Abstract
A red/purple coloration of lower (abaxial) leaf surfaces is commonly observed in deeply-shaded understorey plants, especially in the tropics. However, the functional significance of red abaxial coloration, including its role in photosynthetic adaptation, remains unclear. The objective of this study was to test the back-scatter hypothesis for abaxial leaf coloration, which posits that red pigments internally reflect/scatter red light transmitted by the upper leaf surface back into the mesophyll, thereby enhancing photon capture in light-limited environments. Abaxially red/non-red variegated leaves of Begonia heracleifolia (Cham. & Schltdl.) were used to compare reflectance spectra and chlorophyll fluorescence profiles of abaxially anthocyanic (red) and acyanic (non-red) tissues under red light. Photosynthetic gas exchange in response to red light was also compared for abaxially red/non-red leaf sections. The results did not support a back-scattering function, as anthocyanic leaf surfaces were not more reflective of red light than acyanic surfaces. Anthocyanic tissues also did not exhibit any increases in the mesophyll absorbance of red light, or increased photosynthetic gas exchange under red light at any intensity, relative to acyanic tissues. These results suggest that abaxial anthocyanins do not significantly enhance the absorption of red light in the species tested, and alternative functions are discussed.
Keywords: Abaxial, anthocyanin, back-scatter, Begonia, photosynthesis, photoprotection, shade, understorey
Introduction
Anthocyanins are vacuolar pigments commonly responsible for red, purple, and blue coloration of plant tissues. They can be found in plant species across a broad range of habitats, and are especially common in understorey plants of the tropics (for species surveys see Forsyth and Simmonds, 1954; Lee and Collins, 2001; Dominy et al., 2002). Among tropical species, leaf coloration may be transient, occurring in upper (adaxial) and/or lower (abaxial) cell layers during development and senescence or, alternatively, coloration may be permanent (Lee and Collins, 2001). In permanently pigmented leaves of the tropics, anthocyanins are most commonly abaxial (Lee et al., 1979; Lee and Collins, 2001). Despite the widespread distribution of abaxial coloration among tropical taxa, very little is known regarding the functional significance of this trait. Previous studies on the ecophysiology of foliar anthocyanin have focused almost exclusively on plants with pigmentation in the upper (adaxial) surface of leaves. In high-light environments, for example, it has been demonstrated that adaxial pigmentation may function in light-attenuation, protecting underlying cells from photoinhibition through the absorption of high energy blue-green wavelengths (Gould et al., 2002; for reviews see Chalker-Scott, 1999; Close and Beadle, 2003; Gould, 2004). Abaxial anthocyanins have also been implicated to function in photoprotection in plants where exposed abaxial leaf surfaces are vulnerable to high-incident light (Drumm-Herrel and Mohr, 1985; Sherwin and Farrant, 1998; Hughes and Smith, 2007). However, several studies have also yielded results that do not support a photoprotective function (Burger and Edwards, 1996; Dodd et al., 1998; Lee et al., 2003; Kyparissis et al., 2007). In addition to functioning in light attenuation, anthocyanins in the upper cell layers have also been suggested to function in insect avoidance and/or deterrence (Stone, 1966; Hamilton and Brown, 2001; Archetti and Brown, 2004; Lev-Yadun et al., 2004; Karageorgou and Manetas, 2006), act as a fungicide (Coley and Aide, 1989), function as a carbon sink (Arnold et al., 2004), or as antioxidants during periods of high photo-oxidative stress (Neill et al., 2002; Neill and Gould, 2003). However, none of these hypotheses seem appropriate for explaining the occurrence of anthocyanin in the abaxial cells of understorey species.
Smith (1909) proposed the first functional explanation for abaxial coloration in understorey plants, suggesting that anthocyanin pigments function to elevate leaf temperatures by increasing sunlight absorption, possibly increasing transpiration, nutrient uptake, metabolism, and growth rates in understorey plants. The idea that anthocyanins increase leaf temperature has not received experimental support, as field measurements have shown no differences in leaf temperature between abaxially anthocyanic and acyanic leaves (Lee et al., 1979, 1987; Gould et al., 1995). Lee et al. (1979) observed that the abaxial leaf surfaces of some abaxially anthocyanic species appeared to reflect more red light than green surfaces. This observation led to the idea that anthocyanin pigments near the lower epidermis may function to reflect adaxially-transmitted red light back into the mesophyll, possibly maximizing the capture of red photons in environments where light is limiting. This ‘back-scatter’ hypothesis has yet to receive experimental validation beyond these initial observations, although it is still frequently invoked to explain abaxial coloration in understorey plants. In the current study, abaxially red/green variegated leaves of Begonia heracleifolia (Cham. & Schltdl.) (Fig. 1) were used, together with recently-developed, non-destructive optical techniques for measuring light absorbance within leaf tissues, to test the back-scatter hypothesis. The light response of photosynthesis under red light for abaxial anthocyanic and acyanic leaf sections was also compared to evaluate whether abaxial anthocyanins confer a significant photosynthetic advantage in leaf tissues.
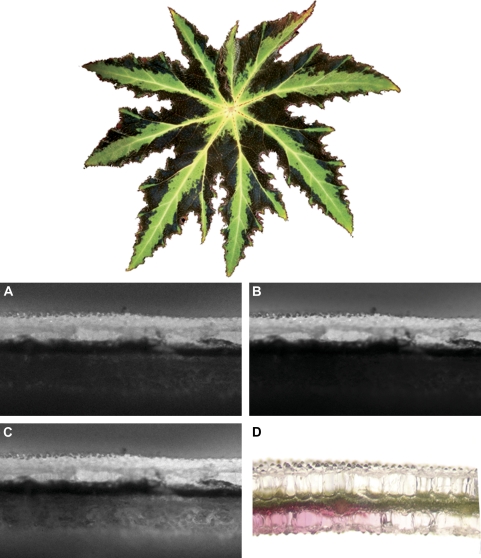
Fig. 1.
Top: adaxial view of Begonia heracleifolia leaf. Bottom: chlorophyll fluorescence in partially red and green variegated leaf sections with upper (adaxial) surface illuminated. In all pictures, the adaxial surface is facing upwards and anthocyanins are present only in the left half of each photograph, as shown in (D). Images represent chlorophyll fluorescence under red (A), blue (B), and green (C) light. Note that, while fluorescence is only emitted by chlorophyll-containing cells, it may also be apparent via scatter within adjacent clear, epidermal lens cells (see text). Scale bar represents 0.5 mm.
Materials and methods
Plant material
Begonia heracleifolia plants were grown in shaded greenhouse conditions with periodic exposure to lower intensity, short-lived sunflecks to longer lasting, more intense sun-patches (Smith et al., 1989). Photosynthetic photon flux densities (PPFDs) in the shade averaged c. 5 0 μmol m−2 s−1, while irradiance during sunflecks and sun-patches ranged from c. 150–1500 μmol m−2 s−1. In all measurements comparing abaxially red and non-red tissues, adjacent variegated sections of individual leaves were used.
Leaf sections for light microscopy were cut from fresh leaves using a razor blade, and mounted on a Zeiss Axioplan upright microscope (Carl Zeiss Inc., Thornwood, NY, USA). Sections were viewed using differential interference contrast (DIC) microscopy, and images were captured using a Hamamatsu C5810 three-chip cooled colour CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan). Photographs of leaf sections were rotated and adjusted for brightness and image sharpness using Adobe Photoshop CS (Adobe Systems, San Jose, CA, USA).
Spectral scans
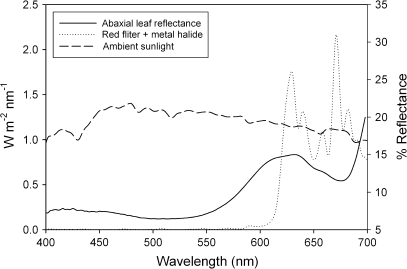
Five leaves were removed from individual plants, kept under cool, moist conditions, and analysed within 8 h using a Li-Cor 1800 spectroradiometer with an external integrating sphere (Li-Cor, Inc., Lincoln, Nebraska, USA). Measurements were taken at 2 nm intervals, and absorbance was calculated as (1 – reflectance – transmittance). Spectral data were compared by calculating 95% confidence intervals for five replicates; areas of error-bar overlap were considered not to be significantly different. For ambient sunlight measurements, the spectroradiometer was positioned horizontally on a flat surface on a clear, sunny day in Winston-Salem, NC, USA. For transmission scans of the red optical filter used in photosynthesis experiments (transmittance spectrum shown in Fig. 2) (Schott Glass, Grünenplan, Germany), the filter was placed in the water bath beneath a 1000 W metal halide bulb, and the spectroradiometer was placed at the same height below the bulb as the cuvette used in the experiments.
Fig. 2.
Spectral scans. The solid line represents the percentage mean leaf reflectance for the red abaxial surface of Begonia heracleifolia leaves. The dotted line represents the transmission spectra for the red filter used in photosynthesis measurements. The ambient solar spectrum is represented by the dashed line.
Pigments
Chlorophyll was extracted from the same leaves for which spectral curves had been measured. Chlorophylls were extracted by removing three 0.33 cm2 leaf discs from either abaxially anthocyanic or acyanic leaf sections, and extracting the tissues overnight in 3 ml N,N′-dimethylformamide. Chlorophyll a and b concentrations were determined spectrophotometrically using the equations described by Porra (2002). Chlorophyll a/b ratios were calculated to assess relative emphasis on light capture versus processing for red and green sections, as higher chlorophyll a/b ratios reflect both an increase in core (exclusively chl a) relative to light–harvesting (both chl a and b) complexes, and/or higher ratios of photosystem I (4/1 ratio of chl a/b) to photosystem II (1.2/1 ratio) (Cui et al., 1991; Demmig-Adams, 1998; Hopkins and Hüner, 2004). Pigment concentrations of adjacent red and green sections were compared using a one-tailed, paired t test, with P < 0.05 used to determine significance.
Chlorophyll fluorescence profiles
Spatial distribution of light absorption within leaf tissues was determined as described in Vogelmann and Han (2000). Briefly, cm2 sections were cut from six different variegated leaves, such that one half of each section contained abaxial anthocyanin and the other half did not (Fig. 1D). The sections were then mounted on an inverted microscope and upper (adaxial) surfaces were irradiated using filtered blue, green, and red light (intensity of the beam was c. 500 μmol m−2 s−1 for blue, 300 for green, and 200 for red light). Resulting leaf chlorophyll fluorescence was filtered through a narrow band-pass filter (680 nm, half band width = 16 nm, Corion Corp., Holliston, MA), and captured using a cryogenically cooled charged-coupled device (CCD) camera (CH270 camera head, CF200A 16/40 camera electronics unit, AT200 controller board, 35 mm shutter; all from Photometrics, Tuscon, AZ). The intensity of chlorophyll fluorescence was used to quantify light absorbance by chlorophylls (with the highest intensities corresponding to the greatest absorbance). Chlorophyll fluorescence according to leaf depth for each image was quantified using Image Pro Plus software (version 4.1, Media Cybernetics, Silver Springs, MD). It is important to note that chlorophyll fluorescence in all cases was emitted exclusively by cells containing chlorophyll (i.e. the mesophyll), although most of this light appeared to scatter in the adjacent clear epidermal lens cells (as described in Brodersen and Vogelmann, 2007). Mean percentage chlorophyll fluorescence for abaxially anthocyanic and acyanic tissues was plotted versus leaf depth (from adaxial surface) and 95% confidence intervals were calculated. Areas of confidence interval overlap were not significantly different.
Light response of photosynthesis
To test the idea that abaxial anthocyanins enhance capture of red photons for use in photosynthesis, photosynthetic light response curves were measured for abaxially anthocyanic/acyanic leaf sections under red light using a Li-Cor 6400 infra-red gas analyser with clear-top chamber (Li-Cor, Lincoln, Nebraska, USA). Light was supplemented by a 1000 W metal halide lamp equipped with a UV filter and water bath, and irradiance was filtered through a red optical filter (transmittance spectrum shown in Fig. 2) (Schott Glass, Grünenplan, Germany). Leaves were positioned horizontally, with the upper surfaces facing the light source. Because variegated leaf sections were smaller in area than the leaf chamber, an opaque tape with a 2 cm2 opening was used to cover the areas of leaves not being measured on both adaxial and abaxial surfaces. The tape effectively reduced stomatal gas exchange to negligible amounts (i.e. <1–2%), as determined by experiments on leaf surfaces entirely covered in tape. Neutral density shade cloths were used to obtain the desired PPFDs, which ranged from 0–600 μmol m−2 s−1 (the latter representing roughly the maximum intensity of red light in full sunlight; Lee and Downum, 1991). The quantum yield of photosynthesis was determined as the slope of the linear portion of the curve, and compared using a paired t test for adjacent red and green sections on the same leaf. The sample size for gas exchange experiments was five leaves. The light response curves for red and green leaf sections were compared using repeated measures ANOVA.
Results and discussion
Optical effects of abaxial anthocyanin
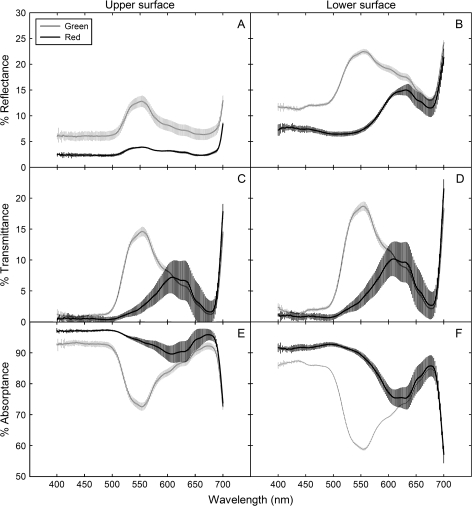
In contrast to the findings of Lee et al. (1979), red (anthocyanic) abaxial surfaces of Begonia heracleifolia were not more reflective of red wavelengths than green (acyanic) abaxial surfaces, but rather, acyanic leaf surfaces were significantly more reflective of all wavelengths than anthocyanic (Fig. 3A, B). This was probably due both to the presence of anthocyanin and higher chlorophyll concentrations in anthocyanic tissues (both of which increase leaf absorbance, decreasing reflectance) (Table 1; Fig. 3). An increased chlorophyll content has been observed in several anthocyanic species relative to acyanic phenotypes (Gould et al., 1995; Manetas et al., 2003; Hughes et al., 2005), and has been attributed to shading effects of anthocyanin (discussed in further detail below). Therefore, although not ideal for quantifying the optical effects of anthocyanin alone (due to the confounding effects of chlorophyll), these scans are probably representative of the realistic effects of anthocyanin on leaf optics in vivo. Furthermore, important information relevant to the back-scatter question can still be garnered from these scans. For example, absorbance spectra showed high absorbance efficiency (∼90%) of red light (650–700 nm) in both tissue types, regardless of the presence of anthocyanin, with less than 5% being lost via transmittance through the leaf lamina (Fig. 3). This degree of efficiency in photon absorption is not unique to Begonia, as shade plants are generally known to increase the allocation of resources towards a light-harvesting capacity, resulting in a relatively high quantum yield of photosynthesis under low irradiances (Larcher, 2003). If abaxial surfaces of understorey plants have evolved to maximize light capture via red-light back-scattering, they seem to have done so for a narrow waveband that is already being (relatively) efficiently captured. Instead, it would seem that a white reflective layer (which is fairly common in the plant kingdom) would be of greater benefit than one that reflects red alone, as this would enhance the capture of wavelengths more readily lost through transmittance, such as green and yellow (Woolley, 1971; Lin and Ehleringer, 1983; DeLucia et al., 1986).
Fig. 3.
Optical properties (absorbance, transmittance, and reflectance) of the adaxial and abaxial surfaces of anthocyanic (black lines) and acyanic (grey lines) tissues of Begonia heracleifolia. The lines represent the means of five leaves; error bars are 95% confidence intervals. The waveband depicted represents the visible spectrum, with violet-blue wavelengths depicted between 400–500 nm, green 500–580, yellow-orange 580–630, and red 630–700 nm.
Table 1.
Chlorophyll data for abaxially anthocyanic (red) and acyanic (green) portions of Begonia heracleifolia leaves
| Abaxial coloration | Chl a (μg cm2)a | Chl b (μg cm2)a | Total Chl (μg cm2)a | Chl a/ba |
| Red | 17.9 a (0.33) | 7.97 a (0.30) | 25.8 a (0.39) | 2.25 a (0.040) |
| Green | 13.7 b (1.0) | 5.44 b (0.21) | 19.1 b (1.5) | 2.51 b (0.096) |
Values are means for five replicates (±SD). Means within columns not followed by the same letter are different at P <0.05.
Comparing these scans to those published for other species, the lack of increased reflectance of red wavelengths in anthocyanic versus acyanic tissues has also been reported in other studies (Gould et al., 1995; Hughes and Smith, 2007). Slight (1–2%) increases in reflectance of red wavelengths have also been reported for anthocyanic tissues relative to acyanic (Burger and Edwards, 1996; Liakopoulos et al., 2006), although differences in the order of magnitude of those reported in Lee et al. (1979) (i.e. 20% higher reflectance of red wavelengths by red tissues) have not been reported. It is possible that repeated analyses of the species tested by Lee et al. (1979) using newer equipment may yield different results (DW Lee, personal communication).
Effects of abaxial anthocyanin on light capture and photosynthesis
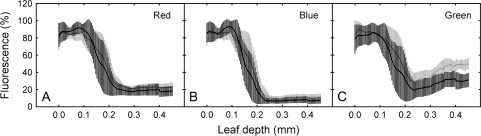
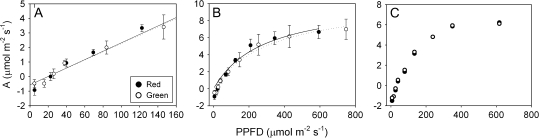
The chlorophyll fluorescence profiles also did not support a back-scattering function of the abaxial anthocyanin layer. If back-scattering was occurring, increased fluorescence should have been observed in anthocyanic tissues relative to acyanic tissues under red light, especially in the most abaxial mesophyll cells. Instead, chlorophyll fluorescence was similar at all depths in anthocyanic and acyanic leaf sections under red light, with very little fluorescence observed in abaxial cells layers in either case (Figs 1A, 4A). This effect was even more pronounced under blue light (Fig. 1B), where virtually no fluorescence was observed to be emitted from abaxial mesophyll cells. This indicates that most of the incident red and blue light was absorbed by chloroplasts in the adaxial mesophyll, and that very little was transmitted to abaxial cells (consistent with our interpretation of spectral scans). Because the light levels used here (i.e. 200 μmol m−2 s−1 for red light) were substantially more intense than PPFDs reported for the shade of tropical forest understoreys (total PPFDs ranging from c. 2–45 μmol m−2 s−1; Lee, 1987), it is unlikely that ambient red wavelengths in the field would be sufficient to penetrate mesophyll cells unabsorbed, much less penetrate them, then strike the anthocyanin layer and be back-scattered. Furthermore, because understorey species photosynthetically saturate at relatively low PPFDs (i.e. 200–300 μmol m−2 s−1; Björkman and Demmig, 1987; Larcher, 2003), irradiance greater than 200 μmol m−2 s−1, such as that experienced during longer-lasting, more intense sunflecks or sun-patches, would probably be sufficient to saturate photosynthesis without the assistance of back-scattering. Consistent with this interpretation, no significant difference in photosynthesis between abaxial red and green leaf sections was observed either under low intensities of red light (0–200 μmol m−2 s−1; Fig. 5A), or when higher irradiances were included (0–600 μmol m−2 s−1; Fig. 5B) (P=0.73 and 0.68, respectively), even despite the higher chlorophyll content in red tissues. At higher light intensities, some variation in photosynthetic gas exchange was observed between leaves (most probably due to differences in stomatal conductance, not shown), although within-leaf comparisons of adjacent red and green sections consistently showed nearly identical rates of photosynthesis at all intensities (Fig. 5C).
Fig. 4.
Percentage chlorophyll fluorescence versus leaf depth for abaxial anthocyanic (black lines) and acyanic (grey lines) tissues of Begonia heracleifolia under red, blue, and green light. Error bars represent 95% confidence intervals for six replicates.
Fig. 5.
Photosynthetic light response curves of abaxial acyanic/green (open circles, dotted lines) and anthocyanic/red (closed circles, solid lines) leaf tissues under red light. (A) Linear portion of the light response curves shown in (B); (B) light response curves from five leaves (with each having a single red and green section tested), and (C), a representative pair of light response curves derived from the same leaf. Error bars represent SE; n=5.
Alternative functions of the anthocyanic layer
Although no difference in mesophyll absorbance of red light between abaxial red and green tissues was observed, significant differences in green light absorption were apparent in our study. Spectral scans showed that anthocyanic tissues absorbed significantly more blue-green light (500–600 nm) than acyanic tissues (Fig. 3E), corresponding with the absorbance peak of anthocyanin. Perhaps more interestingly, chlorophyll fluorescence profiles of abaxial red tissues showed significantly reduced absorption of green light by mesophyll cells (i.e. reduced chlorophyll fluorescence) compared to acyanic tissues, with the effects being greatest in cells most proximal to the anthocyanic layer (Figs 1C, 4C). These results are consistent with an alternative explanation for abaxial coloration proposed by Gould et al. (1995), which purport that abaxial anthocyanins in understorey plants function in light attenuation (similar to their function in adaxial cells) during intermittent exposure to high-intensity sunlight (i.e. sun-patches). Gould et al. (1995) based this hypothesis on the observation that abaxial anthocyanic leaves had a significantly higher maximum quantum yield efficiency of photosystem II (Fv/Fm) than acyanic leaves, as well has a higher chlorophyll content and a higher maximum photosynthesis. However, no details were presented to explain how abaxial pigments might protect leaves in this way.
From the chlorophyll fluorescence profiles presented here, we suspect that attenuation of green wavelengths by anthocyanins may be occurring (i) as adaxial incident light passes through the anthocyanic spongy mesophyll (reducing absorbance by subjacent chloroplasts), and/or (ii) when the remaining green light reaches anthocyanic abaxial lens cells, it is being absorbed before internal scattering may occur (i.e. the opposite of back-scattering). This photoprotective function may be especially adaptive in shade-adapted understorey plants, which photosynthetically saturate (and thus photoinhibit) at relatively low irradiances, and yet frequently encounter potentially damaging irradiance via high-intensity sunflecks and sun-patches. The occurrence of the pigment on the abaxial surface rather than the adaxial might prevent interference of light absorption by anthocyanin at low light intensities, providing photoprotection only when intensities are high enough to penetrate the mesophyll (which would probably correspond to damaging levels of photons). This photoprotective function is further supported by the chlorophyll data (Table 1). As shown in previous studies on anthocyanic versus acyanic phenotypes (Gould et al., 1995; Manetas et al., 2003; Hughes et al., 2005), the abaxial red tissues measured here had a significantly higher total chlorophyll content, and lower chlorophyll a/b ratios than non-red tissues (P <0.01 for both). High chlorophyll content and low chl a/b ratios generally reflect an increased emphasis on light capture relative to processing, a characteristic associated with more shade-adapted tissues (Demmig-Adams, 1998). These shade-like qualities make sense for adaxial anthocyanic tissues (which are directly shaded by the overlying anthocyanic layer), and also for abaxial anthocyanic tissues when abaxial attenuation is considered. However, it is also possible that this effect may be due to increased shading due to higher chlorophyll content, although this too might be an indirect result of shading by anthocyanin (as mentioned above).
Conclusion
In summary, the results reported here clearly do not support a back-scattering function of abaxial red coloration. Red abaxial surfaces were not more reflective of red light than non-red abaxial surfaces, and the presence of a red abaxial layer did not result in increased mesophyll absorption of red light, or an increase in photosynthetic gas exchange. However, these results do appear consistent with a photoprotective function of abaxial anthocyanin, although a more detailed comparison of photoinhibition of photosynthesis for abaxially anthocyanic versus acyanic tissues is needed to substantiate this explanation further.
Acknowledgments
We thank Craig Brodersen and Nate Poirier (University of Vermont) for technical assistance, Dr Howard Neufeld (Appalachian StateUniversity) for use of optical filters and the Li-1800, and also Tim Anderson (PalmHammock Orchid Estate) for gracious donation of plant materials. We also thank the anonymous reviewers for their insightful comments and suggestions.
Glossary
Abbreviations
- PPFD
photosynthetic photon flux density
- chl
chlorophyll
References
- Archetti M, Brown S. The coevolution theory of autumn colours. Proceedings of the Royal Society of London, Series B, Biological Sciences. 2004;271:1219–1223. doi: 10.1098/rspb.2004.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold T, Appel H, Patel V, Stocum E, Kavalier A, Schultz J. Carbohydrate translocation determines the phenolic content of Populus foliage: a test of the sink–source of plant defense. New Phytologist. 2004;164:157–164. doi: 10.1111/j.1469-8137.2004.01157.x. [DOI] [PubMed] [Google Scholar]
- Björkman O, Demmig B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta. 1987;170:489–904. doi: 10.1007/BF00402983. [DOI] [PubMed] [Google Scholar]
- Brodersen CR, Vogelmann TC. Do epidermal lens cells facilitate the absorptance of diffuse light? American Journal of Botany. 2007;94:1061–1066. doi: 10.3732/ajb.94.7.1061. [DOI] [PubMed] [Google Scholar]
- Burger J, Edwards GE. Photosynthetic efficiency, and photodamage by UV and visible radiation, in red versus green leaf Coleus varieties. Plant and Cell Physiology. 1996;37:395–399. [Google Scholar]
- Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochemistry and Photobiology. 1999;70:1–9. [Google Scholar]
- Close DC, Beadle CL. The ecophysiology of foliar anthocyanin. The Botanical Review. 2003;69:149–161. [Google Scholar]
- Coley PD, Aide TM. Red coloration of tropical young leaves: a possible antifungal defense? Journal of Tropical Ecology. 1989;5:293–300. [Google Scholar]
- Cui M, Vogelmann TC, Smith WK. Chlorophyll and light gradients in sun and shade leaves of Spinacia oleracea. Plant, Cell and Environment. 1991;14:493–500. [Google Scholar]
- DeLucia EH, Nelson K, Vogelmann TC, Smith WK. Contribution of intercellular reflectance to photosynthesis in shade leaves. Plant, Cell and Environment. 1996;19:159–170. [Google Scholar]
- Demmig-Adams B. Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiology. 1998;39:474–482. [Google Scholar]
- Dodd I, Critchley C, Woodall G, Stewart G. Photoinhibition in differently coloured juvenile leaves of Syzygium species. Journal of Experimental Botany. 1998;49:1437–1445. [Google Scholar]
- Dominy NJ, Lucas PW, Ramsden LW, Riba-Hernandez P, Stoner KE, Turner IM. Why are young leaves red? Oikos. 2002;98:163–176. [Google Scholar]
- Drumm-Herrel H, Mohr H. Photosensitivity of seedlings differing in their potential to synthesize anthocyanin. Physiologia Plantarum. 1985;64:60–66. [Google Scholar]
- Forsyth WGC, Simmonds NW. Survey of the anthocyanins of some tropical plants. Proceedings of the Royal Society of London, Series B, Biological Sciences. 1954;142:549–564. doi: 10.1098/rspb.1954.0043. [DOI] [PubMed] [Google Scholar]
- Gould KS. Nature's swiss army knife: the diverse protective roles of anthocyanins in leaves. Journal of Biomedicine and Biotechnology. 2004;5:314–320. doi: 10.1155/S1110724304406147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KS, Kuhn DN, Lee DW, Oberbauer SF. Why leaves are sometimes red. Nature. 1995;378:241–242. [Google Scholar]
- Gould KS, Vogelmann TC, Han T, Clearwater MJ. Profiles of photosynthesis within red and green leaves of Quintinia serrata. Physiologia Plantarum. 2002;116:127–133. doi: 10.1034/j.1399-3054.2002.1160116.x. [DOI] [PubMed] [Google Scholar]
- Hamilton WD, Brown SP. Autumn tree colours as a handicap signal. Proceedings of the Royal Societyof London, Series B, Biological Sciences. 2001;268:1489–1493. doi: 10.1098/rspb.2001.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WG, Hüner NPA. Photosynthetic electron transport. In: Hopkins WG, Hüner NP, editors. Introduction to plant physiology. New York: John Wiley; 2004. pp. 68–71. [Google Scholar]
- Hughes NM, Neufeld HS, Burkey KO. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytologist. 2005;168:575–587. doi: 10.1111/j.1469-8137.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Smith WK. Attenuation of incident light in Galax urceolata (Diapensiaceae): concerted influence of adaxial and abaxial anthocyanic layers on photoprotection. American Journal of Botany. 2007;94:784–790. doi: 10.3732/ajb.94.5.784. [DOI] [PubMed] [Google Scholar]
- Karageorgou P, Manetas Y. The importance of being red when young: anthocyanins and the protection of Quercus coccifera from insect herbivory and excess light. Tree Physiology. 2006;26:613–621. doi: 10.1093/treephys/26.5.613. [DOI] [PubMed] [Google Scholar]
- Kyparissis I, Grammatikopoulos G, Manetas Y. Leaf morphological and physiological adjustments to the spectrally selective shade imposed by anthocyanins in Prunus cerasifera. Tree Physiology. 2007;27:849–857. doi: 10.1093/treephys/27.6.849. [DOI] [PubMed] [Google Scholar]
- Larcher W. The light response of photosynthesis. In: Larcher W, editor. Physiological plant ecology. Berlin: Springer Publishing; 2003. pp. 113–114. [Google Scholar]
- Lee DW. The spectral distribution of radiation in two neotropical rainforests. Biotropica. 1987;19:161–166. [Google Scholar]
- Lee DW, Brammeier S, Smith AP. The selective advantages of anthocyanins in developing leaves of mango and cacao. Biotropica. 1987;19:40–49. [Google Scholar]
- Lee DW, Collins TM. Phylogenetic and ontogenetic influences on the distribution of anthocyanins and betacyanins in leaves of tropical plants. International Journal of Plant Sciences. 2001;162:1141–1153. [Google Scholar]
- Lee DW, Downum KR. The spectral distribution of biologically active solar radiation at Miami, Florida, USA. International Journal of Biometeorology. 1991;35:48–54. doi: 10.1007/BF01040963. [DOI] [PubMed] [Google Scholar]
- Lee DW, Lowry JB, Stone BC. Abaxial anthocyanin layer in leaves of tropical rainforest plants: enhancer of light capture in deep shade. Biotropica. 1979;11:70–77. [Google Scholar]
- Lee DW, O'Keefe J, Holbrook NM, Feild TS. Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA. Ecological Research. 2003;18:677–694. [Google Scholar]
- Lev-Yadun S, Dafni A, Flaishman MA, Inbar M, Izhaki I, Katzir G, Ne'eman G. Plant coloration undermines herbivorous insect camouflage. BioEssays. 2004;26:1126–1130. doi: 10.1002/bies.20112. [DOI] [PubMed] [Google Scholar]
- Liakopoulos G, Nikolopoulos D, Klouvatou A, Vekkos K, Manetas Y, Karabourniotis G. The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera) Annals of Botany. 2006;98:257–265. doi: 10.1093/aob/mcl097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZF, Ehleringer J. Epidermis effects on the spectral properties of leaves of four herbaceous species. Physiologia Plantarum. 1983;59:91–94. [Google Scholar]
- Manetas Y, Petropoulou Y, Psaras GK, Drinia A. Exposed red (anthocyanic) leaves of Quercus coccifera display shade characteristics. Functional Plant Biology. 2003;30:265–270. doi: 10.1071/FP02226. [DOI] [PubMed] [Google Scholar]
- Neill SO, Gould KS. Anthocyanins in leaves: light attenuators or antioxidants? Functional Plant Biology. 2003;30:865–873. doi: 10.1071/FP03118. [DOI] [PubMed] [Google Scholar]
- Neill SO, Gould KS, Kilmartin PA, Mitchell KA, Markham KR. Antioxidant activities of red versus green leaves in Elatostema rugosum. Plant, Cell and Environment. 2002;25:539–547. [Google Scholar]
- Porra RJ. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynthesis Research. 2002;73:149–156. doi: 10.1023/A:1020470224740. [DOI] [PubMed] [Google Scholar]
- Sherwin H, Farrant JM. Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscosa. Plant Growth Regulation. 1998;24:203–210. [Google Scholar]
- Smith AM. On the internal temperature of leaves in tropical insolation, with special reference to the effects of their colour on temperature; also observations on the periodicity of the appearance of young coloured leaves of trees growing in Peradeniya Gardens. Annals of the Royal Botanical Gardens. Peradeniya. 1909;5:229–298. [Google Scholar]
- Smith WK, Knapp AK, Reiners WA. Penumbral effects on sunlight penetration in plant communities. Ecology. 1989;70:1603–1609. [Google Scholar]
- Stone BC. Pandanus Stickm. in the Malayan Peninsula, Singapore, and lower Thailand. Malaysian Nature Journal. 1966;19:291–301. [Google Scholar]
- Vogelmann TC, Han T. Measurement of gradients of absorbed light in spinach leaves from chlorophyll fluorescence profiles. Plant, Cell and Environment. 2000;23:1303–1311. [Google Scholar]
- Woolley JT. Reflectance and transmittance of light by leaves. Plant Physiology. 1971;47:656–662. doi: 10.1104/pp.47.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]