Abstract
In the South Australian wheat belt, cyclic drought is a frequent event represented by intermittent periods of rainfall which can occur around anthesis and post-anthesis in wheat. Three South Australian bread wheat (Triticum aestivum L.) cultivars, Excalibur, Kukri, and RAC875, were evaluated in one greenhouse and two growth-room experiments. In the first growth-room experiment, where plants were subjected to severe cyclic water-limiting conditions, RAC875 and Excalibur (drought-tolerant) showed significantly higher grain yield under cyclic water availability compared to Kukri (drought-susceptible), producing 44% and 18% more grain compared to Kukri, respectively. In the second growth-room experiment, where plants were subjected to a milder drought stress, the differences between cultivars were less pronounced, with only RAC875 showing significantly higher grain yield under the cyclic water treatment. Grain number per spike and the percentage of aborted tillers were the major components that affected yield under cyclic water stress. Excalibur and RAC875 adopted different morpho-physiological traits and mechanisms to reduce water stress. Excalibur was most responsive to cyclic water availability and showed the highest level of osmotic adjustment (OA), high stomatal conductance, lowest ABA content, and rapid recovery from stress under cyclic water stress. RAC875 was more conservative and restrained, with moderate OA, high leaf waxiness, high chlorophyll content, and slower recovery from stress. Within this germplasm, the capacity for osmotic adjustment was the main physiological attribute associated with tolerance under cyclic water stress which enabled plants to recover from water deficit.
Keywords: Cyclic drought, osmotic adjustment, wheat
Introduction
Drought is the most important limiting factor for crop production and it is becoming an increasingly severe problem in many regions of the world. In addition to the complexity of drought itself (Passioura, 1996, 2007), plant responses to drought are complex and different mechanisms are adopted by plants when they encounter drought (Levitt, 1980; Jones et al., 1981; Jones, 2004). These mechanisms can be (i) drought escape by rapid development which allows plants to finish their cycle before severe water stress, (ii) drought avoidance by, for instance, increasing water uptake and reducing transpiration rate by the reduction of stomatal conductance and leaf area, (iii) drought tolerance by maintaining tissue turgor during water stress via osmotic adjustment which allows plants to maintain growth under water stress, and (iv) resisting severe stress through survival mechanisms. However, this last mechanism is typically not relevant to agriculture (Tardieu, 2005). The maintenance of high plant water status and plant functions at low plant water potential, and the recovery of plant function after water stress are the major physiological processes that contribute to the maintenance of high yield under cyclic drought periods (Blum, 1996).
Understanding the physiology and genetic control of these mechanisms using physiological and molecular genetic tools will assist breeding programmes seeking to improve drought resistance in crop plants. Physiological studies help to establish the precise screening techniques necessary to identify traits which are related to plant productivity. Recent studies suggest that selection of physiological traits have the potential to improve grain yield under drought in wheat (Richards, 1989, 2004; Reynolds et al., 2001; Chaves et al., 2002, 2003; Reynolds, 2002; Richards et al., 2002; Condon et al., 2004; Olivares-Villegas et al., 2007). Therefore, understanding the physiological responses of crops under drought, and the underlying complex genetic control of different mechanisms of drought tolerance, is crucial to enhance screening for drought tolerance.
Different types of drought exist which are related to the latitude, temperature, and seasonal precipitation of the cropping zone (Fischer and Turner, 1978). A combination of several climatic factors such as heat stress, water deficit, vapour pressure deficit, salinity, and hostile subsoils can produce different types of drought. In South Australia, the target region for the present work, the pattern of drought resembles that of a Mediterranean-type environment, where most precipitation falls in winter and vapour pressure deficits rise with temperature in the spring. Wheat production in this environment relies predominantly upon rainfall during the growing season. Fluctuation in rainfall during the growing season results in cyclic water availability, predominantly around anthesis and post-anthesis. In general, rainfall in the winter months (June–August) is usually adequate for plant growth and exceeds crop demand because of mild temperatures, low evaporation, slow growth rates, and the relatively high reliability of the rain events. During the spring, rainfall becomes less frequent, with intermittent rainy periods. Temperature and vapour pressure deficits increase and moisture stress of unpredictable severity, duration, and timing can occur any time from September. This period coincides with stem elongation, flowering, and grain-filling (Turner, 2004). In addition, drought under South Australian conditions is complicated by strong genotype-by-environment interactions. Most growing areas have a hostile subsoil with salinity and nutrient deficiencies and toxicities being present (Rodriguez et al., 2006). Simulation modelling suggests that a shallow root system is advantageous in this type of environment where there are fluctuations in rainfall during the growing season (Schwinning and Ehleringer, 2001; Sadras and Angus, 2006). Shallow roots would also be advantageous in avoiding the more toxic conditions in the subsoils.
In the field, there are additional factors such as high wind, low humidity, high irradiance, soil-related constraints, and biotic stresses which can confound field experiments. Conducting drought experiments under controlled conditions (pot experiments), however, enables precise control of many of these environmental variables. Therefore, pot experiments are more reproducible, various treatments are easier to apply, and the results are easier to interpret. Unfortunately, pot experiments can have several serious disadvantages that make the results difficult to extrapolate to the field (Passioura, 2006). In this study, our knowledge of the South Australian environment has been used to replicate cyclic water stress in the growth room. On the basis of differences between three wheat cultivars (Excalibur, Kukri, and RAC875) in their performance under South Australian dry environments, it is assumed that a broad palette of traits, acting in concert, may fine-tune the responses to drought in drought-adapted cultivars under such unpredictable and dynamic dry environments. Therefore, the aim was to evaluate the morpho-physiological responses of these cultivars to drought, and to identify the possible palette of traits which enable these wheat cultivars to maintain higher yields under cyclic drought conditions.
Materials and methods
Plant material
Three South Australian wheat cultivars, Excalibur, Kukri, and RAC875, were evaluated. Excalibur (RAC177/‘Monoculm’//RAC311S, released in 1991 by the University of Adelaide) is a drought-adapted cultivar that yields well in South Australian wheat regions, but has low grain quality and is susceptible to rust. Excalibur has a good level of resistance to the root lesion nematode, Pratylenchus neglectus, confirmed by a gene located on chromosome 7AL (Williams et al., 2002). Excalibur is considered to be a relatively Zn-efficient genotype, able to exploit subsoil reserves effectively. Zubaidi et al. (1999) suggested that the better root growth of Excalibur under harsh conditions might be a contributing factor to its success for this environment.
Kukri (76ECN44/76ECN36//MADDEN/6*RAC177), which was released in 1999 by the University of Adelaide, is a hard white wheat which has excellent grain quality and is rust resistant, but its yield production is low to moderate in low-rainfall environments. It is susceptible to root lesion nematodes.
RAC875 (RAC655/3/Sr21/4*LANCE//4*BAYONET), a breeding line from Roseworthy Agricultural Campus, is a white wheat with high grain quality. It is a high-yielding cultivar under South Australian dry environments, but it is also susceptible to rust and root lesion nematode. None of the cultivars carry any known boron-tolerance genes. All three cultivars possess the Rht2 semi-dwarfing gene and have very similar phenology (3–5 d difference in heading time) under field conditions.
Data from a series of drought trials covering three years (2003, 2004, and 2006) and ten sites across South Australian drought environments, which were conducted by Australian Grain Technology (AGT), showed that Excalibur and RAC875, on average, out-yielded Kukri by 24%. Data were kindly provided by Steve Jefferies of AGT.
Greenhouse experiment for water status measurement (Experiment OA)
In order to determine the water status of the three different cultivars in response to water stress, a greenhouse experiment (Experiment OA) was conducted. Six plants (two of each cultivar) were grown together in black plastic pots (10-inch diameter) containing the same volume (3 kg) of standard potting mix (coco peat). Greenhouse temperatures were maintained at 24±1 °C during the day and 18±1 °C during the night (13/11 h day/night). Average relative humidity was 50% and 80% during the day and night, respectively. Plants were well watered until the stage of flag leaf emergence and then water was withheld. One day before withholding water (non-stress measurements), relative water content (RWC) and osmotic potential (OP) were destructively determined using flag leaf blades. From the third day after withholding water, water status parameters of the plants were measured on a daily basis. At all time points, two flag leaves from each plant were cut simultaneously. One flag leaf was placed in liquid nitrogen for OP and the other was used for RWC measurements. Plants from each pot were only used once for measurements and were then discarded from the experiment. Permanent wilting of plants occurred 14 d after withholding water from pots. All leaves were sampled 3.5–4 h after sunrise. For the two parameters (OP and RWC), four replicates (flag leaf blades) per cultivar and sampling date were taken.
Relative water content (RWC)
RWC of flag leaves was determined by the standard method (Barr and Weatherley, 1962). Leaves were cut, and collected at midday to determine fresh weight (FW). Leaf blades were then placed with their cut end pointing down into a Falcon tube containing about 15 ml of 1 mM CaCl2. The CaCl2 was used to try to increase leaf cell integrity, with the aim of reducing cell lysis as a result of excessive rehydration. The turgid weight (TW) was then recorded after overnight rehydration at 4 °C. For dry weight (DW) determination, samples were oven-dried at 70 °C for 48 h. RWC was calculated according to the following equation:
Osmotic potential (OP)
The OP was determined on excised flag leaves. Flag leaves were cut and placed into 10 ml screw-cap tubes (Sarstedt, Australia), immediately frozen in liquid nitrogen and stored at –80 °C until measured. The 20 μl aliquot of cell sap, which was extracted by pressing the total leaf tissue in a 5 ml syringe containing a cellulose filter, was used for osmotic potential measurements. Osmotic potential was measured using a freezing point micro-osmometer (Fiske 210, Massachusetts, USA). Osmolality measurements in mOsm kg−1 water were converted to osmotic potential in MPa from the Van't Hoff relation at 20 °C (Nobel, 1999).
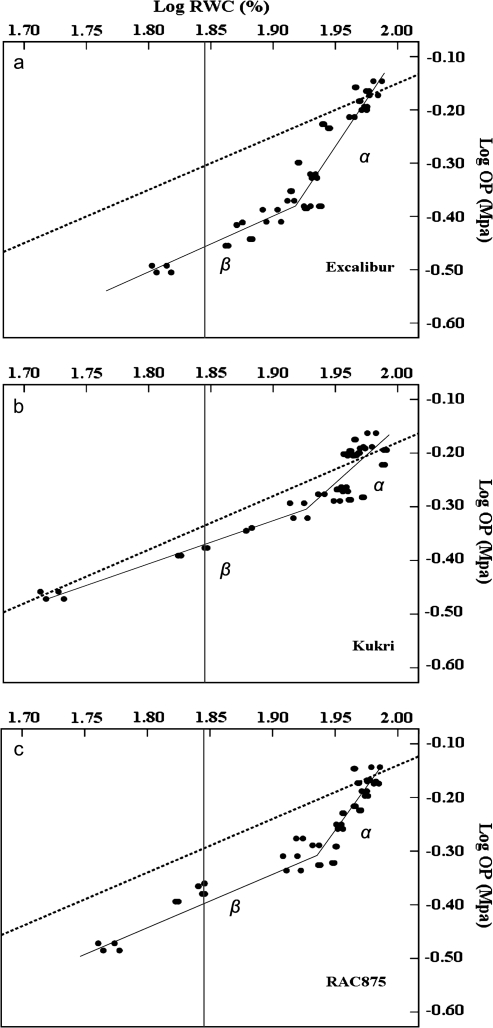
Osmotic adjustment (OA) was calculated using a derivation of the formula for OP (Morgan, 1992). Morgan's regression method is based upon the relationship between OP and RWC. This method compares the observed response with an expected response from an ideal osmometer. OA was estimated from the linear regressions of RWC on OP as derived from consecutive measurements during a drought-stress cycle. Two regression lines were fitted for all measurements taken during the drying cycle in all plants belonging to a given replication. One regression line was derived between RWC and the measured OP, and the other between RWC and calculated OP to account for the concentration of solutes due to water loss (concentration effect). A plot of log RWC and log OP for the three varieties showed two linear phases (α and β; Fig. 4). Log conversion of RWC and OP was conducted to improve the linearity of the relationship. The linear form of the working formula is:
where OPo and RWCo are the initial osmotic potential and the initial relative water content, respectively. RWCo and OPo data were taken after the last irrigation on well-watered plants with no history of water stress. Cultivars ranged in RWCo from 94.5% to 97.5%. The log (OPo RWCo) is the intercept with the slope set to one. The slope of the observed response of log π to log RWC was evaluated. Osmotic adjustment was calculated from the two regressions as the difference between the measured OP and predicted OP at a RWC of 70% (Morgan, 1980, 1992; Babu et al., 1999). In this study, the slope β was used for the calculation of OA.
Fig. 4.
The linear regressions of osmotic potential (OP) and relative water content (RWC) in log scale. Relationship between RWCand OPfor flag leaves of Excalibur (a), Kukri (b), and RAC875 (c). The dashed line is the response of an ideal osmometer and the solid line is the actual fit for RWCversus OP. The vertical grey line is the logarithm of RWC=70%. There are two linear phases: in the first phase (α), there was little change in RWCas OPdeclined, while in the second phase (β), RWCdeclined linearly with OP. Three cultivars were grouped according to their response to high (a), low (b), and medium (c) osmotic adjustment (OA).
Growth room experiments with cyclic drought (Experiment I and II)
Experiments I and II were conducted in a growth room with a refrigerated cooling system at the Australian Centre for Plant Functional Genomics (ACPFG), the University of Adelaide, Australia. A mix of 400 W high pressure sodium (HPS) and metal halide (MH) lamps provided a photon irradiance of 600–700 μmol m−2 s−1. Temperature and humidity were controlled, and conditions were selected to replicate the average conditions found in the northern South Australian wheat belt during the wheat growing season. The temperature regime for these experiments was derived from Bureau of Meteorology climatic data collected at Minnipa (northern South Australia) over the period 1915 to 2004 (http://www.bom.gov.au/climate/averages/tables/ca_sa_names.shtml). Minnipa is a dry environment with the long-term average annual rainfall of ∼327 mm, and the average growing season rainfall is about 242 mm (http://www.sardi.sa.gov.au). Temperature was adjusted throughout plant growth. Table 1 shows the mean of monthly maximum and minimum temperatures at Minnipa, and the adjustments of temperature over time during the plant growth period under controlled conditions. Daylength was 12 h.
Table 1.
Environmental conditions at a representative field site in the target region of interest, and the mean temperature and relative humidity in the two growth rooms during the time of the two experiments (mean ±SE)
| The average temperature (°C) |
Temperature and relative humidity (RH) in growth room (cyclic drought) in the field: Minnipa (1915–2004) |
|||||||
| Month | Max/min | Temperature (°C) | Growth period | Day/night (12/12 h) | Temperature (°C) |
RH (%) |
||
| Exp. I | Exp. II | Exp. I | Exp. II | |||||
| Jun–July | Max. | 16.4 | Weeks 1–4 | Day | 15.8±0.1 | 16.5±0.5 | 68.3±1.1 | 50.0±0.5 |
| Min. | 6.9 | Night | 3.7±0.2 | 6.0±0.3 | 82.2±1.3 | 83.9±0.1 | ||
| August | Max. | 17.3 | Weeks 5–8 | Day | 17.8±0.2 | 18.3±0.2 | 74.0±0.7 | 45.0±0.5 |
| Min. | 6.7 | Night | 5.8±0.2 | 7.5±0.3 | 81.1±0.4 | 72.3±0.4 | ||
| Sept–Oct | Max. | 22.3 | From week 9 to maturity | Day | 24.7±0.2 | 23.8±0.2 | 63.2±0.9 | 55.6±0.4 |
| Min. | 9.1 | Night | 9.6±0.1 | 9.5±0.3 | 78.7±0.7 | 81.4±0.8 | ||
| Average | Max. | 18.7 | Average | Day | 18.2±0.3 | 19.5±0.3 | 66.1±0.6 | 50.2±0.4 |
| Min. | 7.6 | Night | 7.4±0.2 | 7.7±0.3 | 81.6±0.6 | 79.2±0.5 | ||
Soil was collected from the field at the Roseworthy Agricultural Campus, the University of Adelaide, and air dried. It was mixed with Waikerie sand (50:50 w/w) and basal nutrients were added to each pot at the start of the experiment. The pH measured in a 1:5 dilution of soil: 0.01 M CaCl2 was 6.6. Pots were made from black plastic tubing, 15 cm diameter and 40 cm high sealed at one end and filled with 6 kg of the soil–sand mix. To minimize evaporation from the pot surface, the soil surface was covered with a layer of white gravel.
Each experiment comprised two watering regimes: WW, well-watered (field capacity = 13.4%); and RW; droughting and re-watering to provide a cyclic drought treatment which is detailed below. In Experiment I, the level of water stress in the RW treatment was severe, while in Experiment II it was mild. Seeds were surface-sterilized with 0.5% sodium hypochlorite (NaOCl) for 1 min and soaked in water overnight. Seeds were then germinated on moist filter paper in Petri dishes for 24 h. Four germinated seeds were planted in each pot and thinned to three plants after establishment. In this experiment, growth-room temperature was lower during the nights over the first 4 weeks (Table 1). Plants in Experiment I took 140 d and 130 d from planting to maturity for WW and RW treatments, respectively.
Experiment II was conducted in a different growth room. In contrast to Experiment I, germinated seed in this experiment was pretreated with low temperature (4 °C) for 2 weeks. As a result of this vernalization treatment, the night temperature during the first 2 months of this experiment was maintained at a higher level (6±0.3 °C and 7.5±0.3 °C for the first and second months, respectively) than in Experiment I (Table 1). Plants in Experiment II were grown for 130 d and 122 d for WW and RW treatments, respectively. Maturity in Excalibur was 10 d later than in Kukri and RAC875 in this experiment.
Field capacity of the soil was determined by placing sieved (2 mm) air-dry soil into a G4 sintered glass funnel holding a 100 cm water column (ψm = –10 kPa = –0.1 bar). The soil was thoroughly wetted and allowed to drain for 48 h. A soil sample of approximately 30 g was oven-dried at 105 °C for 24 h. The sample was placed in a desiccator and weighed to determine the water content (Marshall and Holmes, 1979; Klute, 1986). Wilting point was determined using a ceramic pressure plate (ψm = –1500 kPa = –15 bar). The percentage water content of the soil:sand mix were 13.4%, 7.6%, 6.9%, and 4.8% at water potentials of –0.1 bar, –1 bar, –5 bar, and –15 bar (wilting point), respectively.
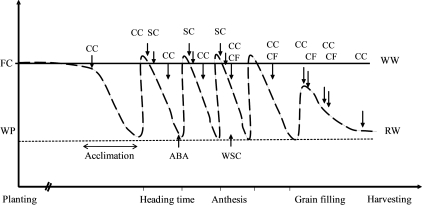
In both experiments (Experiment I and II), all plants were watered to weight daily with field capacity being maintained to the stage of flag leaf emergence. At the beginning of flag leaf emergence (at Zadoks scale = 37, when 1–5 cm of the flag leaf blade was visible, until the awns of apical florets emerged from the flag leaf sheath), water stress was imposed by gradually decreasing the amount of water to the pot for about 10 d to allow for acclimation (Fig. 1). For the cyclic drought treatment, plants were re-watered (RW) to the level of field capacity. Water was withheld until the symptoms of wilting appeared in the morning and also VSWC was decreased to about 7%, then plants were re-watered. Four consecutive drying cycles were imposed (60 DAP and 55 DAP for Experiments I and II, respectively), affecting anthesis and grain-filling of all cultivars, and water stress occurred in these experiments during the reproductive stages from heading to grain-filling. Similar numbers of plants were maintained under non-stress treatment with regular watering (WW). Development of water stress was monitored visually (observation of leaf rolling and leaf drying) and by measurement of leaf relative water content. In addition, volumetric soil water content (VSWC) was determined with a soil moisture meter (TDR 100 Spectrum Technologies, Illinois, USA). The total weight of the pot and all its contents was recorded when the soil was first brought to field capacity. When the plants were re-watered, the pots were weighed and water was added to bring the pot to field capacity again.
Fig. 1.
Schematic diagram of cyclic drought application and the time of trait measurements throughout the experiment. FC, field capacity; WP, wilting point; CC, chlorophyll content; CF, chlorophyll fluorescence; SC, stomatal conductance; ABA, abscisic acid, and WSC, water-soluble carbohydrates.
Measurements of cyclic drought experiments:
Water consumption was recorded by weighing and watering the pots every second day at the early stage of development, and daily at later stages (after flag leaf emergence). Volumetric water content was measured during the entire water-stress period. To control conditions in the growth room, a data logger monitored temperature and relative humidity. A dehumidifier was used to keep the relative humidity constant (Table 1). Trait measurements were taken throughout plant growth and during the cyclic water stress. Figure 1 shows how the water stress was applied and the time of measurements which were taken during the cyclic water stress.
Chlorophyll content and fluorescence:
Chlorophyll content was measured (mean of four measurements) using a portable chlorophyll meter (SPAD-502, Minolta, Tokyo, Japan). Chlorophyll content was measured on the same flag leaf every 3–6 d to monitor the chlorophyll retention after water stress was imposed. The effect of drought on the photosynthetic apparatus of leaves was studied by measurements of chlorophyll fluorescence. Chlorophyll fluorescence parameters were determined (mean of four measurements) four times during the grain-filling stages (94, 97, 103, and 109 DAP) with a fluorimeter (PAM-2000, Walz, Germany). The chlorophyll fluorescence ratio (Fv/Fm) was used to study effects of water stress on photosystem II (PSII) in wheat plants. To measure Fv/Fm, plants were kept in darkness for 20 min to allow full dark adaptation. Measurements were performed on flag leaves between approximately 3–5 h after the lights were turned on (Araus et al., 1998).
Stomatal conductance:
Measurements were made on two consecutive days after re-watering between 3–5 h after the lights were turned on. Stomatal conductance was measured on fully expanded flag leaves from three plants in each pot with a dynamic diffusion porometer (Delta-T AP4, Delta-T Devices Ltd, UK) during the middle of the day. Two measurements from both adaxial and abaxial surfaces of the leaf were taken. The porometer was calibrated at the start of each measurement session.
ABA content:
During the second period of the cyclic drought treatment, when water stress became severe (75 DAP at VSWC of 10.0±0.4%, 8.8±0.5% for Kukri and RAC875, respectively, and 83 DAP for at VSWC of 9.5±1.0% for Excalibur; Fig. 2), xylem sap and spikes were taken for ABA measurements. Xylem sap was collected by pressurizing the main stem of the plant in a pressure chamber. 30–50 μl of sap was collected using a pipette and transferred to an Eppendorf tube. The sap was immediately frozen in liquid nitrogen and stored at –80 °C for ABA analysis (Liu et al., 2005). Simultaneously, the spike of the main stem was sampled from control and water-stressed plants. Awns were removed from the spike and the spikelets immediately frozen in liquid nitrogen and kept at –80 °C. Frozen tissues were ground with a grinding mill covered with liquid nitrogen. Approximately 100–250 mg of ground tissue was used for extraction. ABA was extracted in hot water and quantified using high pressure liquid chromatography (HPLC) (Soar et al., 2004). The ABA measurements were done by Dr Brian Loveys, CSIRO Plant Industry, Waite Campus, Adelaide.
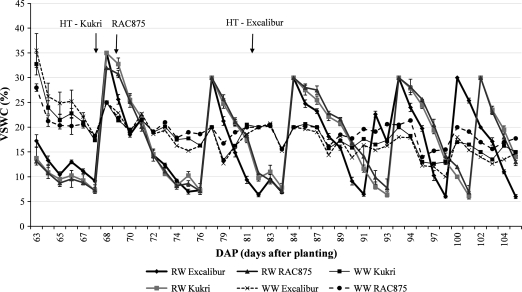
Fig. 2.
The volumetric soil water content (Vw/V) during RW and WW treatments (data from the second experiment). Water stress was started from 53 DAP for Kukri and RAC875, while it was started from 61 DAP for Excalibur. To synchronize the time of watering, all cultivars were watered at 68 DAP. VSWC measurements were started 63 DAP. HT is the heading time.
Water-soluble carbohydrates (WSC):
Samples for stem WSC determination were taken 5 d after anthesis. Stems without sheaths and leaf blades were initially freeze-dried then oven-dried again at 70 °C for 48 h and ground in a mill to pass a 1 mm screen. WSC content was determined using the anthrone method (Yemm and Willis, 1954) with some modifications (van Herwaarden et al., 1998) as follows: carbohydrates were extracted from 50 mg of dried material with 4 ml of 80% (v/v) ethanol at 80 °C followed by two extractions with 4 ml of water at 60 °C.
Carbon isotope discrimination (Δ13C):
Grains were sampled and processed for determination of carbon isotope composition. Approximately 10 g of grain of each cultivar was ground to a fine powder to pass a 0.5 mm sieve and the carbon isotope composition of each cultivar was determined by mass spectrometry (Condon et al., 1987).
Leaf traits:
Leaf glaucousness, leaf rolling, and leaf colour were visually assessed using a 1–5 scale. 1 represented no visible wax on the back of the flag leaf and an unrolled leaf, while 5 indicated the highest level of wax deposition and a tightly rolled leaf (O'Toole and Cruz, 1980; Clarke and Richards, 1988; Clarke et al., 1991).
In Experiment I, the flag leaf area (FLA) was measured using a portable Leaf Area Meter (CI-202, USA). Leaf turgid weight (TW) was recorded after rehydration overnight (at 4 °C). Finally, the leaves were oven-dried (48 h at 80 °C) and dry weight (DW) was obtained. The specific leaf area (SLA, leaf area per unit leaf dry weight) and leaf dry mass content (LDMC, the ratio of leaf dry weight to its turgid weight) were calculated. Leaf thickness was then estimated from the ratio (SLA×LDMC)−1 as described by Vile et al. (2005).
In Experiment II, excised leaf water loss (ELWL) was measured on flag leaves at 95 DAP. The excised leaves were weighed immediately after sampling to record fresh weight (FW), the incubation weight (IW) was recorded after 6 h incubation at 28 °C at 50% relative humidity, and then dry weight (DW) was obtained after 24 h oven-drying at 70 °C as proposed by Clarke and McCaig (1982). ELWL was then calculated from the following formula
Corresponding relative water content on a different set of leaves was also measured.
Plant dry weight determinations:
The number of days from planting to heading was recorded when 50% of the plants had their ears completely emerged. At maturity, total above-ground biomass and grain yield (GY) were recorded by weighing the bulked harvested plants (three plants per pot) and seeds from each pot. Four replicate pots per cultivar and treatment were sampled for dry weight determination. Roots were sampled and carefully washed to determine dry weight after oven-drying at 60 °C for 48 h. Harvest index (HI) was calculated as HI=GY/total biomass (shoot mass+root mass). Grain number and grain weight per spike (determined on three main spikes), the number of spikes per plant, and the number of spikelets per spike were also determined.
Data analysis
The experiments were designed in a randomized complete block. The analysis of variance (ANOVA) was performed using GenStat (Version 6.1 for Windows). Values are given as means and the standard error (SE) of the means which were calculated by SPSS (Version 13.0 for Windows). Graphs were constructed using SPSS and MS-Excel.
Results
Water consumption
In Experiments I and II, total water consumed during plant growth was, on average, 15.7 and 9.8 l per pot under WW and RW treatments, respectively. Under WW conditions, RAC875 used less water (14.7±0.6 l per pot) than Excalibur (16.2±0.4 l per pot) and Kukri (16.1±0.4 l per pot). The total quantity of water applied during the growth period, including before and after water stress, for the two experiments is given in Table 2. In Experiment I, the three cultivars used similar volumes of water before water stress. However, after the imposition of water stress, the volume of water consumed differed considerably between cultivars. During the cyclic water stress, Excalibur used significantly more water than RAC875 and Kukri (15.5% and 18.3%, respectively, P < 0.01). There were no significant differences between Kukri and RAC875 for total water consumption. In Experiment II, Excalibur used approximately 35% more water than RAC875 and Kukri before water stress was imposed. However, this could be attributed to the later heading time of Excalibur in this experiment (see below). Analysis of VSWC during the cyclic water stress also revealed that Excalibur depleted the soil water profile faster than Kukri and RAC875 (Fig. 2).
Table 2.
Average water consumption during the plant growth period
| Treatments | WW |
RW |
||||||||||||||||
| Experiments | I |
II |
Average I & II |
Average I & II |
I (Severe) |
II (Mild) |
||||||||||||
| Traits | Excalibur | Kukri | RAC875 | Excalibur | Kukri | RAC875 | Excalibur | Kukri | RAC875 | Excalibur | Kukri | RAC875 | Excalibur | Kukri | RAC875 | Excalibur | Kukri | RAC875 |
| Water use before stress (l pot−1) | 2.9±0.2 | 2.8±0.3 | 2.8±0.3 | 4.5±0.4 | 3.4±0.1 | 3.8±0.1 | 3.7±0.5 | 3.1±0.4 | 3.3±0.2 | 4.5±0.2 | 3±0.1 | 3.2±0.2 | 3±0.2 | 2.8±0.2 | 3.1±0.3 | 5.1±0.2 | 3.2±0.1 | 3.3±0.1 |
| Water use during stress (l pot−1) | 13±0.3 | 12.4±0.1 | 11.5±0.3 | 12.0±0.3 | 13.6±0.4 | 11.3±0.5 | 12.5±0.5 | 13±0.5 | 11.4±0.7 | 7.5±0.3 | 6.1±0.3 | 6.0±0.3 | 7.1±0 | 5.8±0.2 | 6.0±0.3 | 7.0±0.2 | 6.4±0.1 | 6.0±0.1 |
| Total water used (l pot−1) | 15.9±0.5 | 15.1±0.4 | 14.3±0.5 | 16.5±0.6 | 17±0.5 | 15.1±0.6 | 16.2±0.4 | 16.1±0.4 | 14.7±0.6 | 11.2±0.3 | 9.1±0.3 | 9.2±0.5 | 10.3±0.2 | 8.6±0.2 | 9.1±0.5 | 12.1±0.4 | 9.6±0.1 | 9.3±0.1 |
| WUEa (g l−1 pot−1) | 3.8±0.1 | 4.2±0.1 | 4.2±0.1 | 5.3±0.2 | 6.3±0.1 | 7.2±0.6 | 4.5±0.3 | 5.3±0.4 | 5.7±0.6 | 4.6±0.5 | 5.1±0.5 | 5.1±0.5 | 3.3±0.1 | 3.7±0.2 | 3.8±0.2 | 5.5±0.2 | 6.4±0 | 6.4±0.1 |
Total amount of water applied to each pot during the plant growth before and during the water stress imposition and the estimated water use efficiency (WUE) are given (each value represents the mean ±SE).
WUE=Total biomass (g)/total water consumed (l).
Agronomic traits
Heading time:
In both growth-room experiments, the watering regime (WW and RW) had no significant effect on the heading time. Cultivars under RW treatment flowered at a similar date to those under WW treatment (Table 3). However, there were differences between cultivars for heading time. RAC875 and Kukri had, on average, a similar heading time under both WW and RW treatments (73 DAP and 75 DAP for Kukri and RAC875). In Experiment I (severe water stress), Excalibur flowered 3 d and 2 d later than Kukri and RAC875, respectively. In Experiment II (mild stress), however, these differences were more pronounced, with Excalibur flowering 14 d and 12 d later than Kukri and RAC875, respectively.
Table 3.
Mean value of agronomic traits of Excalibur, Kukri, and RAC875 under controlled growth room conditions
| Treatments | WWa |
RW (cyclic drought)a |
|||||||||||
| Experiments | I |
II |
I. Severe stress |
II. Mild stress |
|||||||||
| Traits/Cultivar | Excalibur | Kukri | RAC875 | Excalibur | Kukri | RAC875 | Excalibur | Kukri | RAC875 | Excalibur | Kukri | RAC875 | |
| Heading time (DAP) | 80.3±0.8* | 75.8±1.5 | 79±0.4 | 86.8±0.3** | 68.8±0.5 | 71.9±0.4 | 80.6±0.4* | 77.6±0.9 | 78±0.6 | 84.5±0.1** | 69.1±1.1 | 73.3±1.1 | |
| Height | Plant height (cm) | 61±0.8 | 69.8±1.1* | 65.7±1.6 | 76.8±1.6 | 80.3±1.7** | 75.1±0.9 | 49.9±1.8 | 51.6±1.5 | 52.3±1.2 | 62.5±0.5 | 67.2±1.4* | 63.1±1.1 |
| Peduncle length (cm) | 20.3±0.7 | 30.8±0.8** | 24.5±1.8 | 26.7±0.6 | 40.8±0.7** | 33.6±0.5 | 15.9±0.7 | 19.4±0.6 | 18.5±0.3 | 19.5±0.7 | 31±0.7* | 23.2±0.5 | |
| Spike length (cm) | − | − | − | 11.3±0.2 | 11.7±0.3 | 11.7±0.1 | − | − | − | 13.1±0.1 | 11.2±0.2 | 11.5±0.1 | |
| Yield and its components | GY (g pot−1) | 20.7±0.8 | 21.3±0.8 | 19.9±1.5 | 27.7±1.6 | 34.9±1.9 | 31.5±1 | 4.5±0.2* | 3.5±0.6 | 6.5±0.8** | 11.9±1.2 | 17.8±0.8* | 20.3±0.3** |
| Grain weight.spike−1 (g) | − | − | − | 5.6±0.2 | 5.5±0.3 | 6.2±0.1 | − | − | − | 7.3±0.3 | 5.8±0.2 | 7.9±0.5 | |
| Spikelet no. | − | − | − | 21.8±1.9 | 20.3±1.6 | 19.4±1.5 | − | − | − | 26.7±0.2** | 18.5±0.2 | 19±0.2 | |
| Grains/spike | 71.1±7.2 | 93.6±1.2 | 82.9±4.7 | 59±2.6 | 60.2±2.3 | 46.3±0.6 | 66.7±2.8** | 44.5±11.6 | 64.3±4.7** | 53.3±2.4 | 53.7±1.7 | 55.3±2.5 | |
| TGW (g) | 40±0.9 | 36.9±0.7 | 48.6±1.1 | 31.7±2 | 30.2±0.7 | 44.7±0.7 | 37±0.8 | 34.8±2.4 | 45.3±1.3 | 45.8±2.7 | 35.8±1.1 | 47.9±1.3 | |
| Tillers plant−1 | 8.5±0.5 | 6.9±0.1 | 5.6±0.3 | 8.5±0.4 | 8.8±0.4 | 7±0.2 | 8±0.4 | 7±0.3 | 4.8±0.3 | 4.9±0.2 | 5.3±0.1 | 3.6±0.1 | |
| Aborted tillers plant−1 | 1.3±0.5 | 0.8±0.2 | 0.6±0 | 0.2±0.1 | 0.1±0.1 | 0.2±0.2 | 6.2±0.3 | 4.5±0.2 | 2.6±0.4 | 2.1±0.4 | 0.9±0.4 | 0.2±0.1 | |
| Abortion (%) | 14.5±5.2 | 10.8±2.2 | 11.4±1.3 | 2.1±1.2 | 1±1 | 2.5±2.5 | 77.8±0.7 | 64.4±2 | 53.4±6.4 | 75.6±15.7 | 20.8±7.7 | 4.9±3.4 | |
| Biomass | Biomass (g.pot-1)b | 59.6±1.7 | 64.2±1.6 | 59.9±2.6 | 90.5±3.1 | 111.2±3.8 | 112.3±4.8 | 33.7±1.7* | 32.1±0.5 | 34.6±0.8* | 66.9±2.5** | 61.9±1.4 | 58.1±1.4 |
| Root mass (g) | 5.6±0.5 | 7.9±0.3 | 6.7±0.3 | 10.8±0.3 | 10.7±1.1 | 9.4±0.5 | 6.5±0.2 | 6.1±0.4 | 6.4±0.2 | 12.6±0.9 | 7.6±0.3 | 7.1±0.4 | |
| Root/shoot ratio (%) | 10.8±0.8 | 13.97±0.3 | 12.67±0.4 | 11.95±0.53* | 9.61±0.8 | 8.4±0.2 | 23.98±1.1 | 23.5±2.1 | 22.9±0.5 | 17.8±0.97** | 12.3±0.6 | 12.2±0.91 | |
| HIc | 0.35±0.02 | 0.33±0.01 | 0.33±0.01 | 0.31±0.01 | 0.31±0.01 | 0.28±0.01 | 0.13±0.01 | 0.11±0.02 | 0.18±0.02 | 0.18±0.02 | 0.29±0.01 | 0.35±0.01 | |
Booting, heading time, and anthesis (maturity-related traits), plant height, peduncle length, and spike length (height-related traits), grain yield, grain weight per spike, number of spikelets per spike, number of grains per spike, thousand grain weight, number of tillers per plant, the proportion of tiller abortion (yield and its components), above-ground biomass, root mass, root-to-shoot ratio, and harvest index for three cultivars grown under well-watered (WW) and re-watering (RW) treatments are given (each value represents the mean ±SE).
*,** Significant at P < 0.05 and P < 0.01.
Total biomass = shoot mass+root mass.
HI = GY/total biomass (shoot mass+root mass).
Plant height and spike length:
Under the WW treatment, Kukri was significantly (P < 0.001) taller than Excalibur and RAC875 (on average 75.0±2.2 cm), while Excalibur was the shortest (68.9±3.1 cm) cultivar in both experiments (Table 3). In Experiment I, where water stress was severe, there were no significant differences in plant height between cultivars under RW treatment. Kukri, which had a greater plant height under WW treatment, was similar in height compared to the other two cultivars (Table 3). Plant measurements revealed that the differences in plant height resulted from a reduction in peduncle length of all cultivars when exposed to drought stress. In Kukri, peduncle length was reduced, on average, by 37% under water stress, whilst peduncle length in Excalibur and RAC875 was less affected by water limitation, with reductions of 20.2% and 24.5%, respectively. In Experiment II, where the water stress was mild, all three cultivars were approximately 10 cm taller than plants in Experiment I.
Spike length was measured in Experiment II. Under the WW treatment there was no significant difference in average spike length between the three cultivars. Conversely, under the RW treatment the spikes of Excalibur were significantly longer (P < 0.01) than those of Kukri and RAC875 (Table 3). The most significant finding was Excalibur's capacity to increase spike length under the RW treatment. In addition, the number of spikelets per spike differed significantly between cultivars under the RW treatment. Measurements of spikelet number in spikes of primary tillers revealed that Excalibur contained approximately 30% more spikelets per spike than Kukri and RAC875 (Table 3).
Grain yield and its components:
Under well-watered conditions, in both experiments, Kukri, Excalibur, and RAC875 were found to have comparable grain yields (Table 3). By contrast, cyclic water stress treatment resulted in significantly different grain yields between cultivars. In Experiment I (severe water stress) the reduction in grain yield under water deficit was 83.1%, 74.7%, and 67.8% for Kukri, Excalibur, and RAC875, respectively. In this experiment the drought-intolerant cultivar, Kukri, yielded 44% less grain than RAC875 and 18% less grain than Excalibur. Under the milder drought-stress conditions of Experiment II, the RW treatment resulted in grain losses of 49%, 57%, and 36% for Kukri, Excalibur, and RAC875, respectively. Notably, whilst RAC875 had the highest grain yield in both experiments (an average of 13.3±2.7 g pot−1), under the RW treatment of Experiment II, Excalibur produced less grain (11.9±1.2 g plot−1) than Kukri. Under the RW treatment, there was a significant interaction between experiments and varieties. In Experiment I, RAC875 and Excalibur showed significantly higher grain yield compared to Kukri. In contrast, in Experiment II, Excalibur had the lowest grain yield. In both experiments, RAC875 showed significantly higher grain yield under cyclic drought stress.
In Experiment I (severe water stress), performance of the cultivars was clearly comparable to their performance in the field. Excalibur and RAC875 produced more grains than Kukri under dry conditions. In Experiment II (mild stress), Excalibur produced less grain yield compared to RAC875 and Kukri. This discrepancy may be explained by variation in developmental stages between cultivars in the second experiment. Excalibur flowered much later than Kukri and RAC875 in Experiment II. Even though, water stress was imposed at the same phenological stages. In Experiment I, cultivated plants were exposed to low temperature (3–4 °C at night) during the first 4 weeks in the growth room. In contrast, in Experiment II, to speed up plant growth, before cultivation, germinated seed were pretreated in a cold room at 4 °C for 2 weeks in the dark. Two weeks pretreatment of the germinated seed at 4 °C may not have provided the vernalization requirement in Excalibur; or alternatively, the transplanting procedure might have resulted in devernalization of the young seedlings.
Tiller abortion and the number of grains per spike were the components that had the greatest impact upon grain yield under RW conditions. Indeed, RAC875, the highest yielding cultivar in both experiments, produced fewer tillers and had a lower tiller abortion rate than Excalibur and Kukri (Table 3). Under severe conditions (Experiment I), the number of grains per spike recorded for Excalibur and RAC875 was significantly higher than in Kukri (P < 0.01). Under mild drought stress (Experiment II), however, the differences between cultivars were not significant. The reduction in grain yield under RW treatment was also closely associated with a reduction in the grain weight of the main tillers. Kukri recorded a lower grain weight (5.8±0.2 g) in the main stems compared to RAC875 and Excalibur (7.9±0.5 g and 7.3±0.3 g), respectively.
Grain size was another yield component that was affected under cyclic drought. The drought-tolerant cultivars, Excalibur and RAC875, produced larger grains on the three main tillers compared to Kukri. Although RAC875 produced the largest grain under both watering regimes and in both experiments (Table 3), grain produced by Excalibur was only significantly (P < 0.01) larger than Kukri under the RW treatment.
There were no differences in HI between cultivars under non-stress conditions, but Kukri had the smallest HI (0.11±0.02) compared with Excalibur (0.13±0.01) and RAC875 (0.18±0.02) under cyclic drought (Experiment I). The HI in Experiment II was, in total, higher than HI in Experiment I, but Excalibur had the smallest HI (0.18±0.02) of all cultivars in Experiment II. RAC875, on average, had the highest HI under cyclic drought conditions (0.27±0.03). As HI was calculated from GY divided by total biomass, including the shoot and root mass, the lower HI in Excalibur is contributed mainly due to larger root mass. Although the root mass was significantly (P < 0.001) lower in Experiment I than II, under WW treatment, there were no significant differences among cultivars in root mass in both experiments. Under RW treatment, however, Excalibur showed larger root mass compared to Kukri and RAC875 (Table 3).
The major determinants of yield under water stress were number of grains per spike and the percentage of aborted tillers. This finding is supported by the negative correlation between GY and the percentage of tiller abortion found in Experiment I and II (r = –0.71 and –0.87, P < 0.01) and the positive correlation between GY and number of grains per spike (r=0.64 and 0.87, P < 0.01) under the RW treatment. There was no significant correlation between GY and grain size in both experiments.
Leaf traits:
There are differences in leaf morphology between cultivars. RAC875 produces erect, rough, and dark green leaves with heavy wax deposition in the abaxial surface of the leaf under both stressed and non-stressed conditions and it appears on the adaxial surface of the leaf under severe water stress. Excalibur has narrower leaves compared to RAC875 and Kukri but produces moderate amounts of waxes on the abaxial surface of the leaf under water stress. Kukri, by contrast, is a non-waxy cultivar with broad, non-erect, pale green and smooth leaves. These cultivars significantly (P < 0.01) differed in their level of leaf waxiness. Wax deposition typically started on the flag leaf sheath and the abaxial surface of the flag leaf blade and its expression increased with timing and water stress. Under water stress, RAC875 displayed the highest level of wax deposition (Table 4) with the abaxial surface completely covered with wax (score of 5.0). Kukri produced the least amount of wax in response to water deficit (score of 1.5) whilst Excalibur plants displayed an intermediate level of wax deposition.
Table 4.
Leaf traits from Experiment I and II, where plants were subjected to well-watered (WW) and cyclic drought (RW) treatments
| Traits | WWa |
RWa |
||||
| Excalibur | Kukri | RAC875 | Excalibur | Kukri | RAC875 | |
| ChlC (SPAD unit) | 49.1±1.7 | 51.1±0.4 | 57±0.7** | 54.8±1.7 | 54±0.9 | 61.7±1.4** |
| Waxiness (1–5) | 1.5±0.1 | 1.0 | 4.0±0.1** | 3.0±0.1* | 1.5±0.2 | 5.0±0.2** |
| Leaf rolling (1–5) | 1.0 | 1.0 | 1.0 | 4.0±0.1** | 3.0±0.2a | 1.4±0.1 |
| Retained green leaves | 3.6±0.2* | 2.8±0.09 | 3.7±0.1* | 3.3±0.1* | 2.2±0.05 | 3.2±0.1* |
| FLA (cm2) | 32.6±1.3 | 39.2±1.3** | 34.6±1.3 | 29.8±0.9** | 26.8±2.5** | 21.6±1.1 |
| SLA (cm2 g-1) | 243.6±12.4 | 250.1±11.8 | 234.1±11.8 | 258.9±9.4 | 231.4±7.7 | 209.9±8 |
| LDMC (mg g-1) | 268.4±11.0 | 273.5±10.8 | 260.5±10.1 | 261.7±7.0** | 255.1±7.1* | 221.1±4.8 |
| LT | 15.6±1.5 | 14.9±1.4 | 16.7±1.6 | 15.1±1 | 16.9±1.0 | 21.8±1.3** |
| ELWL (%) | 43.9±1.4 | 49.0±0.6** | 41.7±1.1 | 35.3±3.4 | 57.7±3.0** | 47.6±2.6 |
Values for chlorophyll content (ChlC), leaf waxiness, leaf rolling, and the average number of green leaves on 95 DAP of Excalibur, Kukri, and RAC875 representing averages of combined Experiment I and Experiment II. Values for flag leaf area (FLA), specific leaf area (SLA), leaf dry matter content (LDMC), and leaf thickness (LT) were measured in Experiment I, while excised leaf water loss (ELWL) was measured in Experiment II (mean ±SE).
*,** Significant differences at P <0.05 and at P <0. 01 levels, respectively.
Leaf rolling was scored on the flag leaves of stressed plants during cyclic drought stress. As water stress progressed, Excalibur started leaf rolling faster than Kukri and RAC875 and had the highest value of leaf rolling during the RW treatment (score of 4). Leaf rolling was observed in Kukri (score of 3) at higher levels of water deficit than those required to elicit rolling in Excalibur, while RAC875 showed almost no leaf rolling (Table 5). In RAC875 the symptoms of leaf rolling were only induced when the soil water content decreased to very low levels (approximately 6% in pots, at ∼ –10 bar).
Table 5.
Predicted osmotic potential (OP) at 70% relative water content for the concentration effect and expressed sap osmotic potential
| Cultivars | OA (MPa) | OP due to concentration effect (MPa) | OP (MPa) | Regression model for phase β (RWC and OP) | R2 |
| Excalibur | 0.846 | –0.310 (–2.040)a | –0.460 (–2.885) | y = 1.083x–2.459 | 0.845 |
| Kukri | 0.097 | –0.352 (–2.249) | –0.370 (–2.346) | y = 0.788x–1.824 | 0.971 |
| RAC875 | 0.364 | –0.312 (–2.052) | –0.383 (–2.416) | y = 1.047x–2.315 | 0.896 |
Data in parenthesis are anti logarithmic values of OPs.
Water stress affected individual flag leaf area (FLA). Plants under RW treatment showed significantly (P < 0.001) smaller FLA compared to plants under WW treatment (Table 4). Significant differences between cultivars under RW and WW treatments were observed. Under WW treatment, Kukri showed significantly (P < 0.001) higher FLA (39.2±1.3 cm2) compared to Excalibur and RAC875 (32.6±1.3 and 34.6±1.3 cm2, respectively). Under RW, however, RAC875 had the smallest FLA (21.6±1.1 cm2. Under WW, although the LT for RAC875 was bigger than Excalibur and Kukri, the difference was not statistically significant. Under RW, however, RAC875 showed significantly (P < 0.001) higher LT compared to Excalibur and Kukri (Table 4). Water stress had no effect on leaf thickness in Excalibur, whilst it led to a 13.4% and 30.5% increase in leaf thickness in Kukri and RAC875, respectively. Water loss from the excised flag leaf was significantly (P < 0.001) higher in Kukri compared to Excalibur and RAC875 under both WW and RW treatments. Although RWC was higher (95.1±0.2%) in non-stressed plants than stressed ones (90.8±0.5%), there were no significant differences between cultivars at these levels of RWC.
Physiological traits
Water status measurements from Experiment OA:
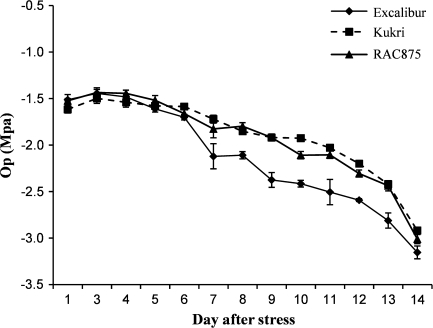
The average osmotic potential (OP) of leaves of all cultivars at the initiation of withholding water was –1.55 MPa and decreased to –3.03 MPa by the end of the experiment. After 6 d of drying, the differences between cultivars became apparent; with OP in Kukri and RAC875 becoming progressively less negative than Excalibur in successive drying conditions (Fig. 3). After withholding water from the pot, it took 14 d to reach to the level of RWC=60%. It seems that the soil water depleted gradually since the coco-peat retains water for longer compared with the sandy-clay soil in the field.
Fig. 3.
Decrease in osmotic potential with successive water stress in Excalibur, Kukri, and RAC875.
To determine the OA for the three cultivars, two regression lines were calculated based on logarithmic conversion of RWC and OP values (see Materials and methods). Regressions of RWC on OP for the three cultivars are presented in Fig. 4. Figure 4 shows the relationship between RWC and OP which represents a biphasic (phase α and β in Fig. 4) response (Wright et al., 1983). The magnitude of the first phase differed substantially between cultivars. There was a clear response in Excalibur and RAC875. Both showed small changes in RWC as OP declined. The initial response did not follow the behaviour of an ideal osmometer (dashed line), which can be mainly attributed to OA (Fig. 4). In the second phase, however, OP declined linearly with RWC. Therefore, decline in OP in the second phase (β) can be attributed to an increase in solute concentration due to water loss (Morgan, 1980; Wright et al., 1983). The OA value for Excalibur (0.846 MPa) was greater than for RAC875 (0.364 MPa) and Kukri (0.097 MPa), indicating a greater degree of solute accumulation (Table 5). The calculation of OA based on Morgan's method showed that Excalibur had the highest, and Kukri the lowest, OA; RAC875 was intermediate (Table 5).
Stomatal conductance:
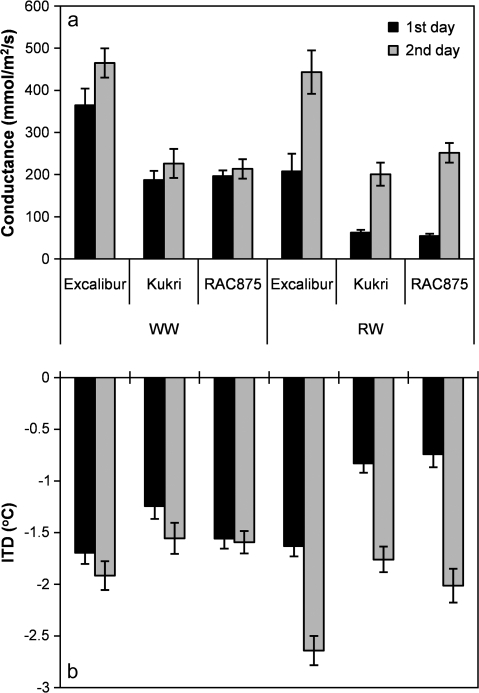
Stomatal conductance was measured once in Experiment I and three times in Experiment II 24 h and 48 h after re-watering to investigate plant recovery. Average stomatal conductance and leaf temperature measurements from Experiments I and II are given in Fig. 5. These measurements revealed that Excalibur has an intrinsically higher stomatal conductance than Kukri and RAC875. Excalibur showed significantly higher stomatal conductance than Kukri and RAC875 at both sampling time points and under both watering regimes (P < 0.01). 24 h after re-watering the stomatal conductance of plants in the RW treatment was lower than those in the WW treatment. However, by 48 h after re-watering, stomatal conductance of all cultivars had returned to levels exhibited by plants in the WW treatments. Excalibur had the highest stomatal conductance under RW treatment (P < 0.001). Kukri had the lowest stomatal conductance on the second day after re-watering. Excalibur recovered much more rapidly after re-watering than the other two cultivars. 24 h after re-watering the stomatal conductance of RW Excalibur was about 57% of the level of the WW Excalibur, whereas the stomatal conductance of RW Kukri and RAC875 stomatal conductance was only about 33% and 28% of the WW plants, respectively. The recovery rates after 48 h of re-watering were 95%, 88%, and 118% for Excalibur, Kukri, and RAC875, respectively.
Fig. 5.
The average stomatal conductance of plants in Experiments I and II for the first and second days after re-watering. In Experiment I, measurements were done in one period, but in Experiment II three periods of measurements were performed (a). Leaf initial temperature differences (ITD) after re-watering (b). ITD was calculated by subtracting the temperature of the ambient air of the leaf temperature. Error bars are SE of means.
Plants subjected to cyclic drought showed lower stomatal conductance during stress and recovery of stomatal function occurred 2 d after re-watering. Our data show that stressed plants had more stomatal closure in the first day after re-watering and they had recovered on the second day. The stomatal conductance results from all series of measurements showed that Excalibur had the highest stomatal conductance on the first and second days after re-watering. RAC875 had similar response to Kukri on the first day, but showed slightly higher stomatal conductance on the second day after re-watering.
The leaf temperature of re-watered Excalibur plants was significantly lower than Kukri and RAC875 at both time points reflecting the higher transpiration rate of Excalibur following re-watering. The relationship between stomatal conductance and leaf temperature was highly significant (P < 0.01) under cyclic drought treatment (r = –0.72 and r = –0.73, 24 h and 48 h after re-watering, respectively), but there were no significant correlations between stomatal conductance and leaf temperature under WW treatment.
Chlorophyll content and fluorescence:
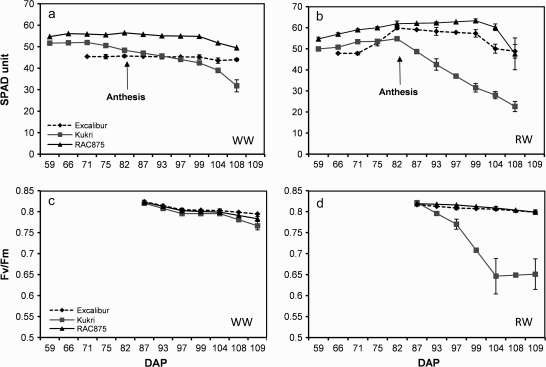
Chlorophyll content and fluorescence of plants under WW and RW treatments were investigated in Experiment II. RAC875 had the highest chlorophyll content (55–65 SPAD units) under both stressed and non-stressed conditions. In all cultivars the imposition of drought stress resulted in an increase in chlorophyll content until plants reached anthesis (Fig. 6). Following anthesis (80 DAP), the chlorophyll content of droughted Kukri and Excalibur plants decreased, whilst the chlorophyll content of RAC875 leaves continued to increase for a further 19 d. Drought had the most pronounced effect on chlorophyll content in Kukri plants, with leaves of drought-treated plants containing approximately 29% less chlorophyll than well-watered plants at 108 DAP. Conversely, at 108 DAP the chlorophyll content of drought-stressed RAC875 and Excalibur plants was similar to well-watered plants at the same stage of development. A significant positive correlation was found only under the water-stress treatment between chlorophyll content and grain size (r=0.52 and r=0.87, P < 0.001). RAC875 with a higher chlorophyll content had higher grain size, while Kukri with a lower chlorophyll content had small and more shrunken grains.
Fig. 6.
Chlorophyll content and fluorescence (Fv/Fmratio) in Excalibur, Kukri, and RAC875 for Experiment II. The measurements were done on the same flag leaves during plant growth under and during the grain-filling period. The chlorophyll content under WW and RW treatments (a, b); large error bar on day 108 for RAC875 under RW treatment resulted from developing senescence in flag leaves. Fv/Fm under WW and RW treatments (c, d). Error bars are SE of means.
The Fv/Fm ratio, which is an indication of the maximum yield of photosystem II photochemistry, ranged between 0.82 and 0.78. There were no significant differences over time among cultivars under WW treatment (Fig. 6c). Under RW treatment, Fv/Fm did not change (0.81) at the first measurement date (87 DAP) and cultivars displayed similar photosynthetic responses. Although, Fv/Fm did not change in Excalibur and RAC875 at the time of measurements 97, 104, and 109 d after planting, it decreased dramatically in Kukri plants (from 0.82 to 0.65) under the RW treatment (Fig. 6d). This decrease was nearly 6% on day 97, 20% on day 104 and remained steady until day 109. Despite considerable differences in the dynamics of leaf chlorophyll content under WW conditions, the fluorescence measurements revealed no significant differences between cultivars over a period of 27 d post anthesis (Fig. 6c).
Water-soluble carbohydrates (WSC):
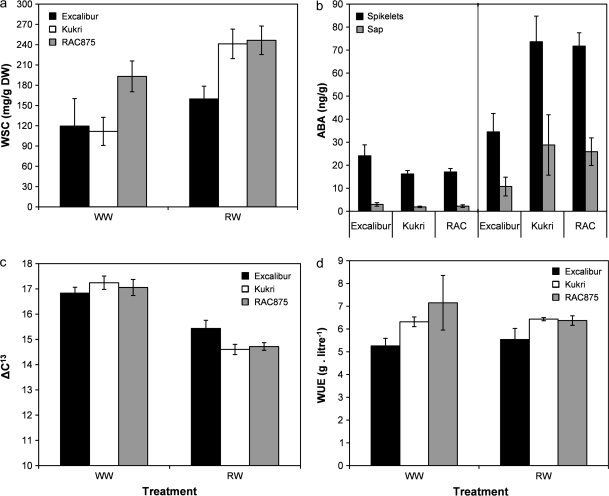
Water-soluble carbohydrates (WSC) concentration in the stem at the stage of 5 d after anthesis is shown in Fig. 7a. Stem carbohydrates of all cultivars increased under water stress relative to the WW treatment (P < 0.01). Under the WW treatment, RAC875 had the highest WSC concentration (193.1±22.8 mg g−1 DW), whereas the WSC content of Kukri and Excalibur was similar (111.7±20.8 and 119.6±40.8 mg g−1 DW, respectively). Under the RW treatment, however, the WSC content of RAC875 and Kukri increased to a similar level (246.4±21.0 and 241.1±21.8 mg g−1 DW, respectively), whilst Excalibur recorded a lower WSC concentration (159.7±18.7 mg g−1 DW). WSC in Excalibur did not change in stressed plants compared to non-stressed ones. Comparing the three cultivars, RAC875 and Kukri had higher WSC values compared to Excalibur under stressed conditions.
Fig. 7.
Drought-related traits for Excalibur, Kukri, and RAC875 under WW and RW treatments in Experiment II. (a) Values for water-soluble stem carbohydrates (WSC); stem samples were taken 5 d after anthesis. (b) ABA concentration in floral tissue and xylem sap; when tissue was collected, plants/lines experienced severe water stress during RW treatment whereas plants under WW conditions remained unstressed. (c) Values for carbon isotope discrimination on grains, pos-harvest and (d) values for agronomic WUE for Excalibur, Kukri, and RAC875 under WW and RW treatments. Error bars are SE of means.
ABA assay:
ABA levels were markedly higher in droughted plants compared with WW plants (P < 0.001, Fig. 7b). The ABA concentration in xylem sap and floral tissues of all cultivars increased under the RW treatment for the three cultivars in both xylem sap and floral tissues. However, the level of ABA was 3-fold higher in floral tissues compared to xylem sap. ABA content increased 3.8 and 11.4 times in spikes and xylem sap due to water stress, respectively. Although, there were no differences between cultivars under WW treatment for ABA content, the cultivars showed significant differences (P < 0.05) under RW treatment. Excalibur showed lower ABA content in spikelets and xylem sap compared to Kukri and RAC875. One data point in Excalibur was removed from the analysis as it was an outlier observation (181.5 μg g−1, versus the mean of 34.5±8.0 μg g−1). Water stress caused a 3.3-fold increase in the ABA content of spikes, but no correlation was found between ABA content and grain set and increased levels of ABA did not induce low grain number in this germplasm (Dembinska et al., 1992). This result shows that Excalibur accumulates less ABA under RW treatment, indicating that ABA may be a primary controller of stomatal behaviour in these plants.
Carbon isotope discrimination (Δ13C):
Carbon isotope discrimination (Δ13C) values from grain were highly affected by water stress. In Experiment II, there were no genotypic differences among cultivars for Δ13C under WW and RW treatments. Plants under WW treatment had significantly (P < 0.001) higher Δ13C values compared to plants under the RW treatment. The three cultivars exhibited similar Δ13C values under WW treatment. Under RW treatment, however, Excalibur showed a slightly greater Δ13C value, but the differences were not statistically significant (Fig. 7c). In general, there were no clear genotypic differences between cultivars under both WW and RW treatment in Experiment II. Highly significant correlations (r=0.87, P < 0.01) were found between Δ13C and total water consumed, and Δ13C and stomatal conductance at the first day after re-watering when plants had experienced water stress under RW treatment (r=0.8, P < 0.01). Cultivars which showed higher stomatal conductance used more water and had higher Δ13C. Condon et al. (1992) pointed out that variation in Δ13C among genotypes may be attributed to genotypic variation in the greater extent of soil water extraction at near anthesis and variation in the response of stomata to soil water depletion and/or to increasing VPD. Condon et al. (1992) acknowledged that the measurement of Δ13C, despite its positive application, also has several shortcomings. It does not provide information on the magnitude of either the assimilation rates or transpiration at the stomata, nor whether variation in Δ13C is being driven by variation in stomatal conductance or photosynthetic capacity. For example, a variety with high stomatal conductance and high photosynthetic capacity would give similar Δ13C to a variety with low conductance and low capacity.
Water use efficiency (WUE), as the ratio between the volume of water consumed and the total biomass (shoot and root) produced, is shown in Table 3. ANOVA showed that in Experiment I, WUE was significantly (P < 0.0001) lower compared to Experiment II. In Experiment I, there was a significant difference (P < 0.001) between treatments, while the difference was not significant in Experiment II (Fig. 7d). In both experiments, Excalibur showed significantly (P < 0.01) lower WUE compared to Kukri and RAC875. Correlations between WUE and Δ13C were not found under both WW and RW treatments in Experiment II.
Discussion
Grains per spike and tiller abortion are the major yield components for higher grain yield under cyclic water stress
The main yield components that associated with yield reduction across the experiments were grain number per spike and number of fertile tillers. Reduction in grain number and in the number of fertile tillers was mainly associated with floret sterility and tiller abortion under water stress. Water stress occurring during the later stages of development caused a greater reduction in grains per spike and in the total number of tillers per plant (Blum and Pnuel, 1990). Blum et al. (1990) suggested that maintaining the potential grain number per spike under stress is more important than tillering ability. In our study, RAC875 produced fewer tillers and maintained higher numbers of grains per tiller. Excalibur produced potentially a high number of tillers, but while encountering water stress, large numbers of tiller were aborted. The ability of rapid recovery under cyclic water stress in Excalibur, therefore, might allow this cultivar to produce more grains on recovered tillers. Kukri did not possess any of these mechanisms. It had a high number of tillers and did not recover after water stress to produce grains, resulting in high numbers of aborted tillers as well as lower grain number per spike under RW treatment.
Osmotic adjustment and its role in conferring drought tolerance
The results from the greenhouse experiment showed that there are different responses among the genotypes for OA capacity. Excalibur showed high, RAC875 moderate, and Kukri low OA. However, at this stage it cannot be fully ruled out that the maturity differences between Excalibur and the other two cultivars also influenced differences for OA. Differences in OA capacity contribute to maintain plant productivity under water stress (Morgan, 1980; Blum et al., 1999). At anthesis, pollen mother cells during meisosis are more sensitive to water stress, and water deficit during these stages significantly reduces grain set as a result of male sterility (Saini and Aspinall, 1982). The capacity to adjust osmotically may enhance spikelet fertility due to pollen development. Under severe drought stress, plants with the ability to adjust osmotically can maintain turgor when leaf water potential is reduced (Morgan, 1980). Morgan and Condon (1986) found a positive relationship between seed set and turgor maintenance and concluded that genotypes with low turgor maintenance produced less seed. OA appears to be associated with extended root growth (Sharp et al., 2004), sustained leaf gas exchange, sustained cellular membrane and protein function, as well as chloroplast volume and function (reviewed by Blum, 1988; Zhang et al., 1999). OA, in addition to maintaining turgor and sustaining cellular function for a longer time under drought conditions, might allow plants to recover faster from water stress.
The genotypic variation in stomatal response in these cultivars may be partly explained by their variation in OA capability. Excalibur with a high level of OA showed higher stomatal conductance after recovery, while Kukri had very low OA and kept stomata more closed. Osmotic adjustment in leaves has been demonstrated to maintain stomatal opening (see Turner, 1986, for a review). Wright (1983) observed that sorghum genotypes with higher OA capability had the ability to maintain stomatal conductance compared to those with low OA. OA resulted in a lower osmotic potential for a given level of leaf RWC at a given level of soil water content. Hence it sustained leaf turgor pressure during soil drying (Turner, 1986). Therefore, genotypes with high OA and more leaf turgor maintenance may become less stressed at a given level of water stress. Consequently, they produce or accumulate less ABA, resulting in higher stomatal activity relative to those genotypes with lower leaf turgor (Ali et al., 1999). In this study ABA was measured in stressed and non-stressed plants. In Kukri and RAC875, samples for ABA measurements were taken on 75 DAP at a VSWC of 10.0±0.4% and 8.8±0.5%, respectively. Excalibur samples were taken on day 83 when the VSWC for this line was 9.5±1.0%. This indicated that the pots were very similar in soil water content, however, cultivars showed significantly (P < 0.05) different ABA contents in both xylem sap and spikelets. Although the cultivars were at the same stress level in terms of water availability, their ABA response appeared different. It might be concluded that the higher OA capability in Excalibur allowed these plants to become less stressed which resulted in low ABA contents, higher stomatal conductance, and rapid recovery after stress. Turgor maintenance might also delay irreversible cell membrane damage and sustain cell functions which are critical for rapid recovery after relief from severe drought stress (Elmi and West, 1995).
Source activity and source strength-related traits and their effects on grain-filling under drought stress
Source activity could be partitioned into two components, namely, current photosynthetic assimilates from different plant parts (e.g. leaves, ears, and awns) and stem water-soluble carbohydrates (WSC) accumulation and remobilization during the grain-filling period (Bewley and Black, 1994). Ehdaie et al. (2008) found no correlation between the amount of stem reserves and the amount of current assimilates that contributed to grain yield. They concluded that selecting genotypes that simultaneously remobilize relatively greater stem reserves and current assimilates to grain yield under drought could reduce the adverse effect of drought.
The result from chlorophyll content measurements during plant growth indicates that cultivars with a high chlorophyll content seem to stay green for longer. In this study, the pale leaves with a lower chlorophyll content in Kukri senesced early, while the dark green leaves with a high chlorophyll content in RAC875 and Excalibur consistently stayed green. These data suggest that the large decrease in chlorophyll content of Kukri leaves post-anthesis under cyclic water stress might be preprogrammed leaf senescence (Lu and Zhang, 1998; Lu et al., 2002). Under the RW treatment, chlorophyll fluorescence measurements were found to reflect the chlorophyll content observations. Chlorophyll fluorescence of RAC875 and Excalibur were unaltered by the drought treatment, consistent with our observation that both cultivars maintained chlorophyll content under these conditions. By contrast, chlorophyll florescence of Kukri was significantly reduced under the RW treatment indicating that leaf senescence was accelerated under drought. This may be associated with a programmed (drought-induced) leaf senescence phenomenon (Munné-Bosch and Alegre, 2004). Although, programmed leaf senescence and leaf abscission contribute to plant survival under drought conditions in nature (Munné-Bosch and Alegre, 2000, 2004), this strategy can lead to yield loss in economically important annual crops (Borrell et al., 2000; Jiang et al., 2004; Rivero et al., 2007). On the basis of the assumption that leaf senescence is a type of programmed cell death (PCD) that is inappropriately activated during drought stress, Rivero et al. (2007) generated transgenic tobacco plants expressing an isopentenyltransferase gene driven by a stress- and maturation-induced promoter. They found that the suppression of drought-induced leaf senescence resulted in outstanding drought tolerance in tobacco. The transgenic plants maintained a relatively high water content and retained photosynthetic activity during the drought compared with control plants. In addition, the transgenic plants grown under restrictive water supply showed a minimal yield loss when watered with only 30% of the amount of water used under control conditions.
The possible explanation for the stay-green characteristic in RAC875 and Excalibur might be their higher degree of OA capability that helped the leaves to stay green for longer and to maintain photosynthetic activity relative to Kukri. High chlorophyll content and stay-green probably contributed to the high-yielding capacity. Borrell et al. (2000) found associations between stay-green and higher leaf chlorophyll content as well as improved yield and transpiration efficiency under post-anthesis drought conditions. Stay-green sorghum hybrids also maintained more photosynthetically active leaves than senescent hybrids under post-anthesis drought. Strong associations between chlorophyll content, photosynthesis rate, and yield were reported in wheat cultivars grown under well-irrigated and drought conditions (Gutierrez-Rodriguez et al., 2004) and in wild wheat (Aegilops geniculata) grown under heat and drought conditions (Zaharieva et al., 2001) Nevertheless, reduced leaf chlorophyll content has been reported to be an important trait in heat stress avoidance. Pale green leaves can decrease radiation absorbed by the leaf surface, thereby reducing the risk of desiccation (Blum, 1988; Reynolds et al., 2005).
Our results from chlorophyll fluorescence measurements as a reflection of photosynthetic capacity shows that the flag leaf of the stay-green cultivar remained functional during the grain-filling period. Differences in photosynthetic capacities during the grain-filling period could be due to different amounts of chloroplasts per unit leaf area. The retention of photosynthetic capacity under water-stress conditions of stay-green cultivars ensures the continued availability of new assimilates and is associated with increased nitrogen uptake during grain-filling in sorghum (Borrell and Hammer, 2000), and can potentially improve grain size. Spano et al. (2003) reported a 10–12% increase in grain size of the stay-green mutants of durum wheat. They concluded that the extended period of flag leaf photosynthetic capacity during the phase of grain-filling is associated with the production of larger grains. Richards et al. (2001) pointed out that the stay-green capability might be a useful trait in environments with a high probability of rainfall during grain-filling. Plants with stay-green characteristics and more photosynthetic tissue may produce more assimilates and extract more water from the soil. Foulkes et al. (2007) found a positive phenotypic correlation between green flag-leaf area retention and yield under drought under the UK drought conditions.
Differences in heading time were observed between Excalibur and the other two cultivars in Experiment II. Thus, differences in chlorophyll content and fluorescence between Excalibur and the other two cultivars might be affected by flowering time. However, significant differences between RAC875 and Kukri, which showed similar heading time, were observed for these same traits.
The stem WSC can serve as a buffer between the supply of current photosynthesis assimilates and the demand by the sink (Borras et al., 2004). Although, the stay-green trait may increase the risk of severe drought and heat stress during the later stages of the grain-filling period, remobilization of a large amount of WSC to grain can complement the longer grain-filling time (Blum, 1998). In this study, the average WSC in the stem of stressed plants was 1.5-fold higher than in the stem of non-stressed plants. These results are in agreement with Goggin and Setter (2004), who found significantly higher average concentrations of total carbohydrates (1.8-fold) and fructans (2.5-fold) in the stems of rain-fed compared to irrigated plants. They speculated that the fructan accumulation in rain-fed stems increased in response to water deficit (Goggin and Setter, 2004). Our results show that RAC875 had a constantly greater capacity for WSC storage under both stress and non-stress treatments. Irrespective of the environment during grain-filling, RAC875 had more available WSC than Excalibur and Kukri. While under stress treatment, both RAC875 and Kukri showed higher WSC concentration compared to Excalibur. Nevertheless, WSC accumulation is very much affected by environmental conditions such as light interception, nitrogen as well as tillering capacity, plant height, and flowering time (Ruuska et al., 2006; Ehdaie et al., 2008). In RAC875, however, the greater WSC concentration might be associated with low tillering capacity in this cultivar, whereas in Excalibur the large number of tillers and late flowering might have resulted in low amounts of WSC under both stress and non-stress conditions. In this study, it was impossible to evaluate the proportion of available WSC that contributed to grain-filling. It is speculated that cultivars with high WSC at anthesis remobilize a high proportion of WSC to the grain during grain filing even under non-stress conditions. The amount of stem WSC and its contribution to the grain for these cultivars needs further investigation.
Leaf morphology and its role in the reduction of transpiration
Under water stress, RAC875 showed a higher chlorophyll content, a greater leaf waxiness, a smaller flag leaf area, and thicker leaves compared to Kukri and Excalibur. The high chlorophyll content in RAC875 might be a reflection of leaf thickness in this cultivar. Thicker leaves would have a higher density of chloroplasts per unit area, and therefore chlorophyll content per unit leaf area (Araus et al., 1986). Excalibur, on the other hand, showed more leaf rolling, moderate leaf waxiness, retained more green leaves as similar as RAC875, bigger leaf area, and less leaf thickness. Both drought-tolerant cultivars had lower excised-leaf water loss, reflecting lower residual transpiration.
The reduction in leaf size in RAC875, which results in a smaller transpiring leaf area, is an adaptive response to water deficit (Tardieu, 2005). Tardieu (2005) pointed out that short-term reduction in leaf area has a similar role to stomatal closure, which allows plant to avoid damaging leaf water potential in leaves by reducing the water flow through the leaf surface. In the longer term, a reduced leaf area can save soil water for the later stages of plant development via a reduction in transpiration. Leaf rolling, which was high in Excalibur, can also reduce the effective leaf area and, hence, reduce radiation interception and, consequently, reduce transpiration under water stress (Loss and Siddique, 1994).
Both drought-tolerant cultivars had a lower excised-leaf water loss which is reflecting low residual transpiration; this might be the result of leaf waxiness. An important function of leaf waxiness is to increase the efficiency of stomatal control by reducing water loss after stomatal closure (Clarke and Richards, 1988). Leaf waxiness, and pubescence have been reported to contribute to heat stress avoidance, by reducing radiation absorbed by the plant (Blum, 1988). The leaf waxiness and leaf angle (erectness) in combination may improve radiation use efficiency (RUE). Whilst waxiness increases radiation reflectance, and thereby reduces leaf and spike temperature it increases leaf and floret survival (Johnson et al., 1983; Richards et al., 1986). Erectophile leaf canopy can use radiation more efficiently while intercepting less radiation (Reynolds et al., 2000).
Cyclic water stress in pot experiments
The three cultivars showed a different response to cyclic water stress under controlled conditions. RAC875 showed a consistently higher yield relative to Kukri under both cyclic water-stress experiments. Excalibur, however, showed strong interaction with experimental conditions. It out-yielded Kukri only under severe stress conditions (Experiment I). These data from the growth-room experiments showed that, with target environment considerations, drought experiments under controlled conditions can relate to field performance, as plants were grown in 35 cm long pots and cyclic water stress during the reproductive stages of development. In addition to reducing the unpredictable variables under controlled conditions, it is possible to determine the extent of the drought stress. It also makes it possible to measure extensive traits throughout the experiment. Although the cyclic drought experiment in the growth room worked for a few cultivars, there are limitations. In particular, controlling watering regimes is very labour intensive, which makes it difficult to assess a large number of genotypes or mapping populations. Also, the described conditions might not work for all genotypes, for example, genotypes which rely on a deep root system to exploit water from depth, unless deep soils are mimicked with 1–2 m long PVC cylinders.
Conclusions
Cyclic drought is a frequent event in South Australian environments, occurring during pre-anthesis, post-anthesis, and the grain-filling period. It is shown that cyclic water stress under growth-room conditions mimicking the drought of the target environment, worked consistently to distinguish drought-tolerant and -intolerant cultivars. Therefore, data from pot experiments based on this system can be extrapolated with some confidence to the field.
Although Excalibur and RAC875 are evidently more drought-tolerant cultivars compared to Kukri, it would not mean that Kukri (a drought-susceptible cultivar) does not possess any ‘drought tolerance traits’. It must be borne in mind that the cultivars which were evaluated in this study (Excalibur, Kukri, and RAC875) have been bred under South Australian dry conditions. Kukri is also an adapted cultivar which may show some drought-tolerance traits. However, what makes the other two cultivars more productive in their target environment is the successful combination of several advantageous traits.
Based on the results of this study, it can be concluded that different adaptive mechanisms are involved to confer drought tolerance in RAC875 and Excalibur. RAC875 out-yielded Kukri on average by 24.3%; it possesses mechanisms which help to maintain a high tissue water status by reduced water loss through the leaf surface by reducing leaf area, reducing residual transpiration and, therefore, avoiding deleterious leaf water potential in leaves. RAC875 is a more conservative and restrained cultivar which shows the lowest tiller number per se, moderate OA, low stomatal conductance, slower recovery after re-watering, thick green leaves, stronger leaf waxiness, a stay-green phenotype, and high WSC. However, RAC875 is potentially sink-limited due to its lower tiller number.
Excalibur, on the other hand, seems to be a more responsive cultivar under drought conditions. It showed a strong interaction with environmental conditions. Excalibur out-yielded Kukri on average by 18% in Experiment I, a similar advantage to RAC875. Excalibur produced more tillers (high pre-anthesis biomass) in the first place and aborted tillers under stress and concentrated on the main stems (a higher number of spikelets per spike). It produced a greater total biomass and had a higher root:shoot ratio under water stress than Kukri and RAC875. It showed leaf rolling and moderate leaf waxiness under stress. Excalibur showed the highest OA capability, the highest stomatal conductance, the lowest ABA contents under stress, and rapid recovery after re-watering. Excalibur possesses mechanisms which allow the plant to endure low tissue osmotic potential and to recover rapidly after re-watering.
These three cultivars with different responses to cyclic water stress were selected in order to develop mapping populations for genetic studies and to identify the genomic regions controlling drought tolerance in wheat.
Acknowledgments
The authors would like to thank Steve Jefferies and Haydn Kuchel, Australian Grain Technology (AGT) for providing information and the seeds of wheat cultivars, Glenn McDonald for his advice and providing equipment to conduct the experiments, Colin Rivers who did the soil water potential measurements, Brian Loveys for the ABA assays, Colin Jenkins for the WSC measurements, and Juan Juttner and Ute Baumann for useful discussion, comments, and reading of the manuscript. While conducting this research, A Izanloo was supported by a PhD scholarship from the Ministry of Science, Research and Technology of Iran (MSRTI), and T Schnurbusch was partly supported by a Research Fellowship, Feodor-Lynen-Program, from the Alexander-von-Humboldt Foundation, Bonn-Bad Godesberg, Germany, and partly by the Australian Centre for Plant Functional Genomics (ACPFG), Adelaide, Australia. We would like to thank GRDC, ARC, and SA State Government for funding this project.
Glossary
Abbreviations
- DAP
days after planting
- ABA
abscisic acid
- RWC
relative water content
- OP
osmotic potential
- OA
osmotic adjustment
- LWP
leaf water potential
- VSWC
volumetric soil water content
- Δ13C
carbon isotope discrimination
- WSC
water-soluble carbohydrates
References
- Ali M, Jensen CR, Mogensen VO, Andersen MN, Henson IE. Root signalling and osmotic adjustment during intermittent soil drying sustain grain yield of field grown wheat. Field Crops Research. 1999;62:35–52. [Google Scholar]
- Araus JL, Alegre L, Tapia L, Calafell R. Relationship between leaf structure and gas exchange in wheat leaves at different insertion levels. Journal of Experimental Botany. 1986;37:1323–1333. [Google Scholar]
- Araus JL, Amaro T, Voltas J, Nakkoul H, Nachit MM. Chlorophyll fluorescence as a selection criterion for grain yield in durum wheat under Mediterranean conditions. Field Crops Research. 1998;55:209–223. [Google Scholar]
- Babu RC, Pathan MS, Blum A, Nguyen HT. Comparison of measurement methods of osmotic adjustment in rice cultivars. Crop Science. 1999;39:150–158. [Google Scholar]
- Barr HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Australian Journal of Biological Science. 1962;15:413–428. [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. 2nd edn. New York: Plenum Press; 1994. [Google Scholar]
- Blum A. Plant breeding for stress environments. Boca Raton, Florida: CRC Press; 1988. [Google Scholar]
- Blum A. Crop responses to drought and the interpretation of adaptation. Plant Growth Regulation. 1996;20:135–148. [Google Scholar]
- Blum A. Improving wheat grain-filling under stress by stem reserve mobilization. Euphytica. 1998;100:77–83. [Google Scholar]
- Blum A, Pnuel Y. Physiological attributes associated with drought resistance of wheat cultivars in a Mediterranean environment. Australian Journal of Agricultural Research. 1990;41:799–810. [Google Scholar]
- Blum A, Ramaiah S, Kanemasu ET, Paulsen GM. Wheat recovery from drought stress at the tillering stage of development. Field Crops Research. 1990;24:67–85. [Google Scholar]
- Blum A, Zhang J, Nguyen HT. Consistent differences among wheat cultivars in osmotic adjustment and their relationship to plant production. Field Crops Research. 1999;64:287–291. [Google Scholar]
- Borras L, Slafer GA, Otegui ME. Seed dry weight response to source–sink manipulations in wheat, maize and soybean: a quantitative reappraisal. Field Crops Research. 2004;86:131–146. [Google Scholar]
- Borrell AK, Hammer GL. Nitrogen dynamics and the physiological basis of stay-green in sorghum. Crop Science. 2000;40:1295–1307. [Google Scholar]
- Borrell AK, Hammer GL, Douglas ACL. Does maintaining green leaf area in sorghum improve yield under drought? I. Leaf growth and senescence. Crop Science. 2000;40:1026–1037. [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought: from genes to the whole plant. Functional Plant Biology. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML, Carvalho I, Faria T, Pinheiro C. How plants cope with water stress in the field? Photosynthesis and growth. Annals of Botany. 2002;89:907–916. doi: 10.1093/aob/mcf105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JM, McCaig TN. Excised-leaf water retention capability as an indicator of drought resistance of Triticum genotypes. Canadian Journal of Plant Science. 1982;62:571–578. [Google Scholar]
- Clarke JM, Richards RA. The effects of glaucousness, epicuticular wax, leaf age, plant height, and growth environment on water-loss rates of excised wheat leaves. Canadian Journal of Plant Science. 1988;68:975–982. [Google Scholar]
- Clarke JM, Romagosa I, Depauw RM. Screening durum-wheat germplasm for dry growing conditions: morphological and physiological criteria. Crop Science. 1991;31:770–775. [Google Scholar]
- Condon AG, Richards RA, Farquhar GD. Carbon isotope discrimination is positively correlated with grain yield and dry matter production in field-grown wheat. Crop Science. 1987;27:996–1001. [Google Scholar]
- Condon AG, Richards RA, Farquhar GD. The effect of variation in soil water availability, vapour pressure deficit and nitrogen nutrition on carbon isotope discrimination in wheat. Australian Journal of Agricultural Research. 1992;43:935–947. [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. Breeding for high water-use efficiency. Journal of Experimental Botany. 2004;55:2447–2460. doi: 10.1093/jxb/erh277. [DOI] [PubMed] [Google Scholar]
- Dembinska O, Lalonde S, Saini HS. Evidence against the regulation of grain set by spikelet abscisic acid levels in water-stressed wheat. Plant Physiology. 1992;100:1599–1602. doi: 10.1104/pp.100.3.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehdaie B, Alloush GA, Waines JG. Genotypic variation in linear rate of grain growth and contribution of stem reserves to grain yield in wheat. Field Crops Research. 2008;106:34–43. [Google Scholar]
- Elmi AA, West CP. Endophyte infection effects on stomatal conductance, osmotic adjustment and drought recovery of tall fescue. New Phytologist. 1995;131:61–67. doi: 10.1111/j.1469-8137.1995.tb03055.x. [DOI] [PubMed] [Google Scholar]
- Fischer RA, Turner NC. Plant productivity in the arid and semiarid zones. Annual Review of Plant Physiology. 1978;29:277–317. [Google Scholar]
- Foulkes MJ, Sylvester-Bradley R, Weightman R, Snape JW. Identifying physiological traits associated with improved drought resistance in winter wheat. Field Crops Research. 2007;103:11–24. [Google Scholar]
- Goggin DE, Setter TL. Fructosyltransferase activity and fructan accumulation during development in wheat exposed to terminal drought. Functional Plant Biology. 2004;31:11–21. doi: 10.1071/FP03123. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Rodriguez M, Reynolds MP, Escalante-Estrada JA, Rodriguez-Gonzales MT. Association between canopy reflectance indices and yield and physiological traits in bread wheat under drought and well-irrigated conditions. Australian Journal of Agricultural Research. 2004;55:1139–1147. [Google Scholar]
- Jiang GH, He YQ, Xu CG, Li XH, Zhang Q. The genetic basis of stay-green in rice analyzed in a population of doubled haploid lines derived from an indica by japonica cross. Theoretical and Applied Genetics. 2004;108:688–698. doi: 10.1007/s00122-003-1465-z. [DOI] [PubMed] [Google Scholar]
- Johnson D, Richards R, Turner N. Yield, water relations, gas exchange, and surface reflectances of near-isogenic wheat lines differing in glaucousness. Crop Science. 1983;23:318–325. [Google Scholar]
- Jones H. What is water use efficiency? In: Bacon MA, editor. Water use efficiency in plant biology. Oxford: Blackwell; 2004. pp. 27–41. [Google Scholar]
- Jones MM, Turner NC, Osmond CB. Mechanisms of drought resistance. In: Paleg LG, Aspinall D, editors. The physiology and biochemistry of drought resistance in plants. Academic Press; 1981. pp. 15–35. [Google Scholar]
- Klute A. Water retention: laboratory methods. In: Klute A, editor. 1986. [Google Scholar]
- Levitt J. Stress terminology. In: Turner NC, Kramer PJ, editors. Adaptation of plants to water and high temperature stress. New York: Wiley; 1980. pp. 473–439. [Google Scholar]
- Liu FL, Jensen CR, Shahanzari A, Andersen MN, Jacobsen SE. ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Science. 2005;168:831–836. [Google Scholar]
- Loss SP, Siddique KHM. Morphological and physiological traits associated with wheat yield increases in mediterranean environments. Advances in Agronomy. 1994;52:229–276. [Google Scholar]
- Lu CM, Zhang JH. Changes in photosystem II function during senescence of wheat leaves. Physiologia Plantarum. 1998;104:239–247. [Google Scholar]
- Lu Q, Lu C, Zhang J, Kuang T. Photosynthesis and chlorophyllafluorescence during flag leaf senescence of field-grown wheat plants. Journal of Plant Physiology. 2002;159:1173–1178. [Google Scholar]
- Marshall TJ, Holmes JW. Soil physics. Cambridge: Cambridge University Press; 1979. [Google Scholar]
- Morgan JM. Osmotic adjustment in the spikelets and leaves of wheat. Journal of Experimental Botany. 1980;31:655–665. [Google Scholar]
- Morgan JM. Osmotic components and properties associated with genotypic differences in osmoregulation in wheat. Australian Journal of Plant Physiology. 1992;19:67–76. [Google Scholar]
- Morgan JM, Condon AG. Water use, grain yield, and osmoregulation in wheat. Australian Journal of Plant Physiology. 1986;13:523–532. [Google Scholar]
- Munné-Bosch S, Alegre L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta. 2000;210:925–931. doi: 10.1007/s004250050699. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L. Die and let live: leaf senescence contributes to plant survival under drought stress. Functional Plant Biology. 2004;31:203–216. doi: 10.1071/FP03236. [DOI] [PubMed] [Google Scholar]
- Nobel PS. Physicochemical and environmental plant physiology. San Diego, USA: Academic Press; 1999. [Google Scholar]
- O'Toole JC, Cruz RT. Responses of leaf water potential, stomatal resistance, and leaf rolling to water stress. Plant Physiology. 1980;65:428–432. doi: 10.1104/pp.65.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Villegas JJ, Reynolds MP, McDonald GK. Drought-adaptive attributes in the Seri/Babax hexaploid wheat population. Functional Plant Biology. 2007;34:189–203. doi: 10.1071/FP06148. [DOI] [PubMed] [Google Scholar]
- Passioura JB. Drought and drought tolerance. Plant Growth Regulation. 1996;20:79–83. [Google Scholar]
- Passioura JB. The perils of pot experiments. Functional Plant Biology. 2006;33:1075–1079. doi: 10.1071/FP06223. [DOI] [PubMed] [Google Scholar]
- Passioura JB. The drought environment: physical, biological and agricultural perspectives. Journal of Experimental Botany. 2007;58:113–117. doi: 10.1093/jxb/erl212. [DOI] [PubMed] [Google Scholar]
- Reynolds MP. Physiological approaches to wheat breeding. In: Curtis BC, Rajaram S, Macpherson HG, editors. Bread wheat. Rome: FAO; 2002. [Google Scholar]
- Reynolds MP, Mujeeb-Kazi A, Sawkins M. Prospects for utilising plant-adaptive mechanisms to improve wheat and other crops in drought- and salinity-prone environments. Annals of Applied Biology. 2005;146:239–259. [Google Scholar]
- Reynolds MP, Trethowan RM, Ginkel Mv Rajaram S. Application of physiology in wheat breeding. In: Reynolds MP, Ortiz-Monasterio JI, McNab A, editors. Application of physiology in wheat breeding. CIMMYT; 2001. pp. 2–10. [Google Scholar]
- Reynolds MP, van Ginkel M, Ribaut J-M. Avenues for genetic modification of radiation use efficiency in wheat. Journal of Experimental Botany. 2000;51:459–473. doi: 10.1093/jexbot/51.suppl_1.459. [DOI] [PubMed] [Google Scholar]
- Richards RA. Breeding for drought resistance: physiological approaches. In: FwG Baker., editor. Drought resistance in cereals. CAB International; 1989. pp. 65–80. [Google Scholar]
- Richards RA. Physiological traits used in the breeding of new cultivars for water-scarce environments. In: Fischer T, Turner N, Angus J, McIntyre L, Robertson M, Borrell A, Lloyd D, editors. Proceedings of the 4th International Crop Science Congress. Australia, Brisbane: 2004. [Google Scholar]
- Richards RA, Condon AG, Rebetzke GJ. Traits to improve yield in dry environments. In: Reynolds MP, Ortiz-Monasterio JI, McNab A, editors. Application of physiology in wheat breeding. Mexico, DF: CIMMYT; 2001. [Google Scholar]
- Richards RA, Rawson HM, Johnson DA. Glaucousness in wheat: its development and effect on water-use efficiency gas exchange and photosynthetic tissue temperatures. Australian Journal of Plant Physiology. 1986;13:465–473. [Google Scholar]
- Richards RA, Rebetzke GJ, Condon AG, van Herwaarden AF. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Science. 2002;42:111–121. doi: 10.2135/cropsci2002.1110. [DOI] [PubMed] [Google Scholar]
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proceedings of the National Academy of Sciences, USA. 2007;104:19631–19636. doi: 10.1073/pnas.0709453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez D, Nuttall J, Sadras VO, Rees HV, Armstrong R. Impact of subsoil constraints on wheat yield and gross margin on fine-textured soils of the southern Victorian Mallee. Australian Journal of Agricultural Research. 2006;57:355–365. [Google Scholar]
- Ruuska SA, Rebetzke GJ, van Herwaarden AF, Richards RA, Fettell NA, Tabe L, Jenkins CLD. Genotypic variation in water-soluble carbohydrate accumulation in wheat. Functional Plant Biology. 2006;33:799–809. doi: 10.1071/FP06062. [DOI] [PubMed] [Google Scholar]
- Sadras VO, Angus JF. Benchmarking water-use efficiency of rain-fed wheat in dry environments. Australian Journal of Agricultural Research. 2006;57:847–856. [Google Scholar]
- Saini HS, Aspinall D. Sterility in wheat (Triticum aestivum L.) induced by water deficit or high temperature: possible mediation by abscisic acid. Australian Journal of Plant Physiology. 1982;9:529–537. [Google Scholar]
- Schwinning S, Ehleringer JR. Water use trade-offs and optimal adaptations to pulse-driven arid ecosystems. Journal of Ecology. 2001;89:464–480. [Google Scholar]
- Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT. Root growth maintenance during water deficits: physiology to functional genomics. Journal of Experimental Botany. 2004;55:2343–2351. doi: 10.1093/jxb/erh276. [DOI] [PubMed] [Google Scholar]
- Soar CJ, Speirs J, Maffei SM, Loveys BR. Gradients in stomatal conductance, xylem sap ABA and bulk leaf ABA along canes of Vitis vinifera cv. Shiraz: molecular and physiological studies investigating their source. Functional Plant Biology. 2004;31:659–669. doi: 10.1071/FP03238. [DOI] [PubMed] [Google Scholar]
- Spano G, Di Fonzo N, Perrotta C, Platani C, Ronga G, Lawlor DW, Napier JA, Shewry PR. Physiological characterization of ‘stay green’ mutants in durum wheat. Journal of Experimental Botany. 2003;54:1415–1420. doi: 10.1093/jxb/erg150. [DOI] [PubMed] [Google Scholar]
- Tardieu F. Plant tolerance to water deficit: physical limits and possibilities for progress. Comptes Rendus Geoscience. 2005;337:57–67. [Google Scholar]
- Turner NC. Adaptation to water deficits: a changing perspective. Australian Journal of Plant Physiology. 1986;13:175–190. [Google Scholar]
- Turner NC. Sustainable production of crops and pastures under drought in a Mediterranean environment. Annals of Applied Biology. 2004;144:139–147. [Google Scholar]
- van Herwaarden AF, Angus JF, Richards RA, Farquhar GD. ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser. II. Carbohydrate and protein dynamics. Australian Journal of Agricultural Research. 1998;49:1083–1093. [Google Scholar]
- Vile D, Garnier E, Shipley B, et al. Specific leaf area and dry matter content estimate thickness in laminar leaves. Annals of Botany. 2005;96:1129–1136. doi: 10.1093/aob/mci264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Taylor S, Bogacki P, Pallotta M, Bariana H, Wallwork H. Mapping of the root lesion nematode (Pratylenchus neglectus) resistance gene Rlnn1 in wheat. Theoretical and Applied Genetics. 2002;104:874–879. doi: 10.1007/s00122-001-0839-3. [DOI] [PubMed] [Google Scholar]
- Wright GC, Smith RCG, Morgan JM. Differences between two grain sorghum genotypes in adaptation to drought stress. III. Physiological responses. Australian Journal of Agricultural Research. 1983;34:637–651. [Google Scholar]
- Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharieva M, Gaulin E, Havaux M, Acevedo E, Monneveux P. Drought and heat responses in the wild wheat relative Aegilops geniculata Roth: potential interest for wheat improvement. Crop Science. 2001;41:1321–1329. [Google Scholar]
- Zhang J, Nguyen HT, Blum A. Genetic analysis of osmotic adjustment in crop plants. Journal of Experimental Botany. 1999;50:291–302. [Google Scholar]
- Zubaidi A, McDonald GK, Hollamby GJ. Shoot growth, root growth and grain yield of bread and durum wheat in South Australia. Australian Journal of Experimental Agriculture. 1999;39:709–720. [Google Scholar]