfer technology is severely limited by the requirement to modify the protein of interest covalently and then reconstitute the complex of interest with the modified protein. The inefficient cross-linking chemistry commonly used in such systems (usually photolysis of aryl azides) also complicates the characterization of partner proteins3.
We report here a new type of label transfer system that eliminates the need for covalent modification of the protein of interest (see Scheme 1). It involves tagging the “bait” protein with a tetracysteine-containing peptide (CCPGCC, here called FRP for FlAsH Receptor Peptide) that binds with high affinity and specificity to a biarsenical derivative of fluorescein called FlAsH.4 We have synthesized a FlAsH derivative that also contains tethered biotin and DOPA residues. The latter, when oxidized with sodium periodate, is transformed into a reactive ortho-quinone that can cross-link with nearby nucleophilic amino acids with yields far higher than are typically observed for photo-activated cross-linkers5. Extensive control reactions have demonstrated that DOPA-protein cross-linking occurs only when the reactive partners are held in close proximity6. After cross-linking, the FlAsH-FRP complex can be dissociated by boiling in an excess of dithiol, resulting in transfer of the biotin label to nearby partner proteins.
Scheme 1.
We first assessed the efficiency of this new label transfer reaction in a relatively simple system by examining the interaction of a Glutathione-S-Transferase-Gal80-binding-peptide (GST-G80BP) fusion protein with the His6-Gal80 protein. The 20-residue G80BP peptide7, selected by phage display binds His6-Gal80 with a KD value of ≈ 300 nM, a value typical of many moderate affinity protein-protein interactions. An FRP site was inserted either between GST and G80BP or at the C-terminal end of the protein and the resultant constructs (GST-FRP-G80BP and GST-G80BP-FRP) were expressed and purified. Two control GST fusions lacking either the G80BP (GST-FRP) or the FRP (GST-G80BP) were also prepared. Each GST fusion (1 μM) was incubated with compound the DOPA-biotin-FlAsH conjugate (DBF; see Scheme 1) (0.1 μM, at which non-specific labeling was minimal, Fig. S1, supporting information) and His6-Gal80 protein (1 μM) in an excess E. coli lysate such that the GST fusion and Gal80 proteins represented less than 4% of the total protein mass. Sodium periodate (5 mM) was then added to trigger the cross-linking reaction. After two minutes, the reaction was quenched with buffer containing 100 mM dithiolthreitol (DTT) and 2 mM British anti-Lewisite (BAL; 2,3-dimercaptopropanol). The samples were boiled, subjected to SDS-PAGE and the gels were transferred and blotted with NeutrAvidin-HRP conjugate to detect biotinylated proteins.
Strong bands representing biotinylated proteins with a molecular mass corresponding to the GST fusion protein were observed in all cases where the fusion included the FRP tag (lanes 3, 5, 6, 7, 9 and 10, Figure 1, top panel). This represents self-labeling through reaction of the FRP-tethered DBF with a nucleophilic residue in the fusion protein. When the GST protein lacked the FRP tag, little or no self-labeling was observed (lane 4 and 8, top panel, Figure 1).
Figure 1.
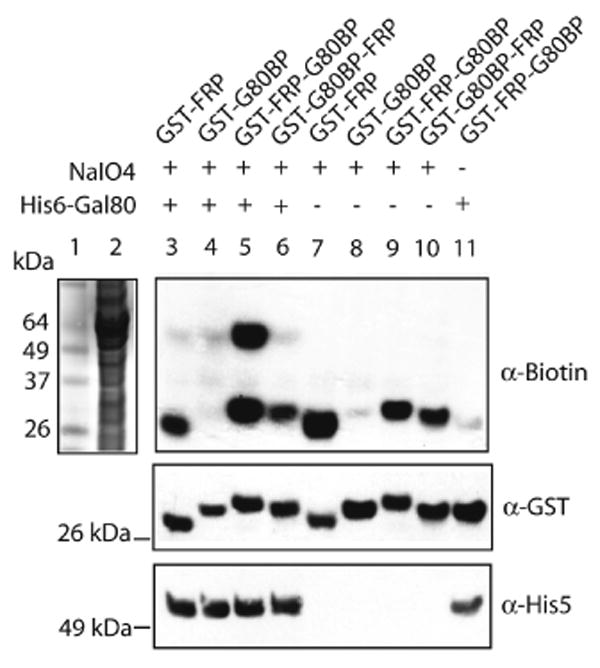
DBF (0.1 μM) transfer from GST-FRP-G80BP (1 μM) to His6-Gal80 (1 μM) probed by Western blotting. Lane 1: Protein standards. Lane 2: E. coli lysate containing 1 μM His6-Gal80 stained by Coomassie blue. Lane 3-6: Reaction mixtures with His6-Gal80. Lane 7-10: Reaction mixtures without His6-Gal80. Lane11: Same as lane 3 but without NaIO4 induction.
In the solution containing both GST-FRP-G80BP and His6-Gal80, addition of periodate resulted in the production of a distinct band with an apparent mass of ≈ 60 kDa (lane 5, top panel, Figure 1). This band represents biotinylated His6-Gal80, as demonstrated by blotting with an anti-His5 antibody (bottom panel, Figure 1). This band was absent when His6-Gal80 (lane 9, top panel, Figure 1) or NaIO4 (lane 11, top panel, Figure 1) was excluded from the reaction. These results are consistent with specific label transfer of the DBF compound from GST-FRP-G80BP to His6-Gal80 upon periodate oxidation and boiling in dithiol. We estimated the yield of the label transfer reaction as at least 85% with a modified “pull down” assay (see Fig. S2), far higher than is normally observed for similar reactions using photo-activated label transfer agents.3 Also, note that in all of the reactions, although the GST fusion protein and Gal80 constitute less than 4% of the total protein present, there is no evidence for non-specific biotinylation of the bacterial proteins. Unexpectedly, when GST-G80BP-FRP was incubated with His6-Gal80 and periodate, little or no label transfer to His6-Gal80 was detected (lane 6, top panel, Figure 1). “Pull-down” assays confirmed that both GST-FRP-G80BP and GST-G80BP-FRP fusions bound to His6-Gal80 with similar affinities and that DBF binding to the two GST fusions did not compromise the protein-protein interactions (Fig. S3). Thus, the lack of product is a result of low cross-linking yield. Given the requirement for templating of the DOPA-derived ortho-quinone intermediate and a reactive nucleophile,5,6 we speculate that placing the FRP tag on the C-terminal side positions the bound DBF reagent beyond the reach of an appropriately nucleophilic Gal80 residue.
We next attempted to apply this method to a more complex system. Acidic activation domains (ADs) from transactivators bind directly to proteins in the 19S regulatory particle of the 26S proteasome8-10 and this interaction is important for efficient transcription of many eukaryotic genes11. The proteasome is a >2 MDa complex containing more than 35 different proteins12. To determine the direct binding partners of the potent viral VP16AD13 with this methodology, GST-Gal4(1-147)-VP16-FRP was incubated with DBF, 26S proteasome and NaIO4, followed by boiling in dithiol to effect label transfer. SDS-PAGE followed by gel transferring and blotting with NeutrAvidin-HRP revealed that two proteins, in addition to the FRP-tagged activator, were labeled by DBF (labeled as a and b, left panel, Figure 2). These two bands were absent when 26S proteasome or periodate was omitted (lanes 3 and 4, left panel, Figure 2), or when GST-Gal4(1-147)-VP16-FRP was substituted with GST-FRP (lane 5, Fig. S4) or GST-Gal4(1-147)-VP16 (lane 5, Fig. S5) Western blotting using anti-Sug1/Rpt6 antibody confirmed that band a was Sug1/Rpt6 (middle panel, Figure 2), one of the six AAA-ATPases located in the 19S regulatory particle of the proteasome14. This protein has previously been shown to be a target of several acidic activators10. This result argues that the VP16 AD-Sug1/Rpt6 interaction appears to be physiologically relevant and Sug1/Rpt6 is likely a general target for many acidic activators. Based on the mobility of band b, it likely represented Rpn11.15 The important point is that incubation of an FRP-tagged protein with a very large macromolecular complex selectively labels only two proteins, arguing that these are nearest neighbors to the C-terminus of the VP16AD.
Figure 2.
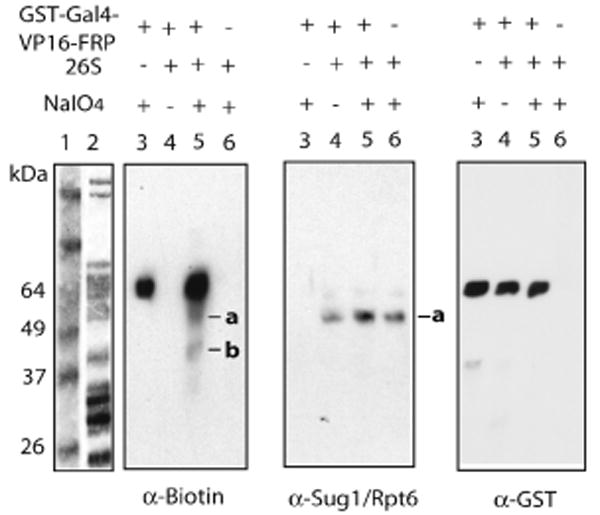
DBF (0.1 μM) transfer from GST-Gal4(1-147)-VP16-FRP (1 μM) to 26S proteasome (0.1 μM). Lane 1: Protein standards. Lane 2: 26S proteasome stained by Coomassie blue. Lane 3-6: Label transfer reactions probed by Western blotting.
This work demonstrates the utility of a novel label transfer system that does not require covalent modification of the protein of interest and that employs an efficient oxidative cross-linking reaction. We anticipate that this procedure will be useful for the characterization of protein-protein interactions in their native environments. Efforts are underway to combine LC/MS/MS analysis with this label transfer technique to obtain higher-resolution information about these interactions and to allow more facile identification of unknown protein-binding partners in cell lysates.
Supplementary Material
Synthesis of DOPA-biotin-FlAsH and all experimental details. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgments
This work was supported by a contract from the National Heart, Lung, and Blood Institute (NO1-HV-28185) and grants from the National Institutes of Health (GM066380) and the Welch Foundation (I-1299).
References
- 1.Chen Y, Ebright YW, Ebright RH. Science. 1994;265:90–92. doi: 10.1126/science.8016656. [DOI] [PubMed] [Google Scholar]
- 2.Koh SS, Ansari AZ, Ptashne M, Young RA. Mol Cell. 1998;1:895–904. doi: 10.1016/s1097-2765(00)80088-x. [DOI] [PubMed] [Google Scholar]
- 3.Lam YA, Lawson TG, Velayutham M, Zweier JL, Pickart CM. Nature. 2002;416:763–767. doi: 10.1038/416763a. [DOI] [PubMed] [Google Scholar]
- 4.Griffin BA, Adams SR, Jones JG, Tsien RY. Methods Enzymol. 2000;327:565–578. doi: 10.1016/s0076-6879(00)27302-3. [DOI] [PubMed] [Google Scholar]
- 5.Burdine L, Gillette TG, Lin HJ, Kodadek T. J Am Chem Soc. 2004;126:11442–11443. doi: 10.1021/ja045982c. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Burdine L, Kodadek T. J Am Chem Soc. 2006;128:15228–15235. doi: 10.1021/ja065794h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han Y, Kodadek T. J Biol Chem. 2000;275:14979–14984. doi: 10.1074/jbc.275.20.14979. [DOI] [PubMed] [Google Scholar]
- 8.Melcher K, Johnston S. Mol Cell Biol. 1995;15:2839–2848. doi: 10.1128/mcb.15.5.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez F, Delahodde A, Kodadek T, Johnston SA. Science. 2002;296:548–550. doi: 10.1126/science.1069490. [DOI] [PubMed] [Google Scholar]
- 10.Archer C, Burdine L, Kodadek T. Mol BioSyst. 2005;1:366–372. doi: 10.1039/b510019d. and references therein. [DOI] [PubMed] [Google Scholar]
- 11.Sikder D, Johnston SA, Kodadek T. J Biol Chem. 2006;281:27346–27355. doi: 10.1074/jbc.M604706200. [DOI] [PubMed] [Google Scholar]
- 12.Baumeister W, Walz J, Zuhl F, Seemuller E. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 13.Sadowski I, Ma J, Triezenberg S, Ptashne M. Nature. 1988;335:563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- 14.Russell SJ, Sathyanarayana UG, Johnston SA. J Biol Chem. 1996;271:32810–32817. doi: 10.1074/jbc.271.51.32810. [DOI] [PubMed] [Google Scholar]
- 15.Verma R, Aravind L, Oania R, McDonald WH, Yates JRI, Koonin EV, Deshaies RJ. Science. 2002;298:611–615. doi: 10.1126/science.1075898. The characterization of this product will be presented elsewhere. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthesis of DOPA-biotin-FlAsH and all experimental details. This material is available free of charge via the internet at http://pubs.acs.org.