Abstract
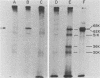
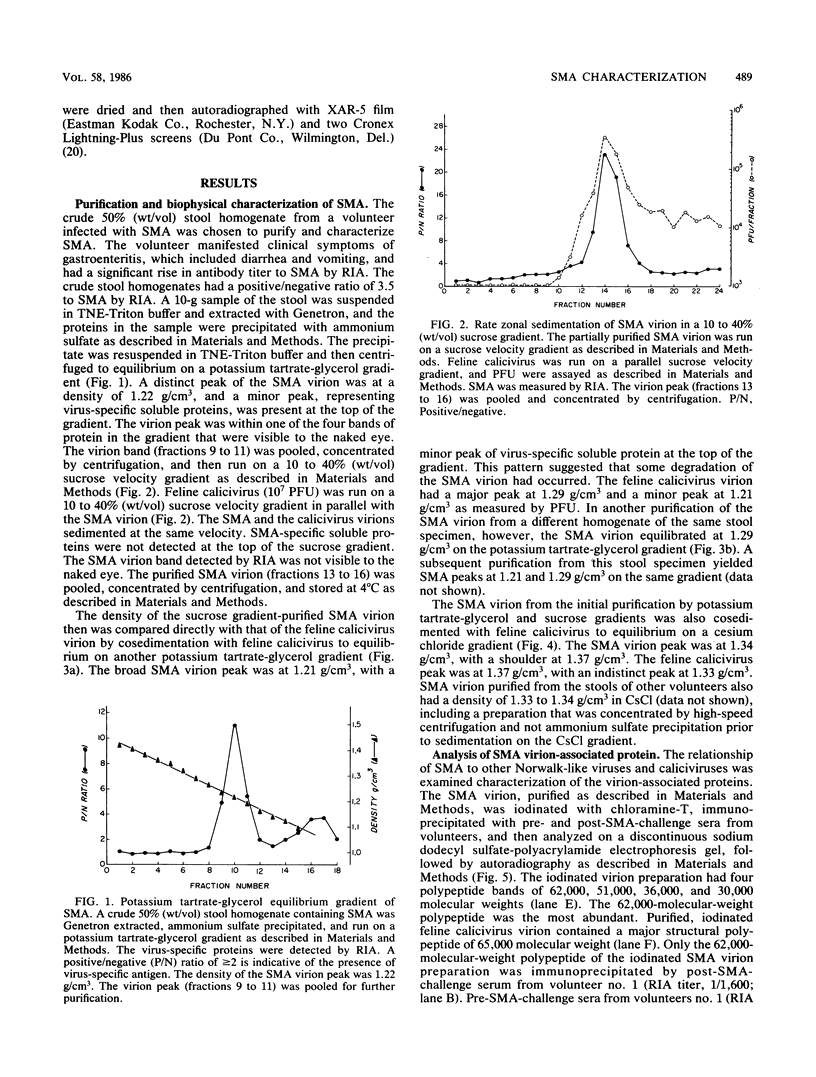
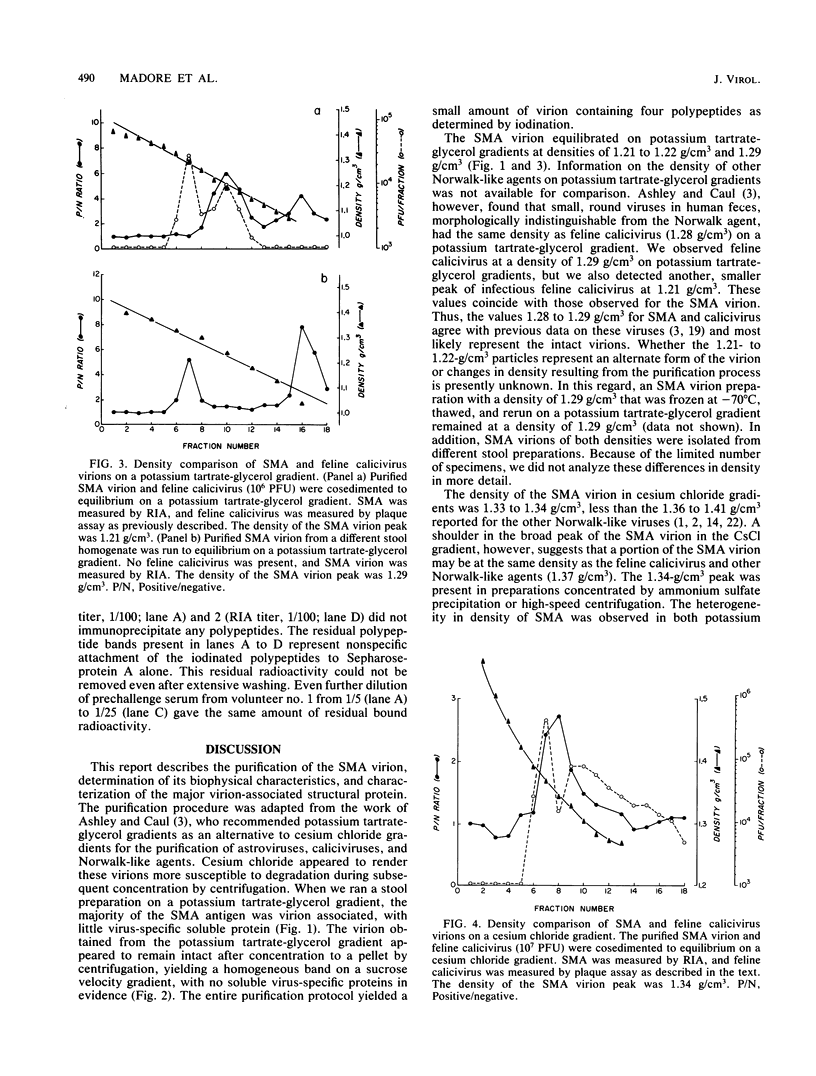
Snow Mountain agent (SMA) is a 27- to 32-nm virus which is the etiologic agent of outbreaks of acute gastroenteritis in Colorado and Vermont. SMA is morphologically similar to but antigenically distinct from the Norwalk and Hawaii agents of viral gastroenteritis but, like those agents, has not been cultivated in vitro. We purified and characterized SMA directly from human stool specimens containing the virus. The density of the SMA virion was 1.29 g/cm3 and 1.21 to 1.22 g/cm3 on potassium tartrate-glycerol gradients and 1.33 to 1.34 g/cm3 on cesium chloride gradients. SMA had an S value of 170 to 183S on a sucrose velocity gradient. The purified virion was iodinated, immunoprecipitated with acute and convalescent sera from volunteers challenged with SMA, and analyzed on polyacrylamide gels. The virion contains one major structural protein of 62,000 molecular weight, which is similar in size to the 59,000-molecular-weight protein found in the Norwalk virion. The biophysical properties and single structural protein of SMA most closely resemble those of the calicivirus group.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acute infectious nonbacterial gastroenteritis: etiology and pathogenesis. Ann Intern Med. 1972 Jun;76(6):993–1008. doi: 10.7326/0003-4819-76-6-993. [DOI] [PubMed] [Google Scholar]
- Appleton H., Buckley M., Thom B. T., Cotton J. L., Henderson S. Virus-like particles in winter vomiting disease. Lancet. 1977 Feb 19;1(8008):409–411. doi: 10.1016/s0140-6736(77)92614-9. [DOI] [PubMed] [Google Scholar]
- Appleton H., Pereira M. S. A possible virus aetiology in outbreaks of food-poisoning from cockles. Lancet. 1977 Apr 9;1(8015):780–781. doi: 10.1016/s0140-6736(77)92960-9. [DOI] [PubMed] [Google Scholar]
- Ashley C. R., Caul E. O. Potassium tartrate-glycerol as a density gradient substrate for separation of small, round viruses from human feces. J Clin Microbiol. 1982 Aug;16(2):377–381. doi: 10.1128/jcm.16.2.377-381.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondum J., Spitalny K. C., Vogt R. L., Godlewski K., Madore H. P., Dolin R. Snow Mountain agent associated with an outbreak of gastroenteritis in Vermont. J Infect Dis. 1985 Oct;152(4):834–837. doi: 10.1093/infdis/152.4.834. [DOI] [PubMed] [Google Scholar]
- Clarke S. K., Cook G. T., Egglestone S. I., Hall T. S., Miller D. L., Reed S. E., Rubenstein D., Smith A. J., Tyrrell D. A. A virus from epidemic vomiting disease. Br Med J. 1972 Jul 8;3(5818):86–89. doi: 10.1136/bmj.3.5818.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin R., Blacklow N. R., DuPont H., Formal S., Buscho R. F., Kasel J. A., Chames R. P., Hornick R., Chanock R. M. Transmission of acute infectious nonbacterial gastroenteritis to volunteers by oral administration of stool filtrates. J Infect Dis. 1971 Mar;123(3):307–312. doi: 10.1093/infdis/123.3.307. [DOI] [PubMed] [Google Scholar]
- Dolin R., Levy A. G., Wyatt R. G., Thornhill T. S., Gardner J. D. Viral gastroenteritis induced by the Hawaii agent. Jejunal histopathology and serologic response. Am J Med. 1975 Dec;59(6):761–768. doi: 10.1016/0002-9343(75)90461-1. [DOI] [PubMed] [Google Scholar]
- Dolin R. Norwalk agent-like particles associated with gastroenteritis in human beings. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):615–619. [PubMed] [Google Scholar]
- Dolin R., Reichman R. C., Roessner K. D., Tralka T. S., Schooley R. T., Gary W., Morens D. Detection by immune electron microscopy of the Snow Mountain agent of acute viral gastroenteritis. J Infect Dis. 1982 Aug;146(2):184–189. doi: 10.1093/infdis/146.2.184. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J. R., Kalica A. R., Wyatt R. G., McAuliffe V. J., Kapikian A. Z., Chanock R. M. Proteins of Norwalk virus. J Virol. 1981 Mar;37(3):994–999. doi: 10.1128/jvi.37.3.994-999.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Wyatt R. G., Valdesuso J., Kalica A. R., London W. T., Chanock R. M., Kapikian A. Z. Solid-phase microtiter radioimmunoassay for detection of the Norwalk strain of acute nonbacterial, epidemic gastroenteritis virus and its antibodies. J Med Virol. 1978;2(2):97–108. doi: 10.1002/jmv.1890020204. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Gerin J. L., Wyatt R. G., Thornhill T. S., Chanock R. M. Density in cesium chloride of the 27 nm "8FIIa" particle associated with acute infectious nonbacterial gastroenteritis: determination by ultra-centrifugation and immune electron microscopy. Proc Soc Exp Biol Med. 1973 Mar;142(3):874–877. doi: 10.3181/00379727-142-37135. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Dolin R., Thornhill T. S., Kalica A. R., Chanock R. M. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972 Nov;10(5):1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morens D. M., Zweighaft R. M., Vernon T. M., Gary G. W., Eslien J. J., Wood B. T., Holman R. C., Dolin R. A waterborne outbreak of gastroenteritis with secondary person-to-person spread. Association with a viral agent. Lancet. 1979 May 5;1(8123):964–966. doi: 10.1016/s0140-6736(79)91734-3. [DOI] [PubMed] [Google Scholar]
- Oshiro L. S., Haley C. E., Roberto R. R., Riggs J. L., Croughan M., Greenberg H., Kapikian A. A 27-nm virus isolated during an outbreak of acute infectious nonbacterial gastroenteritis in a convalescent hospital: a possible new serotype. J Infect Dis. 1981 Jun;143(6):791–795. doi: 10.1093/infdis/143.6.791. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Bachrach H. L., Brown F., Gillespie J. H., Burroughs J. N., Madin S. H., Madeley C. R., Povey R. C., Scott F., Smith A. W. Caliciviridae. Intervirology. 1980;14(1):1–6. doi: 10.1159/000149155. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Terashima H., Chiba S., Sakuma Y., Kogasaka R., Nakata S., Minami R., Horino K., Nakao T. The polypeptide of a human calicivirus. Arch Virol. 1983;78(1-2):1–7. doi: 10.1007/BF01310853. [DOI] [PubMed] [Google Scholar]
- Thornhill T. S., Wyatt R. G., Kalica A. R., Dolin R., Chanock R. M., Kapikian A. Z. Detection by immune electron microscopy of 26- to 27-nm viruslike particles associated with two family outbreaks of gastroenteritis. J Infect Dis. 1977 Jan;135(1):20–27. doi: 10.1093/infdis/135.1.20. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., Dolin R., Blacklow N. R., DuPont H. L., Buscho R. F., Thornhill T. S., Kapikian A. Z., Chanock R. M. Comparison of three agents of acute infectious nonbacterial gastroenteritis by cross-challenge in volunteers. J Infect Dis. 1974 Jun;129(6):709–714. doi: 10.1093/infdis/129.6.709. [DOI] [PubMed] [Google Scholar]