Abstract
In multicellular epithelial tissues, the orientation of polarity of each cell must be coordinated. Previously, we reported that for Madin–Darby canine kidney cells in three-dimensional collagen gel culture, blockade of β1-integrin by the AIIB2 antibody or expression of dominant-negative Rac1N17 led to an inversion of polarity, such that the apical surfaces of the cells were misorientated towards the extracellular matrix. Here, we show that this process results from the activation of RhoA. Knockdown of RhoA by short hairpin RNA reverses the inverted orientation of polarity, resulting in normal cysts. Inhibition of RhoA downstream effectors, Rho kinase (ROCK I) and myosin II, has similar effects. We conclude that the RhoA–ROCK I–myosin II pathway controls the inversion of orientation of epithelial polarity caused by AIIB2 or Rac1N17. These results might be relevant to the hyperactivation of RhoA and disruption of normal polarity frequently observed in human epithelial cancers.
Keywords: β1-integrin, epithelial polarity, myosin II, RhoA, ROCK I
Introduction
Epithelial cells are highly polarized. In a simple tube or cyst, all of the epithelial cells are orientated so that their apical surfaces face a central lumen (O'Brien et al, 2002), and basolateral surfaces are in contact with other cells and the basement membrane. The establishment and maintenance of polarity are crucial to the development and function of epithelial tissues. Disruption of polarity is an important feature of epithelial cancers that accounts for more than 90% of fatal malignancies in adults. Although how the orientation of epithelial polarity is determined is a crucial issue, its recognition as a process that is distinct from the establishment of polarity has been poorly appreciated. One reason is that in conventional culture experiments, cells are usually on an exogenous support such as glass or plastic that provides a strong cue for the cell to orientate its apical surface away from the support. Although genetic screens have identified many genes, the disruption of which leads to loss of epithelial polarity, few if any mutations have been identified in which polarity is present but misorientated (Shin et al, 2005; Goldstein & Macara, 2007). By contrast, chemotaxing cells have long been recognized to be able to rapidly change the orientation of their polarity (Weiner, 2002).
To study the orientation of epithelial polarity, we grew cells in three-dimensional culture in gels of extracellular matrix (ECM) such as collagen I (COLI). When Madin–Darby canine kidney (MDCK) cells are plated in gels, they form cysts consisting of a monolayer of epithelial cells with the apical surfaces facing the central lumen (Montesano et al, 1991). Cells are initially in an isotropic environment that lacks external cues for orientation; therefore, this system is more sensitive to perturbations that alter the orientation of polarity. Previously, we reported that a dominant-negative allele of Rac1 (Rac1N17) caused inversion of polarity such that the apical surface of the cells was misorientated towards the ECM (O'Brien et al, 2001). By contrast, when MDCK cells were grown as a two-dimensional layer on filters, expression of Rac1N17 had only minor effects on polarity. The inversion of polarity in Rac1N17 cysts in three-dimensional culture is different from partial or complete loss of polarity. For example, perturbation of atypical protein kinase C (aPKC) or cell division cycle 42 (Cdc42) inhibits formation of an apical surface and causes cells to accumulate apical proteins in intracellular vacuoles (Martin-Belmonte et al, 2007) in contrast to the mislocalization of apical proteins to the periphery of Rac1N17 cysts.
The orientation of polarity depends on the interaction of cells with the ECM. Cells normally form basolateral surfaces where the ECM is located, and the apical surface is orientated away from the ECM. In order for the cells to sense the ECM, they require integrins, heterodimers of α- and β-subunits. Previously, we reported that interaction of MDCK cells with COLI causes activation of Rac1 (Yu et al, 2005). AIIB2, a β1-integrin function-blocking antibody, prevented activation of Rac1 and led to the inversion of polarity orientation. This suggested that in the presence of AIIB2, the surface interacting with the ECM did not receive the integrin signal from the ECM and thus became the apical surface. The AIIB2 phenotype is similar to that of Rac1N17, suggesting that Rac1 and β1-integrin are needed for normal orientation of polarity. In many systems, Rac1 acts in opposition to RhoA (Schmitz et al, 2000), and therefore decreased Rac1 activity might be expected to lead to increased RhoA activity. Increased RhoA activity is found in many human cancers (Sahai & Marshall, 2002; Gomez del Pulgar et al, 2005), and elevated RhoA activity causes the fibroblast to fail to establish polarity during wound healing (Arthur & Burridge, 2001; Omelchenko et al, 2003). Here, we provide evidence that inversion of polarity caused by the blockade of β1-integrin results from the inappropriate activation of RhoA and can be restored by inhibition of RhoA or its downstream effectors, Rho kinase (ROCK I) or myosin II.
Results
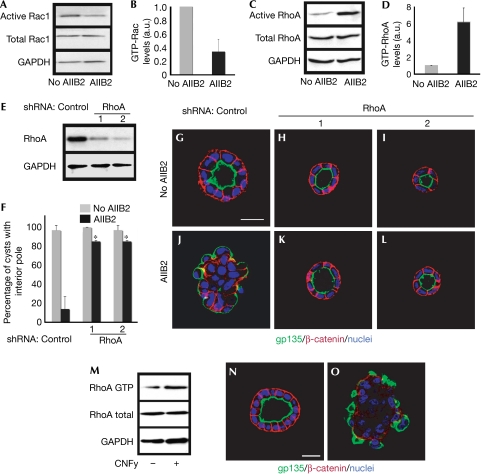
MDCK cells grown in COLI matrix form hollow cysts with an apical interior pole. Blocking of β1-integrin function by AIIB2 treatment causes an inversion of polarity (Yu et al, 2005) and a decrease in Rac1 activity in cysts (Fig 1A,B). We also observed that AIIB2 added within 3 days, but not after 4 days, resulted in inverted cysts (data not shown). Knockdown of β1-integrin by using RNA interference (RNAi) also caused inverted cysts (supplementary Fig S1 online). Expression of a constitutively active form of Rac1 (Rac1V12) decreased the RhoA GTP level (supplementary Fig S2 online); therefore, we proposed that blocking of β1-integrin by AIIB2 might in turn activate RhoA. AIIB2 gave a sixfold increase in RhoA GTP level (Fig 1C,D); this suggested the potential involvement of RhoA in AIIB2-induced inversion of polarity. To test this, we knocked down RhoA by stable short hairpin RNA (shRNA), choosing clones in which endogenous RhoA was reduced to 23% or 13% of control levels (Fig 1E, clones 1 and 2). In COLI, these cells formed cysts with normal polarity, as indicated by gp135 at the apical luminal surface and β-catenin at the basolateral surface (Fig 1H,I), which was similar to control cysts (Fig 1G). Depletion of RhoA had no effect on Rac1 activity in three-dimensional cultures of these cells (data not shown). In the presence of AIIB2, about 85% of RhoA-depleted cysts (clones 1 and 2) had normal polarity (Fig 1F,K,L); however, only 14% of AIIB2 cysts without RhoA RNAi had normal polarity (Fig 1F,J). Knockdown of RhoA by RNAi thus largely restored the inverted polarity of AIIB2. These data are consistent with the hypothesis that blocking of β1-integrin increases RhoA activity, which, in turn, results in cysts with inverted polarity.
Figure 1.
Loss of RhoA function rescues the phenotype caused by AIIB2 treatment. (A,B) Treatment with AIIB2 decreases Rac1 activity, measured by GST-Pak3-CRIB pull-down assay. (C,D) RhoA activity is assessed by a GST-RBD-Rhotekin pull-down assay. (E) Reduction of RhoA with shRNA in two clones shown by immunoblot. (F) Quantification of cysts with normal polarity in cells transfected with control shRNA or RhoA shRNA in the absence or presence of AIIB2. A total of 100 cysts per condition were counted from three experiments; *P<0.001. (G–L) Representative confocal images. The two selected clones were plated in COLI for 5 days. Normal polarity was determined by gp135 (green) at the luminal surface and by β-catenin (red) at the BL surface; nuclei are shown in blue. Scale bars 20 μm. (M–O) Activation of RhoA causes inverted cysts. (M) Activation of RhoA by CNFy treatment is assessed by GST-Rhotekin pull-down assay. CNFy results in cysts with inverted polarity, which was indicated by gp135 at the peripheral surface (green) and β-catenin (red) at the BL surface (O, compare normal cyst in N); nuclei are shown in blue. a.u., arbitrary units; BL, basolateral; COLI, collagen I; CNFy, Yersinia pseudotuberculosis cytotoxic necrotizing factor; CRIB, Cdc42/Rac interactive binding; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GST, glutathione S-transferase; Pak3, p21 activated kinase 3; RBD, Ras binding domain; shRNA, short hairpin RNA.
We proposed that the activation of RhoA mimics the AIIB2 phenotype. The RhoA-activating toxin, Yersinia pseudotuberculosis cytotoxic necrotizing factor (CNFy), was added to cells at the time of plating in COLI (Hoffmann et al, 2004). RhoA activation was confirmed by pull-down assay (Fig 1M). Overall, 80% of CNFy-treated cysts were inverted (Fig 1O), which was similar to that seen with AIIB2 treatment. RhoA activity also had an effect on cell number, consistent with the known effects of RhoA on the cell cycle (Villalonga & Ridley, 2006). Here, we focused on the effects of RhoA on polarity.
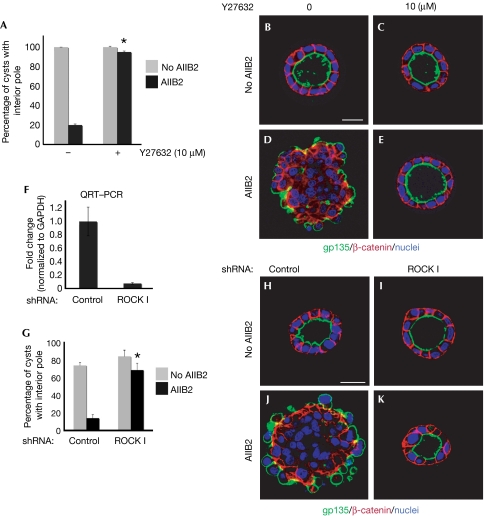
A principal class of RhoA effectors is ROCK (RhoA kinase). We tested whether inhibition of ROCK phenocopied RhoA depletion. Pharmacological inhibitor of ROCK I/II (Y27632, 10 μm) did not affect normal cyst formation (Fig 2A,C) but almost completely restored polarity in the presence of AIIB2—that is, 95% of cysts treated with Y27632 had normal polarity with a central lumen (Fig 2E, quantification in A) compared with 20% in the absence of Y27632 (Fig 2A,D). The addition of Y27632 to 3-day-old AIIB2 cysts also resulted in cysts with an interior pole (data not shown). There are two ubiquitous ROCK family members, ROCK I and ROCK II (Yoneda et al, 2005, 2007). To test respective functions, we depleted ROCK I or ROCK II by stable shRNA. Reduction of endogenous ROCK I and ROCK II was confirmed by using quantitative reverse transcription–PCR (QRT–PCR; Fig 2F) or immunoblot analysis (supplementary Fig S3A online). Knockdown of ROCK I or ROCK II resulted in cysts with normal polarity, as indicated by gp135 at the central lumen and β-catenin at the basolateral surface (Fig 2I; supplementary Fig S3C online). With AIIB2, 68% of ROCK I knockdown cells formed cysts with normal polarity (Fig 2G,K), whereas ROCK II knockdown cells formed inverted cysts (supplementary Fig S3E online), indicating that ROCK I but not ROCK II is downstream from RhoA in polarity orientation.
Figure 2.
Depletion of ROCK I rescues AIIB2 polarity. (A) Quantification of cysts with normal polarity in cells treated with Y27632; *P<0.001. (B–E) Representative confocal images of cysts stained with gp135 (green) and β-catenin (red); nuclei are shown in blue. (F–K) Depletion of ROCK I rescues AIIB2-induced phenotype. (F) Reduction of ROCK I expression level by RNAi is confirmed by QRT–PCR. (G) Quantification of cysts with normal polarity in cells transfected with shRNA to ROCK I; n=3; *P<0.001. (H–J) Confocal images of representative cysts. Cells infected with lentivirus for control or ROCK I were plated in COLI for 5 days and stained with gp135 (green) and β-catenin (red); nuclei are shown in blue. Scale bars, 20 μm. COLI, collagen I; QRT–PCR, quantitative reverse transcription–PCR; RNAi, RNA interference; ROCK, Rho kinase; shRNA, short hairpin RNA.
ROCK enhances myosin II activity by phosphorylating the regulatory myosin light chain (MLC) and inhibiting MLC phosphatases, which inhibit MLC phosphorylation. We tested whether inhibition of β1-integrin affects MLC activity and localization in cysts. In AIIB2 cysts, phosphorylation of MLC (p-MLC) was increased 1.6-fold (Fig 3A,B). In agreement with previous studies, in unperturbed cysts, p-MLC was localized mainly at the basal membrane; some were along the lateral membrane and near the tight junction (Fig 3C; Yu et al, 2003). In AIIB2-treated cysts, p-MLC was instead mostly in small puncta throughout much of the cytoplasm (Fig 3D).
Figure 3.
Inhibition of myosin activity rescues the phenotype induced by AIIB2 treatment. (A,B) MLC activity was analysed by Western blot of cysts for p-MLC against rabbit p-MLC at Ser 19. GAPDH acts as a loading control. (C,D) Confocal micrographs of p-MLC. (C) In normal cysts, p-MLC is localized mainly at the basal plasma membrane, some p-MLC are in the region of TJ. (D) In AIIB2-treated cysts, p-MLC is mislocalized. (E–J) Inhibition of myosin activity by blebbistatin rescues AIIB2-induced phenotype, as indicated by the apical marker gp135 (green in H) located at the luminal surface, and β-catenin (red in H) located peripherally and at the cell–cell contact (compare F). Note that blebbistatin treatment results in normal distribution of p-MLC in the presence of AIIB2 (J). Scale bars, 20 μm. MLC, myosin light chain; p-MLC, phosphorylation of MLC; TJ, tight junction.
To test whether inhibition of myosin II activity could rescue the AIIB2 phenotype, we treated AIIB2 cysts with blebbistatin, an inhibitor of myosin II (Straight et al, 2003). In the presence of blebbistatin, 70% of AIIB2 cysts showed normal polarity with a central lumen lined by apical surfaces, as indicated by gp135 (Fig 3H), whereas in the absence of blebbistatin only 20% of AIIB2 cysts had normal polarity (Fig 3F). Blebbistatin alone at 50 μM had no effect on cyst polarity (Fig 3G) or localization of p-MLC (Fig 3I). Blebbistatin rescued localization of p-MLC in AIIB2 cysts (compare Fig 3C and J); this suggests that the localization of p-MLC might be a marker of polarity. We also found that the depletion of RhoA and ROCK I by shRNA did not affect the localization of p-MLC (supplementary Fig S4A–D online). These data support the hypothesis that myosin II acts downstream from ROCK and is involved in the inversion of polarity owing to β1-integrin blockade.
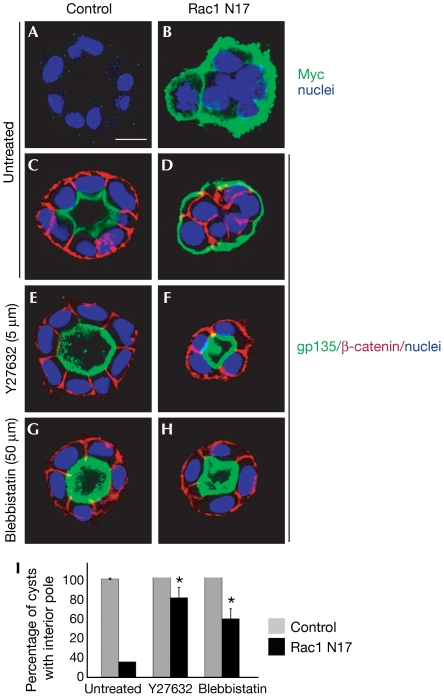
Loss of β1-integrin or Rac1 function results in inversion of polarity. As inhibition of ROCK (Y27632) or myosin II (blebbistatin) could rescue the inversion of polarity caused by β1-integrin blockade, we tested whether Y27632 or blebbistatin could rescue the inversion of polarity caused by Rac1N17, which is controlled by the TET-off system. The expression of Rac1N17 was confirmed by staining for the Myc tag (Fig 4A,B) and Western blotting (data not shown). As reported previously, 98% of control cysts (suppression of Rac1N17) showed normal polarity (luminal gp135, basolateral β-catenin; Fig 4C). The induction of Rac1N17 expression (removal of doxycycline) produced 82% of cysts with inverted polarity (peripheral gp135, no peripheral β-catenin; Fig 4D,I) and diffuse p-MLC distribution (supplementary Fig S4F online). When treated with a ROCK inhibitor (Y27632, 5 μM), about 80% of Rac1N17 cysts showed normal polarity (Fig 4F,I). Similarly, blebbistatin also restored the orientation of polarity in Rac1N17 cysts, with about 60% cysts having normal polarity (Fig 4H,I).
Figure 4.
Inhibition of ROCK or myosin II reverts Rac1N17 cysts. MDCK cells were stably transfected with tetracycline-repressible Myc-Rac1N17, which is expressed by removal of doxycycline in (B,D,F,H). (A,B) Immunostaining of Myc (green) confirms expression of exogenous Rac1N17 in (B) compared with negative control in (A). Inverted polarity is indicated by gp135 at the periphery and β-catenin at the cell–cell contact when Rac1N17 is expressed (D), but not in control cysts (C). Inhibition of ROCK by Y27632 (F) or myosin activity by blebbistatin (H) reverts the inverted orientation of polarity. (C–H) gp135 is green, β-catenin is red and nuclei are blue. (I) Quantification of polarity in Rac1N17 cysts by treatment with Y27632 or blebbistatin; *P<0.001. Scale bar, 10 μm. MDCK, Madin–Darby canine kidney cells; ROCK, Rho kinase.
Phosphatidylinositol 3,4,5-trisphosphate (PIP3) is found at the basolateral surface in MDCK cysts (Yu et al, 2003) and is important in regulating this surface. First, we tested whether AIIB2 treatment disrupts the basolateral distribution of PIP3. In cysts expressing a fluorescent reporter for PIP3 (green fluorescent protein (GFP)-PH-Akt), we found that GFP-PH-Akt was excluded from the periphery in the presence of AIIB2 (supplementary Fig S5B,B′ online, compare with A,A′). This is consistent with the inversion of polarity caused by AIIB2 treatment—that is, the peripheral surface of the cyst lacks basolateral markers. Next, we also found that inhibition of ROCK (Y27632) or myosin II (blebbistatin) rescued the inverted localization of PIP3, so that GFP-PH-Akt localized at the basolateral plasma membrane (supplementary Fig S5C,D′ online).
We also examined the localization of PIP3 in Rac1N17 cysts. In control cysts, GFP-PH-Akt was localized at the basolateral surface (supplementary Fig S6A,A′ online) and absent from the luminal surface. In Rac1N17 cysts, GFP-PH-Akt was absent from the peripheral surface and was observed only at cell–cell contacts (supplementary Fig S6B,B′ online). However, in Rac1N 17 cysts that were treated with Y27632 or blebbistatin, GFP-PH-Akt was at the basolateral surface (supplementary Fig S6C,D′ online) and absent from the luminal surface, where gp135 is usually found instead (Fig 4H,J).
Discussion
Orientation of polarity has long been appreciated in chemotaxing cells, which rapidly change their orientation as the source of chemoattractant moves. However, for epithelial cells, the orientation of polarity has been considered to be linked to the establishment of polarity, which is initiated by the interaction of the cell with the ECM and other cells. Recently, we have shown that PIP3, which is the first biochemical marker of polarity in chemotaxing cells, also functions to determine the location of the basolateral surface of the cell (Gassama-Diagne et al, 2006). By studying epithelial cells grown in three-dimensional ECM gels, we have been able to uncouple the establishment of polarity from the orientation of polarity (O'Brien et al, 2001). Specifically, the expression of Rac1N17 or the blockade of β1-integrin results in MDCK cysts with an inverted polarity—that is, with the apical surface of the cells orientated towards the ECM. Inversion of epithelial polarity is also observed in MDCK cysts grown in suspension without contact with the ECM or supports. When suspension-grown cysts are placed into the ECM, they reorientate their polarity to form cysts with central lumens lined by apical surfaces (Wang et al, 1990; Liu et al, 2007). Taking into consideration these and previous work, we can see the emergence of a pathway that controls the orientation of polarity. COLI in the ECM interacts with β1-integrin, causing the activation of Rac1. Eventually, this leads to the orientation of polarity such that the apical surface and lumen are positioned at the opposite end of the cell from the ECM. Much of our understanding of this pathway comes from conditions that lead to the inversion of orientation of polarity—that is, Rac1N17 or β1-integrin blockade.
Here, we have shown that β1-integrin blockade causes activation of RhoA. Activation of RhoA by this mechanism or by CNFy causes an inversion of orientation of polarity, as does the expression of Rac1N17. The inhibition of RhoA or ROCK I (but not ROCK II) or myosin II abolishes the effects of β1-integrin blockade or expression of Rac1N17, thus restoring the normal orientation of polarity. These data suggest that the pathway of RhoA → ROCK I → myosin II is activated when Rac1 or β1-integrin is inhibited, and activation of this pathway is responsible for the inversion of polarity. This is consistent with the findings from many other systems that RhoA and Rac1 are often in opposition.
RhoA activity is often increased in many types of epithelial cancer. One cause of increased Rho activity is loss of heterozygosity of DLC1 (deleted in liver cancer 1), a GTPase-activating protein for RhoA and a tumour suppressor (Wong et al, 2005; Durkin et al, 2007). Loss of DLC1 is frequently found in many cancers, and leads to increased proliferation and neoplasia. Another RhoA GAP, p190RhoAGAP, downregulates RhoA GTP in normal breast development. These observations suggest that the suppression of RhoA is a normal part of polarity development. Here, we have shown that the activation of RhoA by β1-integrin blockade, knockdown or by CNFy treatment produces cysts with several characteristics of malignancy, including increased proliferation and loss of normal polarization and tissue architecture. Moreover, our findings that the blockade of the Rho–ROCK–myosin II pathway can rescue the effects of RhoA activation and restore normal polarization, are a step towards prophylaxis and therapy of malignancy.
Note that the ROCK inhibitor must be added within 3 days after the cells are placed in ECM with AIIB2 to restore normal polarization. It is possible that inhibition of the ROCK pathway prevents, rather than rescues, misorientation; this distinction might be important mechanistically. A non-mutually exclusive explanation is that after 3 days the cysts have stabilized their polarization, so that the addition of Y27632 can no longer rescue misorientation. In support of this stabilization idea, the addition of AIIB2 alone in the window of 0–3 days results in inverted polarization, but later addition does not.
In AIIB2 cysts, the cells show some heterogeneity of polarity: cells at the periphery of the cyst have their apical surfaces facing outwards. Perhaps when β1-integrin signalling is blocked, these cells sense this as a ‘free' surface. By contrast, cells in the centre are surrounded by cell–cell contacts and might be less able to form an apical surface.
The pathway that controls orientation of polarity must somehow cause the correct positioning of the machinery that forms the apical surface and lumen. Recently, we described a pathway for the formation of the apical surface in MDCK cysts. Phosphatidylinositol 4,5-bisphosphate (PIP2) is enriched at the apical surface, from which PIP3 is excluded. This distribution is established by the lipid phosphatase PTEN (phosphatase and tensin homologue on chromosome 10), which localizes to the apical surface and converts PIP3 to PIP2. The apical PIP2 acts through annexin 2 to recruit Cdc42, which acts in several ways to form the apical surface. For example, Cdc42 controls the subapical actin cytoskeleton. Cdc42 also interacts with Par3, Par6 and aPKC, a master polarity complex. The involvement of Rac1, RhoA and its effectors suggests that actin is important in the orientation of polarity and perhaps in the localization of the Par3–Par6–aPKC complex. Par3 and Par6 were discovered as regulators of polarization of the Caenorhabditis elegans zygote. Actin and myosin cause the movement of the Par3–Par6–aPKC complex to generate their polarized localization after fertilization (Cowan & Hyman, 2007). Perhaps some aspects of this mechanism are conserved in the localization of the Par3–Par6–aPKC complex in epithelia.
Recently, ROCK was shown to phosphorylate Par3, disrupting its interaction with Par6 and PKC (Nakayama et al, 2008). It is possible that the activation of ROCK in our inversion system and the consequent phosphorylation of Par3 and disruption of its interactions with aPKC and Par6 might account for our polarity alterations. However, the inhibition of myosin II by blebbistatin restores inversion of polarity (although not as completely as inhibition of ROCK). As myosin II is downstream from ROCK, it is unlikely that the phosphorylation of Par3 by ROCK accounts for more than a portion of the effects on inversion of polarity reported here.
Methods
Antibodies and reagents have been described previously (Yu et al, 2003, 2005). Mouse anti-RhoA was obtained from Santa Cruz (Santa Cruz, CA, USA) and blebbistatin was from Toronto Research Chem (North York, ON, Canada). Cells were grown and imaged as described previously (Yu et al, 2003, 2005).
Treatment of cysts with reagents. AIIB2, Y27632, blebbistatin and CNFy were added when MDCK cells were plated in COLI; CNFy was used at 0.3 ng/ml.
Rac and RhoA activation assay. To measure GTP-Rac1 and RhoA levels, 1–5 × 106 cells were embedded in COLI gel for 24 h. Samples were rinsed with cold PBS+, weighed to determine total volume and transferred to a vial containing the same volume of Mg2+ lysis buffer (50 mM HEPES, 300 mM NaCl, 20 mM MgCl2, 10% glycerol, 2% Igepal CA-630 and 2 mM EDTA). The samples were incubated on ice with occasional inversion for 20 min, and centrifuged for 5 min at 13,200g to remove collagen fragments and debris. A 50 μl portion of supernatant was set aside for determination of total Rac1 and RhoA separately, and equal volumes of the remaining supernatant were used to determine GTP loading on Rac1 and RhoA by using a pull-down assay with GST-Pak3-CRIB and GST-Rhoteckin-CRIB beads (Upstate, Lake Placid, NY, USA) using the manufacturer's protocols.
RNA interference. The RhoA RNAi construct was made by amplifying a 97-mer oligonucleotide (supplementary Table S1 online). The PCR product was purified, digested with XhoI and EcoRI, and cloned into XhoI and EcoRI sites of the pPRIME-CMV-Neo lentiviral vector (Stegmeier et al, 2005). This was sequenced and used to co-transfect human embryonic kidney 293 FT cells with Virapower lentiviral packaging mix (Invitrogen, Carlsbad, CA, USA). The next day, transfection complexes were removed and cells were allowed to produce virus for 48 h. Media containing virus were collected and used to directly transduce MDCK cells overnight. The cells were allowed to recover for 24 h and then 400 μg/ml geneticin (Invitrogen) was added to select for cells infected with RhoA shRNA lentivirus. RhoA shRNA-positive cells were cloned and screened for RhoA protein levels by immunoblotting.
The ROCK isoform and β1-integrin shRNA constructs were made by annealing and ligating appropriate oligonucleotides (supplementary Table S1 online) into the pLKO.1-puro cloning vector (details at www.addgene.org). The constructs were sequenced before the production of viral particles as described above, except that the cells were infected with lentivirus for 24 h and subjected to puromycin selection (4 μg/ml). Silencing of ROCK and β1-integrin isoforms was confirmed by QRT–PCR and/or western blot.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Figures and Table
Acknowledgments
This study was supported by a fellowship from the National Kidney Foundation to W.Y., National Institutes of Health grants to K.E.M., Susan G. Komen Breast Cancer Fellowship to D.M.B., C.J. Martin Fellowship from the National Health and Medical Research Council Australia to A.M.S. and NIHK08DK68358 to P.B.
Footnotes
The authors declare that they have no conflict of interest.
References
- Arthur WT, Burridge K (2001) RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell 12: 2711–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CR, Hyman AA (2007) Acto-myosin reorganization and PAR polarity in C. elegans. Development 134: 1035–1043 [DOI] [PubMed] [Google Scholar]
- Durkin ME, Yuan BZ, Zhou X, Zimonjic DB, Lowy DR, Thorgeirsson SS, Popescu NC (2007) DLC-1: a Rho GTPase-activating protein and tumour suppressor. J Cell Mol Med 11: 1185–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K (2006) Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8: 963–970 [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG (2007) The PAR proteins: fundamental players in animal cell polarization. Dev Cell 13: 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez del Pulgar T, Benitah SA, Valeron PF, Espina C, Lacal JC (2005) Rho GTPase expression in tumourigenesis: evidence for a significant link. BioEssays 27: 602–613 [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Pop M, Leemhuis J, Schirmer J, Aktories K, Schmidt G (2004) The Yersinia pseudotuberculosis cytotoxic necrotizing factor (CNFY) selectively activates RhoA. J Biol Chem 279: 16026–16032 [DOI] [PubMed] [Google Scholar]
- Liu KD, Datta A, Yu W, Brakeman PR, Jou TS, Matthay MA, Mostov KE (2007) Rac1 is required for reorientation of polarity and lumen formation through a PI 3-kinase-dependent pathway. Am J Physiol Renal Physiol 293: F1633–F1640 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K (2007) PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128: 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Schaller G, Orci L (1991) Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell 66: 697–711 [DOI] [PubMed] [Google Scholar]
- Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K (2008) Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell 14: 205–215 [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE (2001) Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol 3: 831–838 [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Zegers MMP, Mostov KE (2002) Opinion: building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3: 531–537 [DOI] [PubMed] [Google Scholar]
- Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM (2003) Rho-dependent formation of epithelial ‘leader' cells during wound healing. Proc Natl Acad Sci USA 100: 10788–10793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ (2002) RHO-GTPases and cancer. Nat Rev Cancer 2: 133–142 [DOI] [PubMed] [Google Scholar]
- Schmitz AA, Govek EE, Bottner B, Van Aelst L (2000) Rho GTPases: signaling, migration, and invasion. Exp Cell Res 261: 1–12 [DOI] [PubMed] [Google Scholar]
- Shin K, Straight S, Margolis B (2005) PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J Cell Biol 168: 705–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ (2005) A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc Natl Acad Sci USA 102: 13212–13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ (2003) Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299: 1743–1747 [DOI] [PubMed] [Google Scholar]
- Villalonga P, Ridley AJ (2006) Rho GTPases and cell cycle control. Growth Factors 24: 159–164 [DOI] [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ (1990) Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell–cell and cell–substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci 95: 137–151 [DOI] [PubMed] [Google Scholar]
- Weiner OD (2002) Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr Opin Cell Biol 14: 196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CM, Yam JW, Ching YP, Yau TO, Leung TH, Jin DY, Ng IO (2005) Rho GTPase-activating protein deleted in liver cancer suppresses cell proliferation and invasion in hepatocellular carcinoma. Cancer Res 65: 8861–8868 [DOI] [PubMed] [Google Scholar]
- Yoneda A, Multhaupt HA, Couchman JR (2005) The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol 170: 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Ushakov D, Multhaupt HA, Couchman JR (2007) Fibronectin matrix assembly requires distinct contributions from Rho kinases I and II. Mol Biol Cell 18: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, O'Brien LE, Wang F, Bourne H, Mostov KE, Zegers MM (2003) Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol Biol Cell 14: 748–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O'Brien L, Mak G, Jou T, Matlin KS, Mostov KE, Zegers M (2005) β1-integrin orients epithelial polarity via Rac 1 and laminin. Mol Biol Cell 16: 433–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Figures and Table