Abstract
Periderm is a tissue of secondary origin that replaces damaged epidermis. It can be found in underground plant organs, as an above-ground tissue of woody species (cork), and as a wound-healing tissue. Its outer layers are composed of phellem cells with suberized walls that constitute a protective barrier, preventing pathogen invasion and fluid loss. In potato, a model for periderm studies, periderm tissue replaces the epidermis early in tuber development and the suberized phellems constitute the tuber's skin. To identify factors involved in phellem/skin development and that play a role in its defensive characteristics, two-dimensional gel electrophoresis was used to compare the skin and parenchymatic flesh proteomes of young developing tubers. Proteins exhibiting differentially high signal intensity in the skin were sorted by functional categories. As expected, the differential skin proteome was enriched in proteins whose activity is characteristic of actively dividing tissues such as cell proliferation, C1 metabolism, and the oxidative respiratory chain. Interestingly, the major functional category consisted of proteins (63%) involved in plant defence responses to biotic and abiotic stresses. This group included three isozymes of caffeoyl-CoA O-methyltransferase and five isozymes of peroxidase that may play a role in suberization processes. The differential expression of these proteins in the skin was further verified by RT-PCR of their corresponding transcripts in skin and tuber flesh samples. The results presented here shed light on the early events in skin development and further expand the concept of the periderm as a protective tissue containing an array of plant defence components.
Keywords: Periderm, potato tuber, Solanum tuberosum, suberization
Introduction
Potato skin is composed of suberized phellem cells, the outer component of the tuber periderm. The periderm tissue consists of two additional cell types: a single-cell meristematic layer, the phellogen (cork cambium) that produces the phellem cells and is localized underneath them; and a parenchyma-like phelloderm that is derived from inward cell divisions of the phellogen.
The periderm is a tissue of secondary origin that replaces the epidermis when the latter is damaged. In potato, the periderm replaces the epidermis early in tuber development (Reeve et al., 1969). This type of periderm, designated natural or native periderm, is initiated by divisions of both the epidermal and subepidermal cells, first at the stem end and later in the bud-end region of the tuber (Artschwager, 1924; Reeve et al., 1969). New skin layers are continuously added by cell divisions during tuber maturation. In immature periderm, the actively dividing phellogen is labile and prone to fracture, allowing separation of the suberized phellem/skin from the underlying phelloderm and parenchyma cells (Lulai and Freeman, 2001; Sabba and Lulai, 2002). In the present work, this characteristic was used to obtain homogeneous skin tissue.
The protective characteristics of the periderm are thought to derive from the deposition of suberin on the walls of its phellem/skin cells. Following suberization of their walls, the phellem cells die, thus creating an outer defensive layer: the cell corpses are filled with air and therefore provide thermal insulation, the suberized walls prevent invasion by micro-organisms (mechanically and chemically), and wax deposits that are embedded in the suberin material prevent desiccation of the plant tissue (Kolattukudy, 1977).
Suberin is a macromolecule that consists of aromatic and aliphatic polyester domains and is localized between the primary wall and the plasmalemma (Kolattukudy, 1984). The aromatic domain of potato suberin is composed of hydroxycinnamates and their amide derivatives as well as monolignols (Bernards and Lewis, 1998; Yan and Stark, 2000). The major components of the aliphatic domain are α,ω-diacids and ω-hydroxyacids, with minor amounts of alkan-1-ols and alkanoic acids (Graca and Pereira, 2000). Glycerol has been suggested to be involved in the cross-linking between the corresponding polyesters, allowing the formation of a three-dimensional network (Graca and Pereira, 2000), and recently, the glycerol-3-phosphate acetyltransferase 5 gene has been shown to play a critical role in the biogenesis of suberized cell walls (Beisson et al., 2007). Mechanisms by which suberin may impart disease resistance have been suggested (Kolattukudy, 1984): it may act as a barrier to the diffusion of pathogenic enzymes or toxins into living tissues, as a structural barrier to pathogen ingression, or as a biochemical barrier to microbes due to the high proportion of phenolic materials incorporated into its polymer.
This study shows that the skin tissue is enriched with proteins involved in the plant's responses to biotic and abiotic stresses. This result, together with the suggested role of suberin in resistance and our previous report on the phelloderm as the major producing tissue of tuber toxic steroidal glycoalkaloid (Krits et al., 2007), present the periderm as a ‘battery’ of defence components, and further expand the concept of its protective role.
Materials and methods
Plant material
The experiments were carried out with potato plants (Solanum tuberosum cv. Desirée) grown in pots (20 l) filled with perlite in a greenhouse under natural winter conditions (November 2005 to January 2006, average temperature range of 10–18°C). Tubers were harvested at four time points during the growing period. Immature tubers were collected at the end of the fifth and eighth weeks post-sprout-emergence; at that developmental stage, the skin (3–4 and 8–10 cell layers, correspondingly) can be easily peeled from the tuber flesh with no contamination of the tuber parenchyma cells. The other two sampling dates were 5 d and 2 weeks after cutting the foliage (at 10 weeks post-sprouting) to induce the skin-set process. At those developmental stages, the maturing skin adheres strongly to the tuber flesh but can still be peeled away from it.
The harvested tubers were washed carefully with water to remove the remains of the growing media without damaging the skin. Tuber skin and the parenchymatic tissue of the tuber flesh were sampled, snap-frozen in liquid nitrogen, and stored at –80°C. Samples represent an average of five independent Desirée plants; for each sample, two or three replicates were tested.
Tissue embedding and histological staining
Tuber-surface samples (blocks of 5×3×3 mm) were fixed in FAA, dehydrated in an ethanol–xylene series and embedded in paraplast (Paraplast Plus, Oxford Labware, USA) according to standard methods (Ruzin, 1999). Tissue sections (15–20 μm) were stained with Safranin O/Fast green (Sigma Chemicals, Israel) for examination of tissue morphology, or with haematoxylin (Sigma Chemicals) for observation of cell nuclei (Johansen, 1940). Sections were observed under a light microscope (Leica DMLB, Germany) and images were displayed on a monitor through a CCD camera (Leica DC2000) using the Leica IM1000 program. The same samples were viewed under UV light to detect autofluorescence of suberized cell walls in the skin: the Leica DMLB microscope was configured for epifluorescent illumination using an HBO103W/2 mercury lamp, excitation filter BP 340–380, chromatic beam-splitter FT 400 and barrier filter LP 425.
Protein extraction
Frozen tissues from separate skin and parenchyma (700 mg) samples were ground in liquid nitrogen using a mortar and pestle. Total proteins were extracted in 2.5 ml ice-cold buffer containing 0.1 M TRIS-HCl pH 8, 5% (w/v) sucrose, 2% (w/v) SDS, 50 mM dithiothreitol (DTT), and a mixture of protease inhibitors (Complete® tablets, Roche, Germany). An equal volume of saturated phenol (pH 8) was added and the extraction mixture was shaken for 10 min and then centrifuged (10 min, 10 000 g, 4°C). The lower phase was collected and proteins were precipitated by adding of 5 vols of 0.1 M ammonium acetate in methanol overnight. The protein pellet was washed three times with the precipitation solution and a fourth time with 80% acetone. The dried pellet was dissolved in rehydration buffer containing 9 M urea, 2% (w/v) CHAPS, 0.5% (v/v) Pharmalyte, and 100 mM DTT. Protein was quantified using the Total Protein Kit, Micro Lowry (Sigma, Israel).
Two-dimensional gel electrophoresis (2-DE)
The first-dimension electrophoresis was performed using 13-cm-long Immobiline DryStrip gels with a pH range interval of 4–7 (linear), and gels with a broad-range pH interval of 3–11 (non-linear) (Amersham Bioscience, Sweden); the latter was used to gain better coverage of the proteins in the basic region of the strip. Strips were rehydrated overnight with total protein (700 μg or 500 μg, respectively) dissolved in 250 μl rehydration buffer. After rehydration, isoelectric focusing was performed at 18°C in a Multiphor II™ isoelectric focusing system (Amersham Bioscience) using the following conditions: the voltage was increased gradually every 15 min, starting with 300 V, then 500, 1500, 2000, 2500, and 3000 V, for a total time of 1.5 h. The run was continued for a further 4 h at a constant voltage of 3500 V. After isoelectric focusing, the strips were equilibrated at room temperature in 50 mM TRIS-HCl pH 8.8, 6 M urea, 30% (v/v) glycerol, 2% SDS, and 1% DTT for 15 min, then for another 15 min in the same buffer but with 2.5% (w/v) iodoacetamide instead of DTT. The second dimension was run in a 10% SDS-polyacrylamide gel at 35 mA per gel in a PROTEAN® II xi cell (Bio-Rad, USA). Each sample was subjected to 2-DE in replicates and in both pH ranges (4–7 and 3–11). Gels were stained with colloidal Coomassie blue G-250 (Fluka, Switzerland) and gel images were acquired with an Astra 4600 scanner (Umax, Germany). Gel image analyses were performed with Z3 software (Compugen Inc., Israel) set to a minimum spot area of 25 and a minimum spot contrast of 10. Replicated gels of each skin or tuber flesh sample were used for the analyses. Spot pairs were confirmed visually before picking.
Identification of proteins by peptide-mass mapping
Protein identification was performed at the Smoler Proteomics Center at the Technion, Israel. Briefly, protein spots were excised from the gel, treated with 100 mM iodoacetamide and trypsinized with modified trypsin (Promega) at a 1:100 enzyme-to-substrate ratio. The resulting tryptic peptides were resolved by reverse-phase chromatography on 0.1×200 mm fused silica capillaries (100 μm ID, J&W, USA) packed with Everest reverse-phase material (Grace Vydac, USA). The peptides were eluted with linear 50 min gradients of 5–95% (v/v) acetonitrile with 0.1% (v/v) formic acid in water at a flow rate of 0.4 μl min−1. Mass spectrometry (MS) was performed in an ion-trap mass spectrometer (LCQ-DecaXP, Finnigan, USA) in positive mode using a repetitively full MS scan followed by collision-induced dissociation (CID) of the three most dominant ions selected from the first MS scan.
The MS data were clustered and analysed using Sequest (Eng et al., 1994) or Pep-Miner (Beer et al., 2004) software. Briefly, the Sequest software identifies uninterpreted peptides with tandem mass spectra produced under low-energy (10–50 eV) collision conditions by correlating their mass-to-charge ratios for fragment ions to those predicted for known amino acid sequences obtained from the Genepept database. The Pep-Miner software manages large amounts of raw data obtained from the MS by clustering similar spectra from multiple runs, thereby reducing the amount of data to a manageable size and allowing fast and reliable identification of peptides in complex mixtures.
Identifications were carried out using the NCBI non-redundant protein database from September 2006 and the TIGR potato EST database from February 2007. A comparison of the results obtained from the two databases is described in Table S1 of the Supplementary data at JXB online.
Total RNA extraction and RT-PCR
RNA was isolated as described by Chang et al. (1993). After LiCl precipitation, total RNA was washed in 70% cold ethanol, vacuum-dried for 5 min, and washed again in a mixture consisting of equal parts of SSTE (1 M NaCl, 0.1 M TRIS pH 8, 0.5% SDS, 0.001 M EDTA in diethylpyrocarbonate (DEPC)-treated double-distilled water and acidic phenol:chloroform:isoamyl alcohol solutions. After removal of the organic phase, the RNA was re-precipitated overnight in 100% ethanol at –20°C and centrifuged (4°C, 15 min, 18 000 g. The RNA pellet was washed again in 20% cold ethanol, dried on ice for 15 min and redissolved in 30 μl of DEPC-treated double-distilled water. Total RNA was quantified using a NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technology, USA). RNA was further purified by RNeasy Mini Kit (Qiagen) using the On-Column DNase Digestion protocol.
RT-PCR was performed using the Reverse-iT™ One-Step RT-PCR Kit (ABgene, UK) according to the manufacturer's protocol, with RNA samples (around 20 ng per reaction) and specific primers (Table 1). Reaction products were fractionated by electrophoresis on a TAE-agarose gel (2%, w/v) (Sambrook et al., 1989), stained with ethidium bromide, and photographed. Each sample represents five independent plants. Signal intensities were quantified by Image Gauge software, version. 4 (Fuji Photo Film CO. Ltd, Japan), and expression levels were calculated relative to the signal obtained for 18S rRNA product.
Table 1.
List of primers used in the present work
| Protein name | TA numbera | Direct primer | Reverse primer |
| CCoAOMT-3 | TA30485 | GCAAGATCCTAGCAATTGACC | CTTTCTGGTCTCATCAACTTGTC |
| CCoAOMT-5 | TA33337 | CTAGTTACAGCTCTTGCTTTGC | GGATCATTCTCTGAAAGTGC |
| CCoAOMT-6 | TA30542 | GAGAGGGACCTGCTTTACCTG | CATCACCAACCGGAAGCATAC |
| POD (18) | TA25873 | CGACTGCTAATGTTGTTCGGAC | CATTACATGTGTGCACAAACAAAG |
| POD (20) | TA23216 | GTGTTGACTGGAACACAAGGTC | CACTTATGTGTCCACACATAC |
| POD (5) | CV504265 | CAGCCAGACTTTTGTCACTATTC | CCTACAATTGGTAATGAAGGGAC |
| POD (9) | TA39454 | GTGTCTAAGAAAGGGCTATTG | CCACACAATTCTCCGTTCAC |
| POP_A | TA25019 | GGATCAAGTTTTGACCGGGGATG | GCATCAACTGATCATAATACTGAAAG |
| NAC-α type | TA25576 | GTGAAGCAAAGATTGAGGACTTG | CTCTTGAGACATCAAAGAAAGCG |
Potato Transcript Assemblies (http://plantta.tigr.org/) and EST identifiers.
Results
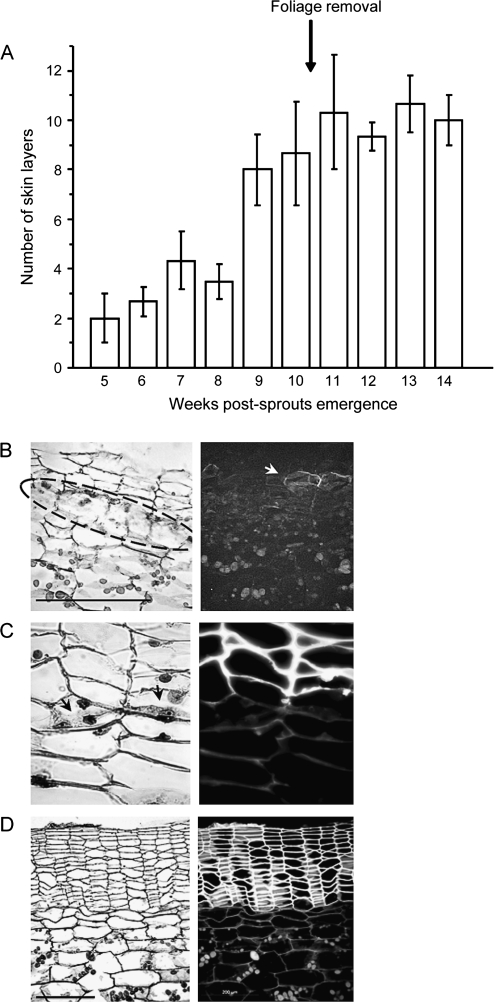
To shed light on tuber skin development and its characteristics as a protective tissue, proteins were extracted from tuber skin and parenchyma (flesh) tissues. Skin samples were collected as early as 5 weeks and 8 weeks post-sprout-emergence, a stage of active cell division (immature skin) (Fig. 1); earlier than that, the skin tissue disintegrates. Tuber flesh samples were also collected at these two time points. Two additional skin samples were collected 5 d and 14 d after the induction of skin set by foliage removal, to identify changes in protein profile during skin maturation.
Fig. 1.
Potato tuber skin development. Cross-sections of tuber surface were stained with Safranin O/Fast green and viewed using light (B–D, left panels) and UV (B–D, right panels) microscopy to examine tissue morphology and autofluorescence of suberized cells, respectively. (A) Number of skin layers (suberized phellem cells) during tuber development; cessation of plant growth and tuber expansion were achieved by pruning of the foliage (arrow). (B) Early stage in periderm development; actively dividing phellogen is encircled, arrow indicates initial suberization of skin cells. (C) Close-up of dividing phellogen cells showing cell plate between daughter cells (arrow) and the suberized skin/phellem cells located above it. (D) Mature skin following foliage removal and the induction of skin set showing columns of suberized phellem cells; the inactive phellogen layer is indistinguishable. Bar=200 μm.
To obtain proteins that are differentially expressed in the immature skin, protein profiles of skin and flesh tissues were compared at 5 weeks and 8 weeks post-sprout-emergence. The 2-DE with pH interval range 4–7, which represents most proteins in these tissues, was used to analyse the total number of spots as well as the number of differential spots in each proteome using the Z3 software. Data indicated a higher number of spots in flesh tissue collected at 5 weeks post-sprout-emergence than in flesh tissue collected 3 weeks later, 432 spots compared to 267 spots, respectively, probably reflecting the greater metabolic activity of the developing tuber. No significant change in the total number of spots was detected for the corresponding skin proteomes (289 and 278 spots, respectively). In the skin proteomes, the Z3 analysis detected spots that did not match those of the corresponding flesh proteomes. The percentage of differential skin spots out of the total number of skin spots was 27 (79 spots) and 18 (50 spots), for the 5 weeks and 8 weeks post-sprout-emergence samples, respectively. In the flesh proteomes sampled at 5 weeks and 8 weeks post-sprout-emergence, 58% (250 spots) and 30% (80 spots) of the spots, respectively, did not match the corresponding skin proteome.
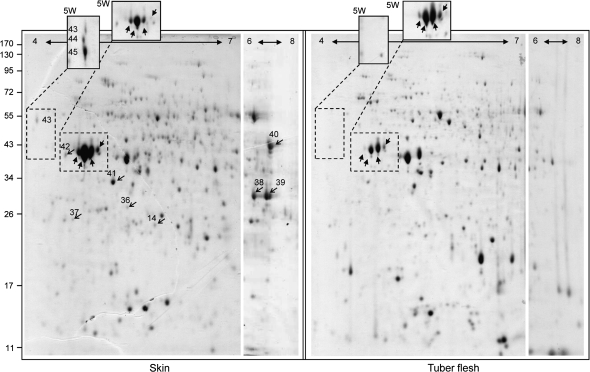
Representative images of the 2-DE (pH interval 4–7) of skin and flesh samples collected at 8 weeks post-sprout-emergence are shown in Fig. 2. This figure also includes the pH 6–8 segment of the 2-DE with pH interval 3–11, in order to show better the proteins that did not separate well at the basic margin of the pH 4–7 strips. In addition, two regions that were taken from the 5 weeks post-sprout-emergence profiles were also inserted into Fig. 2 (a full image of the latter is given in Supplementary Fig. S1 at JXB online).
Fig. 2.
Representative 2-DE images of skin and tuber flesh at the developmental stage of 8 weeks post-sprout-emergence. Left frame: skin proteome. Right frame: flesh proteome. Each frame is a composite of three gels: the larger image represents 2-DE with a (linear) gradient interval of pH 4–7, the narrow gel on the right is a segment of the 2-DE gel with a (non-linear) gradient interval of pH 3–11 showing only the pH range of 6–8. The pH gradient intervals are indicated by a horizontal arrow at the top of each photograph. The third component of each frame is two boxes that protrude above the main image. These boxes were taken from 2-DE (pH 4–7) images of skin and flesh samples that were collected at 5 weeks post-sprout-emergence (5W); dashed lines indicate the corresponding spots in the main image. The left protruding box in the skin frame encloses spots with POP_A sequences (spots 43–45); the right protruding box in each frame encloses patatin spots. Light arrows indicate peroxidase and CCoAOMT isozymes verified by RT-PCR, block arrows indicate major patatin proteins.
For the MS analysis, 60 differential skin spots and three control spots that exhibited equal signals in the skin and flesh samples were picked manually from the gels, and protein identification was carried out using two databases. First, a protein database was used (NR-NCBI); however, some spots could not be identified, and a database of translated potato EST sequences from TIGR was therefore used. The latter database resulted in more specific annotations, for example, to corresponding isogenes, and previously unidentified spots were clearly identified. MS analyses obtained from both databases are compared in Supplementary Table S1 at JXB online. Proteins that accumulated differentially in the skin were categorized by cellular function and are listed in Table 2. Some of the differential skin spots contained two proteins with nearly identical MW and pI values; although their differential accumulation in the skin was questionable, they are listed in Supplementary Table S2 at JXB online.
Table 2.
List of skin proteins that accumulate differentially in potato tuber skin compared to tuber flesh
| Spot no. | Functional categories | Potato TAa/EST | Match peptidesb | Cover (%)c | MW(kDa)/pI |
|
| Experimental | Theoretical | |||||
| Cell proliferation | ||||||
| 1 | Actin (ACT) | TA24741 | 10 | 21 | 43/4.8 | 41.8/5.31 |
| 2d | P23 tumor protein-like (P23/TCTP) | TA24105 | 2 | 10 | 17.84/4.84 | 18.85/4.58 |
| 3d | Proteasome α-7 subunit | TA26206 | 6 | 20 | 27.6/6.3 | 27.1/7 |
| 4 | Proteasome β-2A subunit | TA32826 | 8 | 30 | 21.1/6.48 | 22.1/6.2 |
| 5 | Translation initiation factor 5A-3, eukaryotic (eIF-5A3) | TA23158 | 6 | 30 | 16.25/6.25 | 17.57/5.78 |
| 6 | Tubulin α-chain | TA24049 | 11 | 32 | 49/5.2 | 49.75/4.93 |
| Signal transduction—cell wall | ||||||
| 7 | Remorin (REM) | DN921712 | 4 | 16 | 30.4/6.14 | 21.8/6.14 |
| General metabolism | ||||||
| 8d | UDP-glucose:protein transglucosylase (UPTG2) | TA24497 | 9 | 21 | 36.9/5.8 | 41.15/5.61 |
| 8d | Disulphide-isomerase protein (PDI) | TA25269 | 5 | 17 | 36.9/5.8 | 39.52/5.62 |
| 9d | Triosephosphate isomerase, (TPI) cytosolic isoform | TA25726 | 3 | 16 | 24.4/5.8 | 21.6/5.7 |
| Oxidative respiratory chain | ||||||
| 3d | APFI (hypothetical protein F8G22.2) | TA26786 | 4 | 12 | 27.6/6.3 | 29.5/6.8 |
| 2d | NADH-ubiquinone oxidoreductase 18 kDa subunit, mitochondrial precursor | TA29219 | 2 | 6 | 17.84/4.84 | 19.76/4.94 |
| 10 | NADH:FMN oxidoreductase-like protein | TA30877 | 5 | 22 | 21.1/6.38 | 21.5/6.2 |
| One-carbon (C1) metabolism | ||||||
| 11 | Glutamate-ammonia ligase (GS1) | TA25277 | 5 | 21 | 38/4.5–4.8 | 38.7/5.6 |
| 12 | Serine hydroxymethyltransferase 4 (SHMT4) | TA24707 | 10 | 28 | 53.6/6.4 | 51.7/6.8e |
| 13, 14 | Methionine synthase (MS) | TA24454 | 11–16 | 19–30 | 78.5/6.46–6.57 | 84.6/5.93 |
| Abiotic and biotic stress | ||||||
| 15 | Plasma-membrane polypeptide (DREPP) | TA26238 | 9 | 25 | 26/5.2 | 21.9/5.02 |
| Oxidative stress | ||||||
| 16 | Ascorbate peroxidase 1 (APX1), cytosolic | TA24251 | 12 | 35 | 25.4/5.93 | 27.47/5.56 |
| 17 | Catalase isozyme 2 (CAT2) | TA23780 | 17 | 35 | 53.6/6.4 | 56.5/6.56 |
| 18 | Catechol oxidase B, chloroplast precursor | TA25457 | 6 | 10 | 58.2/5.9 | 66.4/6.3 |
| 19f | Polyphenol oxidase (PPO) | TA24911 | 7 | 11 | 56/6 | 66.7/6.8 |
| Plant defence | ||||||
| 20d | Cysteine protease 1 (CYP1) | CK256743 | 3 | 8 | 38.5/4.86 | 32/5.8 |
| 21 | Elicitor-inducible protein EIG-J7 | TA32425 | 3 | 12 | 16.1/6.57 | 20/6.88 |
| 22f | Elicitor-inducible protein EIG-J7 | TA39811 | 4 | 15 | 15/4.86 | 20.3/6.08e |
| 23 | Endochitinase 2 precursor | TA24214 | 4 | 18 | 28/6.1 | 34.3/5.93 |
| 24 | Endochitinase 2 precursor | CN213814 | 2 | 11 | 27.6/6.3 | 28.6/7.48 |
| 20d,25–28 | Patatin | TA23294 | 9 | 10–27 | 39.6/5.26 | 43.4/5.11 |
| 29 | Patatin putative homolog | TA35081 | 5 | 14 | 43/6 | 43.5/6.5 |
| 30,31 | Patatin protein 07 | TA23344 | 11 | 26 | 36.7–38.5/5.36–5.6 | 42.6/5.2 |
| 32 | Pathogenesis-related protein 10 (PR-10) | CN213132 | 10 | 38 | 16.5/5.45 | 17.4/5.3 |
| 33f | Pathogenesis-related protein 10 (PR-10) | TA22962 | 3 | 13 | 16.9/5.5 | 17.4/5.3 |
| 34 | 2-Oxoglutarate-dependent dioxygenase (SPP2) | TA24517 | 3 | 9 | 36.7/5.6 | 37.9/5.54 |
| Suberization/lignification | ||||||
| 35 | ACP-17 kDa β-hydroxyacyl-acyl carrier protein | TA29588 | 6 | 17 | 17.63/6.15 | 24.6/9.03 |
| 9d | Caffeoyl-CoA O-methyltransferase-5 (CCoAOMT-5) | TA33337 | 6 | 26 | 24.4/5.8 | 26.3/5.51 |
| 36 | Caffeoyl-CoA O-methyltransferase-6 (CCoAOMT-6) | TA30542 | 7 | 29 | 26.4/5.48 | 27.98/5.4 |
| 37 | Caffeoyl-CoA O-methyltransferase-3 (CCoAOMT-3) | TA30485 | 4 | 14 | 26/4.8 | 25.95/5.4e |
| 38,39 | Peroxidase (POD 18) | TA25873 | 10–11 | 27–28 | 32.8/ 6.4–6.7 | 36.3/6.32 |
| 40 | Peroxidase PER9-6 secretory (POD 20) | TA23216 | 12 | 22 | 43/6.8 | 39.7/7.6 |
| 41 | Peroxidase 136, class III, precursor (POD 9) | TA39454 | 10 | 34 | 32.8/5.35 | 35/5.2 |
| 42 | Peroxidase putative (POD 5) | CV504265 | 3 | 12 | 39.6/4.85 | 35.85/9.2e |
| 43-45 | Peroxidase, suberization-associated anionic peroxidase (POP_A) | TA25019 | 5 | 15 | 53.4–39.6/4.15–4.2 | 36.3/6.32 |
| Reference protein | ||||||
| 46 | Nascent polypeptide-associated complex NAC; UBA-like | TA25576 | 4 | 20 | 24.9/4.42 | 21.7/4.38 |
Potato Transcript Assemblies (http://plantta.tigr.org/) and EST identifiers.
Number of peptides identified.
Sequence coverage in between (%).
Spot that contains more than one protein.
Calculated using the most similar protein.
Additional isozymes were found in the skin Table S2.
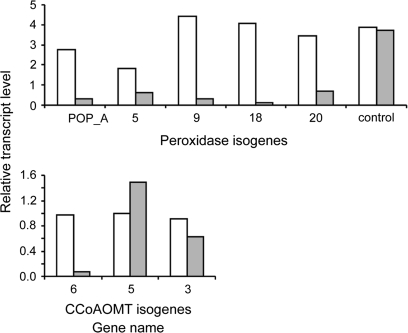
The skin proteins identified fell into three major functional categories: cell proliferation, general metabolism, and stress response (Table 2). The proteins corresponding to stress-related responses amounted to 63% of the total proteins identified and included two to five isozymes for some of the activities. The stress-response proteins include those involved in antioxidative activity, enzymes involved in plant defence or in the synthesis of plant defence compounds, and enzymes that may be involved in suberization processes. The group of suberization-related proteins (n=9) consisted of only three activities: hydroxyacyl-acyl carrier protein (ACP), caffeoyl-CoA O-methyltransferase (CCoAOMT) (three isozymes), and peroxidase (five isozymes). These proteins/isozymes may be unique to suberized potato skin as they were not identified via the NR-NCBI database, or their identification was not accurate due to a lack of matched peptides (see Supplementary Table S1 at JXB online). Most interesting was the identification of the suberization-associated anionic peroxidase (POP_A, spots 43–45) that was initially identified in potato tuber wounding periderm (Roberts and Kolattukudy, 1989). A high signal for spots corresponding to POP_A was obtained in the immature skin sample taken 5 weeks post-sprout-emergence; later sampling indicated a reduction in its level as the skin matured (Fig. 2). In view of their putative involvement in suberization processes, the differential expression of CCoAOMT and peroxidase in the skin was further tested at the transcriptional level by semi-quantitative RT-PCR (Fig. 3). All peroxidase isogenes and two of the three CCoAOMT isogenes showed increased signal in the skin, implying their differential expression in tuber skin versus flesh.
Fig. 3.
Differential expression of peroxidase and CCoAOMT isogenes in the skin compared to tuber flesh. Primers corresponding to peroxidase and CCoAOMT isozymes were designed based on specific peptide sequences obtained from the MS analysis and were used in semi-quantitative RT-PCR with RNA samples of skin (white bars) and tuber flesh (grey bars). The expression levels were monitored relative to 18S rRNA levels in each sample. NAC protein (TA25576, spot 46) was used as reference gene for equal expression in skin and flesh tissues. Signal intensities were quantified by Image Gauge software.
Also notable was the relatively high signal intensity obtained for spots corresponding to patatin protein TA23294 in the skin sample (spots 20, 26, 27, 28; Fig. 2, bold arrows). At the developmental stage of 8 weeks post-sprout-emergence, signal intensities for these spots were higher in the skin than in both the flesh samples and the skin sample collected earlier, at 5 weeks post-sprout-emergence. The signal intensities of these major spots decreased during further skin maturation (5 d and 14 d post-foliage removal, see Supplementary Fig. S1 at JXB online); accordingly, multiple spots containing low-MW degraded patatin peptides were obtained from 8 weeks post-sprouting (data not shown). Contrary to skin patatin, the corresponding spots in the tuber flesh samples exhibited higher signals at 5 weeks post-sprouting than later (Fig. 2).
A comparison of skin proteomes during development, 5 weeks and 8 weeks post-sprout-emergence, and 5 d and 14 d post-skin-set-induction by foliage removal, showed high similarity in the protein profiles (demonstrated in Supplementary Fig. S1 at JXB online). However, analysis with Z3 software indicated a 20% increase in the number of total spots in the mature skin (14 d post-foliage removal). Some of the ‘new’ spots were sent for MS analysis, and all of them consisted of small-size protein fragments. This result suggested that the increase in total spots was due to protein degradation in the dying skin cells at that developmental stage. Hence, no further spots were analysed.
Discussion
Early events in periderm development have not been fully characterized (e.g. genes/enzymes involved in periderm tissue origination and subsequent cell proliferation) and are addressed here using native potato tuber periderm as a model. It is also suggested that, aside from suberin, the skin (phellem cells of the periderm) is enriched with additional defence components against potential pathogen invaders.
To characterize the major processes occurring in the tuber skin, proteins that exhibited higher accumulation in the skin compared to the tuber flesh parenchymatic cells were categorized into three main functional groups: cell proliferation, general metabolism, and stress response.
Proteins involved in cell proliferation
Several proteins identified here may play a role in the intensive cell division activity of the developing periderm. Actin (spot 1) filaments have been suggested to enhance protein synthesis in wounded periderm of potato tubers by providing a scaffold for the assembly of the translational machinery (Morelli et al., 1998). P23/TCTP (spot 2) in association with tubulin (spot 6) has been shown to be preferentially expressed in proliferating mammalian cells (Gachet et al., 1999; Yan et al., 2000). In plants, P23 is expressed in callus cells of alfalfa (Pay et al., 1992), and its transcript level increases following the induction of cell division in root caps (Woo and Hawes, 1997). The proteasome (both α and β type subunits, spots 3 and 4) has been suggested to play an important role in regulating developmental pathways in young and actively dividing tissues by controlling the levels of short-lived regulatory nuclear proteins (Bahrami and Gray, 1999). Finally, the eukaryotic translation-initiation factor 5A (eIF-5A) isoforms regulate cell proliferation, cell growth, and programmed cell death; in Arabidopsis the isoform 5A3 was suggested to activate cell division in primordia (Thompson et al., 2004; Feng et al., 2007). Similarly, it can be speculated that the potato eIF-5A3 (spot 5) is involved in periderm cell proliferation.
Skin samples contained two additional proteins that may be active in proliferating tissues; however, their stronger expression in the skin versus flesh samples has yet to be validated (see Supplementary Table S2 at JXB online). One is a member of the transducin/WD-40 repeat proteins that have been found to be involved in embryogenesis and germination processes (Gallardo et al., 2001; Komatsu et al., 2005), as well as in dividing cells of nodule primordia and in the nodule meristem (McKhann et al., 1997). The other is adenosine kinase isoform 2S (ADK), whose activity has been correlated to cytokinin metabolism with respect to cell division in tobacco BY-2 cells (Kwade et al., 2005).
The process of phellogen cell division and the differentiation of their progeny into suberized skin cells may involve cell–cell signalling. Remorin (spot 7) has been suggested to be a plasmodesma-associated protein involved in cell–cell signalling or transport (Jacinto et al., 1993). RT-PCR indicated that the level of remorin transcript was markedly higher in the skin of the tuber than in its flesh (data not shown); nevertheless its roles in the skin tissue have yet to be clarified.
Proteins categorized in the general metabolism group
The actively developing skin accumulates high levels of proteins belonging to enzymatic complexes of the oxidative respiratory chain and one-carbon (C1) metabolism. NADH:ubiquinone oxidoreductase (spot 2) and NADH:FMN-oxidoreductase (spot 10) are components of the respiratory complex I of plant mitochondria; an additional component of the complex, ATP synthase, was also detected in the skin, but not in a differential manner relative to the flesh sample (see Supplementary Table S2 at JXB online). APFI (spot 3) is a transcription factor that mediates the expression of the mitochondrial complex I genes.
The components of C1 metabolism that accumulated to higher levels in the skin were glutamate-ammonia ligase (GS1, spot 11), serine-hydroxymethyltransferase 4 (SHMT4, spot 12), and methionine synthase (MS, spots 13, 14). The skin SHMT4 identified here is probably a cytosolic protein based on its high homology to the corresponding Arabidopsis isozyme (McClung et al., 2000). In non-photosynthetic cells such as those of the tuber skin tissue, serine–glycine conversion involves cytosolic SHMT (Mouillon et al., 1999).
The serine–glycine conversions utilize tetrahydrofolate as the cofactor for methyl-group transfer (Mouillon et al., 1999); accordingly, two isozymes of MS (spots 13, 14), which produces tetrahydrofolate, accumulate to higher levels in the tuber skin than in the flesh tissue. In addition, lipoamide dehydrogenase, which catalyses the re-oxidation of lipoamide, the cofactor of glycine decarboxylase complex (GDC), was also detected in the skin, although it did not accumulate differentially in that tissue (see Supplementary Table S2 at JXB online).
Glycine conversion by GDC produces ammonia, which is reassimilated by GS activity (spot 11) (Keys, 2006). The skin GS is a cytosolic isoform that is predominant in non-photosynthetic tissues of potato (Teixeira et al., 2005). Thus, identification of SHMT4 and GS1, both characteristic of non-photosynthetic tissues, together with the above-mentioned enzymes, indicates up-regulation of C1 metabolism in developing skin tissue.
Skin enzymes that are also involved in carbon metabolism were the cytosolic isoform of triosephosphate isomerase (cTPI, spot 9), which plays an important role in glycolysis and has been suggested to play a role in growing tissues (Dorion et al., 2005), and UDP-glucose:protein transglucosylase (UPTG2), an autocatalytic glycosyl-transferase involved in the synthesis of cell-wall polysaccharides, the highest expression of which has been observed in actively growing tissues (Wald et al., 2003).
To summarize, all of the above-mentioned proteins represent enzymatic activities that are characteristic of the dynamic metabolism of rapidly developing tissue, in accordance with the processes that are expected to occur during skin development.
Proteins involved in plant responses to abiotic and biotic stresses
Several enzymes with antioxidative activity were differentially expressed in the skin: cytosolic ascorbate peroxidase (cAPX1, spot 16), Class II catalase (CAT2, spot 17), and catechol/polyphenol oxidase (spots 18, 19), all play key roles in the detoxification of reactive oxygen species (ROS) in cells (Niebel et al., 1995; Constabel and Ryan, 1998; Davletova et al., 2005). These antioxidants may also play a role in suberization processes that involve oxidative reactions (Bernards, 2002). This is also true for peroxidase activity, for which five isoenzymes were identified to be differentially expressed in the skin (spots 38–39, 40, 41, 42, 43–45, and Fig. 3). Peroxidase activity has been suggested to play an important role in the polymerization of the aromatic domain of suberin and in its anchorage to the primary cell wall. Of special interest is the potato suberization-associated peroxidase, POP_A (accession number P12437), which was first isolated from the wound periderm of tuber slices (Espelie and Kolattukudy, 1985). In the present work, POP_A exhibited a higher signal in the immature skin of tubers collected at 5 weeks post-sprout-emergence, with a dramatic decrease in its level in the subsequent stages of tuber development (Fig. 2, spots 43–45); its appearance as three distinct spots may result from the glycosylated status of the protein and reduced electrophoretic mobility due to its anionic nature (Roberts et al., 1988). A cationic isoform of peroxidase from tomato has also been suggested to be associated with suberization (Quiroga et al., 2000; Lucena et al., 2003), but was present as well in non-suberizing tissues (Bernards et al., 1999).
An additional gene family of enzymes that may play a role in suberization/lignification processes is the caffeoyl-CoA 3-O-methyltransferases (CCoAOMT, spots 9, 36, 37). CCoAOMT converts caffeoyl-CoA to feruloyl-CoA, and is thought to be involved in plant defence reactions via the synthesis of wall-bound forms of ferulic acid. CCoAOMT-6 transcripts exhibited markedly higher expression in the skin of tubers from 8-week-old plants relative to their parenchyma tissue (Fig. 3).
An additional activity that may take part in suberization processes is the β-hydroxyacyl-acyl carrier protein (ACP) dehydratase (spot 35) which catalyses the third step in the elongation cycle of unsaturated fatty acids (Heath and Rock, 1996). In addition to the general metabolism of fatty acids in potato tuber skin, this enzyme can play a role in synthesizing the unsaturated very long chain fatty acids (VLCFA) components of the suberin aliphatic domain.
The protein identified in spot 15 is a member of a family that contains several plant plasma-membrane proteins termed DREPPs (developmentaly regulated plasma-membrane polypeptides) that were shown to be induced during cold acclimation and drought (Kawamura and Uemura, 2003).
The group of proteins categorized as functioning in plant defence consists of enzymes that play a role in the plant's response to pathogen invasion and synthesis of defence compounds. These are: basic chitinase (spots 23, 24; Takemoto et al., 1997), cysteine protease (CYP1, spot 20; Avrova et al., 1999), the elicitor inducible gene EIG-J7 (spots 21, 22; Takemoto et al., 2001), and the pathogenesis-related protein 10 (PR-10, spots 32, 33; Liu et al., 2006), as well as 2-oxoglutarate-dependent dioxygenase (SPP2, spot 34) that is involved in the biosynthesis of deterrent alkaloids in reproductive tissues (Lantin et al., 1999).
Most interesting was the relatively high level of patatin proteins in the immature skin (Fig. 2) relative to their content in the flesh. As the skin matured, the intensity of the patatin spots decreased dramatically (see Supplementary Fig. S1 at JXB online). In the potato plant, they are mainly expressed in the tuber flesh and are considered to be major storage proteins in the tubers. The patatins are a multigene family and the skin contains at least four different members (Table 2; and see Table S2 in the Supplementary data at JXB online). Two of them, TA23294 and TA23344, are present in multiple spots within a range of pI values but at the same MW, due to charge heterogeneity of the protein (Park et al., 1983).
Patatins have been shown to exhibit antifungal (Sharma et al., 2004) and pesticidal activities (Strickland et al., 1995). Thus, besides being the main storage protein of the potato tuber, they have been suggested to be involved in resistance reactions (Senda et al., 1996) as was shown for tuber storage proteins of other plant species (Shewry, 2003).
Additional components of plant defence were identified in the skin, but their differential accumulation relative to tuber flesh has yet to be clarified (see Supplementary Table S2 at JXB online). Lehesranta et al. (2006) reported an abundance of defence-related proteins in a subset of 150 tuber flesh proteins identified during the tuber's life cycle (i.e. only 27% based on the classification of stress-related proteins used in the present work); some of those proteins were also identified in the present work (PR-10, APX, PPO). Although the experimental design of Lehesranta et al. (2006) differed from ours, it is possible that the same proteins were differentially accumulated in the skin at the developmental stages of 5 weeks and 8 weeks post-sprout-emergence. Alternatively, the skin may contain different isoforms of the corresponding proteins.
In conclusion, the present work expands our understanding of the function of the periderm as a protective tissue. To the best of our knowledge, this is the first proteomic study focusing on native potato tuber periderm, and it indicates that, in addition to its suberized cell walls, the skin is enriched with components of the plant defence response. Moreover, the phelloderm, i.e. the inner cell layers of the tuber periderm, has previously been suggested by us to produce high levels of glycoalkaloids, which are toxic secondary metabolites that are active against pests and pathogens (Krits et al., 2007). Collectively, these findings further illustrate the periderm tissue as a ‘battery’ of defensive elements charged against biotic and abiotic stresses.
The importance of this study is not limited to potato tuber skin. Periderm formation is a common phenomenon in stems and roots of dicotyledons and gymnosperms that increase in thickness by secondary growth, as well as in lenticels and following wounding. Periderm development and anatomy are highly similar across plant species, thus the characterization of potato tuber skin may have implications for other periderm systems.
Supplementary data
Supplementary data can be found at JXB online.
Figure S1. Representative images of 2-DE (pH interval of 4–7) of skin proteomes at four time points during tuber development (see Fig. 1).
Table S1. Comparison of results obtained from TIGR potato EST database as of February 2007 and NCBI non-redundant protein database as of September 2006.
Tables S2. List of skin proteins from differential spots that contained two proteins.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Natan Gollop from the Volcani Center for his assistance in setting up the isoelectrofocusing system. This research was supported by Research Grant No. IS-3581-04 from BARD, The United States–Israel Binational Agricultural Research and Development Fund, and is a contribution of ARO, the Volcani Center No. 138/2007
Glossary
Abbreviation
- 2-DE
two-dimensional gel electrophoresis
References
- Artschwager E. Studies on the potato tuber. Journal of Agricultural Research. 1924;27:809–835. [Google Scholar]
- Avrova AO, Stewart HE, De Jong W, Heilbronn J, Lyon GD, Birch PRJ. A cysteine protease gene is expressed early in resistant potato interactions with Phytophthora infestans. Molecular Plant–Microbe Interaction. 1999;12:1114–1119. doi: 10.1094/MPMI.1999.12.12.1114. [DOI] [PubMed] [Google Scholar]
- Bahrami AR, Gray JE. Expression of a proteasome α-type subunit gene during tobacco development and senescence. Plant Molecular Biology. 1999;39:325–333. doi: 10.1023/a:1006102110889. [DOI] [PubMed] [Google Scholar]
- Beer I, Barnea E, Ziv T, Admon A. Improving large-scale proteomics by clustering of mass spectrometry data. Proteomics. 2004;4:950–960. doi: 10.1002/pmic.200300652. [DOI] [PubMed] [Google Scholar]
- Beisson F, Li YH, Bonaventure G, Pollard M, Ohlrogge JB. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. The Plant Cell. 2007;19:351–368. doi: 10.1105/tpc.106.048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards MA. Demystifying suberin. Canadian Journal of Botany. 2002;80:227–240. [Google Scholar]
- Bernards MA, Fleming WD, Llewellyn DB, Priefer R, Yang XL, Sabatino A, Plourde GL. Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiology. 1999;121:135–145. doi: 10.1104/pp.121.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards MA, Lewis NG. The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry. 1998;47:915–933. doi: 10.1016/s0031-9422(98)80052-6. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter. 1993;11:113–116. [Google Scholar]
- Constabel CP, Ryan CA. A survey of wound- and methyl jasmonate-induced leaf polyphenol oxidase in crop plants. Phytochemistry. 1998;47:507–511. [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. The Plant Cell. 2005;17:268–281. doi: 10.1105/tpc.104.026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorion S, Parveen Jeukens J, Matton DP, Rivoal J. Cloning and characterization of a cytosolic isoform of triosephosphate isomerase developmentally regulated in potato leaves. Plant Science. 2005;168:183–194. [Google Scholar]
- Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. Journal of the American Society for Mass Spectrometry. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Espelie KE, Kolattukudy PE. Purification and characterization of an abscisic acid inducible anionic peroxidase associated with suberization in potato (Solanum tuberosum) Archives of Biochemistry and Biophysics. 1985;240:539–545. doi: 10.1016/0003-9861(85)90060-8. [DOI] [PubMed] [Google Scholar]
- Feng H, Chen Q, Feng J, Zhang J, Yang X, Zuo J. Functional characterization of the Arabidopsis eukaryotic translation initiation factor 5A-2 that plays a crucial role in plant growth and development by regulating cell division, cell growth, and cell death. Plant Physiology. 2007;144:1531–1545. doi: 10.1104/pp.107.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet Y, Tournier S, Lee M, Lazaris-Karatzas A, Poulton T, Bommer UA. The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. Journal of Cell Science. 1999;112:1257–1271. doi: 10.1242/jcs.112.8.1257. [DOI] [PubMed] [Google Scholar]
- Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D. Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiology. 2001;126:835–848. doi: 10.1104/pp.126.2.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graca J, Pereira H. Suberin structure in potato periderm: glycerol, long-chain monomers, and glyceryl and feruloyl dimers. Journal of Agricultural and Food Chemistry. 2000;48:5476–5483. doi: 10.1021/jf0006123. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Rock CO. Roles of the FabA and FabZ beta-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. Journal of Biological Chemistry. 1996;271:27795–27801. doi: 10.1074/jbc.271.44.27795. [DOI] [PubMed] [Google Scholar]
- Jacinto T, Farmer EE, Ryan CA. Purification of potato leaf plasma membrane protein pp34, a protein phosphorylated in response to oligogalacturonide signals for defence and development. Plant Physiology. 1993;103:1393–1397. doi: 10.1104/pp.103.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen DA. Plant microtechniques. New York: McGraw-Hill Book Company, Inc; 1940. [Google Scholar]
- Kawamura Y, Uemura M. Mass spectrometric approach for identifying putative plasma membrane proteins of Arabidopsis leaves associated with cold acclimation. The Plant Journal. 2003;36:141–154. doi: 10.1046/j.1365-313x.2003.01864.x. [DOI] [PubMed] [Google Scholar]
- Keys A. The re-assimilation of ammonia produced by photorespiration and the nitrogen economy of C3 higher plants. Photosynthesis Research. 2006;87:165–175. doi: 10.1007/s11120-005-9024-x. [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE. Lipid polymers and associated phenols, their chemistry, biosynthesis and role in pathogenesis. Recent Advances in Phytochemistry. 1977;77:185–246. [Google Scholar]
- Kolattukudy PE. Biochemistry and function of cutin and suberin. Canadian Journal of Botany. 1984;62:2918–2933. [Google Scholar]
- Komatsu S, Abbasi F, Kobori E, Fujisawa Y, Kato H, Iwasaki Y. Proteomic analysis of rice embryo: an approach for investigating G alpha protein-regulated proteins. Proteomics. 2005;5:3932–3941. doi: 10.1002/pmic.200401237. [DOI] [PubMed] [Google Scholar]
- Krits P, Fogelman E, Ginzberg I. Potato steroidal glycoalkaloid levels and the expression of key isoprenoid metabolic genes. Planta. 2007;227:143–150. doi: 10.1007/s00425-007-0602-3. [DOI] [PubMed] [Google Scholar]
- Kwade Z, Swiatek A, Azmi A, Goossens A, Inzé D, Van Onckelen H, Roef L. Identification of four adenosine kinase isoforms in tobacco BY-2 cells and their putative role in the cell cycle-regulated cytokinin metabolism. Journal of Biological Chemistry. 2005;280:17512–17519. doi: 10.1074/jbc.M411428200. [DOI] [PubMed] [Google Scholar]
- Lantin S, O'Brien M, Matton DP. Pollination, wounding and jasmonate treatments induce the expression of a developmentally regulated pistil dioxygenase at a distance, in the ovary, in the wild potato Solanum chacoense Bitt. Plant Molecular Biology. 1999;41:371–386. doi: 10.1023/a:1006375522626. [DOI] [PubMed] [Google Scholar]
- Lehesranta SJ, Davies HV, Shepherd LVT, Koistinen KM, Massat N, Nunan N, McNicol JW, Karenlampi SO. Proteomic analysis of the potato tuber life cycle. Proteomics. 2006;6:6042–6052. doi: 10.1002/pmic.200600383. [DOI] [PubMed] [Google Scholar]
- Liu XJ, Huang BB, Lin J, Fei J, Chen ZH, Pang YZ, Sun XF, Tang KX. A novel pathogenesis-related protein (SsPR10) from Solanum surattense with ribonucleolytic and antimicrobial activity is stress- and pathogen-inducible. Journal of Plant Physiology. 2006;163:546–556. doi: 10.1016/j.jplph.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Lucena MA, Romero-Aranda R, Mercado JA, Cuartero J, Valpuesta V, Quesada MA. Structural and physiological changes in the roots of tomato plants over-expressing a basic peroxidase. Physiologia Plantarum. 2003;118:422–429. [Google Scholar]
- Lulai EC, Freeman TP. The importance of phellogen cells and their structural characteristics in susceptibility and resistance to excoriation in immature and mature potato tuber (Solanum tuberosum L.) periderm. Annals of Botany. 2001;88:555–561. [Google Scholar]
- McClung CR, Hsu M, Painter JE, Gagne JM, Karlsberg SD, Salome PA. Integrated temporal regulation of the photorespiratory pathway. Circadian regulation of two Arabidopsis genes encoding serine hydroxymethyltransferase. Plant Physiology. 2000;123:381–392. doi: 10.1104/pp.123.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann HI, Frugier F, Petrovics G, Coba de la Peña T, Jurkevitch E, Brown S, Kondorosi E, Kondorosi A, Crespi M. Cloning of a WD-repeat-containing gene from alfalfa (Medicago sativa): a role in hormone-mediated cell division? Plant Molecular Biology. 1997;34:771–780. doi: 10.1023/a:1005899410389. [DOI] [PubMed] [Google Scholar]
- Morelli JK, Zhou W, Yu J, Lu C, Vayda ME. Actin depolymerization affects stress-induced translational activity of potato tuber tissue. Plant Physiology. 1998;116:1227–1237. doi: 10.1104/pp.116.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillon J-M, Aubert S, Bourguignon J, Gout E, Douce R, Rebeille F. Glycine and serine catabolism in non-photosynthetic higher plant cells: their role in C1 metabolism. The Plant Journal. 1999;20:197–205. doi: 10.1046/j.1365-313x.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- Niebel A, Heungens K, Barthels N, Inzé D, Van Montagu M, Gheysen G. Characterization of a pathogen-induced potato catalase and its systemic expression upon nematode and bacterial infection. Molecular Plant–Microbe Interaction. 1995;8:371–378. doi: 10.1094/mpmi-8-0371. [DOI] [PubMed] [Google Scholar]
- Park WD, Blackwood C, Mignery GA, Hermodson MA, Lister RM. Analysis of the heterogeneity of the 40 000 molecular weight tuber glycoprotein of potatoes by immunological methods and by NH2-terminal sequence analysis. Plant Physiology. 1983;71:156–160. doi: 10.1104/pp.71.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pay A, Heberle-Bors E, Hirt H. An alfalfa cDNA encodes a protein with homology to translationally controlled human tumor protein. Plant Molecular Biology. 1992;19:501–503. doi: 10.1007/BF00023399. [DOI] [PubMed] [Google Scholar]
- Quiroga M, Guerrero C, Botella MA, Barcelo A, Amaya I, Medina MI, Alonso FJ, de Forchetti SM, Tigier H, Valpuesta V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiology. 2000;122:1119–1128. doi: 10.1104/pp.122.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve RM, Hautala E, Weaver ML. Anatomy and compositional variation within potatoes. 1. Developmental histology of the tuber. American Potato Journal. 1969;46:361–373. [Google Scholar]
- Roberts E, Kolattukudy PE. Molecular cloning, nucleotide sequence, and abscisic acid induction of a suberization-associated highly anionic peroxidase. Molecular and General Genetics. 1989;217:223–232. doi: 10.1007/BF02464885. [DOI] [PubMed] [Google Scholar]
- Roberts E, Kutchan T, Kolattukudy PE. Cloning and sequencing of cDNA for a highly anionic peroxidase from potato and the induction of its mRNA in suberizing potato tubers and tomato fruits. Plant Molecular Biology. 1988;11:15–26. doi: 10.1007/BF00016010. [DOI] [PubMed] [Google Scholar]
- Ruzin SE. Plant microtechnique and microscopy. New York: Oxford University Press; 1999. [Google Scholar]
- Sabba RP, Lulai EC. Histological analysis of the maturation of native and wound periderm in potato (Solanum tuberosum L.) tuber. Annals of Botany. 2002;90:1–10. doi: 10.1093/aob/mcf147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. 2nd edn. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. Molecular cloning: a laboratory manual. [Google Scholar]
- Senda K, Yoshioka H, Doke N, Kawakita K. A cytosolic phospholipase A2 from potato tissues appears to be patatin. Plant and Cell Physiology. 1996;37:347–353. doi: 10.1093/oxfordjournals.pcp.a028952. [DOI] [PubMed] [Google Scholar]
- Sharma N, Gruszewski HA, Park SW, Holm DG, Vivanco JM. Purification of an isoform of patatin with antimicrobial activity against Phytophthora infestans. Plant Physiology and Biochemistry. 2004;42:647–655. doi: 10.1016/j.plaphy.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Shewry PR. Tuber storage proteins. Annals of Botany. 2003;91:755–769. doi: 10.1093/aob/mcg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JA, Orr GL, Walsh TA. Inhibition of Diabrotica larval growth by patatin, the lipid acyl hydrolase from potato tubers. Plant Physiology. 1995;109:667–674. doi: 10.1104/pp.109.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D, Doke N, Kawakita K. Characterization of elicitor-inducible tobacco genes by differential hybridization. Journal of General Plant Pathology. 2001;67:89–96. [Google Scholar]
- Takemoto D, Furuse K, Doke N, Kawakita K. Identification of chitinase and osmotin-like protein as actin-binding proteins in suspension-cultured potato cells. Plant and Cell Physiology. 1997;38:441–448. doi: 10.1093/oxfordjournals.pcp.a029187. [DOI] [PubMed] [Google Scholar]
- Teixeira J, Pereira S, Canovas F, Salema R. Glutamine synthetase of potato (Solanum tuberosum L. cv. Desiree) plants: cell- and organ-specific expression and differential developmental regulation reveal specific roles in nitrogen assimilation and mobilization. Journal of Experimental Botany. 2005;56:663–671. doi: 10.1093/jxb/eri042. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Hopkins MT, Taylor C, Wang TW. Regulation of senescence by eukaryotic translation initiation factor 5A: implications for plant growth and development. Trends in Plant Science. 2004;9:174–179. doi: 10.1016/j.tplants.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Wald FA, Kissen R, du Jardin P, Moreno S. Characterization of UDP-glucose: protein transglucosylase genes from potato. Plant Molecular Biology. 2003;52:705–714. doi: 10.1023/a:1025061324856. [DOI] [PubMed] [Google Scholar]
- Woo H-H, Hawes MC. Cloning of genes whose expression is correlated with mitosis and localized in dividing cells in root caps of Pisum sativum L. Plant Molecular Biology. 1997;35:1045–1051. doi: 10.1023/a:1005930625920. [DOI] [PubMed] [Google Scholar]
- Yan B, Stark RE. Biosynthesis, molecular structure, and domain architecture of potato suberin: a C-13 NMR study using isotopically labeled precursors. Journal of Agricultural and Food Chemistry. 2000;48:3298–3304. doi: 10.1021/jf000155q. [DOI] [PubMed] [Google Scholar]
- Yan L, Fei K, Bridge D, Sarras MP., Jr A cnidarian homologue of translationally controlled tumor protein (P23/TCTP) Development Genes and Evolution. 2000;210:507–511. doi: 10.1007/s004270000088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.