Abstract
It was previously reported that cryptogein, an elicitor of defence responses, induces an intracellular production of nitric oxide (NO) in tobacco. Here, the possibility was explored that cryptogein might also trigger an increase of NO extracellular content through two distinct approaches, an indirect method using the NO probe 4,5-diaminofluorescein (DAF-2) and an electrochemical method involving a chemically modified microelectrode probing free NO in biological media. While the chemical nature of DAF-2-reactive compound(s) is still uncertain, the electrochemical modified microelectrodes provide real-time evidence that cryptogein induces an increase of extracellular NO. Direct measurement of free extracellular NO might offer important new insights into its role in plants challenged by biotic stresses.
Keywords: Diaminofluorescein, diethylamine NONOate, electrochemical sensor, nitric oxide, plant defence responses
Introduction
A decade of investigation of nitric oxide (NO) functions in plants has led to its characterization as a potent regulator of major processes including germination, root growth, stomatal closure, flowering, and adaptive responses to biotic and abiotic stresses (Lamattina et al., 2003; Delledonne, 2005; Besson-Bard et al., 2008b; Neill et al., 2008). Nitrate/nitrite- as well as L-arginine-dependent enzymatic pathways for NO synthesis have been described. The nitrate/nitrite-dependent route involves a cytosolic nitrate reductase which catalyses the reduction of nitrite to NO both in vitro and in vivo, and an as yet unidentified root-specific plasma membrane nitrite-NO reductase (Besson-Bard et al., 2008a). The occurrence of an L-arginine pathway involving a putative nitric oxide synthase (NOS), the main source for NO in animals (Stuehr et al., 2004), has been hypothesized on the basis of (i) the measurement of NOS-like activities in plant tissues and purified organelles; and (ii) the efficiency of animal NOS inhibitors in suppressing NO synthesis in various physiological contexts (Crawford, 2006; Besson-Bard et al., 2008a). The corresponding enzyme(s) has (have) not been identified yet.
Although many reports highlight a role for NO in plant physiological processes, its detection/quantification is still a major challenge. Commonly developed methods for investigating NO production in plants and plant cell suspensions include the oxyhaemoglobin assay, the Griess reaction, electron paramagnetic resonance spectroscopy, fluorescence, laser photoacoustics, gas-phase chemiluminescence, and CrO3-based conversion of NO to NO2 (Vandelle and Delledonne, 2008; Vitecek et al., 2008). These approaches are based on indirect methods for estimating NO, relying on the measurement of NO-derived species such as dinitrogen trioxide (N2O3), NO2·, nitrite (NO2–), or nitrate (NO3–). In contrast, mass spectrometry and NO-sensitive electrodes allow a direct detection of free diffusing NO. Indeed, similarly to laser photoacoustics and gas-phase chemiluminescence, mass spectrometry-based approaches enable the measurement of NO diffusing from cells into a gas phase. Also, NO-sensitive electrodes enable the detection of NO in solution. In this regard, in animals, the use of chemically modified commercial or home-made microelectrodes is becoming a very attractive approach for tracking NO in biological media. According to numerous studies, this approach constitutes the only absolute real-time proof of the production of free NO in biological media (Bedioui and Villeneuve, 2003; Wadsworth et al., 2006). Notably, nickel phthalocyanine- (or porphyrin) and Nafion®-based NO sensors are now widely used, although they are not commercially available. These home-made microelectrodes consist of a platinum/iridium (Pt/Ir) wire sealed in a glass or plastic capillary in which a thin film of nickel phthalocyanine is electrodeposited. An outermost layer constituted from Nafion® and o-phenylenediamine (o-PD) enhances the selectivity of the electrodes against possible interfering molecules, notably anions such as nitrite, ascorbate, superoxide, peroxynitrite, and hydrogen peroxide (Pontié et al., 2000; Brunet et al., 2003; Griveau et al., 2007a, b). The detection of NO is based on its electrochemical oxidation in a potential range of (+0.6 to +0.9 V versus Ag/AgCl). These NO sensors offer high performance characteristics in term of sensitivity, selectivity, and flexibility, and were successfully used to detect extracellular NO in cultured cells as well as in tissues (Bedioui and Villeneuve, 2003; Pereira Rodriguez et al., 2005, 2006; Griveau et al., 2007b).
All these analytical approaches offer individual advantages but also in some cases disadvantages, including the lack of sensitivity and the risk of interference by NO-unrelated molecules. Accordingly, the use of distinct approaches for detecting NO in similar physiological contexts has led to the publication of conflicting results (Planchet and Kaiser, 2006a, b). Therefore, there is an urgent need to develop sensitive and selective methods for probing NO produced by plants.
Cryptogein is a 10 kDa proteinaceous elicitor produced by the oomycete Phytophthora cryptogea, an avirulent pathogen of tobacco. The transduction pathway by which cryptogein activates defence responses in tobacco has been widely studied (Garcia-Brugger et al., 2006). In particular, using 4,5-diaminofluorescein diacetate (DAF-2DA), a membrane-permeable derivative of the NO-sensitive fluorophore DAF-2, it was previously reported that epidermal cells from tobacco leaves as well as tobacco cultured cells respond to cryptogein through an increase of intracellular NO (Foissner et al., 2000; Lamotte et al., 2004; Besson-Bard et al., 2008a). The elicitor-induced NO production is partly reduced by the mammalian NOS inhibitors Nw-nitro-L-arginine-methyl ester (L-NAME) and S,S′-[1,3-phenylene-bis(1,3-ethanediyl)]bis-isothiourea (PBITU), and completely suppressed by the membrane-permeable NO scavenger cPTIO. Once produced, NO serves as an intracellular signalling messenger by promoting the mobilization of the second messenger Ca2+ (Lamotte et al., 2004, 2006). The ability of cryptogein to trigger NO synthesis in tobacco cells was questioned in further studies in which the extracellular content of NO was analysed using the non-permeable NO-sensitive fluorophore DAF-2 and gas-phase chemiluminescence (Planchet and Kaiser, 2006a; Planchet et al., 2006). Using both methods, no or only a weak NO production from elicited cultured tobacco cells was detected.
Therefore, solving the question of whether NO is released by plant cells challenged by microorganisms or derived elicitors of defence responses is of primary importance to understand its function further in the plant adaptive response to biotic stresses. To this aim, in the present study NO content in the extracellular medium of cryptogein-treated tobacco cell suspensions was investigated using the fluorophore DAF-2 and an NO-sensitive home-made Pt/Ir-based electrochemical microsensor. It is shown that a fast increase of NO concentration can be electrodetected in the medium of elicited cultured tobacco cells and that both methods gave consistent results in term of kinetic and pharmacological sensitivity.
Materials and methods
Cell culture
Nicotiana tabacum cv. Xanthi cell suspensions were cultivated as previously described (Lamotte et al., 2004). Briefly, cell suspensions were maintained in Chandler's medium (Chandler et al., 1972) on a rotary shaker (130 rpm, 25 °C) under continuous light (photon flux rate 30–40 μmol m−2 s−1). Cells were maintained in the growth exponential phase and subcultured 1 d prior to utilization. For experiments, 8-d-old tobacco cell suspensions were used.
Cell culture treatments
Cryptogein was purified according to Bonnet et al. (1996) and dissolved in water as a 100 μM stock solution. L-NAME and PBITU were purchased from ALEXIS® Biochemicals. Catalase, DAF-2, and 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazol-1-yl-oxy-3-oxide (cPTIO) were purchased from Sigma, and diethylamine NONOate (DEA/NO) was purchased from Cayman Chemicals and kept at –80 °C.
All chemicals were dissolved in water, except DAF-2 which was received as a stock solution in 5 mM dimethylsulphoxide (DMSO). DEA/NO was prepared as previously described (Lamotte et al., 2006). Briefly, DEA/NO was dissolved in 0.01 M NaOH as a stock solution (500 mM), stored on ice, and prepared daily. To initiate the release of NO, 10 μl of the stock alkaline solution of DEA/NO was dissolved in 990 μl of 100 mM phosphate buffer, pH 7.2 to give a 5 mM final concentration. Cells were treated with DEA/NO (50 μM final concentration) 20 s after dissolution of the stock alkaline solution of DEA/NO in the phosphate buffer. As a control, cells were treated with the same volume of DEA/NO solubilization buffer without DEA/NO.
Cells were prepared as previously described (Lamotte et al., 2004). Briefly, cells were washed by filtration and resuspended at 0.1 g fresh weight ml−1 in the suspension buffer H10 (10 mM HEPES, 175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4; pH 6), and then equilibrated for 1 h at 24 °C on a rotary shaker (150 rpm) before treatment. Typically, 3×105 cells 0.1 g−1 fresh weight occur in cell suspensions.
Electrochemical apparatus
Electrochemical experiments were carried out at room temperature, in aerobic conditions, using a Quadstat model potentiostat (eDAQ Pty Ltd, Australia) for chronoamperometry. The pseudo-reference Ag/AgCl electrode, serving also as the counter-electrode, was a home-made silver/silver chloride wire (diameter 50 μm).
Home-made NO-sensitive electrode
NO sensors were prepared daily following an already reported procedure (Griveau et al., 2007a, b). Briefly, a length of ∼5 cm of a Teflon-PTFE-insulated Pt/Ir wire (125 μm diameter, from Advent, Oxford, UK) was sharply cut to form a neat disc-shaped area at its end. This disc area (electrode) was then modified by electrodepositing a thin film of nickel phthalocyanine from 2 mM nickel tetrasulphonated phthalocyanine in 0.1 M NaOH aqueous solution, by applying a constant potential of +1.2 V versus Ag/AgCl for 10 min. Following this step, the electrode was rinsed, dried, and then immersed in Nafion® alcoholic solution for 15 s. To ensure good adhesion of the Nafion® layer to the electrode surface, the electrode was placed at 80 °C for 5 min. This protocol was repeated four times. Finally, an o-PD thin film was electrochemically deposited by controlled potential electrolysis at +0.9 V versus Ag/AgCl in 6.5 mM o-PD in phosphate-buffered saline (PBS) for 20 min. This outermost layer was aimed at enhancing the selectivity of the electrode against possible interfering molecules. This final coating of the electrodes was made fresh every day and the electrodes were tested daily in order to check their electrochemical performances.
Calibration of NO sensor
NO standards were prepared by making serial dilutions of saturated NO solutions. Preparation of saturated NO solutions involved meticulous exclusion of O2, as NO is rapidly destroyed by O2. To produce a saturated NO solution, typically containing NO ∼2 mM at 20 °C (Linke, 1965), deionized water solution (2 ml) was bubbled with argon for 20 min to remove oxygen. Then, DEA/NO was added (800 μM) and the flask containing the solution was kept tightly closed at room temperature until the total decomposition of the NO donor (2 h). Standards were made fresh for each experiment and kept in a glass flask with a rubber septum. Dilutions of the saturated solution were made using deoxygenated water samples.
Measurement of NO production
DAF-2 method
After filtration, cell suspensions were incubated with 20 μM DAF-2 for 1 h in the dark at 24 °C on a rotary shaker (150 rpm). Cells were then transferred into 24-well plates (Costar) containing 1 ml of cells per well. Cells were pre-treated by L-NAME, PBITU, or cPTIO 10 min prior to cryptogein addition. Catalase was added 5 min after cryptogein treatment. NO production was measured in the dark using a plate reader fluorometer (Mithras, Berthold) with 485 nm excitation and 535 nm emission filters. Fluorescence was expressed as relative fluorescence units.
Electrochemical detection
For electrochemical measurements, 4 ml of cultured cell suspension were introduced into a well of a 12-well cell culture plate. The electrodes (NO sensor and Ag/AgCl wire) were introduced in the well and positioned just above the cells. The cell suspension was slowly stirred (60 rpm) continuously by means of a magnetic mini barrel. The electrochemical detection of NO was performed by chronoamperometry, by recording the current while applying a constant potential of +0.6 V or +0.9 V versus Ag/AgCl.
Results
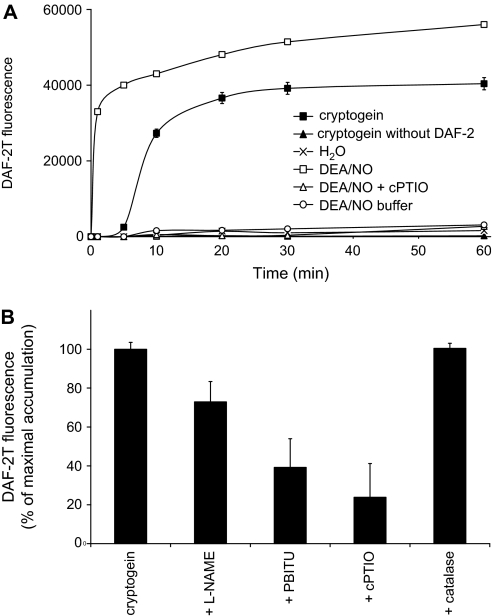
To check whether NO produced by tobacco cells might diffuse in the extracellular space, NO accumulation in the extracellular medium of tobacco cells exposed to cryptogein was first measured using the non-permeable NO-sensitive fluorophore DAF-2. This method was shown to be indirect and might rely on the measurement of reactive nitrogen species derived from NO autoxidation that nitrosate DAF-2 to yield to the highly fluorescent DAF-2 triazole (DAF-2T) (Jourd'heuil, 2002). The efficiency of the fluorophore was verified by monitoring the level of fluorescence of DAF-2-incubated tobacco cell suspensions exposed to the NO donor DEA/NO (Fig. 1A). The NO donor triggered a two-step rise in fluorescence: a fast increase within the first minute, and then a lower enhancement evolving continuously during the next hour. As reported in other studies (see for instance Planchet and Kaiser, 2006a), the NO donor-induced DAF-2T increase of fluorescence was not observed in the presence of cPTIO, thus indicating that this effect was indeed caused by NO released by DEA/NO. When DAF-2-incubated cell suspensions were treated with cryptogein, an increase in fluorescence occurred after 5 min of treatment and reached a maximum within 30 min (Fig. 1A). Here too, the cryptogein-induced rise in fluorescence was strongly reduced when tobacco cell suspensions were co-treated by the NO scavenger cPTIO, suggesting the involvement of NO in this process (Fig. 1B). Furthermore, the rise of DAF-2T fluorescence was suppressed by 27% and 61% by the NOS inhibitors L-NAME and PBITU, respectively, suggesting that NO synthesis might be catalysed, at least partly, by a NOS-like enzyme. These data fit well with those previously reported in which the enzymatic origin of intracellular NO was investigated (Foissner et al., 2000; Lamotte et al., 2004). Finally, no increase of fluorescence was observed in cell suspensions treated with cryptogein without DAF-2. This last result excludes the hypothesis that under exposure to cryptogein, tobacco cells might produce new compounds having fluorescence profiles similar to that of DAF-2T and therefore interfering with the measurements.
Fig. 1.
Measurement of DAF-2T increase in fluorescence in the extracellular medium of tobacco cultured cells exposed to cryptogein or DEA/NO using the DAF method. (A) Time course of cryptogein- and DEA/NO-induced DAF-2T increase of fluorescence. DAF-2-incubated cell suspensions were treated with 100 nM cryptogein or 50 μM DEA/NO in the presence or absence of 1 mM cPTIO. Cells treated with cryptogein in the absence of DAF-2 were pre-incubated with DMSO for 1 h. Each value represents the mean ±SD of nine measurements (three replicates per experiment performed three times). (B) Effects of mammalian NOS inhibitors, cPTIO, and catalase on cryptogein-induced increase of DAF-2T fluorescence. Cells were pre-treated for 10 min with 5 mM L-NAME, 5 mM PBITU, or 1 mM cPTIO before addition of 100 nM cryptogein. Catalase was added 5 min after cryptogein. DAF-2T accumulation was determined after 30 min of cryptogein treatment and expressed as a percentage of the maximal DAF-2T accumulation. Each value represents the mean ±SD of 15 measurements (three replicates per experiment performed five times).
Jourd'heuil (2002) reported that DAF-2 fluorometric assays are prone to misinterpretation when oxidants and NO are co-synthesized. Indeed, the author showed that DAF-2 is oxidized to a stable non-fluorescent intermediate in the presence of both H2O2 and horseradish peroxidase. This oxidation strongly amplifies NO-triggered DAF-2T formation. Because both NO and H2O2 are simultaneously synthesized in response to cryptogein (Foissner et al., 2000; Lamotte et al., 2004), an investigation was carried out to determine whether H2O2 might interfere with DAF-2-based NO measurement. This was accomplished by co-treating cells with cryptogein and catalase which immediately consumed the apoplastic H2O2 produced in response to the elicitor, as previously reported (Lecourieux et al., 2002). Figure 1B shows that the consumption of H2O2 by catalase did not affect the cryptogein-induced DAF-2T increase of fluorescence, indicating that H2O2 and/or H2O2-derived species do not interfere with DAF-2-based measurement.
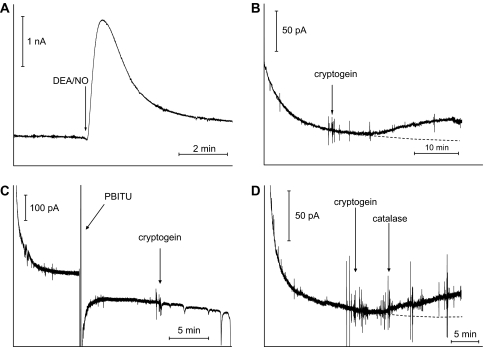
To support further the assumption that cryptogein induces synthesis of NO diffusing out of cells, the presence of NO in the extracellular medium of tobacco cell suspensions was monitored using an NO-sensitive home-made Pt/Ir-based electrochemical microsensor (Supplemental Fig. S1 available at JXB online). The NO sensor is associated with an Ag/AgCl wire as a reference electrode (Griveau et al., 2007b). Recent studies reported that this miniaturized NO sensor is well suited for the rapid real-time detection of NO in vitro, but also in vivo in mammalian biological models (Griveau et al., 2007a, b). Notably, it was successfully used for tracking NO in tumour-bearing mice (Griveau et al., 2007b). As a first step, the efficiency of the electrode system as an NO sensor was checked by electrodetecting NO released by DEA/NO in the extracellular medium of tobacco cell suspensions. The amperometric response of the electrode was measured at +0.9 V versus Ag/AgCl. The obtained amperogram (Fig. 2A) displays a fast and large increase in intensity after DEA/NO addition to the cell suspensions. Then, a relatively slow decrease of the current was observed. This signal resembles those reported by Griveau et al. (2007a) in which the kinetic release of NO from DEA/NO in phosphate buffer solution was investigated using similar microelectrodes, indicating that the NO sensor can be successfully used in tobacco cell suspensions without any complication related to an eventual fouling or passivation of the electrode. Importantly, this study reported that the electrochemical signals measured using the NO-sensitive microelectrodes were directly related to the oxidation of NO and not to interfering by-products of the fully decomposed donor. Furthermore, this two-step response fits well with those shown Fig. 1A in which DAF-2 accumulation following DEA/NO application was measured. Therefore, these data indicate that the NO sensor is suitable for analysing NO evolution in the extracellular medium of tobacco cell suspensions and they clearly show the reversibility of the electrochemical response as a baseline is reached when no more NO is produced from the NO donor. It should be noted that the NO sensor showed an interfering signal from cPTIO, thus precluding its use as a control (data not shown).
Fig. 2.
Measurement of free NO in the extracellular medium of tobacco cultured cells exposed to cryptogein or DEA/NO using the miniaturized electrochemical sensor. (A) Amperometric curve illustrating the kinetic of NO released by DEA/NO (50 μM) in tobacco cell suspensions. Operating potential, +0.9 V. The data are representative of three experiments. (B) Amperometric curve illustrating the increase of extracellular NO concentration in tobacco cell suspensions exposed to cryptogein (100 nM). Operating potential, +0.6 V. The data are representative of six experiments. (C) Amperometric curve illustrating the effect of PBITU on the cryptogein-induced increase of the NO extracellular concentration. Cells were pre-treated for 10 min with 5 mM PBITU before addition of 100 nM cryptogein. Operating potential, +0.6 V. The data are representative of three experiments. (D) Amperometric curve illustrating the effect of catalase on the cryptogein-induced increase of the NO extracellular concentration. Catalase was added 5 min after 100 nM cryptogein. Operating potential, +0.6 V. The data are representative of three experiments.
The electrochemical detection of NO in the extracellular medium of tobacco cells treated with cryptogein was next investigated. The corresponding amperogram shows an increase of the current which occurred 5 min after the addition of the elicitor (Fig. 2B). Then, the current value increased at least during the next 25 min. This result is in accordance with those obtained with DAF-2 (Fig. 1A) and highlights cryptogein's ability to trigger an increase of NO concentration in the extracellular medium of tobacco suspension cells. Here too, the elicitor-triggered increase in current was strongly suppressed by PBITU, the most efficient inhibitor of NO synthesis (Fig. 2C). It should be noted that this last result indicates that cryptogein itself does not produce an interfering signal. Also, it clearly shows that the electrochemical detection is not sensitive to an eventual temperature change due to heat production upon addition of crytogein. Finally, it should be emphasized that the amperometric measurement was stopped voluntarily after 35 min of recording in order to avoid damaging the sensor coating during a long-term run. Indeed, accumulation of the NO electrochemical oxidation products within the membranes might affect the performances of the sensor. The reversibility of this electrochemical response upon cryptogein activation of the cells was checked in an independent parallel experiment (Supplementary Fig. S2 at JXB online).
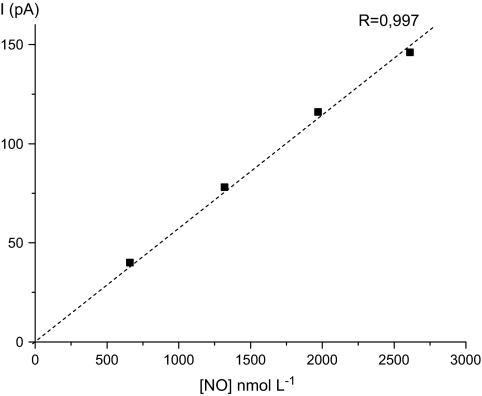
Given the well reported system-to-system variability and the difficulties in absolute measurement of NO concentration in solution due to its high reactivity, the electrochemical measurements reported provide information on relative rates and dynamics. On the basis of calibration of the sensor in HEPES buffer, the NO sensor sensitivity to NO in HEPES buffer is estimated to 0.06 pA nM−1, as can be calculated from the slope of the linear calibration curve shown in Fig. 3. Thus, it can be estimated that the overall concentration of NO to which the electrode was exposed under the experimental conditions employed lies in the order of 300 nM.
Fig. 3.
Relationship between NO concentration in aerobic HEPES buffer and NO sensor current measured by amperometry at +0.9 V.
It should be noted here that the configuration of the home-made sensor makes it free of interference from anions (nitrate, superoxide, peroxynitrite, and nitrite to some extent) and this was shown and quantified in previous published studies (Pontié et al., 2000; Brunet et al., 2003). Also, although the sensor showed interfering signals from highly concentrated NO2– (500 μM) and H2O2 (50 μM) solution, lowering the operating potential of the sensor from +0.9 V to +0.6 V allowed the undesired amperometric current related to both these species (if any appears) to be decreased without affecting the sensitivity of the detector to NO too much.
To check whether H2O2 produced in response to cryptogein might interfere with the electrochemical detection of NO, tobacco cell suspensions were co-treated with cryptogein and catalase in order to consume H2O2. As shown Fig. 2D, the amplitude of the current increase (ΔI = 20±2 pA, i.e. 330±30 nM NO) recorded in this condition is identical to those recorded without catalase, discarding the possibility that H2O2 could be electrodetected in the present conditions. A putative interference by nitrite was also examined. Indeed, cryptogein was shown to trigger a fast and massive nitrate efflux leading to a loss of internal nitrate content of ∼60% within 1 h (Wendehenne et al., 2002). Therefore, it is plausible that part of the apoplastic nitrate might be reduced to nitrite by a putative plasma membrane nitrate reductase, as reported in tobacco roots (Stöhr et al., 2001), or through a chemical-based process. To assess this hypothesis, the extracellular nitrite concentration was measured in cryptogein-treated tobacco cell suspensions. It was not possible to detect the presence of nitrite during the first 3 h of cryptogein treatment (data not shown), excluding putative interference of nitrite with the electrochemical detection of NO in these experimental conditions.
Discussion
Understanding NO functions in specific plant physiological contexts requires detailed analysis of its spatial distribution through sensitive and selective approaches. In the present study, it was first demonstrated that cryptogein triggered a fast and transient increase of extracellular DAF-2T fluorescence. The chemical nature of the DAF-2-reactive compound(s) has been investigated recently (Planchet and Kaiser, 2006a). These authors provided evidence that the increase in DAF-2T fluorescence in the extracellular medium of tobacco cell suspensions exposed to cryptogein, to inhibitors of mitochondrial electron transport, or to anoxia was not due to NO itself but to an as yet unidentified compound released from cells into the supernatant. This compound might be distinct from ascorbate and, as reported in the present study, did not correspond to H2O2 or H2O2-derived species. The observation that the cryptogein-induced increase of DAF-2T fluorescence was suppressed when cell suspensions were pre-treated with cPTIO suggests that this compound is related to NO synthesis. Whether it corresponds to an NO adduct or a DAF-2-reactive compound chemically unrelated to NO but rapidly synthesized (i.e. within 5 min) and/or released into the extracellular medium through an NO-dependent pathway remains to be established. The fact that DEA/NO triggers a fast cPTIO-sensitive increase of DAF-2 fluorescence in the cell culture medium containing (Fig. 1A) or not containing (data not shown) tobacco cells favours the hypothesis of the involvement of an NO adduct. Whatever the scenario, on the basis of these data, it is not possible to conclude that the increase of DAF-2T fluorescence observed in response to cryptogein corresponds to a rise in extracellular NO content.
Interestingly, in a recent in vitro study, Arita et al. (2006) reported that the mixing of 250 μM of the NO donor SNAP and 100 μM of cPTIO (pH 7.4) in a quartz cuvette results in an increase instead of a decrease of DAF-2T fluorescence. Indeed, in these conditions, NO2· resulting from NO oxidation by cPTIO combines with NO (still present in the medium) to yield N2O3, which reacts with DAF-2 to give DAF-2T. In the present assays, it was always observed that cPTIO efficiently suppresses the increase of DAF-2T fluorescence triggered by DEA/NO or cryptogein in cell suspensions, but also in tissues (Foissner et al., 2000). The present data fit well with many studies also reporting the ability of cPTIO to reduce DAF-2T increases in fluorescence induced by exogenously applied or endogenously produced NO in biological samples (see for instance Prado et al., 2004; Hu et al., 2005; Bright et al., 2006; Planchet and Kaiser, 2006a; Zottini et al., 2007). Although speculative, the inconsistencies between the investigation of Arita et al. (2006) and other works might be related to the conditions in which the analyses were performed. Notably, because of the ability of plant cells to activate antioxidative/antinitrosative mechanisms, it is assumed that the process described by Arita et al. might be less pronounced in vivo that in vitro. Taken together, it is believed and has been shown here that the combination of DAF and cPTIO is an informative strategy when investigating NO production in vivo but one must be extremely cautious of interpretation based on this strategy. Therefore, as discussed by Arita et al. (2006), it is suggested to confirm DAF-based NO measurements with another method having distinct detection principles when investigating in vivo NO production.
In contrast to the DAF-2-based method, the electrochemical approach allows a real-time in vivo determination of NO in animal biological systems (Bedioui and Villeneuve, 2003; Wadsworth et al., 2006; Griveau et al., 2007b). Using DEA/NO, it was demonstrated that the home-made electrochemical sensor used here is efficient for probing NO in the extracellular medium of tobacco cell suspensions. Subsequent experiments provided evidence that a rise of NO concentration can be detected in the extracellular medium of tobacco cell suspensions elicited by cryptogein. The corresponding increase of the current was unrelated to H2O2 or nitrite which might interfere with the NO sensor at high concentrations. Interestingly, the evolution of the amperometric signal recorded in cryptogein-treated cell suspensions resembles those obtained with DAF-2 and also DAF-2DA, the membrane-permeable derivative of DAF-2 (Foissner et al., 2000; Lamotte et al., 2004): in all cases, the response occurred within 5 min and was sensitive to the mammalian NOS inhibitors. The findings that the increases of intra- and extracellular NO occur almost simultaneously and display similar pharmacological features suggest that NO is first produced intracellularly via an enzyme sensitive to mammalian NOS inhibitors. Then, part of the synthesized NO diffuses almost immediately outside of the cells. Bovine haemoglobin was not used as an NO absorber (scavenger) since biofouling of the sensor surface is known to occur from macrobiomolecules (such as haemoglobin, serum albumin, and even non-adherent cells) and lead to the passivation of the electrode and thus a decrease in the electrochemical signals (Pontié and Bedioui, 2002; Pereira Rodrigues et al., 2006).
Using a gas-phase chemiluminescent assay based on the chemiluminescent properties of NO2· formed from NO that reacts with ozone (Mc Murtry et al., 2000), Planchet et al. (2006) reported that cryptogein caused no or only a weak NO emission which appeared several hours after elicitor addition to the cell suspensions. This result strongly differs from the present electrochemical data. This discrepancy should be discussed in the light of the principle of the two methods: as discussed above, the electrochemical method allows the direct detection of NO in aqueous solution whereas the chemiluminescent assay allows indirect NO detection in gas phases. This statement raises the question of whether NO is emitted in the gas phase from tobacco cell suspensions following cryptogein treatment. The answer was recently provided by Vitecek et al. (2008) who set up a new sensitive detector for measuring NO emitted in the gas phase from plants and plant cell suspensions. This detector relies on the ability of the strong oxidizing agent CrO3 to oxidize NO to NO2, NO2 being subsequently captured by a Griess reagent trap. Using this method, the authors were able to detect a significant NO emission in the gas phase from tobacco cells exposed to cryptogein for 1 h. These data clearly support the present findings that the elicitor triggers an increase of extracellular NO.
To conclude, the feasibility of using a home-made miniaturized electrochemical sensor for measuring extracellular NO in elicited tobacco cell suspensions was demonstrated. Because electrochemical detection constitutes the only real-time proof of the production of NO in biological systems (Bedioui and Villeneuve, 2003), its application to plant cells needs to be further developed. This method should not only help in reinterpreting and solving controversies but, more importantly, also should offer new insights into the role of NO in plant physiological as well as pathophysiological processes. Notably, the demonstration that cryptogein induces a fast increase of the extracellular NO concentration suggests that this radical, in addition of being an intracellular messenger (Besson-Bard et al., 2008b), might also display specific extracellular functions. Whether it acts as a diffusing intercellular messenger or a cytotoxic weapon against invading microorganisms as reported in animals remains to be established.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Schematic representation of the platinum/iridium-based electrochemical NO sensor.
Fig. S2. Amperometric curve illustrating the increase of extracellular NO concentration in tobacco cell suspensions exposed to cryptogein (250 nM). Operating potential, +0.8 V. The data are representative of three experiments.
Acknowledgements
This work was supported by the Conseil Régional de Bourgogne (funding number 07 9201 CPER O2 S 5527) and Agence Nationale de la Recherche (BLAN07-2_184783). AB-B was supported by fellowships from the Ministère de l'Education Nationale, de la Recherche et de la Technologie and from L'Oréal France-UNESCO-Académie des Sciences (‘Pour les Femmes et la Science’ Program, national France award 2007).
Supplementary Material
Glossary
Abbreviations
- cPTIO
2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazol-1-yl-oxy-3-oxide
- DAF-2
4,5-diaminofluorescein
- DEA/NO
diethylamine NONOate
- DMSO
dimethylsulphoxide
- L-NAME
Nw-nitro-L-arginine-methyl ester
- NO
nitric oxide
- NOS
nitric oxide synthase
- PBITU
S,S′-[1,3-phenylene-bis(1,3-ethanediyl)]bis-isothiourea
References
- Arita NO, Cohen MF, Tokuda G, Yamasaki H. Fluorometric detection of nitric oxide with diaminofluoresceins (DAFs): applications and limitations for plant NO research. In: Lamattina L, Polacco JC, editors. Nitric oxide in plant growth. Plant Cell Monographs. Vol. 6. Heidelberg: Springer; 2006. pp. 269–280. [Google Scholar]
- Bedioui F, Villeneuve N. Electrochemical nitric oxide sensors for biological samples—Principle, selected examples and application. Electroanalysis. 2003;15:5–18. [Google Scholar]
- Besson-Bard A, Courtois C, Gauthier A, Dahan J, Dobrowolska G, Jeandroz S, Pugin A, Wendehenne D. Nitric oxide in plants: production and cross-talk with Ca2+ signalling. Molecular Plant. 2008a;1:218–228. doi: 10.1093/mp/ssm016. [DOI] [PubMed] [Google Scholar]
- Besson-Bard A, Pugin A, Wendehenne D. New insights into nitric oxide signaling in plants. Annual Review of Plant Biology. 2008b;59:10. doi: 10.1146/annurev.arplant.59.032607.092830. 1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- Bonnet P, Bourdon E, Ponchet M, Blein JP, Ricci P. Acquired resistance triggered by elicitins in tobacco and other plants. European Journal of Plant Pathology. 1996;102:181–192. [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. The Plant Journal. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Brunet A, Pailleret A, Devynck MA, Devynck J, Bedioui F. Electrochemical sensing of nitric oxide for biological systems: methodological approach and new insights in examining interfering compounds. Talanta. 2003;61:53–59. doi: 10.1016/S0039-9140(03)00359-X. [DOI] [PubMed] [Google Scholar]
- Chandler MT, Tandeau de Marsac N, De Kouchkovsky Y. Photosynthetic growth of tobacco cells in liquid suspension. Canadian Journal of Botany. 1972;50:2265–2270. [Google Scholar]
- Crawford NM. Mechanisms for nitric oxide synthesis in plants. Journal of Experimental Botany. 2006;57:471–418. doi: 10.1093/jxb/erj050. [DOI] [PubMed] [Google Scholar]
- Delledonne M. NO news is good news for plants. Current Opinion in Plant Biology. 2005;8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. The Plant Journal. 2000;23:817–824. doi: 10.1046/j.1365-313x.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Brugger A, Lamotte O, Vandelle E, Bourque S, Lecourieux D, Poinssot B, Wendehenne D, Pugin A. Early signaling events induced by elicitors of plant defenses. Molecular Plant-Microbe Interaction. 2006;19:711–724. doi: 10.1094/MPMI-19-0711. [DOI] [PubMed] [Google Scholar]
- Griveau S, Dumézy C, Goldner P, Bedioui F. Electrochemical analysis of the kinetics of nitric oxide release from two diazeniumdiolates in buffered aqueous solutions. Electrochemistry Communications. 2007a;9:2551–2556. [Google Scholar]
- Griveau S, Dumézy C, Séguin J, Chabot GG, Scherman D, Bedioui F. In vivo electrochemical detection of nitric oxide in tumor-bearing mice. Analytical Chemistry. 2007b;79:1030–1033. doi: 10.1021/ac061634c. [DOI] [PubMed] [Google Scholar]
- Hu X, Neill SJ, Tang Z, Cai W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiology. 2005;137:663–670. doi: 10.1104/pp.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourd'heuil D. Increased nitric oxide-dependent nitrosylation of 4,5-diaminofluorescein by oxidants: implications for the measurement of intracellular nitric oxide. Free Radical Biology and Medicine. 2002;33:676–684. doi: 10.1016/s0891-5849(02)00955-3. [DOI] [PubMed] [Google Scholar]
- Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annual Review of Plant Biology. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D. Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radical Biology and Medicine. 2006;40:1369–1376. doi: 10.1016/j.freeradbiomed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Lamotte O, Gould K, Lecourieux D, Sequeira-Legrand A, Lebrun-Garcia A, Durner J, Pugin A, Wendehenne D. Analysis of nitric oxide signalling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiology. 2004;135:516–529. doi: 10.1104/pp.104.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. The Plant Cell. 2002;14:2627–2641. doi: 10.1105/tpc.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke WF. Solubilities: inorganic and metal organic compounds. 4th edn. vol. II. Washington, DC: ACS; 1965. [Google Scholar]
- Mc Murtry MS, Kira DH, Dinh-Xuan T, Archer SL. Measurement of nitric oxide, nitrite and nitrate using a chemiluminescence assay: an update for the year 2000. Analysis. 2000;28:455–465. [Google Scholar]
- Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. Nitric oxide, stomatal closure, and abiotic stress. Journal of Experimental Botany. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- Pereira Rodrigues N, Bedioui F, Deutsch A, Zurgil N, Afrimzon E, Shafran Y, Deutsch M. Construction and use of an integrated electrochemical device for the detection of biologically relevant compounds released from non adherent cells. Application for the electrochemical determination of nitric oxide produced by human U937 cells. Electrochemistry Communications. 2006;8:341–347. [Google Scholar]
- Pereira Rodrigues N, Zurgil N, Chang SC, Henderson J, Bedioui F, Mc Neil C, Deutsch M. Combined system for the simultaneous optical and electrochemical detection of intra- and extra-cellular NO produced by glioblastoma cells. Analytical Chemistry. 2005;77:2733–2738. doi: 10.1021/ac0483163. [DOI] [PubMed] [Google Scholar]
- Planchet E, Kaiser WM. Nitric oxide (NO) detection by DAF fluorescence and chemiluminescence: a comparison using abiotic and biotic NO sources. Journal of Experimental Botany. 2006a;57:3043–3055. doi: 10.1093/jxb/erl070. [DOI] [PubMed] [Google Scholar]
- Planchet E, Kaiser WM. Nitric oxide production in plants. Facts and fictions. Plant Signaling and Behavior. 2006b;1:46–51. doi: 10.4161/psb.1.2.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchet E, Sonoda M, Zeier J, Kaiser WM. Nitric oxide (NO) as an intermediate in the cryptogein-induced hypersensitive response—a critical re-evaluation. Plant, Cell and Environment. 2006;29:59–69. doi: 10.1111/j.1365-3040.2005.01400.x. [DOI] [PubMed] [Google Scholar]
- Pontié M, Bedioui F. Evaluation of the non biofouling behaviour of nitric oxide electrochemical sensor materials by using sessile drop contact angle measurements and free enthalpy of adhesion calculations. Materials Science and Engineering: C. 2002;21:69–73. [Google Scholar]
- Pontié M, Gobin C, Pauporté T, Bedioui F, Devynck J. Electrochemical nitric oxide microsensors: sensitivity and selectivity characterization. Analytica Chimica Acta. 2000;411:175–185. [Google Scholar]
- Prado AM, Porterfield DM, Feijó JA. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development. 2004;131:2707–2714. doi: 10.1242/dev.01153. [DOI] [PubMed] [Google Scholar]
- Stöhr C, Strube F, Marx G, Ullrich WR, Rockel P. A plasma membrane-bound enzyme of tobacco roots catalyses the formation of nitric oxide from nitrite. Planta. 2001;212:835–841. doi: 10.1007/s004250000447. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Santolini J, Wang ZQ, Wie CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. Journal of Biological Chemistry. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- Vandelle E, Delledonne M. Methods for nitric oxide detection during plant–pathogen interactions. Methods in Enzymology. 2008;437:573–592. doi: 10.1016/S0076-6879(07)37029-8. [DOI] [PubMed] [Google Scholar]
- Vitecek J, Reinohl V, Jones RL. Measuring NO production by plant tissues and suspension cultured cells. Molecular Plant. 2008;1:270–284. doi: 10.1093/mp/ssm020. [DOI] [PubMed] [Google Scholar]
- Wadsworth R, Stankevicius E, Simonsen U. Physiologically relevant measurements of nitric oxide in cardiovascular research using electrochemical microsensors. Journal of Vascular Research. 2006;43:70–85. doi: 10.1159/000089547. [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Lamotte O, Frachisse JM, Barbier-Brygoo H, Pugin A. Nitrate efflux is an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. The Plant Cell. 2002;14:937–951. doi: 10.1105/tpc.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottini M, Costa A, De Michele R, Ruzzene M, Carimi F, Lo Schiavo F. Salicylic acid activates nitric oxide synthesis in Arabidopsis. Journal of Experimental Botany. 2007;58:1397–1405. doi: 10.1093/jxb/erm001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.