Abstract
Brassica juncea BjCHI1 is a plant chitinase with two chitin-binding domains. Its expression, induced in response to wounding, methyl jasmonate treatment, Aspergillus niger infection, and caterpillar Pieris rapae feeding, suggests that it plays a role in defence. In this study, to investigate the potential of using BjCHI1 in agriculture, Pichia-expressed BjCHI1 and its deletion derivatives that lack one or both chitin-binding domains were tested against phytopathogenic fungi and bacteria. Transplastomic tobacco expressing BjCHI1 was also generated and its extracts assessed. In radial growth-inhibition assays, BjCHI1 and its derivative with one chitin-binding domain showed anti-fungal activities against phytopathogens, Colletotrichum truncatum, C. acutatum, Botrytis cinerea, and Ascochyta rabiei. BjCHI1 also inhibited spore germination of C. truncatum. Furthermore, BjCHI1, but not its derivatives lacking one or both domains, inhibited the growth of Gram-negative bacteria (Escherichia coli, Ralstonia solanacearum, Pseudomonas aeruginosa) more effectively than Gram-positive bacteria (Micrococcus luteus and Bacillus megaterium), indicating that the duplicated chitin-binding domain, uncommon in chitinases, is essential for bacterial agglutination. Galactose, glucose, and lactose relieved agglutination, suggesting that BjCHI1 interacts with the carbohydrate components of the Gram-negative bacterial cell wall. Retention of chitinase and bacterial agglutination activities in transplastomic tobacco extracts implicates that BjCHI1 is potentially useful against both fungal and bacterial phytopathogens in agriculture.
Keywords: Bacterial agglutination, chitin-binding domain, chloroplast transformation, Indian mustard, lectin, phytopathogens, Pichia-expressed proteins, transplastomic tobacco
Introduction
Many classes of plant lectins have been identified and they have been implicated in plant defence (Peumans and Van Damme, 1995). One well-characterized group structurally consists of chitin-binding domains, of which one such domain occurs in the mature form of hevein (Van Parijs et al., 1991; Beintema, 1994). Hevein-like chitin-binding domains have also been identified at the N-terminus of many members belonging to family 19 chitinases, particularly from Classes I and IV (Collinge et al., 1993; Beintema, 1994). The precursor of Urtica dioica agglutinin (UDA) consists of two chitin-binding domains at its N-terminus and a C-terminal chitinase domain (Beintema and Peumans, 1992; Lerner and Raikhel, 1992). Following post-translational processing, the UDA precursor is cleaved to generate UDA which lacks the chitinase catalytic domain (Lerner and Raikhel, 1992). Wheat germ agglutinin consists of four chitin-binding domains (Wright et al., 1991). The majority of plant lectins are comprised of hololectins that contain two or more chitin-binding domains which confer agglutination properties (Peumans and Van Damme, 1995).
Since a chitin-binding domain is present in the N-terminus of many members belonging to family 19 chitinases (Collinge et al., 1993), they may be considered as a class of lectins that are structurally linked to an unrelated domain (Van Damme et al., 2004). Chitinases are pathogenesis-related proteins that catalyse the random cleavage of internal β-1,4 glycosidic linkages in chitin, a major constituent of fungal cell walls and insect exoskeletons (Boller, 1985). Chitinases exhibit antifungal activity (Schlumbaum et al., 1986) by lysing fungal tips and inhibiting growth (Mauch et al., 1988).
Brassica juncea (Indian mustard) BjCHI1 encodes an unusual chitinase with two, almost identical, chitin-binding domains (Zhao and Chye, 1999). Its induced mRNA expression upon wounding, methyl jasmonate treatment, Aspergillus niger infection, and caterpillar Pieris rapae feeding, suggests it has a role in defence (Zhao and Chye, 1999; Fung et al., 2002). Further, extracts from transgenic tobacco and transgenic potato plants overexpressing BjCHI1 showed activity against Trichoderma viride (Chye et al., 2005). Transgenic potato plants were conferred enhanced protection to infection by soil-borne Rhizoctonia solani (Fung et al., 2002; Chye et al., 2005). BjCHI1 showed strongest (50–60%) homology to the catalytic domains of family 19 (class I) enzymes, but was structurally distinct from other plant chitinases by the presence of a second chitin-binding domain (Zhao and Chye, 1999). BjCHI1 and UDA are the only known plant proteins that contain two N-terminal chitin-binding domains in tandem. Using Pichia-expressed recombinant BjCHI1, it was demonstrated that its chitin-binding domains confer agglutination of rabbit erythrocytes, a property first reported in chitinases (Tang et al., 2004). Both chitin-binding domains were deemed essential for erythrocyte agglutination, since this was notably absent in derivatives of BjCHI1 lacking one and both chitin-binding domains. In contrast, the removal of one or both chitin-binding domains in BjCHI1 did not adversely affect chitinase activity (Fung et al., 2002; Tang et al., 2004), consistent with findings from site-directed mutagenesis analysis (Tang et al., 2004) and X-ray crystallography of the catalytic domain (Ubhayasekara et al., 2007).
In this study, BjCHI1 was tested against some common phytopathogens to establish if BjCHI1 could be further applied in crop protection. The phytopathogens tested included Colletotrichum truncatum, C. acutatum, Botrytis cinerea, and Ascochyta rabiei. Colletotrichum is the causative agent of anthracnose, a worldwide disease affecting fruit and plantation crops, including papaya, strawberry, tomato, kiwi, pepper, peach, grape, and pecan. B. cinerea causes blight (grey mould) and infects many ornamental crops including bulbs and soft fruit crops like grape and strawberry. Ascochyta rabiei causes ascochyta blight of chickpea. The bacterial pathogen Ralstonia solanacerum was also tested since it causes bacterial wilt on many tropical plants like banana, tomato, pepper, eggplant, and potato. Finally, the use of BjCHI1 in plastid transformation was explored by testing transplastomic tobacco extracts for chitinase activity followed by bacterial agglutination assays.
Materials and methods
Construction of yeast expression plasmids
BjCHI1 (Zhao and Chye, 1999; GenBank accession no. AAF02299) and its deletion derivatives lacking one or both chitin-binding domains were cloned into the Pichia pastoris expression vector pPIC9K (Invitrogen, San Diego, CA, USA), in-frame to the pPIC9K N-terminal secretory signal peptide (Tang et al., 2004). Polymerase chain reaction (PCR) was used to generate the DNA fragments corresponding to BjCHI1 and its derivatives for expression of recombinant proteins that would be secreted into the growth medium (Tang et al., 2004).
Production of secreted chitinases expressed in P. pastoris
Transformation of P. pastoris strain KM71 was carried out by electroporation (Tang et al., 2004). Transformants were verified using PCR and Southern blot analyses before use in protein expression according to the instructions specified in the Multi-copy Pichia Expression Kit (Invitrogen). Protein production in Pichia was initiated by inoculating an overnight culture grown at 30 °C in 10 ml of BMGY medium [1% (w/v) yeast extract, 2% (w/v) peptone, 100 mM potassium phosphate, pH 6.0, 1.34% (w/v) yeast nitrogen base, 4×10−5% (w/v) biotin, 1% (v/v) glycerol], to 800 ml of BMGY medium until the optical density (OD) at 600 nm reached at least 2.0. Subsequently, the cells were harvested by centrifugation at 5000 g for 5 min. The cell pellet was then resuspended in 80 ml of BMMY medium [1% (w/v) yeast extract, 2% (w/v) peptone, 100 mM potassium phosphate, pH 6.0, 1.34% (w/v) yeast nitrogen base, 4×10−5% (w/v) biotin, 0.5% (v/v) methanol] and grown at 30 °C for a further 2 d, during which time methanol was added to a final concentration of 0.5% (v/v) every 24 h. Cells were then harvested by centrifugation at 5000 g for 15 min at 4 °C. The Pichia-expressed protein in the supernatant was precipitated overnight using 65% (w/v) ammonium sulphate, following which the precipitated protein was resuspended in 10 mM sodium phosphate buffer, pH 7.2, and dialysed against the same buffer. The dialysed sample was centrifuged at 5000 g at 4 °C for 15 min, filtered through a membrane filter (0.2 μm, Nalgene) and concentrated using a Centricon concentrator (10 kDa MW cut-off; Millipore). The protein was purified on a gel filtration Superdex HiLoad 16/60 column in a Fast Protein Liquid Chromatography (FPLC) system (Amersham Pharmacia Biotechnology, Uppsala, Sweden) according to Tang et al. (2004). Fractions (2 ml), were collected and analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE; Laemmli, 1970) followed by staining with Coomassie Brilliant Blue to check purity. The purest fractions containing the desired band were pooled and stored at –80 °C.
Protein concentrations were determined using the method of Bradford (1976). These solutions and the control (BSA in 10 mM sodium phosphate buffer, pH 7.2) were filter (0.22 μm) sterilized before use for in vitro tests. Pichia-expressed crude proteins and FPLC-purified recombinant proteins were loaded on to SDS-PAGE to check protein quality after the determination of protein concentration and before further use for in vitro tests.
Anti-fungal activities using plate growth inhibition assays
Anti-fungal activities of Pichia-expressed, filter-sterilized, FPLC-purified recombinant BjCHI1 and its derivative with one chitin-binding domain were tested in vitro using potato dextrose agar (PDA) plates following the method of Fung et al. (2002). Phytopathogens C. truncatum and C. acutatum were each inoculated at the centre of the plate and incubated in the dark at 28 °C. Subsequently, various amounts of filter-sterilized recombinant protein (5 μg or 20 μg) dissolved in sodium phosphate buffer (10 mM, pH 7.2) in a total volume of 100 μl, were added to wells 0.5 cm from the growing hyphal front. The plates were placed in the dark at 28 °C and photographed after 72 h. C. acutatum and B. cinerea were tested using larger amounts of filter-sterilized BjCHI1 (10, 20, 30, 40, or 50 μg). For C. acutatum, plates were incubated in the dark at 28 °C followed by photography after 48 h and for B. cinerea, plates were incubated in the dark at 20 °C followed by photography after 24 h. A. rabiei was inoculated on the centre of a PDA plate for 10–12 d following which BjCHI1 (0, 4, 8, 12, 16, and 20 μg), dissolved in sodium phosphate buffer (10 mM, pH 7.2) in a total volume of 100 μl, were added to wells 0.5 cm from the growing hyphal front. The plate was placed in the dark at 20 °C and photographed after 5 d. Plate assays were repeated at least five times with reproducible results.
Determination of IC50 value
Antifungal activity of BjCHI1 was expressed as the IC50 value of C. truncatum. Hyphal growth inhibition assays (Broekaert et al., 1989) were used to determine the concentration required for 50% growth inhibition (IC50). Spore suspensions (500 μl of a stock solution of 2×104 spores ml−1) in potato dextrose broth were incubated in flat bottom wells of a microtitre plate at room temperature for 2 h. BjCHI1 was added to a final concentration of 0, 0.5, 1, 2, 4, 8, or 16 μg ml−1 and the plates were placed at room temperature until the control germlings (incubated in broth lacking BjCHI1) attained an average length of 500 μm. The average length of 50 individual hyphae from each well was determined from photomicrographs observed with an inverted microscope. The IC50 value was estimated from the length of the hyphae. Relative hyphal growth was expressed as a percentage of the hyphal growth of control cultures incubated in media lacking BjCHI1. Altogether, three independent experiments were conducted with at least 50 samples taken from each.
Anti-bacterial assays
Bacteria tested in anti-bacterial assays were E. coli, R. solanacearum, Pseudomonas aeruginosa, Micrococcus luteus, and Bacillus megaterium. Inhibition assays on bacterial growth (Larrick et al., 1993) were initiated by inoculation of a single bacterial colony in 50 ml of Luria Broth (for E. coli, P. aeruginosa, M. luteus, and B. megaterium) or TCC medium (for R. solanacearum). TCC medium contained 0.25% (w/v) dextrose, 1% (w/v) peptone, 0.1% (w/v) Casamino acid (Difco), and 0.005% (w/v) triphenyl tetrazolium chloride. The cultures were grown overnight at 37 °C for E. coli and P. aeruginosa, 32 °C for R. solanacearum, 30 °C for M. luteus, and 28 °C for B. megaterium. Each overnight culture (50 μl) was further diluted 1:1000 using 50 ml fresh medium to a final concentration of 105 colony-forming units ml−1. To a microtitre well containing 80 μl of diluted bacterial culture (105 colony-forming units ml−1), BjCHI1, its deletion derivatives or BSA (control) was added to obtain final concentrations of 10–60 μg ml−1. Sodium phosphate buffer (10 mM, pH 7.2) was added to a total volume of 100 μl. An OD600 reading was recorded after 30 min and a second reading was taken following incubation for 24 h at the appropriate incubation temperatures. An increase in OD600 would indicate bacterial growth. Three independent experiments, with three replicates in each, were performed altogether.
Bacterial agglutination assays
Agglutination assays were tested using Gram-negative bacteria (E. coli, R. solanacearum, P. aeruginosa) and Gram-positive bacteria (M. luteus and B. megaterium). In bacterial agglutination assays (Saito et al., 1995), 25 μl suspension of each bacterial culture (OD600=10) was added to 25 μl of BjCHI1 solution to obtain final concentrations of 0, 7.8, 15.6, 31.3, 62.5, 125, and 250 μg ml−1, in wells on a microtitre plate, and left for 24 h at room temperature. Agglutinating activities were observed and expressed as the minimum agglutinating concentration (Saito et al., 1995).
To investigate the inhibition of BjCHI1 binding activity by free monosaccharides (galactose and glucose) and disaccharides (lactose), 25 μl of BjCHI1 solution (at a final concentration of 250 μg ml−1) was preincubated with 50 μl of galactose, glucose or lactose, at 37 °C for 1 h, before addition of 25 μl of a R. solanacearum culture. The mixtures, in wells of a microtitre plate, were incubated at room temperature for 24 h. Each sugar was tested at final concentrations of 0, 3.125, 6.25, 12.5, 25, 50, and 100 nM. The inhibition of BjCHI1 binding activity by each monosaccharide or disaccharide was expressed as the minimum inhibitory concentration (Hirata et al., 1990).
The agglutinating activity of purified chitinase from transplastomic tobacco lines was similarly tested against R. solanacearum. Affinity column-purified BjCHI1 chitinase from transplastomic tobacco was used at final concentrations of 0, 125, 250, and 500 μg ml−1 in wells on a microtitre plate, and left to incubate with R. solanacearum at room temperature for 24 h.
Construction of plastid transformation vector for BjCHI1 expression
Plastid transformation vector pMLVHisBj was constructed from plasmid pMLVHisA (Zhou et al., 2006), a derivative of pVSR326 (Reddy et al., 2002). Plasmid pVSR326 utilizes the tobacco plastid genome sequences spanning rbcL-accD to target the reporter gene encoding β-glucuronidase (GUS) into the chloroplast genome by homologous recombination (Reddy et al., 2002). The promoter and terminator for GUS are derived from the rice plastid gene psbA, which encodes a 32 kDa photosystem II protein. The selectable marker gene aadA, which specifies spectinomycin-resistance, is expressed from the promoter of the rice plastid 16S rRNA operon (rrn) and aadA is located adjacent to GUS (Reddy et al., 2002). In the pMLVHisA vector, transcription of the transgene is under the control of the rice plastid psbA promoter and the psbA terminator. In the construction of pMLVHisA, other than unique restriction sites introduced to pSRV326 to facilitate the cloning of the transgene for transplastomic expression, a start codon plus a (His)5-tag was incorporated to tag the recombinant protein. This enables recognition of the recombinant protein using antisera against the (His)5-tag in western blot analysis and easy purification of the recombinant protein, if and when required, using Ni-NTA Agarose (Qiagen) affinity columns.
A 1.3 kb ApaI fragment encoding BjCHI1, including 7 bp of the 5′-untranslated region, was cloned into the ApaI site of pMLVHisA. In the resultant derivative, designated pMLVHisBj, amino acids 1-393 of BjCHI1 were fused in-frame to 18 pMLVHisA-derived residues, including the ‘ATG’ start codon and a (His)5-tag. The presence of flanking rbcL and accD sequences from the tobacco plastid genome enabled the incorporation of BjCHI1 (and aadA) into the tobacco plastid genome via homologous recombination.
Tobacco plastid transformation
Sterilized tobacco seeds were germinated on Murashige and Skoog (MS) medium (Murashige and Skoog, 1962), supplemented with agar (5 g l−1) and sucrose (30 g l−1) for about 6 weeks to obtain the leaf material for transformation. Plastid transformation (Svab et al., 1993) was carried out by bombardment of tobacco leaves with tungsten particles (M 17) coated with pMLVHisBj plasmid DNA using the particle delivery system, PDS 1000-He (Bio-Rad) and its accessories. For bombardment, leaves were placed abaxial side up on RMOP medium consisting of MS salts, 6-benzylaminopurine (1 mg l−1), α-naphthalene acetic acid (0.1 mg l−1), thiamine (1 mg l−1), myo-inositol (100 mg l−1), agar (5 g l−1) at pH 5.7, and sucrose (30 g l−1). Following bombardment, leaves were cut into small pieces and placed on RMOP selection medium containing spectinomycin dihydrochloride (500 mg l−1). The regenerated tobacco plantlets obtained following plastid transformation were subjected to five more cycles on selection medium to obtain homoplastomic plastid-containing plants.
Plantlets were tested by PCR analysis using a primer pair consisting of a BjCHI1-specific primer and a pMLVHisBj vector-specific primer to confirm integration of an intact insert via homologous recombination. Subsequently, a pair of primers that flank the inserted DNA in the tobacco plastome was used in PCR to estimate the percentage of homoplastomic genomes in each transplastomic lines. Each PCR reaction (25 μl) consisted of 250 ng template, 5 pmol of each primer, 0.75 U AmpliTaq Gold (Applied Biosystems), 2.5 μl 10× PCR reaction buffer, 1.5 μl of 25 mM MgCl2 and 0.25 μl of each 10 mM dNTP. PCR-amplification was initiated with denaturation at 95 °C for 3 min, followed by 35 cycles of 95 °C for 1 min, 58 °C for 1 min, and 72 °C for 1 min.
Southern blot, northern blot, and western blot analyses on transplastomic tobacco lines
Following preliminary analysis by PCR, the transplastomic tobacco lines were tested using Southern blot, northern blot, and western blot analyses. For Southern blot analysis, 20 μg DNA isolated according to Dellaporta et al. (1983) was digested with BamHI, separated by electrophoresis in 0.7% agarose gel, and blotted onto Hybond-N (Amersham) membranes (Sambrook et al., 1989). Membranes were prehybridized in a solution containing 50% deionized formamide, 6× SSC, 5× Denhardt's, 1% SDS, 100 mg ml−1 denatured salmon sperm DNA at 42 °C for 4–6 h. Random-primed 32P-labelled rbcL probe was added and hybridized overnight. Membranes were washed in 0.1× SSC, 0.1% SDS at 65 °C.
For northern blot analysis, RNA (20 μg) extracted from whole plants (Nagy et al., 1988), was denatured at 50 °C for 30 min in glyoxal, separated by electrophoresis in 1.5% agarose gel, and transferred to Hybond-N membrane (Amersham). Blots, prehybridized for 4–6 h, were hybridized with random-primed 32P-labelled BjCHI1 cDNA (Zhao and Chye, 1999) in a solution containing 50% formamide, 1× Denhardt's, 6× SSPE (1× SSPE: 0.15 M NaCl, 0.01 M NaH2PO4, 0.001 M EDTA, pH 7.4), 0.1% SDS, 100 μg ml−1 denatured salmon sperm DNA, and 10% dextran sulphate at 42 °C overnight. Blots were washed in 0.1× standard saline citrate (1× SSC:150 mM NaCl, 15 mM sodium citrate, pH 7.0) containing 0.1% SDS at 65 °C.
For western blot analysis, total plant protein was prepared from whole plants according to Kush et al. (1990). Twenty μg of total protein was separated by SDS-PAGE and transferred onto Hybond-C (Amersham) membrane as described by Sambrook et al. (1989). In western blot analysis, cross-reacting bands were detected using polyclonal antibodies against BjCHI1 following the procedures described in the Amplified Alkaline Phosphatase Goat Anti-Rabbit Immuno-Blot Assay Kit (Bio-Rad). Polyclonal antibodies were raised in rabbits against a synthetic peptide (YKEEIDKSDPHC) corresponding to amino acids 231–242 of BjCHI1 (Zhao and Chye, 1999).
Chitinase assays
For colorimetric chitinase assays (Wirth and Wolf, 1990) using the substrate carboxymethylchitin-Remazol Brilliant Violet 5R (CM-chitin-RBV; Loewe Biochemica GmbH., Sauerlac, Germany), samples of plant extracts (1–10 μg) were made up to 250 μl with water and incubated with 250 μl 0.1 M sodium acetate buffer, pH 5.0 and 250 μl CM-RBV-chitin at 37 °C for 1 h. The reaction was stopped with 250 μl 1 M HCl, placed on ice for 15 min to precipitate the non-degraded chitin, and the reaction mixture subsequently centrifuged at 20 000 g at 4 °C for 5 min. OD550nm was read and values were corrected for background, which was determined by using values obtained with protein that had been heated for 10 min at 100 °C to destroy activity. Three replicates were performed and average values used. One unit of chitinase was defined as the enzyme activity liberating 1 mg of soluble N-acetylglucosamine (GlnNAc) equivalent h−1 at infinite dilution.
Results
BjCHI1 inhibits growth of C. truncatum, C. acutatum, B. cinerea, and A. rabiei in vitro
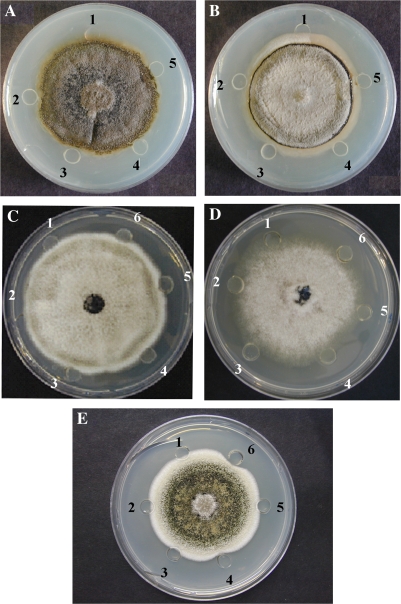
Antifungal activities of BjCHI1 and its deletion derivative with one chitin-binding domain were demonstrated using radial growth-inhibition assays (Schlumbaum et al., 1986). Growth inhibition of C. truncatum (Fig. 1A) and C. acutatum (Fig. 1B) were observed in wells to which BjCHI1 (wells 2 and 3, containing 5 μg and 20 μg, respectively) and its derivative (wells 4 and 5, containing 5 μg and 20 μg, respectively) were added, in contrast to wells containing buffer control (well 1) in which fungal hyphae had grown within the wells.
Fig. 1.
BjCHI1 inhibits growth of phytopathogens Colletotrichum truncatum, C. acutatum, Botrytis cinerea, and Ascochyta rabiei. (A, B) Photographs of the bottom of Petri plates showing the effect of BjCHI1 and its derivative with one chitin-binding domain on the growth of C. truncatum (A) and C. acutatum (B). FPLC-purified proteins were added in wells when the hyphal front was 0.5 cm from the wells. Well 1, control with 10 mM sodium phosphate buffer (pH 7.2); well 2, 5 μg BjCHI1; well 3, 20 μg BjCHI1; well 4, 5 μg BjCHI1 derivative, and well 5, 20 μg BjCHI1 derivative. The photograph was taken 72 h after addition of protein. (C, D) Photographs of the top of the plates showing the effect of BjCHI1 on the growth of C. acutatum (C) and B. cinerea (D). Well 1, control with 10 mM sodium phosphate buffer (pH 7.2); well 2, 10 μg BjCHI1; well 3, 20 μg BjCHI1; well 4, 30 μg BjCHI1; well 5, 40 μg BjCHI1 and well 6, 50 μg BjCHI1. (E) Photograph of the top of the plate showing the effect of BjCHI1 on the growth of A. rabiei. Well 1, control with 10 mM sodium phosphate buffer (pH 7.2); well 2, 4 μg BjCHI1; well 3, 8 μg BjCHI1; well 4, 12 μg BjCHI1; well 5, 16 μg BjCHI1, and well 6, 20 μg BjCHI1.
When the amounts of BjCHI1 were increased beyond 20 μg, significant inhibition of C. acutatum was observed in wells containing 30 μg (Fig. 1C, well 4), 40 μg (Fig. 1C, well 5), and 50 μg (Fig. 1C, well 6) of BjCHI1. Similar results were evident in plate assays on B. cinerea (Fig. 1D) at which BjCHI1 at 0 μg (well 1), 10 μg (well 2), 20 μg (well 3), 30 μg (well 4), 40 μg (well 5), and 50 μg (well 6) were tested. Figure 1E shows results from plate assays on A. rabiei using BjCHI1 at 0 μg (well 1), 4 μg (well 2), 8 μg (well 3), 12 μg (well 4), 16 μg (well 5), and 20 μg (well 6). A. rabiei was noticeably inhibited by BjCHI1 at 20 μg (Fig. 1E, well 6).
These results demonstrate the in vitro activity of BjCHI1 against phytopathogens C. truncatum, C. acutatum, B. cinerea, and A. rabiei. Results of anti-fungal activities on C. truncatum and C. acutatum exhibited by BjCHI1 and its derivative with one chitin-binding domain are consistent with observations of these two proteins on Trichoderma viride in plate assays (Fung et al., 2002). BjCHI1 and its derivatives lacking one or both chitin-binding domains were also observed to inhibit spore germination of T. viride (data not shown), confirming previous observations that chitinase activity is unaffected by removal of either one or both chitin-binding domains (Fung et al., 2002; Tang et al., 2004).
BjCHI1 inhibits spore germination of C. truncatum in vitro
The hyphal growth inhibition assay was used to determine the IC50 value of BjCHI1 against phytopathogen C. truncatum. Growth of hyphae in BjCHI1-containing medium was examined in three independent experiments. The IC50 value of BjCHI1 on C. truncatum was estimated to be approximately 1 μg ml−1. Germination of spores grown in potato dextrose broth as observed by light microscopy, was mimimal (∼15% relative hyphal growth) after 24 h of incubation in potato dextrose broth in the presence of BjCHI1 at 8 μg ml−1. By contrast, the average hyphal length of the spores incubated in a broth lacking BjCHI1 reached an average of 500 μm.
Pichia-expressed recombinant BjCHI1 shows antibacterial activity in vitro
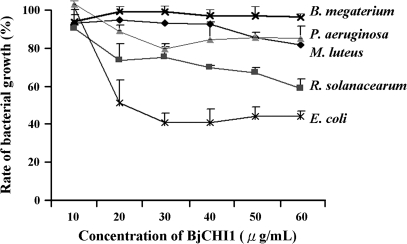
When BjCHI1 and its derivatives lacking one or both chitin-binding domains were tested against E. coli, R. solanacearum, P. aeruginosa, M. luteus, and B. megaterium, only BjCHI1 was observed to inhibit the growth of all bacteria tested. In these tests, BSA was used as a control. Distinct BjCHI1-mediated inhibition (P < 0.1) was observed at various concentrations with different strains, for example, 20 μg ml−1 for E. coli and R. solanacearum, but at higher BjCHI1 concentrations for other bacteria (Fig. 2). In contrast, both BjCHI1 derivatives did not show significant inhibition using the Student's t test.
Fig. 2.
BjCHI1-mediated growth inhibition of bacteria. Inhibition curves of BjCHI1 on B. megaterium, M. luteus, P. aeruginosa, R. solanacearum, and E. coli. Relative bacterial growth (%) in the presence of varying amounts of BjCHI1 in comparison to BSA (100%). The results were derived from three independent experiments (each carried out in triplicate) and are mean values +SD.
A comparison of growth inhibition using BjCHI1 at 60 μg ml−1 for all bacteria tested revealed that E. coli was most susceptible (Fig. 2), followed by R. solanacearum and P. aeruginosa. The inhibition of M. luteus was less significant, and it was negligible for B. megaterium. The most BjCHI1-susceptible bacteria (E. coli, R. solanacearum, and P. aeruginosa) are Gram-negative, while the other least BjCHI1-susceptible bacteria (M. luteus and B. megaterium) are Gram-positive.
BjCHI1 agglutinates Gram-negative bacteria
Since the data from bacterial growth inhibition assays suggested that BjCHI1 was more effective on Gram-negative bacteria, which differed in cell wall composition from Gram-positive bacteria, further investigations were then carried out to check if inhibition was related to BjCHI1-mediated bacterial agglutination.
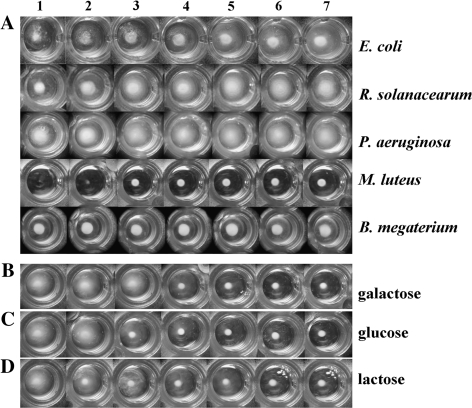
Agglutination assays were carried out and agglutinating activities were observed and expressed as the minimum agglutinating concentration (MAC). These assays on E. coli, R. solanacearum, and P. aeruginosa revealed that higher concentrations of BjCHI1 culminated in greater agglutination (Fig. 3A). By contrast, agglutination was absent for M. luteus and B. megaterium with increasing amounts of BjCHI1 (Fig. 3A). The MAC values (Table 1) when compared to the BSA control were consistent with results from bacterial growth inhibition. Enhanced agglutination of E. coli and R. solanacearum was correlated with stronger inhibition (56% and 40%, respectively, in Table 1) as shown in the bacterial growth inhibition assays in Fig. 2. The slight self-agglutination of P. aeruginosa resulting from basal agglutination could account for the comparably lower degree of inhibition seen in the bacterial growth inhibition assays. Lack of agglutination with Gram-positive bacteria M. luteus and B. megaterium was consistent with results of a lack of BjCHI1-mediated growth inhibition for M. luteus and B. megaterium (Table 1).
Fig. 3.
BjCHI1-mediated agglutination of bacteria. (A) Bacteria, E. coli, R. solanacearum, P. aeruginosa, M. luteus, and B. megaterium, were tested using BjCHI1 at final concentrations of 0 (well 1), 7.8 (well 2), 15.6 (well 3), 31.3 (well 4), 62.5 (well 5), 125 (well 6), and 250 (well 7) μg ml−1. (B–D) Effect of galactose (B), glucose (C) or lactose (D) on the agglutination of R. solanacearum. BjCHI1 (125 μg ml−1) was preincubated with final sugar concentrations at 0 nM (well 1), 3.125 nM (well 2), 6.25 nM (well 3), 12.5 nM (well 4), 25 nM (well 5), 50 nM (well 6), and 100 nM (well 7) before the addition of R. solanacearum.
Table 1.
BjCHI1-mediated agglutination and anti-bacterial activity
| MACa (μg ml−1) | MPCb | RIAc (%) | |
| E. coli | 7.8 | Self-precipitated | 56 |
| R. solanacearum | 7.8 | Self-precipitated | 40 |
| P. aeruginosa | 15.6 | Self-precipitated | 17 |
| M. luteus | > 250 | 31.25 μg ml−1 | 14 |
| B. megaterium | > 250 | Self-precipitated | 4 |
Minimum agglutinating concentration.
Minimum precipitating concentration.
Relative inhibitory activity (RIA) at 60 μg ml−1: RIA = [1– the growth of bacteria in 60 μg ml−1 BjCHI1/growth of bacteria in control group]×100%.
Free monosaccharides or disaccharides relieve binding activity of BjCHI1
Inhibition of carbohydrate-binding activity was performed following Hirata et al. (1990). Three types of carbohydrates (galactose, glucose, and lactose) were tested, and each result was expressed as the minimum inhibitory concentration against 125 μg ml−1 of BjCHI1. Since R. solanacearum demonstrated the most consistent agglutination response on addition of BjCHI1, it was used as the sample bacterium in the agglutination inhibition assays.
Progressive inhibition of agglutination was observed when the added amount of carbohydrate was increased (Fig. 3B–D). Both free galactose and glucose inhibited agglutination of R. solanacearum, although the latter displayed a slightly stronger inhibitory effect (Fig. 3B, C). The result is consistent with the structure of lipopolysaccharide, of which the outer core contains a pentasaccharide of glucose, galactose, and N-acetylglucosamine, and the O-antigen consists of mannose (with sugar specificity similar to glucose), and rhamnose, amongst others. Remarkably, most chitin-binding lectins containing hevein domains are N-acetlyglucosamine-specific (Beintema, 1994). This provides an insight on the mechanism through which BjCHI1 agglutinates Gram-negative bacterial cells.
Analyses on transplastomic tobacco lines expressing BjCHI1
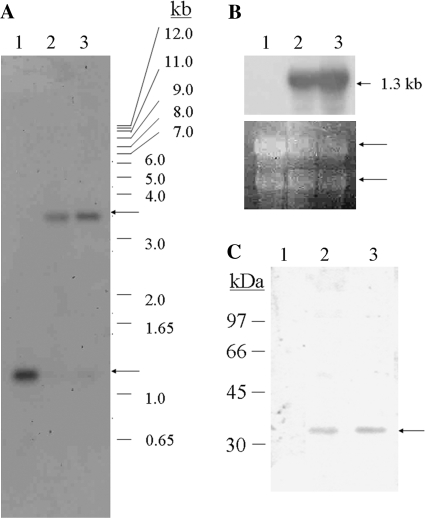
Following preliminary PCR analysis using BjCHI1-specific and plastid vector-specific primer pairs, as well as primer pairs flanking the inserted DNA in the tobacco plastome, Southern blot analysis was used to confirm the integration of BjCHI1 by digestion of DNA from the transplastomic tobacco lines, with BamHI. Southern blot analysis was carried out with wild-type tobacco as a control by using a 32P-labelled rbcL probe. The results (Fig. 4A) showed the presence of a 1.2 kb hybridizing band in wild-type (lane 1) tobacco and a strong 3.4 kb hybridizing band in the two transplastomic tobacco lines (His-BjCHI1-3 in lane 2 and HisBjCHI1-4 in lane 3) confirming integration of the BjCHI1 cDNA in the tobacco plastome of these transplastomic lines. The 3.4 kb band was clearly absent in wild type (Fig. 4A, lane 1). The 1.2 kb hybridizing rbcL BamHI band corresponds to the BamHI fragment (nucleotide positions 58051–59288) within rbcL in the tobacco plastome (Accession No. Z00044). The 3.4 kb BamHI recombinant band resulting from homologous recombination, is due to cleavage corresponding to the BamHI restriction sites at rbcL (nucleotide position 58051 in the tobacco plastome) and at accD (nucleotide position 60656), but inclusive of the pMLVHisBj vector DNA between them. Upon homologous recombination at the plastid flanking sequences rbcL and accD regions, these two BamHI sites are expected to be 3.4 kb apart. In the BjCHI1 plastid transformation vector pMLVHisBj, the BjCHI1 cDNA was cloned between the plastid flanking sequences (rbcL and accD) to enable BjCHI1 to be inserted into the tobacco plastome.
Fig. 4.
Analysis of transplastomic tobacco expressing BjCHI1. (A) Southern blot analysis of BamHI-digested DNA (20 μg) from wild-type (lane 1) and BjCHI1 transplastomic tobacco (HisBjCHI1-3 in lane 2 and HisBjCHI1-4 in lane 3) using 32P-labelled rbcL probe. Arrows indicate hybridizing rbcL bands. In wild type, the 1.2 kb hybridizing rbcL-hybridizing BamHI band corresponds to nucleotide positions 58051–59288 (within rbcL) in the tobacco plastome (Accession No. Z00044). The 3.4 kb band in the transplastomic lines is a recombinant band arising from homologous recombination of BjCHI1 cDNA flanked by rbcL and accD plastid DNA. (B) Northern blot analysis of RNA from wild-type (lane 1), and BjCHI1 transplastomic tobacco lines HisBjCHI1-3 and -4 (lanes 2 and 3, respectively) hybridized to 32P-labelled BjCHI1 cDNA. The arrow denotes the 1.3 kb BjCHI1 mRNA band. Below, ethidium bromide-stained gel with rRNA bands (arrowed). (C) Western blot analysis of crude total proteins from BjCHI1 transplastomic tobacco plants using antibodies against BjCHI1. Total whole plant protein from wild-type (lane 1), and transplastomic lines HisBjCHI1-3 and -4 (lanes 2 and 3, respectively). The arrow denotes the 37 kDa cross-reacting band.
Following the confirmation of gene integration into the plastid genome, northern blot analysis was carried out on transplastomic tobacco plants. Results of northern blot analysis (Fig. 4B) using 32P-labelled BjCHI1 cDNA showed the presence of a 1.3 kb hybridizing band in both lines (HisBjCHI1-3 in lane 2 and HisBjCHI1-4 in lane 3), whereas it was absent in the wild type (lane 1), confirming efficient transcription of BjCHI1 from the plastid genome. Ethidium bromide-stained rRNA bands (Fig. 4B, bottom) showed even loading of total RNA.
Following Southern blot and northern blot analyses, transplastomic tobacco plants were further tested by western blot analysis using anti-BjCHI1 specific antibodies. Western blot analysis using these antibodies (Fig. 4C) on crude protein from BjCHI1 transplastomic tobacco (HisBjCHI1-3 in lane 2 and HisBjCHI1-4 in lane 3) showed a cross-reacting BjCHI1 band with an apparent molecular mass of 37 kDa in the transplastomic lines, while no such band was evident in the wild type (lane 1).
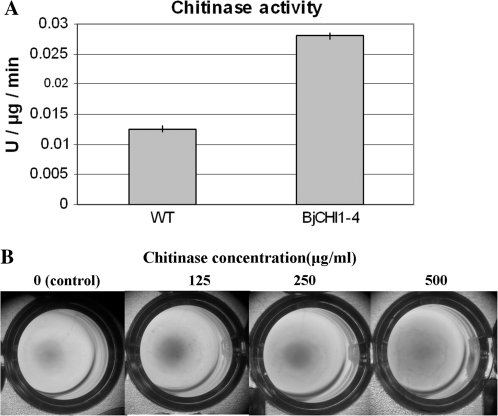
Figure 5A indicates that transplastomic tobacco line HisBjCHI1-4 (0.028 U μg−1 min−1) showed greater than a 2-fold increase in chitinase activity compared to the wild-type tobacco line (0.013 U μg−1 min−1). When agglutinating activity of affinity-purified chitinase from transplastomic tobacco lines against R. solanacearum was examined, a gradual increase of agglutinating activity was observed with an increase in the chitinase final concentration from 0 μg to 500 μg ml−1; clear agglutination activity was observed at the highest concentration tested (Fig. 5B).
Fig. 5.
Analyses of plastid-expressed BjCHI1 in tobacco leaf extracts. (A) Graphical representation of BjCHI1 chitinase activity expressed in U μg−1 min−1 for WT tobacco and transplastomic line HisBjCHI1-4. (B) Agglutination assays on affinity-column-purified BjCHI1 chitinase from transplastomic tobacco against R. solanacearum. Affinity-column purified chitinase was used at final concentrations of 0, 125, 250, and 500 μg ml−1, to test the agglutination of R. solanacearum in wells on a microtitre plate. The plate incubated for 24 h at room temperature before photography.
Discussion
Initial attempts to express (His)6-BjCHI1 in E. coli resulted in problems with poor bacterial growth (Zhao and Chye, 1999). Subsequently, BjCHI1 and its deletion derivatives lacking one or both chitin-binding domains were expressed in Pichia for efficient mass production of the protein (Tang et al., 2004). This investigation further shows that Pichia-expressed BjCHI1 inhibits growth of Gram-negative bacteria, providing an eventual explanation of its adverse effect on E. coli growth in a previous report (Zhao and Chye, 1999). The importance of the second chitin-binding domain in conferring anti-bacterial activity on Gram-negative bacteria is also demonstrated because BjCHI1 derivatives lacking one or both chitin-binding domains did not confer anti-bacterial properties. It has been previously shown that only BjCHI1 with both chitin binding domains (but not its derivatives) has the ability to agglutinate rabbit erythrocytes. This study further showed that BjCHI1 agglutinates the Gram-negative bacterial pathogen, R. solanacearum, thus extending anti-fungal action. Since agglutination is largely an extracellular response, the different biochemical nature of the cell wall of Gram-positive and Gram-negative bacteria, could to some extent dictate the interaction involving the duplicated chitin (carbohydrate)-binding domain of BjCHI1. In contrast, the removal of one or both chitin-binding domains did not adversely affect its catalytic activity in chitinase assays using Pichia-derived BjCHI1 (Tang et al., 2004). These results are consistent with the agglutination function of other known carbohydrate-binding proteins (Peumans and Van Damme, 1995).
Plant lectins are defined as plant proteins possessing at least one non-catalytic domain, which binds reversibly to a specific mono- or oligosaccharide (Peumans and Van Damme, 1995). Monovalent lectins, possessing only one carbohydrate-binding domain, do not precipitate glycoconjugates or agglutinate cells. Previous studies have shown that hololectins may effectively agglutinate cells because they contain two or more carbohydrate binding domains which are identical or very homologous (Van Damme et al., 1998). Interestingly, the amino acid sequence of the two chitin-binding domains of BjCHI1 shows 95% identity with each other (Zhao and Chye, 1999). The bivalent structure of BjCHI1 may be a prototype for plant pathogenesis-related proteins that are able to agglutinate and inhibit bacteria cell growth. The precursor of UDA resembles BjCHI1 by the presence of two chitin-binding domains (Zhao and Chye, 1999) and has been classified as a hololectin (Van Damme et al., 1998). However, UDA differs from BjCHI1 because its catalytic domain is eliminated from the two chitin-binding domains during post-translational cleavage. Observation reported herein of inhibition from agglutination by free monosaccharides (galactose and glucose) and disaccharide (lactose) further suggests lectin-lipopolysaccharide interaction between BjCHI1 and Gram-negative bacterial cells.
The IC50 value of BjCHI1 on the growth of C. truncatum was approximately 1 μg ml−1. In comparison, this is lower than most other plant chitinases with an average value of 2 μg ml−1. The IC50 value against T. viride for UDA, which lacks the catalytic domain, is 50 μg ml−1 (Broekaert et al., 1989). However, addition of low concentrations of UDA to other tobacco chitinases increased the inhibitory potency of the chitinase by about 4-fold, indicating a synergistic action of UDA and chitinases. UDA enhances accessibility to the active sites with its two chitin-binding domains, and the chitinase confers the catalytic function (Broekaert et al., 1989). BjCHI1, a functional protein that includes both the double chitin-binding domains and the catalytic domain in one molecule, potentially resembles the combinatory action of UDA acting in synergism with other chitinases. BjCHI1 exhibited combined anti-microbial activity in combating fungi and in conferring anti-bacterial properties in defence against Gram-negative phytopathogens. In the current study, the BjCHI1 derivative with one chitin-binding domain, also showed the ability to inhibit fungal hyphae growth in in vitro plate assays against C. truncatum and C. acutatum, in accordance with previous studies that demonstrated similar levels of chitinase activities in BjCHI1 and this derivative using standard colorimetric chitinase assays (Fung et al., 2002; Tang et al., 2004).
BjCHI1 as a chimera comprising an N-terminal duplicated chitin-binding domains conferring agglutination-mediated anti-bacterial properties and a C-terminal chitinase domain providing anti-fungal activity, is structurally an effective anti-microbial agent. An intriguing question relates to the presence of the second chitin-binding domain in BjCHI1, which may be redundant in antifungal activity, as the majority of chitinases consist of at most only one chitin-binding domain and a catalytic domain (Collinge et al., 1993). Based on the non-antibacterial nature of the BjCHI1 derivatives lacking chitin-binding domains, the bivalent structure of the carbohydrate-binding domains in BjCHI1 now appears especially significant in defence against bacteria. Molecular and structural studies have shown that cross-linking interactions between certain bivalent and multivalent lectins result in the formation of unique two- and three-dimensional supermolecular assemblies (Brewer, 1997). Mobile infecting bacterial cells could be immobilized and inhibited by such supermolecular assemblies as a result of BjCHI1 binding bacterial cells. In addition, the appearance of a second chitin-binding domain may enhance the ability of BjCHI1 to access the reactive sites on fungal hyphae.
Chitinolytic activity against insects, nematodes, and fungi raises the use of chitinases as alternative pesticides which are environmentally-friendly compared to chemical pesticides. In this study, BjCHI1 showed dual roles as a defence protein against both pathogenic bacteria, including R. solanacearum, as well as fungal phytopathogens, C. truncatum, C. acutatum, B. cinerea, and A. rabiei. Given that extracts from BjCHI1-overexpressing transplastomic tobacco conferred both chitinase and agglutination activities, BjCHI1 could be potentially promising for future applications in plant protection in agriculture. However, despite the many advantages that plastid transformation has over nuclear transformation in the expression of foreign proteins, this relatively new technology is applicable to only a few plant species (Maliga, 2004). Perhaps, ultimately a combination of nuclear and plastid transformation approaches using multiple genes may be necessary to attain disease resistance in genetically-engineered plants. Limitations encountered in the production of disease-resistant plants also arise from the diversity in phytopathogens, ranging from bacteria, and fungi to algal Oomycetes, and the complexities in their lifestyles, which may involve biotrophy, hemibiotrophy, and nectrophy, making it difficult for any one approach to be effective (Collinge et al., 2008). Indeed, useful levels of protection have not been accomplished from strategies involving single pathogenesis-related proteins (Gurr and Rushton, 2005; Collinge et al., 2008). Perhaps the use of synthetic ‘designer’ and ‘lifestyle-specific’ promoters may be necessary to alleviate some of these problems currently hindering genetic engineering in disease protection (Gurr and Rushton, 2005).
Acknowledgments
We thank Professor CY Ma for the provision of FPLC facilities. This work was supported the University of Hong Kong (10205761 and 10208034). MLC was recipient of a Dyason Universitas 21 Fellowship at the University of Melbourne.
References
- Beintema JJ. Structural features of plant chitinases and chitin-binding proteins. FEBS Letters. 1994;350:159–163. doi: 10.1016/0014-5793(94)00753-5. [DOI] [PubMed] [Google Scholar]
- Beintema JJ, Peumans WJ. The primary structure of stinging nettle (Urtica dioica) agglutinin, A two-domain member of the hevein family. FEBS Letters. 1992;299:131–134. doi: 10.1016/0014-5793(92)80231-5. [DOI] [PubMed] [Google Scholar]
- Boller T. Induction of hydrolases as a defence reaction against pathogens. In: Key JL, Kosuge T, editors. Cellular and molecular biology of plant stress. New York: Alan R Liss; 1985. pp. 247–262. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brewer CF. Cross-linking activities of galectins and other multivalent lectins. Trends in Glycoscience and Glycotechnology. 1997;9:155–165. [Google Scholar]
- Broekaert WF, Van Parijs J, Leyns F, Joos H, Peumans WJ. A chitin-binding lectin from stinging nettle rhizomes with antifungal properties. Science. 1989;245:1100–1102. doi: 10.1126/science.245.4922.1100. [DOI] [PubMed] [Google Scholar]
- Chye ML, Zhao KJ, He ZM, Ramalingam S, Fung KL. Expression of an agglutinating chitinase BjCHI1 with two chitin-binding domains is R. solani-inducible and confers fungal protection in transgenic potato. Planta. 2005;220:717–730. doi: 10.1007/s00425-004-1391-6. [DOI] [PubMed] [Google Scholar]
- Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K. Plant chitinases. The Plant Journal. 1993;3:31–40. doi: 10.1046/j.1365-313x.1993.t01-1-00999.x. [DOI] [PubMed] [Google Scholar]
- Collinge DB, Lund OS, Thordal-Christensen H. What are theprospects for genetically engineered, disease resistant plants? European Journal of Plant Pathology. 2008;121:217–231. [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA mini-preparation, version II. Plant Molecular Biology Reporter. 1983;1:19–21. [Google Scholar]
- Fung KL, Zhao KJ, He ZM, Chye ML. Tobacco-expressed Brassica juncea chitinase BjCHI1 shows antifungal activity in vitro. Plant Molecular Biology. 2002;50:283–294. doi: 10.1023/a:1016067200148. [DOI] [PubMed] [Google Scholar]
- Gurr SJ, Rushton PJ. Engineering plants with increaseddisease resistance: how are we going to express it? Trends in Biotechnology. 2005;23:283–290. doi: 10.1016/j.tibtech.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Hirata M, Yoshida M, Inada K, Kirikae T. Investigation of endotoxin binding cationic proteins from granulocytes: agglutination of erythrocytes sensitized with Re-LPS. Advances in Experimental Medicine and Biology. 1990;256:287–299. doi: 10.1007/978-1-4757-5140-6_25. [DOI] [PubMed] [Google Scholar]
- Kush A, Goyvaerts E, Chye ML, Chua NH. Laticifer-specific gene expression of Hevea brasiliensis (rubber tree). Proceedings of the National Academy of Sciences. USA. 1990;87:1787–1790. doi: 10.1073/pnas.87.5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner DR, Raikhel NV. The gene for stinging nettle lectin (Urtica dioica agglutinin) encodes both a lectin and a chitinase. Journal of Biological Chemistry. 1992;267:11085–11091. [PubMed] [Google Scholar]
- Larrick JW, Hirata M, Shimomoura Y, Yoshida M, Zheng H, Zhong J, Wright SC. Antimicrobial activity of rabbit CAP18-deived peptides. Antimicrobial Agents Chemotherapy. 1993;37:2534–2539. doi: 10.1128/aac.37.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga P. Plastid transformation in higher plants. Annual Review of Plant Biology. 2004;55:289–313. doi: 10.1146/annurev.arplant.55.031903.141633. [DOI] [PubMed] [Google Scholar]
- Mauch F, Mauch-Mani B, Boller T. Antifungal hydrolases in pea tissue. Plant Physiology. 1988;88:936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Nagy F, Kay SA, Chua NH. Analysis of gene expression in transgenic plants. In: Gelvin SB, Schilperoot RA, editors. Plant molecular biology manual. B4. Dordrecht: Kluwer Academic Publishers; 1988. pp. 1–29. [Google Scholar]
- Peumans WJ, Van Damme EJM. Lectins as plant defence proteins. Plant Physiology. 1995;109:347–352. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VS, Leelavathi S, Selvapandiyan A, Raman R, Giovanni F, Shukla V, Bhatnagar RK. Analysis of chloroplast transformed tobacco plants with cry1Ia5 under rice psbA transcriptional elements reveal high level expression of Bt toxin without imposing yield penalty and stable inheritance of transplastome. Molecular Breeding. 2002;9:259–269. [Google Scholar]
- Saito T, Kawabata S, Hirata M, Iwanaga S. A novel type of limulus lectin-L6. Journal of Biological Chemistry. 1995;270:14493–14499. doi: 10.1074/jbc.270.24.14493. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schlumbaum A, Mauch F, Vögeli U, Boller T. Plant chitinases are potent inhibitor of fungal growth. Nature. 1986;324:365–367. [Google Scholar]
- Svab Z, Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proceedings of the National Academy of Sciences, USA. 1993;90:913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CM, Chye ML, Ramalingam S, Ouyang SW, Zhao KJ, Ubhayasekera W, Mowbray S. Functional analysis of the chitin-binding domains and the catalytic domain of Brassica juncea chitinase BjCHI1. Plant Molecular Biology. 2004;56:285–298. doi: 10.1007/s11103-004-3382-1. [DOI] [PubMed] [Google Scholar]
- Ubhayasekara W, Tang CM, Ho SWT, Berlund G, Bergfors T, Chye ML, Mowbray SL. Crystal structures of a family 19 chitinase from Brassica juncea show flexibility of binding cleft loops. FEBS Journal. 2007;274:3695–3703. doi: 10.1111/j.1742-4658.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- Van Damme EJM, Barre A, Rouge P, Peumans WJ. Potato lectin: an updated model of a unique chimeric plant protein. The Plant Journal. 2004;37:34–45. doi: 10.1046/j.1365-313x.2003.01929.x. [DOI] [PubMed] [Google Scholar]
- Van Damme EJMV, Peumans WJ, Pusztai A, Bardocz S. Handbook of plant lectins: properties and biomedical applications. Chichester: John Wiley and Sons; 1998. [Google Scholar]
- Van Parijs J, Broekaert WF, Goldstein IJ, Peumans WJ. Hevein: an antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta. 1991;183:258–264. doi: 10.1007/BF00197797. [DOI] [PubMed] [Google Scholar]
- Wirth SJ, Wolf GA. Dye-labelled substrates for the assay and detection of chitinase and lysozyme activity. Journal of Microbiological Methods. 1990;12:197–205. [Google Scholar]
- Wright HT, Sandrasegaram G, Wright CS. Evolution of a family of N-acetylglucosamine binding proteins containing the disulfide-rich domain of wheat germ agglutinin. Journal of Molecular Evolution. 1991;33:283–294. doi: 10.1007/BF02100680. [DOI] [PubMed] [Google Scholar]
- Zhao KJ, Chye ML. Methyl jasmonate induces expression of a novel Brassica juncea chitinase with two chitin-binding domains. Plant Molecular Biology. 1999;40:1009–1018. doi: 10.1023/a:1006266407368. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lee MYT, Ng JMH, Chye ML, Yip WK, Zee SY, Lam E. A truncated hepatitis E virus ORF2 protein expressed in tobacco plastids is antigenic in mice. World Journal of Gastroenterology. 2006;12:306–312. doi: 10.3748/wjg.v12.i2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]