Abstract
Transcytosis of the polymeric immunoglobulin receptor (pIgR) is stimulated by binding of its ligand, dimeric IgA (dIgA). During this process, dIgA binding at the basolateral surface of the epithelial cell transmits a signal to the apical region of the cell, which in turn stimulates the transport of dIgA–pIgR complex from a postmicrotubule compartment to the apical surface. We have previously reported that the signal of stimulation was controlled by a protein-tyrosine kinase (PTK) activated upon dIgA binding. We now show that this signal of stimulation moves across the cell independently of pIgR movement or microtubules and acts through the tyrosine kinase activity by releasing Ca++ from inositol trisphosphate–sensitive intracellular stores. Surprisingly we have found that a second independent signal is required to achieve dIgA-stimulated transcytosis of pIgR. This second signal depends on dIgA binding to the pIgR solely at the basolateral surface and the ability of pIgR to dimerize. This enables pIgR molecules that have bound dIgA at the basolateral surface to respond to the signal of stimulation once they reach the postmicrotubule compartment. We propose that the use of two signals may be a general mechanism by which signaling receptors maintain specificity along their signaling and trafficking pathways.
INTRODUCTION
In recent years we have seen major advances in our understanding of the complex signaling pathways that regulate cell function. Concomitant with this understanding has come an appreciation that these pathways are both compartmentalized and intimately tied to the processes that regulate traffic between membrane compartments (Seaman et al., 1996; Roth and Sternweis, 1997). This compartmentalization exists at several levels and serves multiple functions. Within the plasma membrane, certain signaling molecules may be segregated to small subdomains, such as caveolae or rafts enriched in glycosphingolipids and cholesterol (Lisanti et al., 1994). Such segregation has been proposed to contribute to the specificity of signaling interactions by bringing selected signaling components together. This might facilitate their interaction and/or avoid undesired interactions with other molecules that are excluded from these subdomains (Lisanti et al., 1994; Harder and Simons, 1997). After ligand binding, most signaling receptors are removed from the plasma membrane by endocytosis. Ligand-induced endocytosis can serve to down-regulate signaling by routing the receptor and/or ligand to lysosomes for degradation, or it can initiate or perpetuate the signaling cascade (Baas et al., 1995). Signaling by a receptor has been shown to continue after it has been internalized. For instance, inhibition of endocytosis of the epidermal growth factor receptor (EGFR)1 or insulin receptor (InsR) by point mutations in their cytoplasmic tail or by use of a dominant negative mutation of dynamin has demonstrated that certain signaling events mediated by these receptors require previous endocytosis (Vieira et al., 1996; Ceresa et al., 1998). Finally, signaling by a receptor at the cell surface can alter the trafficking of another molecule, as exemplified by signaling from the InsR, which promotes the exocytosis of a glucose transporter in insulin-sensitive cells (Rea and James, 1997). Another example is the regulation of the major histocompatibility complex class II molecule–specific compartment by signaling from the B cell receptor (Siemasko et al., 1998).
Polarized cells represent an additional layer of complexity of compartmentalization and spatial transmission of signaling information. In polarized epithelial cells that have separate apical and basolateral plasma membrane domains with distinct compositions, many signaling molecules are specifically associated with either the apical or basolateral surface or with organelles located in the apical or basolateral regions of the cytoplasm (Kim, 1997). A crucial question is how signals are communicated across epithelial cells, i.e., how information moves from the basolateral to the apical pole of the cell. An example of transmission of information across an epithelial cell is found in pancreatic and salivary epithelial cells, where binding of a secretagogue to the basolateral surface causes production of inositol trisphosphate (IP3). This IP3 then rapidly diffuses across the cell and causes the release of Ca++ from intracellular stores located in the apical region of the cytoplasm. The elevated calcium then stimulates the exocytosis of large secretory granules at the apical surface (Gerasimenko et al., 1996; Tanimura and Turner, 1996; van de Put and Elliott, 1997). This example illustrates how a hormonal signal acting at the basolateral surface of the cell produces an action at the apical surface resulting in secretion. Calcium signaling involving waves and spikes of elevated intracellular free Ca++ also occurs in epithelial cells that do not have classical “regulated” secretory pathways (e.g., hepatocytes), suggesting that spatial transmission of signaling information may be indeed a general phenomenon (Thorn et al., 1993, 1996; Thomas et al., 1996; Pfeiffer et al., 1998).
We have used the polymeric immunoglobulin receptor (pIgR) as a model system to study the compartmentalization and spatial transmission of signaling information in polarized epithelial cells (Mostov, 1994). The pIgR is expressed by many types of polarized epithelial cells and transcytoses dimeric IgA (dIgA) from the basolateral to the apical surface. The following model has emerged based largely on studies using Madin–Darby canine kidney (MDCK) cells expressing exogenous rabbit pIgR. Newly made pIgR follows the secretory pathway through the Golgi and trans-Golgi network. From the trans-Golgi network, the pIgR is delivered to the basolateral surface. Basolateral sorting is specified by a basolateral sorting signal consisting of residues 653–670 (Casanova et al., 1991; Aroeti et al., 1993). This sorting signal comprises the membrane-proximal portion of the C-terminal cytoplasmic domain of the pIgR, which encompasses residues 653–755.
Once at the basolateral surface, the pIgR can bind its ligand, dIgA, and undergo transcytosis. Based on biochemical, light microscopic, and most importantly electron microscopic analysis, transcytosing dIgA has been found to move through three intracellular compartments. (Hunziker et al., 1990; Apodaca et al., 1994; Mostov, 1994; Song et al., 1994a,b; Gibson et al., 1998). In the first step, the pIgR is endocytosed (with or without dIgA bound) and delivered to basolateral early endosomes (BEEs). In the second step, the pIgR is found in long, 100-nm-diameter tubules, whose structure is dependent on microtubules. Nocodazole treatment of the cells prevents formation of these tubules. This tubular compartment is accessible to transferrin (Tf) and dIgA endocytosed from both the apical and basolateral surfaces. The third compartment consists of 100-nm cup-shaped vesicles that are distributed immediately beneath the apical plasma membrane and tend to be clustered around the centriole. The cup-shaped vesicles are enriched in dIgA and depleted in Tf. Finally, the pIgR is delivered from the cup-shaped vesicles to the apical surface, where its extracellular, ligand-binding domain is cleaved off and released together with the dIgA. This cleaved fragment is called the secretory component (SC).
Several lines of evidence suggest that transcytosis of pIgR consists of two components: constitutive or baseline transcytosis that occurs when dIgA is not bound and ligand-stimulated transcytosis. First of all, in vivo a considerable fraction of pIgR is often transcytosed in the absence of dIgA, leading to the release of SC without dIgA bound. This process is controlled by phosphorylation of Ser-664, which apparently acts to inactivate the primary basolateral targeting signal and thereby allows the pIgR to be transcytosed after it is endocytosed (Casanova et al., 1990). If phosphorylation is prevented by mutation of Ser-664 to Ala, the pIgR, after endocytosis, recycles back to the basolateral surface instead of undergoing transcytosis.
Second, binding of dIgA to pIgR stimulates transcytosis of the pIgR. This stimulation acts primarily after the nocodazole-sensitive step, i.e., either on the movement of pIgR out of BEE into the tubular compartment and/or at a subsequent step in transcytosis. (Song et al., 1994a). Ligand binding has recently been shown to result in an approximately threefold increase in pIgR transcytosis in rat liver and is therefore not simply an artifact of using cultured cells transfected with pIgR (Giffroy et al., 1998).
Finally, very recent electron microscopy analysis has shown that, at least under certain conditions, formation of the tubular compartment itself is dependent on binding of dIgA to the pIgR (Gibson et al., 1998). This provides a dramatic morphological corollary to the biochemical and functional data on stimulation of transcytosis by dIgA binding to pIgR.
dIgA binding to the pIgR stimulates transcytosis through a signal transduction pathway. We have recently demonstrated an outline of how this pathway works. First of all, binding of dIgA induces dimerization of the pIgR. This was demonstrated by expressing in Jurkat cells a chimera of the pIgR and the cytoplasmic domain of the zeta chain of the T cell receptor as a sensitive reporter of receptor oligomerization induced by polymeric IgA (Singer and Mostov, 1998). Second, in MDCK cells binding of dIgA to pIgR induces tyrosine phosphorylation of several proteins, including the phosphatidylinositol-specific phospholipase Cγ1 (PLCγ1). Binding of dIgA to pIgR also causes membrane translocation of PKCε, release of IP3, and finally an increase in the intracellular concentration of free Ca++, ([Ca++]i) (Cardone et al., 1996; Luton et al., 1998). The pIgR itself, however, is not tyrosine phosphorylated and does not have any intrinsic kinase activity. Using inhibitors of PTKs and pIgR mutants deficient in stimulating the PTK activity upon dIgA binding, we have demonstrated that rapid tyrosine phosphorylation is essential for ligand-stimulated transcytosis (Luton et al., 1998). The profile of pharmacological inhibitors was consistent with the involvement of a member of the src family of tyrosine kinases, which may associate directly or indirectly with the pIgR.
These observations imply that information is somehow transmitted across the epithelial cell from the basolateral surface where pIgR binds dIgA to the apical pole of the cell where pIgR transport is stimulated. We now report that two separate signals or processes are involved in dIgA-stimulated pIgR transcytosis. The first signal is one of “stimulation.” The signal of stimulation requires the activity of a nonreceptor tyrosine kinase, calcium release from IP3 intracellular stores, and can be mimicked by pharmacologically increasing [Ca++]i. The second signal, which we call a process of “sensitization,” enables the pIgR to respond to the first kinase-dependent signal of stimulation. To be sensitized the pIgR must first bind dIgA at the basolateral surface and subsequently must move to the postmicrotubule compartment (PMC), where it can then respond to the signal of stimulation. Sensitization also requires that the pIgR be able to dimerize. We conclude that two different signals, those of sensitization and stimulation, must separately move across the epithelial cell to achieve dIgA-stimulated pIgR transcytosis. These results provide novel insights into two questions of general importance to cell biology, namely, how signals can be propagated across polarized cells, and how specificity can be maintained between receptors using identical signaling molecules.
MATERIALS AND METHODS
Cells
The MDCK strain II cell line and its transfectants were maintained as previously described (Breitfeld et al., 1989). The mutants pIgR-A657, pIgR-725t, and pIgR-GpA G83A have been previously described (Breitfeld et al., 1990; Aroeti et al., 1993; Singer and Mostov, 1998). Cells were grown on 0.4-μm pore size Transwell filters (Corning Costar, Cambridge, MA), and the medium was changed every day. Cells were used 4 or 5 d after plating. All experiments have been reproduced with at least two different clones of each mutant.
Reagents
Trypsin, leupeptin, and soybean trypsin inhibitor were from Sigma (Sigma, St. Louis, MO). The sulfo NHS-biotin was obtained from Pierce (Rockford, IL). NP40, nocodazole, ionomycin, phorbol myristate acetate (PMA), and xestospongin C were from Calbiochem (La Jolla, CA). The protein-tyrosine kinase (PTK) inhibitor 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP1) was purchased from Biomol (Plymouth Meeting, PA). Cells were pretreated with all drugs for 30 min before the experiment, and all drugs were present throughout the assays. At the concentration used none of the drugs had any effect on polarity, as measured by the integrity of the tight junctions, transepithelial resistance, or the restricted basolateral localization of E-cadherin as confirmed by cell surface biotinylation (our unpublished data).
Antibodies
The anti-phosphotyrosine antibody 4G10 and the mixed mAbs against PLC-γ1 were from Upstate Biotechnology (Lake Placid, NY). The anti-mouse IgG HRP secondary antibody was purchased from Bio-Rad (Hercules, CA). The avidin-HRP and the ECL system were obtained from Amersham (Arlington Heights, IL). Purified human dIgA was kindly provided by Prof. J.-P. Vaerman (Catholic University of Louvain, Brussels, Belgium).
dIgA Stimulation, Immunoprecipitation, and Anti-Phosphotyrosine Western Blot
MDCK cells were grown on 75-mm filters for 4–5 d. The filters were washed three times in minimum essential medium (MEM)-BSA (MEM, 6 mg/ml BSA, 0.35 g/l NaHCO3, 20 mM HEPES, pH 7.4, and antibiotics) at 37°C. Five milliliters of MEM-BSA were added into the apical chamber, and the filter was placed onto a 300-μl drop of MEM-BSA with or without 0.3 mg/ml dIgA for different periods. At the indicated time point the filter was submerged into 500 ml of ice-cold PBS. The filter was rapidly placed onto an ice-cold metal plate covered with parafilm, and 1 ml of fresh lysis buffer (1% NP40, 125 mM NaCl, 20 mM HEPES, pH 7.4, 10 mM NaF, 2 mM Na-vanadate, and a mixture of proteases inhibitors) was added into the apical chamber. All the following steps were done at 4°C. The filters were gently shaken for 15 min, and the cells were harvested with a plastic rubber policeman. The lysates were transferred into an Eppendorf tube, vigorously vortexed for 30 s, and placed on a rotator for 15 min. The lysates were spun 20 min at high speed in an Eppendorf microfuge, and the supernatants precleared twice for 30 min each and immunoprecipitated for 4–5 h. The protein concentration in each sample was quantitated using a Bradford assay (Pierce) and standardized before immunoprecipitation. The immunoprecipitates were resolved by SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) in 3-[cyclohexylamino]-1-propanesulfonic acid buffer (2.2 g/l, pH 11). The membrane was blocked with PBS with 5% BSA, probed with the anti-phosphotyrosine antibody 4G10, washed extensively, and revealed by an anti-mouse HRP antibody and ECL.
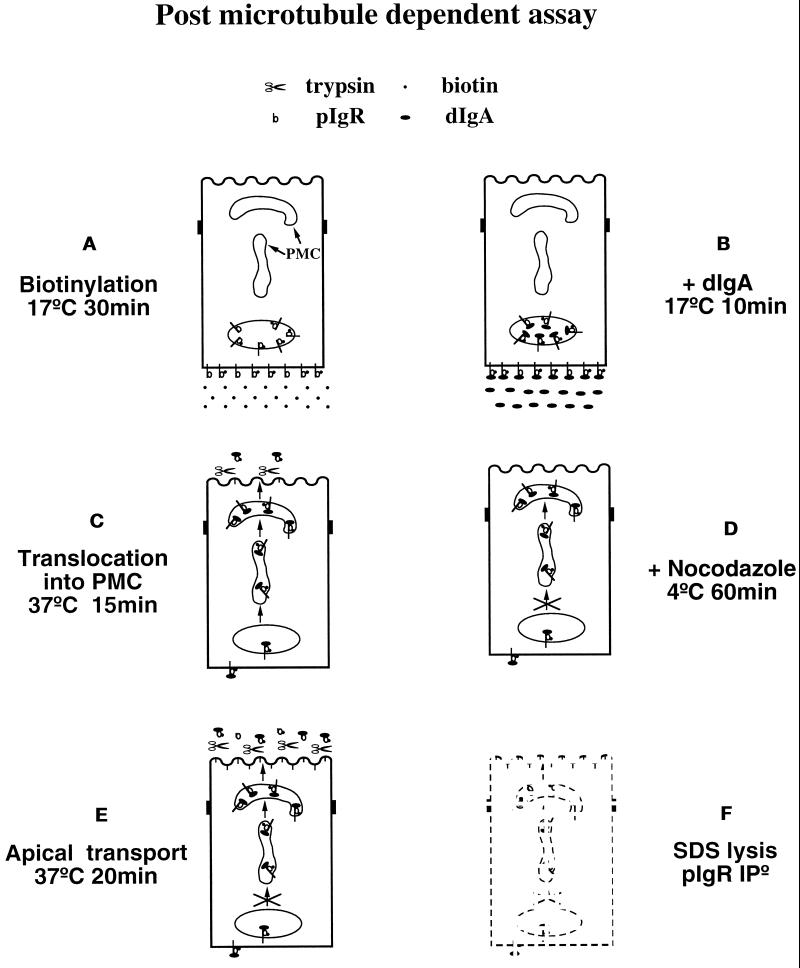
Postmicrotubule-dependent Assay
This assay quantitatively measured the transport of a preloaded, biotinylated pIgR from the PMC to the apical plasma membrane and has been described in detail elsewhere (Song et al., 1994a). The modifications of this assay are described in detail in RESULTS and the figure legends. Cells grown 4–5 d on 12-mm filters were used. The assay consists of six stages, as diagrammed in Figure 1. Stage A: After three quick washes with HBSS containing 25 mM HEPES, pH 7.4, the basolateral cell surface was biotinylated for 30 min at 17°C with 500 μl of a solution containing 0.2 mg/ml sulfo-NHS-biotin dissolved in HBSS. During the biotinylation 300 μl of MEM BSA was present in the apical chamber. Cells were then washed three times with MEM-BSA at 17°C to quench the excess biotin. Stage B: 200 μl of MEM-BSA was added to the apical chambers, and the filter units were placed onto a drop of MEM-BSA containing or lacking 0.3 mg/ml dIgA and incubated at 17°C for 10 min. Stage C: Cells were then chased for 15 min at 37°C with trypsin (15 μg/ml) in the apical medium and with or without dIgA at the basolateral surface. Stage D: The chase was stopped by moving the cells to cold MEM-BSA and by three washes in cold MEM-BSA containing 15% horse serum and further incubated for 1 h at 4°C in the presence of 33 μM nocodazole to depolymerize the microtubules. Stage E: The cells went on to a second chase for 20 min at 37°C with trypsin (15 μg/ml) in the apical medium and soybean trypsin inhibitor (0.125 mg/ml) in the basolateral medium. Note that in the simplified version of this assay used in Figures 6, 7, and 10 the nocodazole treatment (stage D) is omitted and the two chase stages (stages B and E) are combined in one 30-min chase at 37°C. Stage F: At the end of the chase the cells were washed twice in cold MEM-BSA containing 15% horse serum and incubated 5 min in MEM-BSA with soybean trypsin inhibitor (0.125 mg/ml) to quench the trypsin. Cells were washed three more times in MEM-BSA and 15% horse serum and once in PBS before lysis. The lysates were precleared, and the pIgR was immunoprecipitated with a sheep anti-rabbit SC antiserum. The immunoprecipitates were analyzed on SDS-PAGE and transferred to a Millipore PVDF membrane in 3-[cyclohexylamino]-1-propanesulfonic acid buffer (2.2 g/l, pH 11). The biotinylated pIgR was revealed by probing the membrane with streptavidin-HRP and ECL and quantitated with a Molecular Dynamics (Sunnyvale, CA) densitometer. A set of filters was used as a standard and lysed after the nocodazole treatment. The amount of biotinylated pIgR in these samples was considered 100%. In the samples subjected to the second chase at 37°C, the amount of remaining pIgR in the cells was estimated as a percentage of the standard set.
Figure 1.
Diagram of the postmicrotubule-dependent assay. This assay quantitates the amount of pIgR transported from the PMC to the apical surface in the presence or absence of dIgA. See text for a detailed explanation of the protocol.
Figure 6.
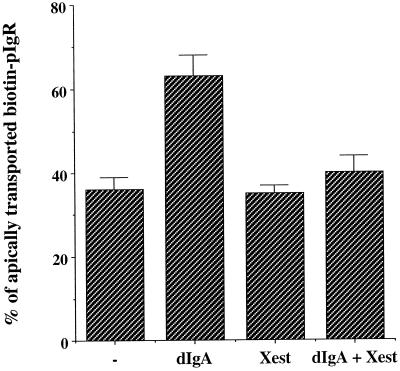
The increase of intracellular calcium concentration stimulated by dIgA is controlled by the IP3-R. MDCK cells expressing pIgR-WT were submitted to the postmicrotubule-dependent assay as presented in Figure 1. As described in MATERIALS AND METHODS, the assay in this figure as well as Figures 6 and 9 was simplified by omitting the nocodazole incubation (stage D) and combining stages C and E into one incubation at 37°C for 30 min. When indicated the cells were exposed basolaterally to dIgA at stage B. When indicated cells were pretreated with xestospongin C (10 μM) 30 min before biotinylation. The drug was also present throughout the assay. n = 3; p < 0.001 for dIgA; p > 0.5 for Xest and dIgA + Xest.
Figure 7.
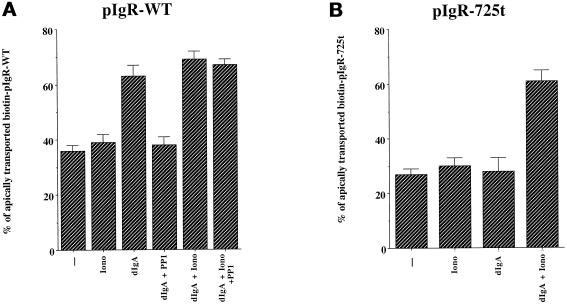
Ionomycin stimulates apical transport of pIgR–dIgA complexes even in the absence of dIgA-induced PTK stimulation. MDCK cells expressing pIgR-WT (A) or pIgR-725t (B) were submitted to the simplified postmicrotubule-dependent assay. Where indicated the cells were exposed basolaterally to dIgA at stage B, to ionomycin (1 μM) at stage C, and/or to PP1 (10 mM) 1 h before and throughout the assay. n = 4; at least p < 0.005 for dIgA, +dIgA + Iono, and dIgA + iono + PP1 in A and +dIgA + Iono in B; at least p > 0.5 for Iono and dIgA + PP1 in A and for Iono and dIgA in B.
Figure 10.
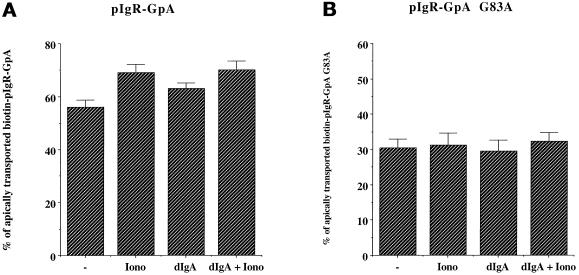
pIgR dimerization is required to respond to the signal of stimulation. MDCK cells expressing pIgR-GpA (A) or pIgR-GpA G83A (B) were submitted to the simplified postmicrotubule-dependent assay. When indicated the cells were exposed basolaterally to dIgA at stage B and/or to ionomycin (1 μM) at stage C. (A) n = 3; p < 0.005 for Iono and dIgA + Iono; p < 0.01 for dIgA. (B) n = 3; p > 0.5 for Iono, dIgA, and dIgA + Iono.
Statistical Analysis
The number of experiments is indicated in the legends (n), and the statistical significance (p) was calculated by Student’s t test.
RESULTS
DIgA-induced Signal of Stimulation of pIgR Transcytosis
For our first set of experiments analyzing signaling during transcytosis we used a strategy of physiologically manipulating the conditions of where and when dIgA was added to the cells. Although morphologically transcytosis involves three intracellular compartments, we decided to use a biochemical assay that permitted us to precisely quantitate changes in transcytosis resulting from these manipulations. We took advantage of the fact that movement out of the BEE into the tubular compartment is dependent on microtubules and can be completely inhibited by the microtubule-depolymerizing agent nocodazole (Hunziker et al., 1990; Gibson et al., 1998). We therefore conveniently divided transcytosis into a premicrotubule-dependent step and a postmicrotubule-dependent step. The postmicrotubule-dependent step probably combines several distinct processes. One of these is the movement of pIgR from the cup-shaped vesicles out to the plasma membrane. The postmicrotubule-dependent step also potentially includes the preceding movement from the tubular compartment into the cup-shaped vesicles, although this step is not well understood. These PMCs are probably equivalent to what we and others have previously termed the apical recycling endosome, but we use the term PMC here to emphasize that it is functionally defined in this paper as being beyond the nocodazole block. We have previously devised an assay for this postmicrotubule-dependent step (Song et al., 1994a; Luton et al., 1998). This assay consists of six stages, which are presented in Figure 1.
A) The pIgR at the basolateral surface is biotinylated by exposure of the basolateral surface to the membrane-impermeant biotinylation reagent sulfo-NHS-biotin for 30 min at 17°C. At this temperature the pIgR can be internalized into BEE, but there is little delivery to the PMC.
B) The basolateral surface of the cells is exposed to dIgA for 10 min, also at 17°C, to allow binding of dIgA to pIgR. In the −dIgA control, cells undergo a mock incubation without dIgA.
C) The cells are incubated at 37°C for 15 min to allow movement of the pIgR and dIgA to the PMC. Trypsin is included in the apical medium during this incubation to cleave any pIgR that reaches the apical plasma membrane.
D) The cells are then incubated at 4°C for 1 h in the presence of nocodazole to depolymerize microtubules and block any further movement of the pIgR from the BEE to the PMC.
E) The cells are incubated for a second time at 37°C for 20 min to allow the third step of transcytosis to occur, i.e., the microtubule-independent transport from the PMC to the apical surface. During this incubation, trypsin is present in the apical medium to cleave all of the pIgR that undergoes this third step.
F) The cells are lysed, and the pIgR is immunoprecipitated and analyzed by a Western blot probed with streptavidin-HRP and ECL. The biotinylated pIgR that remains uncleaved and associated with the cells is measured. The decrease in biotinylated intact pIgR, relative to control cells that did not undergo the second incubation at 37°C, is taken as a measure of the third step of transcytosis.
Although this assay involves multiple manipulations of the cells, it gives highly reproducible and internally consistent results. For example, the assay has been used in previous studies to show that dIgA stimulation acts primarily on the postmicrotubule-dependent step, that this stimulation is abrogated by inhibitors of PTKs and that this stimulation is dependent on a short domain of the pIgR’s cytoplasmic domain encompassing amino-acids 725–737 (Song et al., 1994a,b; Luton et al., 1998).
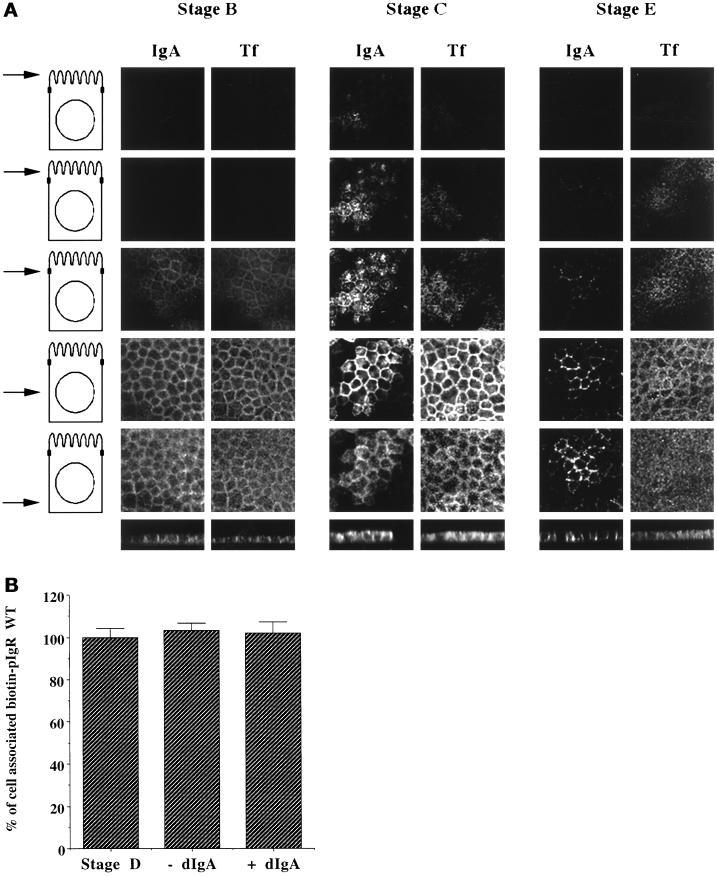
Because this assay is crucial to our analysis of signaling, we performed additional experiments to better characterize the assay. First, we analyzed the localization of dIgA bound to the pIgR at several stages of the assay and compared this with the localization of basolaterally internalized Tf. Figure 2A shows a series of confocal micrographs taken at various planes of sections through the cell, with the level of each section illustrated diagramatically on the left. Vertical or X-Z sections are shown at the bottom. At stage B, both basolaterally internalized dIgA and Tf are confined to the basal and lateral regions of the cytoplasm. No internalized ligands are seen above the level of the tight junctions, i.e., in the top two sections. In contrast, in stage D a considerable amount of dIgA is seen in the upper sections, notably the upper two sections, and some of this material is clustered around the center of the apical surface of the cell, most likely in the pericentriolar cup-shaped vesicles. Considerably less Tf is seen in these upper sections. The Tf that is present is not particularly clustered in the center of the cell, which reflects the partial lack of Tf in the cup-shaped vesicles. In stage E, after the final 37°C chase in the presence of nocodazole, dIgA has almost entirely left the apical regions of the cell, and as previously shown, most of this dIgA has been transcytosed into the apical medium. In contrast, much of the dIgA in the basal and lateral regions of the cytoplasm remain associated with the cell. Similarly, much of the Tf remains associated with the cell. This remaining dIgA and Tf represents the material that did not move from the BEE during stage C, secondary to the nocodazole block.
Figure 2.
Controls for the postmicrotubule-dependent assay. (A) Micrographs of MDCK cells at stage A, D, or E of the postmicrotubule-dependent assay described in Figure 1. Staining for dIgA and Tf are compared. The top five rows are horizontal (X–Y) sections taken at various levels of the cells. On the left side a schematic of an MDCK cell is shown, with an arrow indicating where the section was taken with respect to the nucleus and ZO-1 staining. The two top rows were sections taken 1 and 2 μm, respectively, above the tight junctions, as defined by ZO-1. The third row is at the level of the tight junctions. The fourth row is at the middle of the nucleus, whereas the fifth row is beneath the nucleus. The bottom row shows vertical (X–Z) sections through the cell. (B) The cells were biotinylated at 4°C and then either exposed or not exposed to dIgA at 4°C, corresponding to stages A and B of the postmicrotubule-dependent assay. Stage C was omitted. As in stage D, the cells were then either treated or not treated with nocodazole for 1 h at 4°C. The cells were finally warmed at 37°C for 35 min to allow the apical transport of the pIgR, as in stage E of the postmicrotubule-dependent assay. Usually stage E is a 20-min step, but we chose 35 min to compensate for the 15 min of stage C that was omitted. n = 2; p > 0.5 for all samples.
In our assay it is important to demonstrate that the nocodazole inhibition completely blocks subsequent traffic from the BEE to the apical surface. To focus specifically on the completeness of this blockage, we modified the assay as follows. Cells were biotinylated (stage A) and treated with dIgA (stage B) at 4°C, and step C was omitted, so that no material had a chance to move past the nocodazole block. Stages D–F were performed as previously described. As a control, parallel filters of cells were harvested immediately after stage D to assay the total amount of biotinylated pIgR. Figure 2B shows that in both the absence and presence of added dIgA, there was quantitatively no loss of biotinylated pIgR from the cells during stage E, which, therefore, demonstrates that the nocodazole block was complete.
Taken together, the data in Figure 2 plus previously published data (Apodaca et al., 1994; Song et al., 1994a; Luton et al., 1998) demonstrate that we can use this assay to functionally divide transcytosis into a postmicrotubule-dependent step and premicrotubule-dependent step. The postmicrotubule-dependent assay most likely follows the movement of material that was in the cup-shaped vesicles and the tubular compartment to the apical plasma membrane. It is possible that only a portion of material in the tubular compartment is able to reach the apical plasma membrane under the conditions of our assay. Nevertheless, the assay provides a functional and biochemical division of transcytosis into pre- and postmicrotubule steps that is necessary for analyzing signaling during transcytosis.
We then examined the extent to which the postmicrotubule assay can be used to analyze the stimulation of transcytosis by dIgA binding. Figure 3 shows a typical example of results obtained with the standard version of the assay. (This is similar to previously published data and is presented here as a control for later experiments). Inclusion of dIgA (but not control IgG) during the 17°C incubation after biotinylation stimulates the postmicrotubule assay by ∼30% over the −dIgA control.
Figure 3.
dIgA but not IgG stimulates pIgR transcytosis. (A) Cells were treated according to the protocol presented in Figure 1. (B) Quantitation of the apical transport of pIgR in the presence of dIgA or IgG or medium alone added at stage B. These data are essentially a confirmation of previously published results and are presented here to provide a basis for comparison of subsequent experiments. n = 12; p < 0.002 for +dIgA; p > 0.5 for +IgG.
In contrast, we have previously shown that when the entire process of transcytosis is measured using metabolically labeled pIgR instead of biotinylated pIgR, dIgA can stimulate the rate of transcytosis by ∼2-fold (Luton et al., 1998). Moreover, in vivo transcytosis of pIgR by intact rat liver is increased by approximately threefold (Giffroy et al., 1998). The biotinylation assay causes a smaller effect largely because we must first biotinylate the pIgR for 30 min at 17°C in stage A to obtain adequate labeling. During this time much of the pIgR is endocytosed. The dIgA is added only later in stage B, because inclusion of dIgA during or before biotinylation results in destruction of the ability of dIgA to bind pIgR, because of the sensitivity of dIgA to high levels of biotinylation reagent. Therefore, much of the pIgR is endocytosed in stage A before the dIgA is added in stage B and never has the opportunity to bind dIgA, artificially leading to an underestimation of the potential for dIgA to stimulate transcytosis. Despite this underestimation, we decided to use this assay, because it allowed us to precisely focus on the third step of receptor transcytosis of the receptor, necessary for the analysis of signal transmission across the cells.
We previously found that tyrosine phosphorylation occurs within 10 s after addition of dIgA to the basolateral surface of the cell. Therefore, the signal that controls dIgA-stimulated pIgR transcytosis is most likely generated at the basolateral surface (Luton et al., 1998). There are two possible mechanisms by which dIgA binding to pIgR at the basolateral surface could subsequently stimulate movement in the PMC to the apical surface. In the first, dIgA binding to the pIgR at the basolateral surface produces a signal that is intrinsic to the dIgA–pIgR complex and moves with the complex from the basolateral surface to the tubular compartment and/or the cup-shaped vesicles. Once the dIgA–pIgR complex is in the PMC, the signal stimulates the movement of the complex to the apical surface. A second possible mechanism involves a signal that stimulates pIgR apical transport, which may not be intrinsic to the dIgA–pIgR complex. That is, the signal might act in trans by originating from the dIgA–pIgR complexes present at the basolateral surface, which results in the transmission of the information across the cell to the PMC, where the signal stimulates movement of other dIgA–pIgR complexes to the apical surface.
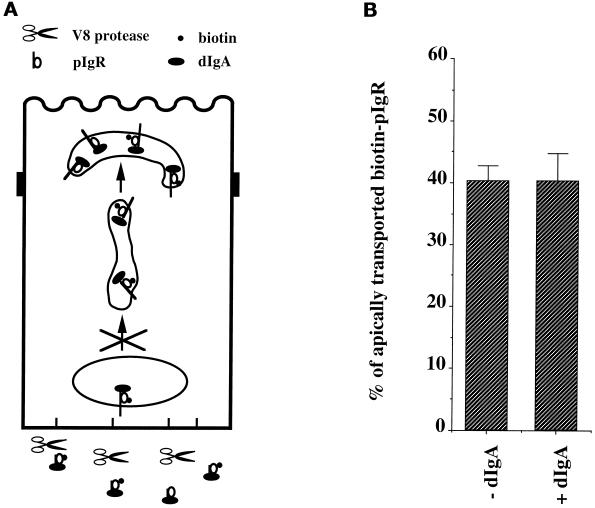
To distinguish between these two possible mechanisms, we included the V8 protease in the basolateral medium during stage E, i.e., the second incubation at 37°C when pIgR is chased from the PMC to the apical surface (Figure 4). As previously shown, these proteolytic conditions efficiently cleave any dIgA–pIgR complexes that are present at (or recycle through) the basolateral surface (Apodaca et al., 1994). It should be noted that during stage E, vesicular traffic of dIgA–pIgR between the BEE and PMC is effectively blocked by the previous depolymerization of microtubules. If dIgA–pIgR complexes in the PMC produced the signal, then transcytosis should still be stimulated, despite the destruction of the basolateral dIgA–pIgR complexes. However, because the V8 protease blocks stimulation (Figure 4), we conclude that the basolateral dIgA–pIgR complexes produce the signal that stimulates the postmicrotubule-dependent step of transcytosis.
Figure 4.
Basolateral V8 protease exposure of the cells prevents dIgA stimulation of pIgR transcytosis. (A) The cells were treated according to the protocol presented in Figure 1 with the modification shown and described in the text. V8 protease was included in stages D and E in all samples. (B) Quantitation of the apical transport of pIgR in the presence of dIgA or medium alone added at stage B. n = 5; p > 0.5 for +dIgA.
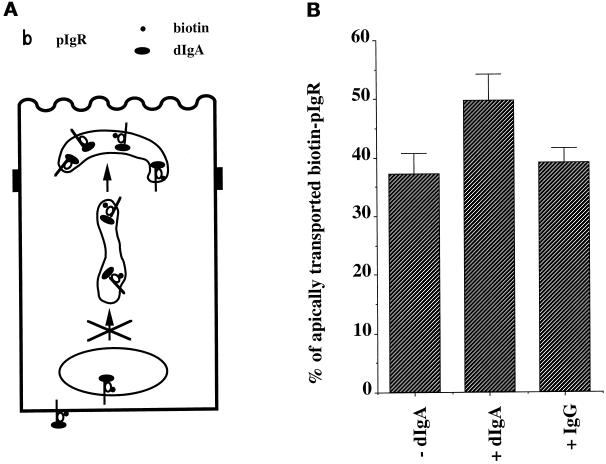
One potential problem with this experiment is that the V8 protease treatment can cleave other proteins at the basolateral surface and thus could possibly have unforeseen effects on the cell. To strengthen our conclusion, we next performed the opposite experiment. Ordinarily, dIgA is included only during stage B, when pIgR at the basolateral surface and/or recycling from the BEE can bind to this dIgA. Stimulation attributable to this standard addition is indicated by +dIgA in Figure 5. We now also included dIgA in the basolateral medium during stage E, i.e., after the nocodazole treatment and during the movement from the PMC to the apical surface of dIgA–pIgR already present in the PMC. The addition of basolateral dIgA during stage E is indicated by ++dIgA in Figure 5 and caused an additional stimulation of transcytosis. An IgG control was also present in both steps (++IgG) had no effect. We therefore conclude that the signal of stimulation originates from the basolateral surface and can travel across the cell to stimulate the apical transport of the pIgR present in the PMC. We have previously reported that stimulation by dIgA added in stage B at the beginning of the assay is blocked by a variety of inhibitors of PTKs, including PP1, which is highly specific for the src family members (Hanke et al., 1996; Luton et al., 1998). Figure 5 shows that the additional stimulation caused by the addition of dIgA in stage E is also blocked by this inhibitor, confirming that tyrosine kinase activity is necessary for this stimulation. Similar results were obtained with genistein or herbimycin (our unpublished results).
Figure 5.
Basolateral dIgA stimulates apical transport of pIgR. (A) The cells were treated according to the protocol presented in Figure 1 with the modifications shown and described in the text. (B) Quantitation of the apical transport of pIgR in the presence or absence of dIgA added at stage B or in the presence of basolateral dIgA or IgG at stages B and E. n = 5; p < 0.003 for +dIgA; p < 0.001 for ++dIgA; p > 0.5 for ++IgG and ++IgA + PP1.
Taken together, Figures 4 and 5 show that dIgA binding to pIgR at the basolateral surface and/or BEE sends a PTK-dependent signal across the cell to stimulate pIgR movement from the PMC to the apical surface. We term this process a “signal of stimulation.” Transmission of this signal originating from the basolateral surface is not blocked by nocodazole-induced microtubule depolymerization and is therefore independent of the movement of pIgR from the basolateral surface to the PMC.
Role of Calcium in the Signal of Stimulation
The signal of stimulation is capable of moving across the cell, even when movement of the pIgR in vesicles is blocked by depolymerization of microtubules. We reported previously that both the PKC activator PMA and the calcium ATPase inhibitor thapsigargin were independently able to stimulate dIgA transcytosis (Cardone et al., 1994, 1996). Additionally, neither PMA nor the calcium ionophore ionomycin, used at low concentrations (20 nM and 500 nM respectively), stimulated transcytosis but when used together gave a synergistic stimulation (Luton et al., 1998). Moreover, we reported that dIgA triggers the activation of PLCγ1, stimulates production of IP3, causes an increase of [Ca++]i, and activates the PKC (Cardone et al., 1996). This suggests that the PLCγ1 signaling pathway is involved in stimulation of transcytosis, but does not establish which elements of the pathway are truly required for stimulation. In this study we attempted to determine whether calcium and IP3 are in fact a required part of the signal of stimulation.
A rise in [Ca++]i can be due to either an influx of calcium from the extracellular medium or release from intracellular stores or both. We previously showed that thapsigargin was able to stimulate transcytosis (Cardone et al., 1996). Even in the presence of low extracellular calcium (50 nM), which presumably reduces influx of calcium from outside the cell, thapsigargin is still capable of stimulating transcytosis (our unpublished data), suggesting that calcium from intracellular stores is capable of supporting stimulation of transcytosis.
Epithelial cells are known to express the two types of channels responsible for intracellular release of calcium, i.e. the ryanodine receptor and IP3 receptor (IP3R) channels. Both receptors are localized to the apical region of the cell and are candidates for the receptor system that is involved in elevation of [Ca++]i (Bush et al., 1994; Haller et al., 1996; Tunwell and Lai, 1996; Lee et al., 1997). The IP3R can be specifically blocked by the new cell-permeable IP3R inhibitor xestospongin C (Gafni et al., 1997). As shown in Figure 6, xestospongin C completely blocked the stimulation of pIgR apical transport by dIgA, suggesting that the IP3R is required for stimulation of transcytosis. As a control we found that xestospongin C did not alter endocytosis of dIgA by pIgR at the basolateral surface (our unpublished data). Consistent with the proposed involvement of the IP3R, we found that several inhibitors reportedly specific for the ryanodine receptor (ryanodine up to 50 μM, caffeine up to 1 mM, or ruthenium red up to 50 μM) had no effect on dIgA stimulation of transcytosis (our unpublished results).
As mentioned above, we have previously shown that a sufficient elevation of [Ca++]i can itself stimulate transcytosis of the ligand. We therefore investigated the possibility that an elevation of [Ca++]i acts specifically as a signal of stimulation of receptor transcytosis. To study just the effect of calcium, we analyzed cells expressing the wild-type pIgR after pretreatment with the PTK inhibitor PP1, which abrogates the production of the signal of stimulation when dIgA is included.
As described earlier, pIgR loaded with dIgA from the basolateral surface is stimulated (dIgA), and this stimulation is blocked by the tyrosine kinase inhibitor PP1 (dIgA + PP1; Figure 7A). Loading with dIgA and then adding ionomycin (dIgA + Iono) does not significantly further stimulate transcytosis, most likely because the maximum level of transcytosis has already been reached. However, PP1 does not block the stimulation from ionomycin for dIgA-loaded pIgR (dIgA + Iono + PP1). This is consistent with the idea that elevation of [Ca++]i by ionomycin is itself sufficient to provide the signal of stimulation by bypassing the requirement for PTK.
Importantly, Figure 7A also shows that raising the [Ca++]i with ionomycin, in the absence of dIgA, does not stimulate pIgR transcytosis (Iono). Rather, stimulation of transcytosis requires both ionomycin and dIgA binding. When both are present, stimulation occurs, even in the presence of PP1. This result is surprising and suggests that in addition to the signal of stimulation provided by increasing the [Ca++]i, a second signal is required to achieve dIgA-stimulated transcytosis of pIgR. This second signal does not seem to require PTK activity, because it can occur even in the presence of PP1 (Figure 7A, dIgA + Iono + PP1).
To investigate this further, we used a mutant pIgR that is truncated after residue 725 (pIgR-725t) and is thus lacking the C-terminal 30 residues. We recently reported that pIgR-725t is unable to cause tyrosine phosphorylation upon dIgA binding, is defective in dIgA transcytosis, and is unable to undergo dIgA-stimulated transcytosis (Luton et al., 1998). As shown in Figure 7B, neither ionomycin nor dIgA alone is capable of stimulating transcytosis of the pIgR-725t kinase-deficient mutant. However, the combination of ionomycin and dIgA does stimulate transcytosis of pIgR-725t. Taken together, these experiments suggest that the signal of stimulation can be provided by raising [Ca++]i with ionomycin, under conditions in which the normal production of this signal is blocked either by PP1 or by a mutation in the pIgR kinase-dependent domain. In addition, these results indicate that dIgA binding provides a second signal that is not dependent on PTK activation, because it is neither sensitive to the PTK inhibitor nor abrogated by the deletion of the domain required for kinase activation. Because this second signal is independent of PTK activation but is necessary for a response to the PTK-dependent signal of stimulation, we propose that it is a signal or process of sensitization. This hypothesis is examined in more detail below.
Sensitization
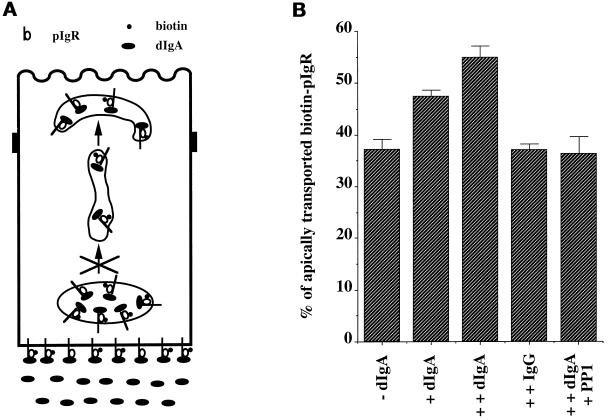
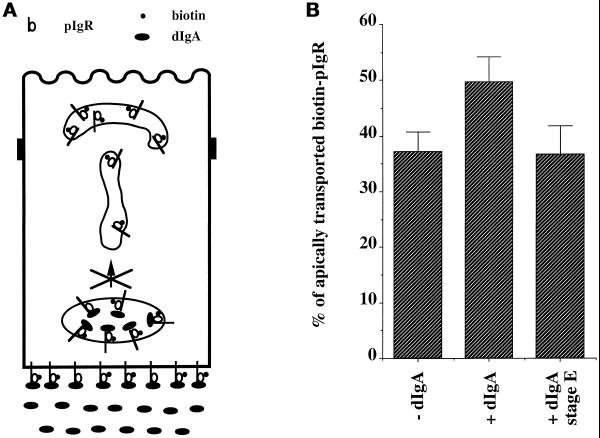
The evidence presented thus far indicates that the kinase-dependent signal of stimulation is not intrinsic to the dIgA–pIgR complex; i.e., it can move across the cell from the basolateral surface to the PMC even when movement of the dIgA–pIgR complex is blocked by nocodazole. This signal is blocked by xestospongin C and can be substituted for by an elevation of [Ca++]i. Moreover, dIgA binding seems to provide an additional signal of sensitization that is kinase-independent. Although dIgA binding to pIgR provides this signal of sensitization, it is not known whether the pIgR molecules present in the PMC, which undergo the stimulation of transcytosis, must be bound to dIgA. We therefore specifically tested in our postmicrotubule-dependent assay whether empty pIgR and dIgA–pIgR complexes present in the PMC were equally competent to respond to the kinase-dependent signal of stimulation. To address this question we modified our assay by omitting dIgA from stage B, resulting in biotinylated pIgR present in the PMC not being bound to dIgA. Instead, dIgA was included only in stage E, in which it could bind to pIgR confined to the basolateral surface and BEE but could not move to the PMC because of to the nocodazole block. Under these conditions (designated +dIgA stage E in Figure 8) there was no stimulation, despite the fact that we showed in Figure 5 that adding dIgA during stage E generated an active signal of stimulation. This confirms that the signal of stimulation is alone not sufficient to cause the stimulation seen in step 3 of transcytosis. Rather, to respond to the signal of stimulation, the pIgR in the PMC must somehow be sensitized by its binding to dIgA. This provides additional evidence for the hypothesis that there is a process of sensitization that requires dIgA binding to pIgR.
Figure 8.
Basolateral dIgA stimulates apical transport of pIgR–dIgA complexes only. (A) Cells were treated according to the protocol presented in Figure 1 with the modification shown and described in the text. (B) Quantitation of the apical transport of pIgR in the presence of dIgA or medium alone added at stage B or dIgA added only at stage E. n = 4; p < 0.001 for +dIgA; p > 0.5 for +dIgA stage E.
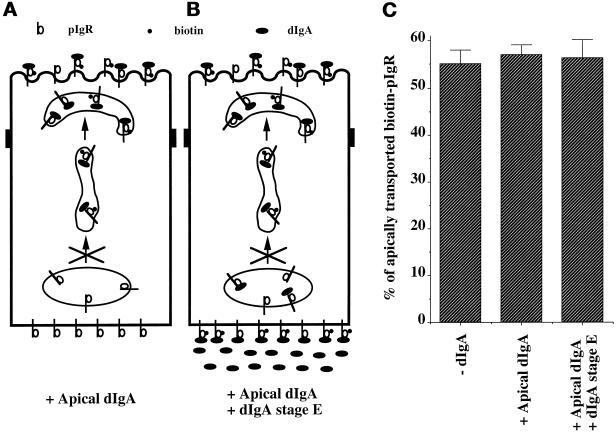
We and others have shown that apically internalized dIgA reaches the PMC. In fact, the apical recycling endosome was originally described as a compartment where the dIgA internalized from both surfaces of the cell meet and from where the apically internalized dIgA recycles to the apical surface (Apodaca et al., 1994; Barroso and Sztul, 1994; Gibson et al., 1998). We therefore investigated whether the movement of apically internalized dIgA–pIgR complexes from the PMC back to the apical surface could respond to the signal of stimulation transmitted from dIgA–pIgR complexes at the basolateral surface. To test this model, we modified our assay as follows. In stage A, pIgR was biotinylated at the apical but not the basolateral surface. In stage B, dIgA was included only in the apical medium so that the apical biotinylated pIgR could bind to dIgA (Figure 9, + Apical dIgA). The −dIgA control omitted dIgA from the apical medium. In stage E dIgA was included in the basolateral medium to provide the signal of stimulation to the dIgA–pIgR present in the PMC (Figure 9, +Apical dIgA + dIgA stage E). As shown in Figure 9, when the pIgR was loaded with dIgA at the apical surface, the dIgA–pIgR complex was unable to respond to the signal of stimulation coming from dIgA–pIgR complexes at either the basolateral surface or BEE. This result is surprising, because dIgA–pIgR complexes coming from both surfaces have been shown to largely colocalize, based on both confocal microscopy and HRP-mediated cross-linking (Apodaca et al., 1994; Barroso and Sztul, 1994; Gibson et al., 1998). Our results show that the dIgA–pIgR complexes coming from the two surfaces are not equivalent. Rather, sensitization of the pIgR requires that it binds to dIgA exclusively at the basolateral surface and then moves across the cell to the PMC to be able to respond to the signal of stimulation. This suggests that the pIgR complexes must undergo some sort of modification (not necessarily covalent) at the basolateral surface. Because sensitization moves with the pIgR, rather than acting in trans, it may be more appropriate to refer to sensitization as a “process” rather than a true signal.
Figure 9.
Basolateral dIgA stimulates apical transport of pIgR–dIgA complexes formed only at the basolateral surface. (A and B) Modifications made to the protocol presented in Figure 1 and described in the text. In this experiment we followed the apical transport of a pool of pIgR biotinylated and endocytosed into the PMC from the apical surface. (C) Quantitations of the apical transport of pIgR in the presence of dIgA, medium alone added apically at stage B, or dIgA added both apically at stage B and basolaterally at stage E. n = 3; p > 0.5 for +Apical dIgA and +Apical dIgA/+dIgA stage E.
Dimerization of pIgR Is Required for the Signal of Sensitization
We have previously demonstrated that binding of dIgA to the pIgR induces dimerization and that this dimerization is necessary to stimulate pIgR transcytosis (Singer and Mostov, 1998). Therefore, we decided to investigate the role of this pIgR dimerization in sensitization. The role of dimerization of the pIgR in transcytosis was analyzed by constructing chimeric pIgRs that either forced the dimerization of pIgR or prevented dimerization. The transmembrane domain (TMD) of glycophorin A has been extensively studied and has been shown to form very stable homodimers, even when transplanted into chimeric membrane proteins. Specific point mutations in the glycophorin A TMD strongly prevent dimerization (Lemmon et al., 1992). We made chimeras of pIgR containing either the wild-type glycophorin TMD or a nondimerizing mutant, designated pIgR-GpA G83A (Singer and Mostov, 1998). As seen in Figure 10B, pIgR-GpA G83A does not undergo stimulated transcytosis in the presence of ionomycin, dIgA, or even dIgA and ionomycin together. In contrast, the chimera with the wild-type TMD (pIgR-GpA) undergoes stimulation by ionomycin, dIgA, or a combination of the two (Figure 10A). The degree of stimulation is small, because of the high level of background transcytosis even in the absence of stimulation, but is nevertheless statistically significant. These results suggest that dimerization is necessary for a response to the bypass signal of elevated [Ca++]i provided by ionomycin and therefore is necessary for sensitization.
Our data are most easily explained by a model in which there are two signals. The signal of stimulation is emitted by dIgA–pIgR at the basolateral surface, depends on a tyrosine kinase activity, and can travel across the cell in a microtubule-independent manner. This signal of stimulation is therefore likely to be independent of movement of the pIgR itself across the cell. This signal is most likely due to an increase in [Ca++]i. In contrast, the signal or process of sensitization requires pIgR binding to dIgA at the basolateral surface (not the apical surface), and depends on the dIgA–pIgR complex being located in the PMC. Sensitized pIgR in the PMC can receive the signal of stimulation sent by dIgA–pIgR located at the basolateral surface. This signal is dependent on pIgR dimerization in response to dIgA binding.
Genetic Analysis and Complementation of the Two Signals
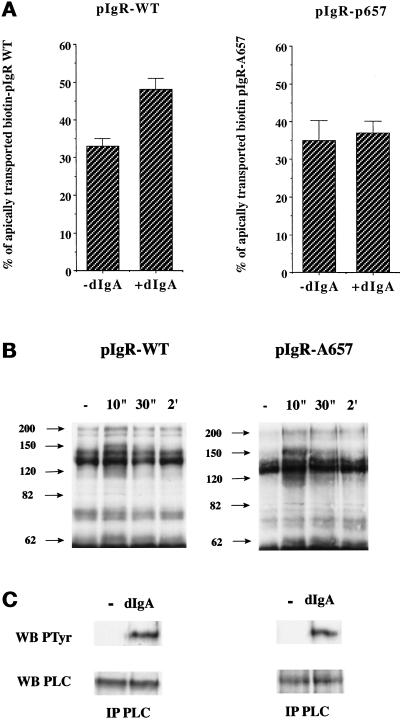
An advantage of the pIgR system is that we have previously constructed and analyzed a large number of mutations in the C-terminal, cytoplasmic domain of the pIgR, which consists of residues 653–755. We recently reported that a receptor truncated after residue 725 (pIgR-725t) is unable to cause tyrosine phosphorylation upon dIgA binding and is defective in dIgA-stimulated pIgR transcytosis (Luton et al., 1998). The mutant pIgR-725t might therefore be defective in producing this signal of stimulation. Our aim then was to identify a mutant pIgR defective in the signal of sensitization. We screened for mutants that would be unable to respond to dIgA stimulation in our postmicrotubule-dependent assay but were still able to stimulate tyrosine phosphorylation upon binding to dIgA. In a preliminary screen using mutants containing large deletions of the cytoplasmic domain, we found a mutant lacking the basolateral targeting signal (deletion of residues 655–670) that met these criteria (our unpublished data). This region has previously been subjected to an Ala scan, which showed that residue Arg-657 was required for basolateral targeting (Aroeti and Mostov, 1994). We found that this point mutant (pIgR-A657) also fulfilled the criteria for a defect in sensitization. The analysis of transcytosis of this mutant by the postmicrotubule-dependent assay showed that the constitutive transport from PMC to apical surface is comparable with that of the wild-type receptor but that dIgA did not stimulate the apical transport of pIgR-A657 (Figure 11A). Figure 11B shows that dIgA binding to pIgR-A657 causes transient tyrosine phosphorylation of the same group of proteins as does the wild-type pIgR. These transiently phosphorylated proteins are identified by the arrows in Figure 11B. Figure 11C shows that pIgR-A657 is also capable of triggering the tyrosine phosphorylation of PLCγ1.
Figure 11.
dIgA binding to pIgR-A657 stimulates PTK activation but not step 3 of transcytosis. (A) Quantitations of the postmicrotubule-dependent assay performed according to the protocol presented in Figure 1 with MDCK cells expressing either pIgR-WT or pIgR-A657. (B) After exposure to dIgA (0.3 mg/ml) for the indicated period, the cells were lysed, and the lysates immunoprecipitated with a specific anti-phosphotyrosine mAb (4G10). The immunoprecipitates were resolved on SDS-PAGE, the gel was transferred to a PVDF membrane, and the membrane was probed with the anti-phosphotyrosine antibody. The arrows indicate bands that exhibit an increase in tyrosine phosphorylation. (C) After a 30-s exposure to dIgA (0.3 mg/ml), the cells were lysed, and the lysates were immunoprecipitated with a mixture of monoclonal anti-PLC-γ1 antibodies. The immunoprecipitates were resolved on SDS-PAGE, the gel was transferred to a PVDF membrane, and the membrane was first probed with the anti-phosphotyrosine antibody (top gels). After stripping, the membrane was reprobed with the same anti-PLC-γ1 mAbs (bottom gels).
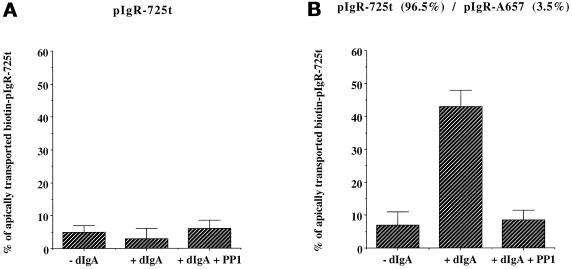
These data are consistent with the hypothesis that pIgR-A657 is deficient in the process of sensitization. This is in contrast to pIgR-725t, which is apparently defective in the signal of stimulation. Because the two signals, sensitization and stimulation, require different portions of the pIgR cytoplasmic domain, this provided an opportunity to test our two signal hypothesis by genetic complementation. We therefore constructed clonal MDCK cell lines simultaneously expressing both pIgR-A657 and pIgR-725t. Our hypothesis predicted that coexpression of these two mutants would permit sensitized pIgR-725t in the PMC to respond to the signal of stimulation produced by pIgR-A657 at the basolateral surface or BEE. If the hypothesis were correct, dIgA-stimulated transcytosis of pIgR-725t would be rescued by coexpression of pIgR-A657. A potential complication is that the pIgR dimerizes upon dIgA binding (Singer and Mostov, 1998), which might result in the assembly of heterodimers consisting of pIgR-A657 and pIgR-725t. These heterodimers could rescue transcytosis by direct association of the two mutant molecules, rather than by transmission of a signal between molecules located at opposite sides of the cell. To circumvent this problem, we chose two clonal MDCK cell lines in which ∼5% of the pIgR on the basolateral surface was pIgR-A657 and the remaining 95% was pIgR-725t. (These values were determined by biotinylation of pIgR at the basolateral surface.) In these clones, at most 5% of the pIgR-725t could be rescued by direct binding to pIgR-A657.
In our assay, we analyzed the ability of pIgR-A657 to send the signal of stimulation to dIgA–pIgR-725t complexes located in the PMC. To distinguish pIgR-A657 from pIgR-725t, we took advantage of the fact that pIgR-725t has lost the epitope recognized by the mAb SC166, which is specific for the C-terminal portion of pIgR. We successively immunoprecipitated pIgR-A657 with SC166 and then immunoprecipitated pIgR-725t with a sheep polyclonal anti-SC serum. The results are shown in Figure 12. In the control cells expressing pIgR-725t alone, dIgA did not stimulate the postmicrotubule step of transcytosis (Figure 12A). In contrast, in cells coexpressing pIgR-725t and pIgR-A657, addition of dIgA led to a dramatic stimulation of transcytosis of pIgR-725t (Figure 12B). This stimulation was completely sensitive to PP1, indicating that it required tyrosine kinase activity, much like the stimulation of transcytosis using the wild-type pIgR (Figure 12B). Similar results were obtained with a second independent MDCK clone coexpressing pIgR-725t and pIgR-A657 (our unpublished results). The stimulation in Figure 12B was in fact greater than that usually seen with the wild-type pIgR. The reason for this is unknown, but it may be that the loss of the C-terminal 30 residues makes the pIgR-725t “supersensitized.” Alternatively it may be that when the pIgR-725t is unable to produce the signal of stimulation, it accumulates excessively in the PMC, and its rate of transcytosis is then highly triggered by the signal from pIgR-A657.
Figure 12.
dIgA stimulation of pIgR-725t is restored by coexpression of pIgR-A657 in a PTK-dependent-manner. MDCK cells expressing pIgR-725t alone (A) or coexpressing both pIgR-725t and pIgR-A657 (B) were submitted to the postmicrotubule-depenedent assay presented Figure 1 with or without 1-h pretreatment of the cells with the PTK inhibitor PP1 (10 μM) before biotinylation (stage A). The drug was also present throughout the assay. In the cotransfected clone 96.5% of total pIgR present at the basolateral surface was pIgR-725t, and 3.5% was pIgR-A657, as indicated in parentheses. n = 8; p < 0.001 for +dIgA in B; p > 0.5 for the other samples in A and B.
DISCUSSION
The pIgR and MDCK cells have long been a system of choice for studying protein sorting in epithelial cells, particularly the transcytosis of proteins across cells. Here we show that they also represent a powerful system for studying compartmentalization and spatial transmission of signals, particularly the movement of information across the cell. In this model system, binding of dIgA to pIgR at the basolateral surface stimulates the transcytosis of dIgA/pIgR complexes from the PMC to the apical surface. We have used a variety of approaches to reveal a system that uses two signals, stimulation and sensitization. These signals move across the cell in very different ways and provide for specific crosstalk between pIgRs located at the basolateral surface and in the PMC.
Stimulation
The signal of stimulation involves a pathway that begins with the binding of dIgA to pIgR, which induces rapid and transient tyrosine phosphorylation of several proteins, including PLCγ1. Because the pIgR itself lacks intrinsic kinase activity and is not phosphorylated on tyrosine, we suggest that it interacts (probably via an intermediate protein) with a nonreceptor tyrosine kinase, most likely the src family. Truncation of the last 30 amino acids of the cytoplasmic domain of the pIgR prevents kinase activation and subsequent signaling steps, along with dIgA-stimulated transcytosis of pIgR. Similarly, the blocking of tyrosine kinase activity by selective inhibitors indicates that a tyrosine kinase activity is required for stimulation of transcytosis (Luton et al., 1998).
As previously shown, phosphorylation of PLCγ1 causes production of IP3, elevation of [Ca++]i, and activation of PKC. We now report that the calcium comes from IP3-sensitive stores, as indicated by the block of stimulation by the specific IP3R calcium channel inhibitor xestospongin C, and that elevation of [Ca++]i is required for stimulation of transcytosis. Furthermore, artificial elevation of [Ca++]i by ionomycin bypasses the lack of tyrosine kinase activation exhibited either by the pIgR-725t or by pharmacological inhibition of tyrosine kinase activity.
Movement of pIgR from the BEE to the PMC (as well as other molecules such as Tf receptor) is blocked by microtubule depolymerization (Hunziker et al., 1990; Apodaca et al., 1994; Gibson et al., 1998). Our modified assay in contrast shows that movement of the signal of stimulation across the cell is not impeded by microtubule depolymerization. Once dIgA–pIgR complexes had reached the PMC and microtubules had been depolymerized, destruction of dIgA–pIgR complexes with basolaterally applied protease blocked the stimulation. Formation of more dIgA–pIgR complexes by addition of further basolateral dIgA increased the stimulation. The most likely mechanism for movement of this signal is therefore the diffusion of IP3 from the basolateral surface and/or BEE, where it is presumably produced, to the apical region of the cytoplasm. Unlike calcium, which cannot rapidly diffuse through the cytosol, IP3 is capable of rapid diffusion (Thomas et al., 1996). The IP3 then acts on IP3Rs that regulate the release of calcium stores, which are located in the apical region of epithelial cells. Indeed, in epithelial cells (including MDCK cells) in addition to the endoplasmic reticulum there exist endosomal and lysosomal calcium stores, which are regulated in a complex manner by at least three IP3R and the two ryanodine receptor isoforms (Tunwell and Lai, 1996; Lee et al., 1997; Yamamoto-Hino et al., 1998).
Sensitization
The most surprising result was that dIgA binding to pIgR resulted in a distinct and novel signal or process, which we call sensitization. This was first suggested by the finding that ionomycin alone failed to stimulate transcytosis of the pIgR in the absence of dIgA binding. Additional evidence was obtained by modifying our assay. We found that the pIgR in the PMC must first be loaded with dIgA for it to respond to the signal of stimulation; i.e., empty pIgR in the PMC is not sensitized. This indicates that the signal of sensitization must be physically carried by the pIgR across the cell, from the basolateral surface and/or BEE to the PMC. Unlike diffusion of IP3, this movement involves microtubule-dependent vesicular traffic. We then used genetics to confirm this conclusion. Whereas the signal of stimulation is eliminated by truncation of the pIgR cytoplasmic tail after residue 725, sensitization is blocked by mutation of residue 657. Coexpression of the two mutants enables the pIgR-A657 to rescue the stimulated transcytosis of pIgR-725t. The finding of distinct structural requirements for the two signals and the ability of the mutants to complement is particularly strong evidence for our two-signal model. This is reminiscent of results from kinase-deficient EGFR, which lacks EGF-induced down-regulation. Down-regulation of this mutant was partially rescued by cotransfection with a kinase-active EGFR (Honegger et al., 1990). Binding of dIgA to pIgR causes dimerization of the pIgR. This was directly shown using a chimera of the pIgR and the cytoplasmic domain of the zeta chain of the T cell receptor, which provided a dimerization sensitive readout (Singer and Mostov, 1998). Moreover, when we prevented dimerization of the pIgR, by using a chimera containing a mutant GlyTMS, sensitization was prevented. This leads us to believe that dIgA-induced dimerization is necessary for the signal of sensitization.
dIgA–pIgR complexes can be endocytosed from either surface of the cell and become colocalized in the PMC. However, we showed that dIgA–pIgR complexes endocytosed from the apical surface did not respond to the signal of stimulation. This implies that in the PMC, dIgA–pIgR complexes that originate at the two surfaces are not equivalent. There are other indications reported previously that dIgA–pIgR complexes originating at the two surfaces are not identical. For instance, transcytosis of dIgA–pIgR from the PMC to the apical surface requires N-ethylmaleimide–sensitive factor but does not use syntaxin 3. In contrast, recycling of apically internalized dIgA–pIgR from the PMC back to the apical surface does use syntaxin 3 but apparently does not require N-ethylmaleimide–sensitive factor (Apodaca et al., 1996; Low et al., 1998). Furthermore, these two processes differ in their sensitivity to brefeldin A (Barroso and Sztul, 1994).
One possible mechanism for our results is that sensitization is due simply to dimerization of the pIgR. However, dimerization alone cannot completely account for these data. For example, binding of dIgA to the pIgR at the apical plasma membrane does not lead to sensitization. There may be a mechanism that allows dIgA to induce pIgR dimerization at the basolateral surface but prevents this dimerization at the apical surface. Such a mechanism might involve, for instance, interaction of the pIgR with a protein or lipid found uniquely at one surface of the cell or the other. In our opinion this would essentially be part of the process of sensitization and, in any case, indicates that dimerization alone is not sufficient to account for sensitization.
We think it is more likely that dimerization occurs equally on both membranes, as it does on the surface of Jurkat cells (Singer and Mostov, 1998). We therefore speculate that the basolateral specificity of the signal of sensitization is provided by a basolateral specific event occurring in response to dIgA binding and pIgR dimerization. One possibility is that pIgR coming from one side or the other undergoes a specific posttranslational modification. Although phosphorylation of pIgR has been shown to control its trafficking, this is unlikely to account for the differences. The two major sites of phosphorylation of the pIgR are at Ser-664 and Ser-726, and mutation of either or both does not block the ability of pIgR to undergo dIgA stimulated transcytosis (Song et al., 1994a; Luton et al., 1998). We cannot rule out a minor site of phosphorylation or a different type of modification. However, we speculate that the cytoplasmic domain of the dIgA–pIgR complex associates with an unidentified protein at the basolateral surface. This associated protein may then travel with the pIgR to the PMC and initiate the signal of sensitization. In this model, dimerization of the pIgR is necessary for association with this as yet unidentified other protein. We note that there are parallels between the pIgR and several other receptors, such as EGFR, platelet-derived growth factor receptor, c-fms, or InsR. In all cases, their intrinsic tyrosine kinase activity is necessary for proper trafficking of the receptor (Felder et al., 1990; Carlberg et al., 1991; Sorkin et al., 1991; Carpentier et al., 1993). However, in all reported cases, at least two distinct regions of the cytoplasmic domain of each receptor are needed for proper trafficking (Carlberg et al., 1991; Carpentier et al., 1993; Joly et al., 1994; Opresko et al., 1995). One of the regions generates a signal of stimulation, which in the case of receptor tyrosine kinases, is a tyrosine kinase domain. In the case of the pIgR, this seems to involve the last 30 amino acids of the cytoplasmic domain, which mediates an interaction with a nonreceptor tyrosine kinase. Another region(s) is needed for the receptor to respond to the signal appropriately, i.e., to undergo endocytosis, down-regulation, or transcytosis, depending on the particular receptor. This may therefore be viewed as a signal of sensitization.
Currently a central question in cell biology is how specificity in signaling can be maintained in the complex signaling networks that exist in an epithelial cell. The involvement of two separate signals of stimulation and sensitization for dIgA-stimulated pIgR transcytosis provides a possible mechanism for increasing specificity in signaling pathways. For instance, we showed previously that although binding of dIgA to basolateral pIgR activates a signaling pathway that can act at the PMC, transcytosis of Tf in the PMC to the apical surface is not stimulated (Apodaca et al., 1994). We believe this is because the Tf receptor was not sensitized to respond to this signal. Similarly, although binding of hepatocyte growth factor to its receptor, the c-Met tyrosine kinase, stimulates its intrinsic tyrosine kinase activity and leads to activation of PLCγ1 and IP3 production (Okano et al., 1993; Ponzetto et al., 1994), it does not stimulate transcytosis of pIgR (our unpublished results). Here again, we speculate that it is presumably because the pIgR is not sensitized by the hepatocyte growth factor. The involvement of two signals in pIgR transcytosis may serve as a model for a more general principle of signal transduction that acts to maintain specificity between the stimulus and the final response.
ACKNOWLEDGMENTS
We thank Drs. Anthony Defranco and Arthur Weiss for valuable discussions and Drs. David Krantz and Joshua Lipschutz for critical reading of the manuscript. A special thanks to Professor Jean-Pierre Vaerman (Catholic University of the Louvain, Brussels, Belgium) for providing us with dIgA. This work was supported by National Institutes of Health grants AI-25144 and AI-36953.
Abbreviations used:
- BEE
basolateral early endosome
- [Ca++]i
intracellular free Ca++ concentration
- dIgA
dimeric immunoglobulin A
- EGFR
epidermal growth factor receptor
- InsR
insulin receptor
- IP3
inositol trisphosphate
- IP3R
IP3 receptor
- MDCK
Madin–Darby canine kidney
- MEM
minimum essential medium
- pIgR
polymeric immunoglobulin receptor
- PLC
phosphatidylinosiol-specific phospholipase C
- PMA
phorbol myristate acetate
- PMC
postmicrotubule compartment
- PP1
4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo-[3,4-d]pyrimidine
- PTK
protein-tyrosine kinase
- PVDF
polyvinylidene difluoride
- SC
secretory component
- Tf
transferrin
- TMD
transmembrane domain
REFERENCES
- Apodaca G, Cardone MH, Whiteheart SW, DasGupta BR, Mostov KE. Reconstitution of transcytosis in SLO-permeabilized MDCK cells: existence of an NSF-dependent fusion mechanism with the apical surface of MDCK cells. EMBO J. 1996;15:1471–1481. [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeti B, Kosen PA, Kuntz ID, Cohen FE, Mostov KE. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J Cell Biol. 1993;123:1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeti B, Mostov KE. Polarized sorting of the polymeric immunoglobulin receptor in the exocytotic and endocytotic pathways is controlled by the same amino acids. EMBO J. 1994;13:2297–2304. doi: 10.1002/j.1460-2075.1994.tb06513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PC, Di Guglielmo GM, Authier F, Posner BI, Bergeron JMJ. Compartmentalized signal transduction by receptor tyrosine kinases. Trends Cell Biol. 1995;5:465–470. doi: 10.1016/s0962-8924(00)89116-3. [DOI] [PubMed] [Google Scholar]
- Barroso M, Sztul E. Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G protein sensitive passage through the apical endosome. J Cell Biol. 1994;124:83–100. doi: 10.1083/jcb.124.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld P, Casanova JE, Harris JM, Simister NE, Mostov KE. Expression and analysis of the polymeric immunoglobulin receptor. Methods Cell Biol. 1989;32:329–337. doi: 10.1016/s0091-679x(08)61178-4. [DOI] [PubMed] [Google Scholar]
- Breitfeld PP, Casanova JE, McKinnon WC, Mostov KE. Deletions in the cytoplasmic domain of the polymeric immunoglobulin receptor differentially affect endocytotic rate and postendocytotic traffic. J Biol Chem. 1990;265:13750–13757. [PubMed] [Google Scholar]
- Bush KT, Stuart RO, Li S-H, Moura LA, Sharp AH, Ross CA, Nigam SK. Epithelial inositol 1,4,5-triphosphate receptors. J Biol Chem. 1994;269:23694–23699. [PubMed] [Google Scholar]
- Cardone MH, Smith BL, Mennit PA, Mochly-Rosen D, Silver RB, Mostov KE. Signal transduction by the polymeric immunoglobulin receptor suggests a role in regulation of receptor transcytosis. J Cell Biol. 1996;133:997–1005. doi: 10.1083/jcb.133.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone MH, Smith BL, Song W, Mochley-Rosen D, Mostov KE. Phorbol myristate acetate-mediated stimulation of transcytosis and apical recycling in MDCK cells. J Cell Biol. 1994;124:717–727. doi: 10.1083/jcb.124.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg K, Tapley P, Haystead C, Rohrschneider LR. The role of kinase activity and the kinase insert region in ligand-induced internalization and degradation of the c-fms protein. EMBO J. 1991;10:877–883. doi: 10.1002/j.1460-2075.1991.tb08020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J-L, Paccaud J-P, Baecker J, Gilbert A, Orci L, Kahn CR. Two steps of insulin receptor internalization depend on different domains of the β-subunit. J Cell Biol. 1993;122:1243–1252. doi: 10.1083/jcb.122.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JE, Apodaca G, Mostov KE. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991;66:65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Casanova JE, Breitfeld PP, Ross SA, Mostov KE. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990;248:742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- Ceresa BP, Kao AW, Santeler SR, Pessin JE. Inhibition of clathrin-mediated endocytosis selectively attenuates insulin receptor signal transduction pathways. Mol Cell Biol. 1998;18:3862–3870. doi: 10.1128/mcb.18.7.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins CR. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Gerasimenko OV, Gerasimenko JV, Belan PV, Petersen OH. Inositol trisphosphate and cyclic ADP-ribose-mediated release of Ca2+ from single isolated pancreatic zymogen granules. Cell. 1996;84:473–480. doi: 10.1016/s0092-8674(00)81292-1. [DOI] [PubMed] [Google Scholar]
- Gibson A, Futter CE, Maxwell S, Allchin EH, Shipman M, Kraehenbuhl JP, Domingo D, Odorizzi G, Trowbridge IS, Hopkins CR. Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells. J Cell Biol. 1998;143:81–94. doi: 10.1083/jcb.143.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffroy D, Langendries A, Maurice M, Daniel F, Lardeux B, Courtoy PJ, Vaerman J-P. In vivo stimulation of polymeric Ig receptor-transcytosis by circulating polymeric IgA in rat liver. Int Immunol. 1998;10:347–354. doi: 10.1093/intimm/10.3.347. [DOI] [PubMed] [Google Scholar]
- Haller T, Volkl H, Deetjen P, Dietl P. The lysosomal Ca2+ pool in MDCK cells can be released by Ins(1,4,5)P3-dependent hormones or thapsigargin but does not activate store-operated Ca2+ entry. Biochem J. 1996;319:909–912. doi: 10.1042/bj3190909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and src family-selective tyrosine kinase inhibitor. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Honegger A-M, Schmidt A, Ullrich A, Schlessinger J. Separate endocytic pathways of kinase-defective and -active EGF receptor mutants in same cells. J Cell Biol. 1990;110:1541–1548. doi: 10.1083/jcb.110.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Mâle P, Mellman I. Differential microtubule requirements for transcytosis in MDCK cells. EMBO J. 1990;9:3515–3525. doi: 10.1002/j.1460-2075.1990.tb07560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly M, Kazlauskas A, Fay FS, Corvera S. Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase. Science. 1994;263:684–687. doi: 10.1126/science.8303278. [DOI] [PubMed] [Google Scholar]
- Kim SK. Polarized signaling: basolateral receptor localization in epithelial cells by PDZ-containing proteins. Curr Opin Cell Biol. 1997;9:853–859. doi: 10.1016/s0955-0674(97)80088-9. [DOI] [PubMed] [Google Scholar]
- Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJH, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. J Biol Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Glanagan JM, Treutlein R, Zhang J, Engelman DM. Sequence specificity in the dimerization of transmembrane α-helices. Biochemistry. 1992;31:12719–12725. doi: 10.1021/bi00166a002. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Tang Z. Caveolae, caveolin and caveolin-rich membrane domains: a signaling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Low S-H, Chapin SJ, Wimmer C, Whiteheart SW, Komuves LG, Mostov KE, Weimbs T. The SNARE machinery in apical plasma membrane trafficking in MDCK cells. J Cell Biol. 1998;141:1503–1513. doi: 10.1083/jcb.141.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luton F, Cardone MH, Zhang M, Mostov KE. Role of tyrosine phosphorylation in ligand-induced regulation of transcytosis of the polymeric Ig receptor. Mol Biol Cell. 1998;9:1787–1802. doi: 10.1091/mbc.9.7.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- Okano Y, Mizuno K, Osada S, Nakamura T, Nozawa Y. Tyrosine phosphorylation of phospholipase C gamma in c-met/HGF receptor-stimulated hepatocytes: comparison with HepG2 hepatocarcinoma cells. Biochem Biophys Res Commun. 1993;190:842–848. doi: 10.1006/bbrc.1993.1125. [DOI] [PubMed] [Google Scholar]
- Opresko LK, Chang CP, Will BH, Burke PM, Gill GN, Willey HS. Endocytosis and lysosomal targeting of epidermal growth factor receptors are mediated by distinct sequences independent of the tyrosine kinase domain. J Biol Chem. 1995;270:4325–4333. doi: 10.1074/jbc.270.9.4325. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F, Sternfeld L, Schmid A, Schulz I. Control of Ca2+ wave propagation in mouse pancreatic acinar cells. Am J Physiol. 1998;274:663–672. doi: 10.1152/ajpcell.1998.274.3.c663. [DOI] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- Roth MG, Sternweis PC. The role of lipid signaling in constitutive membrane traffic. Curr Opin Cell Biol. 1997;9:519–526. doi: 10.1016/s0955-0674(97)80028-2. [DOI] [PubMed] [Google Scholar]
- Seaman MNJ, Burd CG, Emr SD. Receptor signaling and the regulation of endocytic membrane transport. Curr Opin Cell Biol. 1996;8:549–556. doi: 10.1016/s0955-0674(96)80034-2. [DOI] [PubMed] [Google Scholar]
- Siemasko K, Eisfelder BJ, Williamson E, Kabak S, Clark MR. Signals from the B lymphocyte antigen receptor regulate MHC class II containing late endosomes. J Immunol. 1998;160:5203–5208. [PubMed] [Google Scholar]
- Singer KL, Mostov KE. Dimerization of the polymeric immunoglobulin receptor controls its transcytotic trafficking. Mol Biol Cell. 1998;9:901–915. doi: 10.1091/mbc.9.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Apodaca G, Mostov K. Transcytosis of the polymeric immunoglobulin receptor is regulated in multiple intracellular compartments. J Biol Chem. 1994a;269:29474–29480. [PubMed] [Google Scholar]
- Song W, Bomsel M, Casanova J, Vaerman J-P, Mostov KE. Stimulation of transcytosis of the polymeric immunoglobulin receptor by dimeric IgA. Proc Natl Acad Sci USA. 1994b;91:163–166. doi: 10.1073/pnas.91.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Westermark B, Heldin C-H, Claesson-Welsh L. Effect of receptor kinase inactivation on the rate of internalization and degradation of PDGF and the PDGF b-receptor. J Cell Biol. 1991;112:469–478. doi: 10.1083/jcb.112.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Turner RJ. Inositol 1,4,5-trisphosphate-dependent oscillations of luminal [Ca2+] in permeabilized HSY cells. J Biol Chem. 1996;271:30904–30908. doi: 10.1074/jbc.271.48.30904. [DOI] [PubMed] [Google Scholar]
- Thomas AP, Bird GSJ, Hajnoczky G, Robb-Gaspers LD, Putney JJW. Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol triphosphate. Cell. 1993;74:661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- Thorn P, Moreton R, Berridge M. Multiple, coordinated Ca2+-release events underlie the inositol trisphospahte-induced local Ca2+ spikes in mouse pancreatic acinar cells. EMBO J. 1996;15:999–1003. [PMC free article] [PubMed] [Google Scholar]
- Tunwell REA, Lai FA. Ryanodine receptor expression in the kidney and a nonexcitable kidney epithelial cell. J Biol Chem. 1996;271:29583–29588. doi: 10.1074/jbc.271.47.29583. [DOI] [PubMed] [Google Scholar]
- van de Put FH, Elliott AC. The endoplasmic reticulum can act as a functional Ca2+ store in all subcellular regions of the pancreatic acinar cell. J Biol Chem. 1997;272:27764–27770. doi: 10.1074/jbc.272.44.27764. [DOI] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Hino M, Miyawaki A, Segawa A, Adachi E, Yamashina S, Fujimoto T, Sugiyama T, Furuichi T, Hasegawa M, Mikoshiba K. Apical vesicles bearing inositol 1,4,5-trisphosphate receptors in the Ca2+ initiation site of ductal epithelium of submandibular gland. J Cell Biol. 1998;141:135–142. doi: 10.1083/jcb.141.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]