Abstract
Cellular localization determines whether the serine protease HtrA2 exerts pro- or antiapoptotic functions. In contrast to the well-characterized proapoptotic function of cytosolic HtrA2, mechanisms underlying the mitochondrial protective role are poorly understood. Mpv17l is a transmembrane protein previously implicated in peroxisomal reactive oxygen species metabolism and a close homolog of the inner mitochondrial membrane protein Mpv17. Here we demonstrate a previously undescribed direct interaction between Mpv17l and HtrA2 in mitochondria. The interaction is mediated by a PDZ domain and induces protease activation of HtrA2. HtrA2 inhibits mitochondrial superoxide generation, stabilizes mitochondrial membrane potential, and prevents apoptosis at baseline and in response to extracellular inducers of mitochondrial stress. The physiological role of Mpv17l is underscored by the finding that oxidative stress-induced downregulation of Mpv17l is a consistent feature in renal injury models. Our findings identify Mpv17l as a unique interacting protein and regulator of HtrA2 protease mediating antioxidant and antiapoptotic function in mitochondria.
Keywords: reactive oxygen species, proximal tubular epithelial cells, kidney injury, transforming growth factor beta, hypoxia
The serine protease HtrA2, also called Omi, is a mammalian homologue of the bacterial endopeptidases HtrA and DegS (1, 2). HtrA2 exerts either proapoptotic or antiapoptotic activities, depending on its subcellular localization and interacting proteins. For example, HtrA2 is localized to mitochondria in mammalian cells and exists in a premature and mature form, generated by autoproteolysis (3). Under conditions that stimulate mitochondrial apoptotic pathways, the mature form of HtrA2 is released into the cytoplasm where it binds the inhibitor of apoptosis (IAP). The binding of mature HtrA2 to IAPs is thought to contribute to induction of apoptosis by relieving the inhibition of caspases by IAPs (3–5). In addition to IAP, several cytosolic targets of HtrA2 protease have been identified, indicating IAP- and caspase-independent proapoptotic activities (6). Thus, HtrA2 has been considered a proapoptotic protein similar to the Drosophila death-promoting proteins Reaper, Grim, and Hid.
Recent reports provide strong support for an antiapoptotic function of HtrA2. For example, mice with a targeted deletion of HtrA2 or point mutations of its protease domain demonstrate features of a neurodegenerative disorder that resembles Parkinson's disease (5–7). HtrA2-deficient mice manifest loss of striatal cells by 20 days of age, and HtrA2-deficient cells are more susceptible to undergo apoptosis compared with wild-type cells (5). Mutations in the HtrA2 gene in patients with Parkinson's disease cause loss of its protease activity, associated with neuronal cell death as a result of mitochondrial dysfunction (8). A screen of 29 mitochondrial proteins for interaction with HtrA2 identified the PTEN (phosphatase and tensin homologue)-induced putative kinase 1 (PINK1). Similar to HtrA2, mutations in the PINK1 gene have previously been associated with Parkinson's disease (9). PINK1 and HtrA2 function in a shared pathway in neuronal cells, as PINK1 modulates the protease activity of HtrA2 in mitochondria through a p38-dependent phosphorylation of HtrA2 (10).
Mpv17 mitochondrial membrane protein-like (Mpv17l) protein is a member of the Mpv17/PMP22 family of transmembrane proteins (11). Mpv17l has high sequence homology with the Mpv17 gene. This gene encodes a mitochondrial inner membrane protein that is implicated in the metabolism of reactive oxygen species (ROS). Mutations in this gene cause the hepatocerebral form of mitochondrial DNA depletion syndrome (MDDS) in humans (12). In mice, deletion of Mpv17 causes proteinuric kidney disease and alterations in the inner ear that resemble Alport syndrome (13, 14). Murine Mpv17l exists in two alternative splice variants (15). To date, a 20 kDa-isoform (l-Mpv17l) has been characterized as a peroxisomal protein with three transmembrane domains, whereas a 10 kDa isoform (s-Mpv17l) has been localized to the cytosol. Both isoforms are involved in ROS metabolism (15, 16).
Here we demonstrate that both Mpv17l isoforms are localized in mitochondria under homeostatic conditions, though not in peroxisomes as previously reported (15, 17). In mitochondria, Mpv17l isoforms interact with HtrA2 through a PDZ domain-binding motif to enable HtrA2 protease activity. The Mpv17l-dependent protease activity of HtrA2 prevents mitochondrial dysfunction and apoptosis at baseline and in response to extracellular inducers of mitochondrial stress by reducing mitochondrial oxidative stress and stabilizing mitochondrial membrane potential. Our findings suggest that the Mpv17l and HtrA2 complex is critical for mitochondrial oxidative stress sensing and protects against ROS-induced mitochondrial dysfunction.
Results
Expression of Mpv17l in Kidney Tubular Epithelia Is Downregulated in Tgf β1 Transgenic (TG) Mice with Progressive Kidney Disease.
Because deletion of the Mpv17 gene in mice causes proteinuric kidney disease (13), we examined mRNA levels of Mpv17l and Mpv17 in TGF β1 transgenic (TG) mice, which develop lethal kidney disease characterized by progressive tubulointerstitial fibrosis and glomerulosclerosis [see supporting information (SI) Text] (18). Compared with healthy control mice, whole kidney mRNA levels of Mpv17l, and to a lesser extent Mpv17, were drastically decreased in TGF β1 transgenic animals (see Fig. S1A, left]. This TGF β1-induced downregulation of Mpv17l mRNA could be also detected in murine proximal tubular epithelial cells (MCT), whereas D-glucose, cobalt chloride, and antimycin A had no effect on Mpv17l mRNA levels (Fig. S1A, right). Tubular expression of Mpv17l, prominently observed in kidney sections of control mice, was strongly reduced or absent in TG mice, as demonstrated by immunofluorescence labeling of kidney sections or immunoblotting of whole kidney lysates (Fig. 1A). Similar findings were obtained in murine models of diabetic type 1 (Akita) and type 2 (db/db) kidney disease, compared with control mice (Fig. S1B). This demonstrates that the loss of Mpv17l in renal nephron segments is a hallmark of progressive kidney disease.
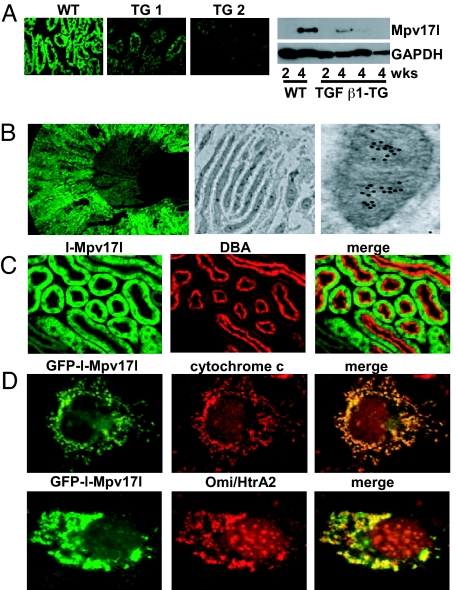
Fig. 1.
Mpv17l is localized in mitochondria and downregulated in the TGF β1 transgenic mouse model. (A) Immunofluorescence of Mpv17l-stained kidney sections from one 4-week-old control and two TGF β1 transgenic mice. Western blot analysis of l-Mpv17l protein levels (20 kDa) in kidney extracts of 2- and 4-week-old wild-type and TGF β1 transgenic mice. GAPDH protein levels are shown for equal loading. (B) (Left) cryosections from mouse kidney were stained with anti-rabbit Mpv17l antibody (see Materials and Methods) and visualized by immunofluorescence microscopy (10×). (Right) electron microscopy with ImmunoGold labeling of Mpv17l in kidney sections. (C) Double labeling of kidney tissue with anti-Mpv17l and Dolichos biflorus agglutinin (DBA), a marker for proximal tubular cells. (D) COS-7 cells, transfected with FLAG-tagged l-Mpv17l, were stained with anti-FLAG and anti-cytochrome c and visualized using confocal microscopy.
Mpv17l Is Localized in Mitochondria of Kidney Proximal Tubular Epithelial Cells.
In normal adult kidney, Mpv17l protein was expressed in the outer medulla and inner cortex, which was shown by immunofluorescence staining on frozen murine kidney sections (Fig. 1B). ImmunoGold electron microscopy revealed an accumulation of gold particles in mitochondria of proximal tubular cells with predominant localization to the inner mitochondrial membrane (Fig. 1B, right). To further verify expression in proximal tubular cells, we double labeled with Dolichos biflorus agglutinin (DBA), a marker for proximal tubular cells and principal cells of the collecting duct (Fig. 1C). Expression of the 20 kDa splice variant (GFP-l-Mpv17l) in COS-7 cells confirmed that l-Mpv17l was confined to mitochondria, where it colocalized with the mitochondrial marker cytochrome c (Fig. 1D). Similarly, the 10 kDa Mpv17l splice variant (GFP-s-Mpv17l) could also be detected in mitochondria (Fig. S1C). Furthermore, l-Mpv17l also colocalized with Omi/HtrA2 but did not colocalize with the peroxisomal marker PMP70 (Fig. S1C). These findings were confirmed by subcellular fractionation assays (Fig. S1D). Taken together, our studies suggest that the l-Mpv17l is localized in mitochondria but not in peroxisomes (16), and s-Mpv17l shows a strong mitochondrial signal but an additional cytosolic distribution.
Inducers of Mitochondrial ROS Generation Downregulate Mpv17l by a ROS-Dependent Mechanism Associated with Apoptosis in Proximal Tubular Cells.
Because ROS generation and mitochondrial dysfunction are hallmarks of acute and chronic kidney diseases (19), we exposed MCT cells to well-characterized inducers of ROS, including D-glucose (Fig. 2A), the hypoxia-mimicking substance cobalt chloride (CoCl2), TGFβ1, and bovine serum albumin (BSA) (SI Text and Fig. S2 A–C). High-ambient D-glucose (30 mM) downregulated Mpv17l protein associated with increased ROS generation and apoptosis (Fig. 2A, left panel). A significant downregulation of Mpv17l after 24 h was preceded by a ROS increase, which was detectable after 15 min of stimulation (Fig. 2A, right panel). These effects were prevented by antioxidants, such as N-acetylcysteine and manganese(III) tetrakis(4-benzoic acid)porphyrin (MnTBAP) (Fig. 2B and Fig. S2C). Stimuli such as TNFα and LPS did not induce ROS or downregulate Mpv17l protein levels (Fig. S2D). These findings suggest that multiple inducers of mitochondrial ROS generation suppress Mpv17l protein in a ROS-dependent manner.
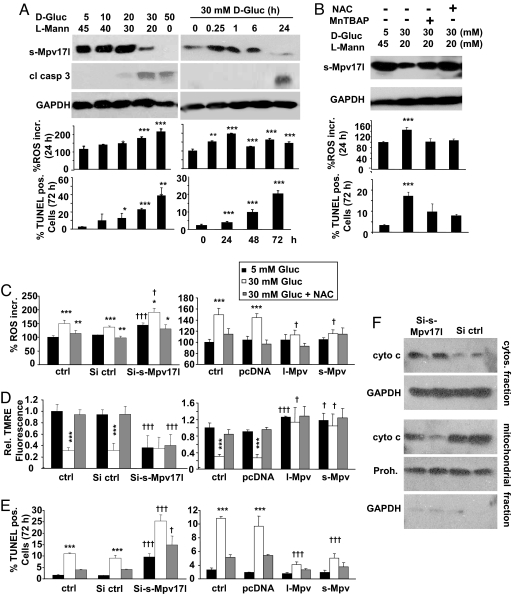
Fig. 2.
Regulation of Mpv17l protein levels is associated with alterations of ROS generation, mitochondrial membrane potential, and apoptosis in murine proximal tubular epithelial (MCT) cells. (A) Immunoblot analysis of Mpv17l and cleaved caspase-3 expression levels. Bar graphs show average ± SEM of ROS generation (percentage ROS increase) and the rate of apoptosis in MCT cells, stimulated with different concentrations of D-glucose and at different time points as indicated. L-Mannitol was added to maintain constant total osmotic pressure of 50 mM. (B) Immunoblot analysis of Mpv17l expression levels, percentage ROS increase, and apoptosis in MCT cells, preincubated for 30 min with two antioxidants (MnTBAP; 50 μM and NAC; 1 mM) before D-glucose (30 mM) stimulation. (C) Bar graphs showing average ± SEM of percentage ROS increase. (D) Loss of ΔΨm, indicated by relative change of tetramethylrhodamine ethyl ester (TMRE) fluorescence. (E) Rate of apoptosis in siRNA clones (three clones averaged) and overexpression clones (three clones averaged), which were stimulated with D-glucose (30 mM) ± preincubation with NAC (1 mM). (F) Representative immunoblot of cytochrome c in subcellular fractions of extracts from two Mpv17l siRNA clones compared with two control clones. GAPDH and prohibitin protein levels were used for equal loading. Statistical analysis was performed by using Student's t test and ANOVA with *, P < 0.05; **, P < 0.01; ***, P < 0.005 (*, compared with unstimulated control; †, compared with equivalent si control).
Mpv17l Increases Resistance to Mitochondrial Dysfunction and Apoptosis.
Silencing of endogenous Mpv17l or overexpression of l-Mpv17l or s-Mpv17l were accomplished by stable transfection of MCT cells and analysis of three individual clones (SI Text and Fig. S2F). Reduction of Mpv17l was associated with increased mitochondrial superoxide generation, which could be partially blocked by antioxidants, whereas overexpression reduced D-glucose-induced mitochondrial ROS levels (Fig. 2C). Furthermore, Mpv17l silencing led to the depolarization of the mitochondrial membrane potential (ΔΨm), which was alleviated by stable overexpression of both forms in MCT cells (Fig. 2D). Additionally, reduction of Mpv17l expression increased apoptosis at baseline conditions and stimulated with D-glucose, which could be partially attenuated by antioxidants, whereas overexpression significantly reduced D-glucose-induced apoptosis in MCT cells (Fig. 2E). siMpv17l clones showed an increased cytosolic cytochrome c fraction compared with control siRNA clones, indicating outer mitochondrial membrane permeabilization (Fig. 2F). Together, these findings demonstrate that the level of mitochondrial Mpv17l is inversely correlated with mitochondrial ROS generation, loss of ΔΨm, and apoptosis. Thus, we conclude that both s-Mpv17l and l-Mpv17l protect against ROS-induced mitochondrial dysfunction and apoptosis.
Mp17l Attenuates Antimycin A-Induced Accumulation of Activated Bax.
It has been shown previously that processed HtrA2 can prevent the translocation of Bax into the outer mitochondrial membrane (20). Therefore, 293T cells were transfected with Mpv17l and stimulated with antimycin A for 2 h and 24 h. In comparison with control transfected cells, less activated Bax could be detected in the Mpv17l-overexpressing cells (SI Text and Fig. S2E). This finding suggests that attenuated accumulation of activated Bax in the outer mitochondrial membrane is involved in the Mpv17l-mediated protection against apoptosis.
Mp17l Isoforms Interact with PDZ Domain Protein HtrA2 Through a PDZ Binding Motif.
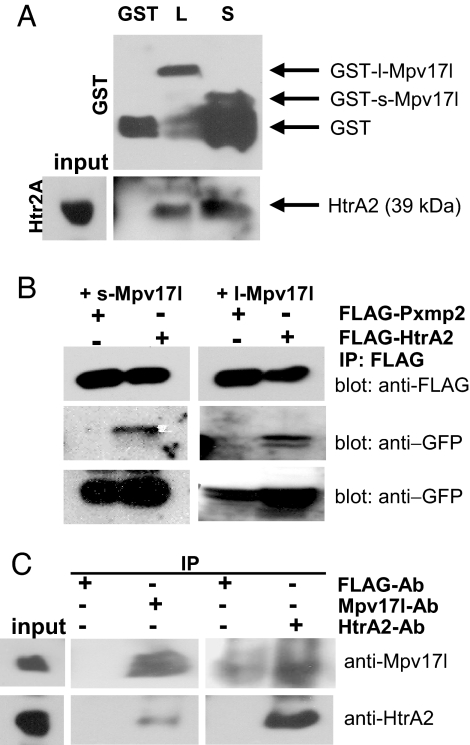
Because of a strict colocalization between HtrA2 and Mpv17l in mitochondria of transfected COS-7 cells, we used whole kidney protein extracts and GST-chimeric proteins of l-Mpv17l and s-Mpv17l for protein–protein interaction studies. Endogenous mature HtrA2 protein (39 kDa) (21) interacted with both Mpv17l-GST proteins, which were in general prone to degradation processes (Fig. 3A). This interaction was confirmed by coimmunoprecipitation studies in transfected 293T cells (Fig. 3B). Endogenous Mpv17l and HtrA2 coimmunoprecipitated from whole murine kidney lysates, whereas a control IgG did not bind to these proteins (Fig. 3C). Thus, l-Mpv17l and s-Mpv17l isoforms interact with HtrA2.
Fig. 3.
Both l-Mpv17l and s-Mpv17l proteins are forming a complex with the mature form of the HtrA2 protein. (A) Immunoblot analysis of a GST-pull-down assay using whole kidney protein extracts on immobilized GST, GST-l-Mpv17l, and GST-s-Mpv17l proteins. (B) FLAG immunoblot showing coimmunoprecipitation between GFP-l-Mpv17l, GFP-s-Mpv17l, and FLAG-HtrA2 or FLAG-Pxmp2. (C) Immunoblot of endogenous coimmunoprecipitation analyses on whole kidney extract as input with either the Mpv17l antibody or the HtrA2 antibody, compared with the FLAG antibody as control.
Sequence analysis revealed a PDZ binding motif in the C-terminus of l-Mpv17l, and HtrA2 contains a C-terminal PDZ domain. Wild-type Flag-l-Mpv17l interacted with GFP-HtrA2, while mutant Flag-l-Mpv17l-DI, where aspartic acid (position 112) and isoleucine (position 114) of the KDDI motif were replaced with alanine residues by site-directed mutagenesis, was unable to bind GFP-HtrA2 (Fig. 4A). These findings demonstrate that the PDZ binding motif of Mpv17l binds HtrA2.
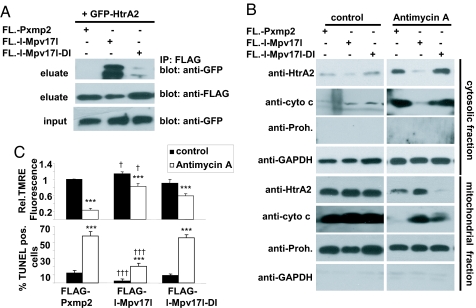
Fig. 4.
The PDZ binding motif of Mpv17l interacts with HtrA2, which is essential for the protective function of Mpv17l. (A) FLAG immunoblot showing coimmunoprecipitation experiments of lysates from 293T cells, transfected with GFP-HtrA2 and FLAG-l-Mpv17l or FLAG-l-Mpv17l-DI (the required amino acids for the PDZ-BM are mutated: D112A, I114A), compared with FLAG-Pxmp2 as control. (B) Representative immunoblot of HtrA2, and cytochrome c showing subcellular fractions of extracts from 293T cells, transfected with FLAG-l-Mpv17l, FLAG-l-Mpv17l-DI, or control FLAG -Pxmp2, stimulated with antimycin A to induce outer mitochondrial membrane permeabilization. (C) Bar graphs showing average ± SEM of the relative TMRE fluorescence indicating mitochondrial membrane potential change and the rate of apoptosis in 293T cells, transfected with either FLAG-l-Mpv17l, the nonbinding mutant FLAG-l-Mpv17l-DI or FLAG-Pxmp2 as control. Statistical analysis was performed by using Student's t test and ANOVA with ***, P < 0.005 (*, compared with unstimulated control; †, compared with equivalent si control).
PDZ Binding Motif-Dependent Mpv17l/HtrA2 Protein Complex Confers Resistance Against Mitochondrial Dysfunction Induced by Mitochondrial Stress.
For the induction of mitochondrial ROS, we used antimycin A, an inhibitor of complex III of the respiratory chain. Transfection of 293T cells with FLAG-l-Mpv17l, but not PDZ-binding motif mutant FLAG-l-Mpv17l-DI, blocked antimycin A-induced translocation of cytochrome c and HtrA2 from the mitochondria into the cytosol (Fig. 4B). Only wild-type FLAG-l-Mpv17l, but not mutant FLAG-l-Mpv17l-DI, was able to mediate resistance to loss of ΔΨm and apoptosis induced by antimycin A (Fig. 4C). These findings suggest that the PDZ binding motif-mediated complex formation of Mpv17l and HtrA2 renders mitochondria resistant against ROS-induced loss of ΔΨm and apoptosis.
Resistance to Mitochondrial Dysfunction Mediated by Mpv17l/HtrA2 Protein Complexes Is Dependent on the Protease Activity of HtrA2.
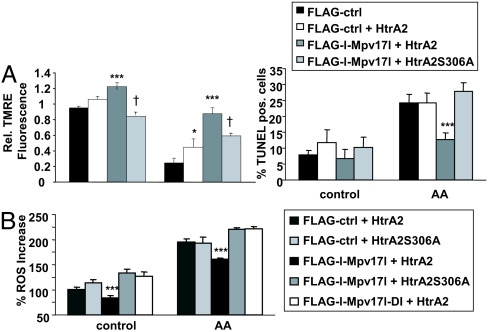
Because the protease activity of HtrA2 depends on protein–protein interactions via its PDZ domain (22), we reasoned that the protease activity of HtrA2 may be critical for the mitochondrial defense function of Mpv17l/HtrA2 complexes. Therefore, we transiently transfected HtrA2−/− MEFs with a ≈30% transfection efficiency. Cotransfection with wild-type Flag-l-Mpv17l and wild-type HtrA2 plasmids, but not the protease-defective HtrA2 mutant HtrA2-S306A plasmid, conferred resistance to mitochondrial dysfunction and apoptosis upon Mpv17l-negative HtrA2−/− MEFs under nonstimulated conditions and stimulation with antimycin A (Fig. 5A). Transient transfection with the protease defective mutant led to a decrease of mitochondrial membrane potential under baseline and stimulated conditions (Fig. 5A). These findings demonstrate that the protease activity of HtrA2 depends on the PDZ-binding motif-dependent complex formation with Mpv17l and is required for mitochondrial membrane stabilization and protection against apoptosis.
Fig. 5.
The serine protease activity of HtrA2 is required for the mitochondrial protective function of Mpv17l. (A) Bar graphs showing average ± SEM of the percentage change of ΔΨm (Left) and the rate of apoptosis (right) of HtrA2−/− MEFs, cotransfected with FLAG-l-Mpv17l and HtrA2 or mutant HtrA2S306A compared with control cells. (B) Bar graphs indicating average ± SEM of percentage ROS generation in HtrA2 −/− MEFs, cotransfected with FLAG-Pxmp2 as control, FLAG-l-Mpv17l, or the mutant FLAG-l-Mpv17l-DI and wild-type HtrA2 or the protease-defective HtrA2S306. Results are representative data from three independent experiments. Statistical analysis was performed using Student's t test and ANOVA with *, P < 0.05; **, P < 0.01; ***, P < 0.005 (compared with untreated control) and †, P < 0.05 (compared with cells transfected with FLAG-l-Mpv17l and HtrA2).
Finally, we reasoned whether the Mpv17l-dependent protease activity of HtrA2 modulates mitochondrial ROS generation as a unique mechanism of the mitochondrial antioxidant defense. HtrA2−/− MEFs were cotransfected with various combinations of wild-type and PDZ-binding motif mutant Mpv17l constructs together with wild-type and protease-defective HtrA2 mutant plasmids. Only the coexpression of wild-type FLAG-l-Mpv17l and wild-type HtrA2 reduced mitochondrial ROS generation under unstimulated and stimulated conditions (Fig. 5B). To underscore these findings, we analyzed ROS generation, ΔΨm, and apoptosis in wild-type and HtrA2−/− MEFs transfected with Mpv17l and subsequently stimulated with rotenone (25 μM for 27 h). Only wild-type cells transfected with Mpv17l showed a significant reduction of ROS levels, a stabilization of ΔΨm, and less apoptosis compared with control transfected cells (Fig. S3 A–C). To further show that rotenone is a ROS inducer similar to antimycin A, we treated MCT cells with both reagents and measured MitoSOX fluorescence (Fig. S4A). To indicate that antimycin causes mitochondrial dysfunction, we measured DYm of cells, treated with either antimycin A or the control stimulus staurosporine (Fig. S4B). Finally, for the validation of the apoptosis studies, Annexin V/PI analysis showed results comparable to TUNEL labeling in 293T cells, transfected with either a control or Mpv171 (Fig. S4C). Our findings suggest that the Mpv17l/HtrA2 complex provides a unique antioxidant defense mechanism in mitochondria against mitochondrial ROS generation.
Discussion
The ability of cells to respond to environmental or internal stress is essential for cell survival and depends in part on mechanisms to detect and remove misfolded or damaged proteins induced by cellular stresses, including oxidative stress. HtrA proteases are widely conserved and contain carboxy-terminal PDZ domains that activate their protease domain when binding to damaged or misfolded proteins (23, 24). The mammalian HtrA2 protease is emerging as an essential regulator of mitochondrial function that protects mitochondria from stress and prevents apoptosis in striatal neurons (5, 7, 25). Defects in HtrA2 protease activity cause a parkinsonian neurodegenerative phenotype in mice and are associated with Parkinson's disease in humans (7, 8). Recent evidence suggests that the MAPK p38 and the PTEN (phosphatase and tensin homologue)-induced putative kinase 1 (PINK1) phosphorylate HtrA2 in an external stress-sensing pathway, resulting in increased activity of HtrA2 protease and protection of mitochondria associated with cell survival (10). Thus, HtrA2 exerts a central role in protection of cells against mitochondria-associated cellular stress.
In this report, we identify Mpv17l as a mitochondrial binding partner of HtrA2 and a regulator of the mitochondrial function of the HtrA2 serine protease to suppress mitochondrial ROS generation. This antioxidant function of HtrA2 protease is essential for stabilization of the inner mitochondrial membrane potential and mitochondrial retention of cytochrome c and HtrA2 itself, thereby protecting cells against mitochondrial apoptosis signals, such as metabolic and cytokine stimuli.
Based on our findings and recent reports, we propose a new mechanism for mitochondrial antioxidant stress protection. The PDZ domain of HtrA2 regulates its catalytic activity (7, 22).
In contrast to previous reports suggesting that Mpv17l isoforms are localized in peroxisomes or cytosol (15, 17), our findings demonstrate that Mpv17l is localized with its three transmembrane domains at the inner mitochondrial membrane in proximity with protein complexes of the respiratory chain, the primary site of mitochondrial ROS generation. By providing a mitochondrial membrane-binding ligand for the regulatory PDZ domain of HtrA2, Mpv17l may localize and activate the catalytic activity of HtrA2 at the inner mitochondrial membrane and intermembrane space through allosteric activation. Thus, we propose that Mpv17l and HtrA2 constitute a multimeric mitochondrial protein complex that senses and regulates oxidative stress, possibly by HtrA2-mediated removal of damaged or misfolded proteins involved in mitochondrial ROS generation and/or degradation.
When mitochondrial ROS generation increases in response to various upstream stress signals, Mpv17l proteins are profoundly downregulated. In the absence of Mpv17l ligands, HtrA2 PDZ domain may become unliganded, and HtrA2 may switch back to a catalytically inactive conformation and release into the cytosol, where it exerts its proapoptotic function. The loss of a local antioxidant barrier may further increase net balance of ROS generation, resulting in collapse of the inner mitochondrial membrane potential, recruitment of Bax into the outer mitochondrial membrane, and subsequent permeabilization with release of HtrA2 and cytochrome c into the cytosol, thereby triggering cellular apoptosis cascades. Though we find that ROS cause a profound decrease of Mpv17l protein levels, the exact mechanism(s) remain(s) to be determined.
In summary, we report that Mpv17l and HtrA2 form a unique mitochondrial protein complex critical for mitochondrial oxidative stress sensing and regulation of ROS net balance in response to internal and external stress.
Materials and Methods
Cell Culture.
293T cells and COS-7 cells were purchased from American Type Culture Collection and cultured according to the vendor's instructions. The mouse proximal tubular cell line MCT was provided by Fuad Ziyadeh (American University, Beirut, Lebanon). Wild-type immortalized and Htr2A knockout mouse embryo fibroblasts (MEFs) (5) were kindly provided by Dr. J. Downward (Cancer Research UK London Research Institute, London, England). Transfections were performed using Effectene and Superfect (Qiagen) according to the manufacturer's protocol.
Determination of ROS Generation.
ROS generation was measured with the fluoroprobes dihydroethidium (DHE) and MitoSOX (Invitrogen), which detects superoxide or, specifically, mitochondrial superoxide generation (validated by immunofluorescence microsopy). Briefly, 3 × 105 cells were trypsinized and centrifuged, and the pellet was resuspended in 1 μM DHE or 1 μM MitoSOX in PBS for 45 min at 37°C. Stock solutions were prepared in DMSO. Cells were then washed with PBS, and emitted fluorescence was determined using a BD FACSCanto (BD Biosciences). Flow cytometry data were analyzed using FACS Diva (BD Biosciences). Results were shown in percentage increase of fluorescence and compared with untreated control cells.
Measurement of the Inner Mitochondrial Membrane Potential (ΔΨm).
To analyze the changes in the mitochondrial membrane potential, we used tetramethylrhodamine ethyl ester (TMRE) (Invitrogen). Briefly, after treatment, 3 × 105 cells were trypsinized and incubated in 25 nM TMRE for 30 min at 37°C. By using this concentration (non quenching mode), depolarization causes a drop of the TMRE fluorescence. Cells were washed with PBS and cells were analyzed by flow cytometry, measuring TMRE fluorescence in FL-2. The relative fluorescence of TMRE was compared with that of the respective untreated control cells in each experiment (26). To ensure the correlation between the mitochondrial TMRE uptake and the mitochondrial membrane potential, staurosporine (0.5 μM for 30 min) was used as a control stimulus that is known to depolarize the mitochondrial membrane potential. All of the experiments described herein were repeated at least three times.
Supplementary Material
Acknowledgments.
The authors thank M. Donnelly and S. Ratner for their technical assistance, and Dr. L.C.F. Mulder for valuable discussions. This work was supported by National Institutes of Health Grants 5U01DK060995, 5R01DK056077, and 5R01DK060043 (to E.P.B.). S.K. was supported by the Feodor-Lynen Fellowship of the Alexander von Humboldt Foundation, Germany, and a National Kidney Foundation (NKF) Basic Research Fellowship. Confocal laser scanning microscopy was performed at the MSSM-Microscopy Shared Resource Facility, supported with funding from National Institutes of Health-National Cancer Institute Shared Resources Grant (5R24 CA095823–04), NSF Major Research Instrumentation Grant (DBI-9724504), and National Institutes of Health Shared Instrumentation Grant (1 S10 RR0 9145–01).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801146105/DCSupplemental.
References
- 1.Faccio L, et al. Characterization of a novel human serine protease that has extensive homology to bacterial heat shock endoprotease HtrA and is regulated by kidney ischemia. J Biol Chem. 2000;275:2581–2588. doi: 10.1074/jbc.275.4.2581. [DOI] [PubMed] [Google Scholar]
- 2.Gray CW, et al. Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur J Biochem. 2000;267:5699–5710. doi: 10.1046/j.1432-1327.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- 3.van Loo G, et al. The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Differ. 2002;9:20–26. doi: 10.1038/sj.cdd.4400970. [DOI] [PubMed] [Google Scholar]
- 4.Verhagen AM, et al. trA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J Biol Chem. 2002;277:445–454. doi: 10.1074/jbc.M109891200. [DOI] [PubMed] [Google Scholar]
- 5.Martins LM, et al. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vande WL, et al. Proteome-wide identification of HtrA2/Omi substrates. J Proteome Res. 2007;6:1006–1015. doi: 10.1021/pr060510d. [DOI] [PubMed] [Google Scholar]
- 7.Jones JM, et al. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature. 2003;425:721–727. doi: 10.1038/nature02052. [DOI] [PubMed] [Google Scholar]
- 8.Strauss KM, et al. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson's disease. Hum Mol Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- 9.Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 10.Plun-Favreau H, et al. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- 11.Iida R, Yasuda T, Tsubota E, Matsuki T, Kishi K. Cloning, mapping, genomic organization, and expression of mouse M-LP, a new member of the peroxisomal membrane protein Mpv17 domain family. Biochem Biophys Res Commun. 2001;283:292–296. doi: 10.1006/bbrc.2001.4769. [DOI] [PubMed] [Google Scholar]
- 12.Spinazzola A, et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet. 2006;38:570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- 13.Weiher H, Noda T, Gray DA, Sharpe AH, Jaenisch R. Transgenic mouse model of kidney disease: Insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell. 1990;62:425–434. doi: 10.1016/0092-8674(90)90008-3. [DOI] [PubMed] [Google Scholar]
- 14.Meyer zum Gottesberge AM, Reuter A, Weiher H. Inner ear defect similar to Alport's syndrome in the glomerulosclerosis mouse model Mpv17. Eur Arch Otorhinolaryngol. 1996;253:470–474. doi: 10.1007/BF00179952. [DOI] [PubMed] [Google Scholar]
- 15.Iida R, et al. A novel alternative spliced Mpv17-like protein isoform localizes in cytosol and is expressed in a kidney- and adult-specific manner. Exp Cell Res. 2005;302:22–30. doi: 10.1016/j.yexcr.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Iida R, et al. M-LP, Mpv17-like protein, has a peroxisomal membrane targeting signal comprising a transmembrane domain and a positively charged loop and up-regulates expression of the manganese superoxide dismutase gene. J Biol Chem. 2003;278:6301–6306. doi: 10.1074/jbc.M210886200. [DOI] [PubMed] [Google Scholar]
- 17.Iida R, et al. Human Mpv17-like protein is localized in peroxisomes and regulates expression of antioxidant enzymes. Biochem Biophys Res Commun. 2006;344:948–954. doi: 10.1016/j.bbrc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson N, et al. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci USA. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 20.Chao JR, et al. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature. 2008;452:98–102. doi: 10.1038/nature06604. [DOI] [PubMed] [Google Scholar]
- 21.Hegde R, et al. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J Biol Chem. 2002;277:432–438. doi: 10.1074/jbc.M109721200. [DOI] [PubMed] [Google Scholar]
- 22.Martins LM, et al. Binding specificity and regulation of the serine protease and PDZ domains of HtrA2/Omi. J Biol Chem. 2003;278:49417–49427. doi: 10.1074/jbc.M308659200. [DOI] [PubMed] [Google Scholar]
- 23.Schlieker C, Mogk A, Bukau B. A PDZ switch for a cellular stress response. Cell. 2004;117:417–419. doi: 10.1016/s0092-8674(04)00453-2. [DOI] [PubMed] [Google Scholar]
- 24.Sohn J, Grant RA, Sauer RT. Allosteric activation of DegS, a stress sensor PDZ protease. Cell. 2007;131:572–583. doi: 10.1016/j.cell.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 25.Yang QH, Church-Hajduk R, Ren J, Newton ML, Du C. Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis (IAP) irreversibly inactivates IAPs and facilitates caspase activity in apoptosis. Genes Dev. 2003;17:1487–1496. doi: 10.1101/gad.1097903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterhouse NJ, et al. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J Cell Biol. 2001;153:319–328. doi: 10.1083/jcb.153.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.