Abstract
We have previously reported that royal jelly (RJ) from honeybees (Apis mellifera) has weak estrogenic activity mediated by interaction with estrogen receptors that leads to changes in gene expression and cell proliferation. In this study, we isolated four compounds from RJ that exhibit estrogenic activity as evaluated by a ligand-binding assay for the estrogen receptor (ER) β. These compounds were identified as 10-hydroxy-trans-2-decenoic acid, 10-hydroxydecanoic acid, trans-2-decenoic acid and 24-methylenecholesterol. All these compounds inhibited binding of 17β-estradiol to ERβ, although more weakly than diethylstilbestrol or phytoestrogens. However, these compounds had little or no effect on the binding of 17β-estradiol to ERα. Expression assays suggested that these compounds activated ER, as evidenced by enhanced transcription of a reporter gene containing an estrogen-responsive element. Treatment of MCF-7 cells with these compounds enhanced their proliferation, but concomitant treatment with tamoxifen blocked this effect. Exposure of immature rats to these compounds by subcutaneous injection induced mild hypertrophy of the luminal epithelium of the uterus, but was not associated with an increase in uterine weight. These findings provide evidence that these compounds contribute to the estrogenic effect of RJ.
Keywords: Apis mellifera, 2-Decenoic acid, 10-Hydroxydecanoic acid, 10-Hydroxy-2-decenoic acid, 24-Methylenecholesterol
Introduction
Post-menopausal women are at increased risk for many health problems, such as hot flashes, reduced bone density, mood swings and cardiovascular diseases (1). The most likely cause of these problems is estrogen deficiency. Menopausal disorders are conventionally treated with synthetic estrogens. However, conventional hormone replacement therapy is suspected to increase the risk of breast cancer and to cause other undesirable side effects, such as breast tenderness and uterine bleeding (2,3). Because of these undesirable side effects, many women seek more natural alternatives, such as the so-called phytoestrogens. These alternatives include soybean products that contain estrogen-like molecules such as daidzein and genistein. Soybeans and soybean-derived products have recently become common additives in health foods and beverages. Phytoestrogens are similar to mammalian estrogens both structurally and functionally, and are considered to prevent menopausal symptoms (4,5).
In addition to soybean products, royal jelly (RJ) has become a common additive in health foods and beverages. RJ is a viscous substance secreted by the hypopharyngeal and mandibular glands of worker honeybees (Apis mellifera) and is an essential food for both the queen and her larvae. RJ is a mixture of many constituents including proteins, free amino acids, lipids, vitamins and sugars (6). 10-hydroxy-trans-2-decenoic acid is a characteristic constituent of RJ. RJ has been reported to exhibit physiological and pharmacological effects in mammals, including vasodilative and hypotensive activities, antihypercholesterolemic activity and anti-inflammatory functions (7). In addition to these activities, RJ has been suggested to improve menopausal symptoms. RJ has been shown to alleviate the so-called autonomic imbalance in menopausal women (8,9), leading to the hypothesis that RJ or its components have effects on gene expression similar to, or mimic, the effects of estrogen. Our previous studies in vitro and in vivo have shown that RJ exhibits weak estrogenic activities mediated by interaction with estrogen receptors (ERs) that leads to changes in gene expression and cell proliferation (10,11). A better understanding of the components of RJ that mediate the induction of estrogenic effects could provide the basis for the development of new natural alternative medicines and health food additives. In the present study, therefore, we isolated four ERβ-binding compounds from RJ and examined estrogenic activity of these compounds.
Methods
Materials
Fresh RJ was collected from A. mellifera L. fed primarily on nectar and pollen from Brassicaceae Brassica campestris L. in the Yangtze valley in the People's Republic of China. 17β-estradiol (E2), 17α-ethynylestradiol (17αEE2), tamoxifen and 10-hydroxydecanoic acid (10HDA), were purchased from Sigma (St Louis, MO, USA); 10-hydroxy-trans-2-decenoic acid (10H2DA) was obtained from Shiono Koryo Kaisha, Ltd (Osaka, Japan); trans-2-decenoic acid (2DEA) was from Wako Pure Chemical Industries, Ltd (Osaka, Japan).
Ligand Binding Assay for Estrogen Receptors (ERs)
The ability of the compounds to compete with E2 for binding to human ERα and ERβ was evaluated and compared with the activity of diethylstilbestrol using the Ligand Screening System Estrogen Receptor (Toyobo Co., Ltd, Osaka, Japan) according to the manufacturer's instruction.
NMR
1H- and 13C-NMR and H-H COSY, HSQC and HMBC spectra were recorded with the MERCURYplus 300NB NMR instrument (Varian, Inc., Palo Alto, CA, USA). The solvent used was deuterium methanol-d4. Tetramethylsilane was used as an internal standard.
Isolation of Active Compounds from RJ
In this study, the following fractionations were performed in combination with the ERβ ligand-binding assay to identify active compounds. A Quantity of 2000 g of fresh RJ with a moisture content of ∼67% was first extracted with 4000 ml of ethanol by stirring for 2 h at room temperature and then filtered to separate the resulting extract into soluble and insoluble fractions. Another 4000 ml of ethanol was then added to the insoluble fraction and stirred for a further 2 h at room temperature. Insoluble precipitates were removed by filtration, and the supernatant was combined with the soluble fraction from the first extraction and evaporated to dryness. The resulting material (385 g) was then extracted with 2000 ml of water : chloroform (1 : 1). After stirring vigorously for 10 min, the mixture was separated into water and chloroform layers. The chloroform layer was collected and evaporated to dryness (72 g) and extracted with 1000 ml of n-hexane. After stirring vigorously for 10 min, the mixture was separated into hexane-soluble and insoluble fractions by centrifugation and each fraction was evaporated to dryness (yield of each fraction was 5.32 g and 66 g, respectively). 5.32 g of the hexane-soluble fraction was dissolved in n-hexane, applied to a column (41 × 300 mm) of silica gel (BW320MH; Fuji Silysia Chemical Ltd, Aichi, Japan) and then successively eluted with 400 ml of n-hexane and 900 ml of chloroform to yield 10 fractions.
The active fractions assessed by ER binding assay were pooled, evaporated to dryness and dissolved in a small amount of chloroform, followed by recrystalization five times using methanol. The resulting crystal was identified as 24-methylenecholesterol (24MET) by NMR analysis. The solution remaining after isolation of the crystallized material was evaporated to dryness, dissolved in chloroform and then applied to a column (34 × 240 mm) of Sephadex LH-20 (Amersham Bioscience Corp., NJ, USA). The column was eluted with 300 ml of chloroform to yield seven fractions. The active fractions were pooled, evaporated to dryness and identified as 2DEA by NMR analysis. Five grams of the evaporated hexane-insoluble fraction was dissolved in 40% methanol and applied to a column (50 × 280 mm) of ODS (Chromatorex; Fuji Silysia Chemical Ltd). The column was eluted with 40% methanol containing 0.1% trifluoroacetic acid to generate seven fractions. We obtained two active fractions and identified the active compound within these fractions as 10H2DA and 10HDA by NMR analysis. The yields of 24MET, 2DEA, 10H2DA and 10HDA were 1.2 g, 3 mg, 1.65 g and 0.53 g, respectively.
Cell Culture and Cell Proliferation Assay
The human breast cancer cell line MCF-7 was provided by the Cell Resource Center for Biomedical Research, Tohoku University, Japan. Cells were maintained in RPMI-1640 (Sigma) containing 10% heat-inactivated FBS (Sigma) at 37°C in a 5% CO2 atmosphere. For cell proliferation assays, cells were seeded in 96-well plates at a density of 10 000 cells/100 μl/well in phenol red-free RPMI-1640 (Sigma) containing 10% charcoal dextran-treated FBS (12). One day later, the medium was changed and cells were incubated with 0.5 nM E2 or RJ components (commercially available 10H2DA, 10HDA and 2DEA; purified 24MET) in the presence or absence of 1 μM tamoxifen. Three to four days after initiation of the treatment, a colorimetric proliferation assay was performed using the MTT Cell Growth Kit as directed by the manufacturer (Chemicon International, Inc., Temecula, CA, USA).
Transfection and Reporter Gene Assay
MCF-7 cells were transfected with pERE-Luc (13; provided by Dr V. C. Jordan of Northwestern University Medical School, Chicago) and the control plasmid pRL-TK Vector (Promega, Madison, WI, USA) as described previously (10). Twenty-four hours after transfection, the cells were washed and incubated in fresh Dulbecco's Modified Eagle Medium (Nissui Pharmaceutical Co., Ltd, Tokyo, Japan) that contained 10% charcoal dextran-treated FBS with 0.1–10 nM E2 or RJ components (commercially available 10H2DA, 10HDA and 2DEA; purified 24MET) for 24 h. A luciferase assay was performed as described previously (10) and the luciferase activities were normalized to control for variations in transfection efficiency using the sea pansy-luciferase internal control and were expressed as fold induction over the control activity. The luciferase activities presented in this study represent the means of the activities obtained following analyses from three independent transfections.
Animal Protocols for In Vivo Studies
Fifteen-day-old female Sprague Dawley rats were obtained from Japan SLC, Inc. (Hamamatsu, Japan) in groups of ∼10 pups per mother. All rats were housed at 23 ± 1°C with 55 ± 10% humidity and fed a standard laboratory rodent diet (CRF-1; Oriental Yeast Co., Ltd, Japan). Starting at post-natal day 20, the female rats were injected subcutaneously with 2 doses of 17αEE2 (0.1 and 3 μg kg−1 day−1) or 1 g kg−1 day−1 RJ or its components (commercially available 10H2DA, 10HDA and 2DEA; purified 24MET) in sesame oil (Nacalai Tesque, Inc., Kyoto, Japan). The rats received 10 ml kg−1 body weight of the control or test solutions once a day for three days. The injections were administered between 10 and 11 A.M. each day. Controls received 10 ml kg−1 of sesame oil once a day for three days. Twenty-seven hours after the last injection on post-natal day 23, the rats were sacrificed and each uterus was rapidly excised by severing just above the vagina, and leaving the ovaries attached. After weighing, the uterus was rapidly fixed in Bouin's solution for histological analysis. All animal studies were conducted in accordance with the internationally accepted principles for laboratory animal use and care (14).
Histology
Uteri from rats in each dose group were fixed in Bouin's solution for 24 h, dehydrated and embedded in paraffin. Serial 8 μm cross sections were made through the uterine horns, and stained with hematoxylin and eosin. The height of the epithelial cells lining the lumen along the uterus was measured in tissue sections from the mid-region of each uterine horn as described previously (15) with slight modification. In brief, the epithelial cell heights in the rats shown in Table 1 were measured in five different areas from five sections at intervals of ∼160 μm from rats shown in Table 1 using a light microscope (Zeiss Axiovert S100TV).
Table 1.
Effects of RJ and its components on uterine wet weight
| Group and dose | N | Body weight (g) |
Uterus (mg) |
Uterus/body weight | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Control | 17 | 48.3 | 4.3 | 26.6 | 4.6 | 0.56 |
| 17αEE2 (0.1 μg kg−1) | 12 | 47.9 | 6.7 | 41.0** | 13.5 | 0.87 |
| 17αEE2 (3 μg kg−1) | 5 | 47.1 | 3.5 | 115** | 11.5 | 2.5 |
| RJ (1 g kg−1) | 12 | 49.1 | 5.5 | 26.9 | 3.6 | 0.55 |
| 10H2DA (1 g kg−1) | 8 | 42.9* | 7.5 | 25.9 | 9.8 | 0.59 |
| 10HDA (1 g kg−1) | 11 | 45.8 | 5.8 | 25.2 | 3.3 | 0.55 |
| 2DEA (1 g kg−1) | 8 | 45.9 | 6.9 | 22.9 | 3.9 | 0.51 |
| 24MET (1 g kg−1) | 8 | 47.5 | 6.2 | 25.9 | 2.9 | 0.55 |
Note: Asterisks indicate values that are significantly different (*P < 0.05; **P < 0.01) from vehicle control values.
Data Analysis
Dunnett's test was performed to assess the significance of differences between control and test groups.
Results
Identification of Estrogenic Compounds from RJ
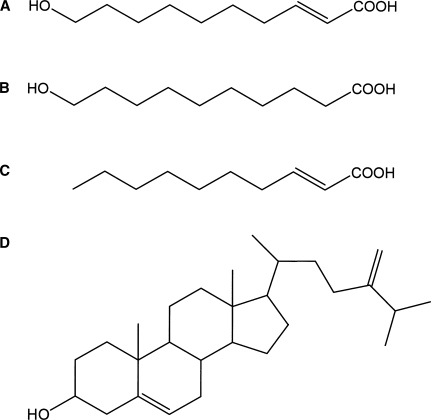
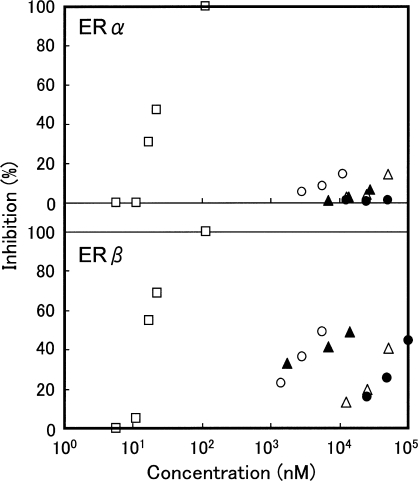
We have previously reported that RJ exhibits estrogenic activities that are mediated by interaction with ERs, leading to changes in gene expressions and cell proliferation (10,11). In this study, we aimed to identify the constituents in RJ responsible for the estrogenic effects using an ERβ ligand-binding assay. We attempted to purify the active constituents in RJ following solvent extraction and distribution, column chromatography and recrystalization. We used NMR analysis to identify four compounds from RJ with affinity for ERβ: 10H2DA, 10HDA, 2DEA and 24MET. The spectra of the purified 10H2DA, 10HDA and 2DEA were consistent with those observed following analysis of the comparable commercially available reagents. The spectrum observed following analysis of the purified 24MET was consistent with that previously reported (16). The structures of the four compounds are shown in Fig. 1. We next evaluated the affinities of 10H2DA, 10HDA, 2DEA or 24MET for ERs by comparing the abilities of these compounds, relative to that of diethylstilbestrol, to compete with E2 for binding to the ERα and ERβ. 10H2DA, 10HDA, 2DEA or 24MET inhibited the binding of E2 to the ERβ in a concentration-dependent manner, but these compounds had little or no effect upon the ability of E2 to bind to ERα (Fig. 2). The IC50 of 10H2DA, 10HDA, 2DEA and 24MET that inhibited binding of E2 to ERβ were estimated to be 90, 140, 17 and 6.0 μM, respectively. The IC50 of diethylstilbestrol to inhibit binding to ERβ under the corresponding conditions was 21 nM. The affinities of RJ components for the ERβ were ∼2–4 orders of magnitude lower than that of diethylstilbestrol.
Figure 1.
Chemical structures of (A) 10-hydroxy-trans-2-decenoic acid (B) 10-hydroxydecanoic acid (C) trans-2-decenoic acid and (D) 24-methylenecholesterol.
Figure 2.
Binding affinities of RJ-derived estrogenic compounds for ERs. The ability of 10H2DA (open triangle), 10HDA (closed circle), 2DEA (closed triangle) and 24MET (open circle) to inhibit E2 binding to ERα and ERβ were evaluated and compared with that of diethylstilbestrol (open square) as described in ‘Methods’. The mean from two experiments is shown.
Enhanced Transcription of a Reporter Gene and Cell Proliferation by RJ Components
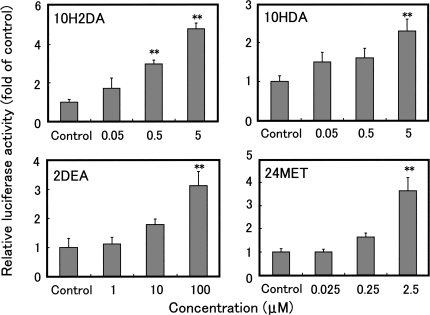
We next examined the effects of 10H2DA, 10HDA, 2DEA and 24MET on the activation of ER(s) using a reporter gene assay. We evaluated the ability of these compounds to stimulate transcription following transient transfection of MCF-7 cells with a reporter plasmid (pERE-Luc) (13) expressing the luciferase gene under the transcriptional control of the Xenopus vitellogenin A2 estrogen response element (ERE). Exposure of transfected cells to various concentrations of the RJ compounds led to increased levels of luciferase activity in a dose-dependent manner (Fig. 3). These results suggest that these compounds activate ER(s) resulting in enhanced transcription from pERE-Luc via the ERE.
Figure 3.
Reporter gene expression assays using MCF-7 cells transfected transiently with pERE-Luc. The transfected cells were incubated with 10H2DA, 10HDA, 2DES or 24MET for 24 h and luciferase activities were measured. Data are expressed as fold induction over the negative control, and are plotted as the mean and SD of three experiments. An asterisk (**) indicates values that are significantly different (P < 0.01) from control values.
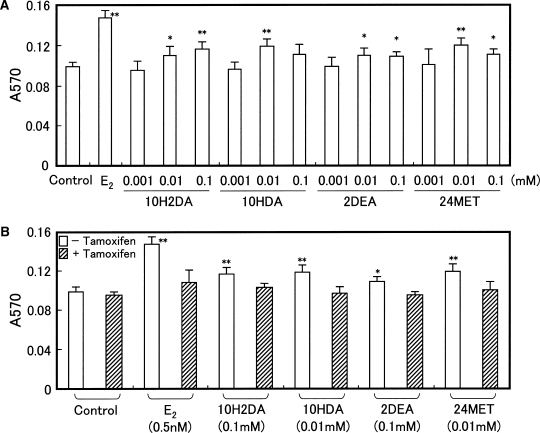
The effects of the RJ components on proliferation of MCF-7 cells were determined using a methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay. The addition of each compound increased the rate of proliferation of MCF-7 cells at concentrations of 0.01–0.1 mM, although the maximal response was lower than that produced by E2 (Fig. 4A). Stimulation of cell proliferation mediated by these compounds was blocked by 1 μM tamoxifen, an estrogen antagonist that also inhibited stimulation of growth by E2, but which had no effect on proliferation when administered alone (Fig. 4B). These results indicate that the estrogenic action of 10H2DA, 10HDA, 2DEA or 24MET on MCF-7 cell proliferation is mediated by signaling through the estrogen receptor.
Figure 4.
Effects of RJ-derived compounds on the proliferation of MCF-7 cells. (A) MCF-7 cells were incubated with 0.001–0.1 mM 10H2DA, 10HDA, 2DEA or 24MET for three days, and then subjected to the MTT assays. As a positive control, cells were treated with 0.5 nM E2. Asterisks indicate values that are significantly different (*P < 0.05; **P < 0.01) from untreated control values. (B) MCF-7 cells were incubated with 0.5 nM E2, 0.1 mM 10H2DA, 0.01 mM 10HDA, 0.1 mM 2DEA or 0.01 mM 24MET with/without 1 μM tamoxifen for three days prior to the MTT assay. Asterisks indicate values that are significantly different (*P < 0.05; **P < 0.01) from untreated control (without tamoxifen) values. Results shown in (A) and (B) represent the mean and SD from six experiments.
Mild Hypertrophy of the Uterine Luminal Epithelium Induced by RJ or its Components
The uterotrophic response in immature rodents and adult ovariectomized rats can be used as a measure of estrogen-like activity of compounds in vivo (17). In the rat, the concentration of E2 is consistently low throughout prepubertal development, but starts to increase after post-natal day 28 with a large increase one day before the first ovulation (18). However, exposure of rats to estrogens during the prepubertal period can induce a uterotrophic response that is characterized by a reversible modification of the morphology and physiology of the uterus. We evaluated the effects on the uterus following exposure of 20-day old female rats to RJ, the estrogenic compounds we purified from RJ (10H2DA, 10HDA, 2DEA or 24MET), and 17αEE2, by subcutaneous injection for 3 days. All treatments were well-tolerated by the rats. We observed no evidence of overt toxicity or clinical signs of toxicity. Premature vaginal opening was not detected in any of the rats. 17αEE2 led to a significant (P < 0.01) increase in uterine wet weight at doses of 0.1 and 3 μg kg−1day−1, producing an increase in wet uterine weight of over 1.5-fold compared with vehicle-treated controls (Table 1). No significant changes in wet uterine weight were observed in rats exposed to RJ or its components (Table 1).
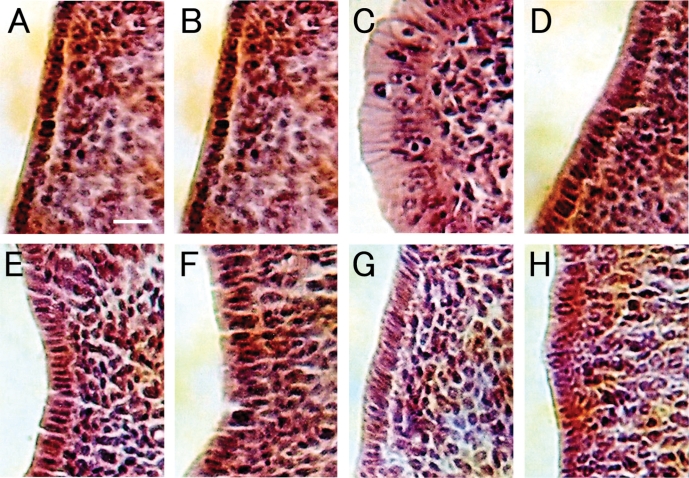
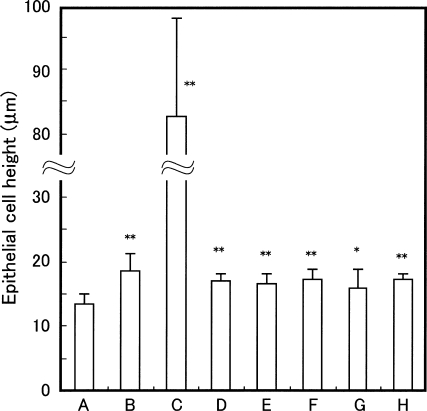
Histological effects of these agents on immature uterine epithelial cell height are shown in Fig. 5, and are quantified in Fig. 6. 17αEE2 at a dose of 3 μg kg−1 day−1 induced marked hypertrophy of the luminal epithelium, whereas only a mild increase in luminal height was observed in uteri treated with 17αEE2 at 0.1 μg kg−1 day−1 (Fig. 5). Although an increase in uterine weight in response to exposure to RJ or its components was not detected, they did induce mild but statistically significant hypertrophy of the luminal epithelium compared with the vehicle-treated controls (Figs 5 and 6). Altogether, the effects of exposure to 17αEE2 at 0.1 μg kg−1 day−1, or RJ or its components, on hypertrophy of the luminal epithelium were indistinguishable from each other at the histological level.
Figure 5.
Uterotrophic response in immature rats injected with vehicle control (A), 0.1 μg kg−1 17αEE2 (B), 3 μg kg−1 17αEE2 (C), RJ (D), 10H2DA (E), 10HDA (F), 2DEA (G) or 24MET (H). Tissue sections from the mid-region of each uterine horn are shown. Bar: 20 μm.
Figure 6.
Luminal epithelial cell height of the uterus from immature rats injected with vehicle control (A), 0.1 μg kg−1 17αEE2 (B), 3 μg kg−1 17αEE2 (C), RJ (D), 10H2DA (E), 10HDA (F), 2DEA (G) or 24MET (H). Tissue sections from the mid-region of each uterine horn were evaluated for epithelial cell height of the epithelial lining of the lumen along the uterus. The epithelial cell height was measured in five different areas comprising five sections at approximately 160 μm intervals from the rats shown in Table 1. Data are expressed as the mean and SD. Asterisks indicate values that are significantly different (*P < 0.05; **P < 0.01) from control values.
Discussion
We have previously shown that RJ has estrogenic activities and suggested it contains estrogenic components (10,11). In the current study, we isolated four compounds from RJ (10H2DA, 10HDA, 2DEA and 24MET) that exhibit ERβ-binding activity. We used several assays to evaluate the estrogenic activity of these compounds. ERE-based reporter gene expression assays suggested that these compounds activated ERs, leading to transcriptional activation via an ERE (Fig. 3). We further observed that these compounds stimulated the proliferation of MCF-7 cells, which have been shown to respond to estrogens (Fig. 4). Concomitant treatment with tamoxifen blocked this effect. Thus, all in vitro data indicate that 10H2DA, 10HDA, 2DEA and 24MET elicit the full sequence of estrogenic action.
RJ comprises 60–70% water, 12–15% proteins, 10–12% carbohydrates, 3–7% lipids (including sterols and fatty acids) and traces of mineral salts and vitamins (19). 24MET constitutes 49–58% of RJ sterols (20). 10H2DA and 10HDA are characteristic fatty acids of RJ, together representing 60–80% of the organic acid content of RJ, whereas 2DEA is present at a much lower level (21). 10H2DA has been reported to have antibacterial (22), antitumor (23) and insulin-like (24) activities. 10H2DA and 10HDA have also been shown to promote collagen production by skin fibroblasts (25). However, little information is available concerning the pharmacological effects of either 24MET or 2DEA. To our knowledge, this is the first report demonstrating the estrogenic effects of 24MET, 2DEA, 10H2DA or 10HDA, although these compounds are not as potent as steroid estrogens.
10H2DA, 10HDA, 2DEA and 24MET have rather weak binding affinities for ERβ compared with diethylstilbestrol (10−2–10−4-fold) (Fig. 2), circulating E2 (10−3–10−5-fold), genistein (10−3–10−4-fold) or daidzein (10−1–10−3-fold) (26). However, the concentrations of these compounds in RJ are much greater than those of the endogenous steroid estrogens found under physiological conditions. The high concentrations of these compounds in RJ could, partly at least, account for the pharmacological effects attributed to RJ, including improvement of menopausal symptoms (8,9), bone formation (27) and prevention of osteoporosis (28), although further analysis is necessary.
The weight increase induced in the immature uterus of the rat by exposure to ethynylestradiol is characteristic of the response expected for a potent ER agonist (17). The uterine wet weight increase induced by 17αEE2 results from hypertrophy and hyperplasia, as well as accumulation of fluid in the lumen (15). Although we did not detect an increase in the uterine wet weight in response to exposure of the immature rat uterus to 10H2DA, 10HDA, 2DEA or 24MET, we did observe mild hypertrophy of the luminal epithelium (Figs 5 and 6; Table 1). These observations may be explained by differences in the pattern of expression of ER subtypes in rat tissues. ERα rather than ERβ is predominantly expressed in the uterus (29). Our data shows that 10H2DA, 10HDA, 2DEA and 24MET bind preferentially to ERβ rather than ERα (Fig. 2), which may explain why we only observe a slight increase in epithelial cell height, and no increase in uterus weight, in response to treatment of immature rats with these compounds.
Earlier studies have shown that unsaturated fatty acids, but not saturated fatty acids, modulate estrogen and/or ER(s) by alterations in estradiol binding to receptors (30), and/or by cleaving native ER(s) (31). Linoleic acid, an unsaturated fatty acid, was isolated from chaste-tree berries as an estrogenic compound utilizing ERβ binding as a monitor (32). Thus, unsaturated fatty acids have been shown to modulate and/or induce some estrogenic effects via interaction with estrogen and/or ERs. Other studies suggest that non-esterified fatty acids may influence cell growth and proliferation by modifying membrane fluidity (33). Our data indicate that 10H2DA, 10HDA, 2DEA and 24MET interact with ER, activate ERs resulting in enhanced transcription of reporter gene via the ERE and enhance MCF-7 cell proliferation. 10H2DA, 10HDA and 2DEA are similar in their molecular structure, although 10HDA is saturated whereas 10H2DA and 2DEA are both unsaturated fatty acids. The results suggest that saturated fatty acids can also exhibit estrogenic activity.
In summary, we isolated and identified four compounds associated with the estrogenic effects of RJ. Further understanding of these compounds should provide a scientific basis for the development of better therapeutic applications of dietary supplement for the improvement of quality of life in menopausal women.
Acknowledgements
We thank Dr Y. Inoh and Miss N. Takeshima of our laboratory for their technical assistance.
References
- 1.Rymer J, Wilson R, Ballard K. Making decisions about hormone replacement therapy. Br Med J. 2003;326:322–6. doi: 10.1136/bmj.326.7384.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez E, Gallus S, Bosetti C, Franceschi S, Negri E, La Vecchia C. Hormone replacement therapy and cancer risk: a systematic analysis from a network of case-control studies. Int J Cancer. 2003;105:408–12. doi: 10.1002/ijc.11083. [DOI] [PubMed] [Google Scholar]
- 3.Humphries KH, Gill S. Risks and benefits of hormone replacement therapy: the evidence speaks. Can Med Assoc J. 2003;168:1001–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Benassayag C, Perrot-Applanat M, Ferre F. Phytoestrogens as modulators of steroid action in target cells. J Chromatogr [B] 2002;777:233–48. doi: 10.1016/s1570-0232(02)00340-9. [DOI] [PubMed] [Google Scholar]
- 5.Beck V, Rohr U, Jungbauer A. Phytoestrogens derived from red clover: an alternative to estrogen replacement therapy? J Steroid Biochem Mol Biol. 2005;94:499–518. doi: 10.1016/j.jsbmb.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 6.Mateescu C, Barbulescu D. Enhanced nutritive, functional and therapeutic action of combined bee products in complex food supplements. Roum Biotechnol Lett. 1999;4:163–72. [Google Scholar]
- 7.Fujii A. Pharmacological effect of royal jelly. Honeybee Sci. 1995;16:97–104. (in Japanese) [Google Scholar]
- 8.Kushima K, Hasegawa N. Menopausal disorder. J Ther. 1972;54:578–84. (in Japanese) [Google Scholar]
- 9.Kushima K, Hasegawa N, Ogawa E. Effects of royal jelly on autonomic imbalance in menopausal women. World Obstet Gynecol. 1973;25:439–43. (in Japanese) [Google Scholar]
- 10.Mishima S, Suzuki KM, Isohama Y, Kuratsu N, Araki Y, Inoue M, et al. Royal jelly has estrogenic effects in vitro and in vivo. J Ethnopharmacol. 2005;101:215–20. doi: 10.1016/j.jep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Mishima S, Miyata T, Suzuki KM, Araki Y, Akao Y, Isohama Y. Estrogenic effects of royal jelly. J Tradit Med. 2005;22(Suppl 1):171–5. [Google Scholar]
- 12.Horwitz KB, McGuire WL. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978;253:2223–8. [PubMed] [Google Scholar]
- 13.Catherino WH, Jordan VC. The biological action of cDNAs from mutated estrogen receptors transfected into breast cancer cells. Cancer Lett. 1995;90:35–42. doi: 10.1016/0304-3835(94)03675-9. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 15.Naciff JM, Overmann GJ, Torontali SM, Carr GJ, Tiesman JP, Richardson BD, et al. Gene expression profile induced by 17 alpha-ethynyl estradiol in the prepubertal female reproductive system of the rat. Toxicol Sci. 2003;72:314–30. doi: 10.1093/toxsci/kfg037. [DOI] [PubMed] [Google Scholar]
- 16.Mclnnes AG, Water JA, Wright JLC. 13C NMR spectra of delta24(28) phytosterols. Org Magn Reson. 1980;13:302–3. [Google Scholar]
- 17.Kanno J, Onyon L, Haseman J, Fenner-Crisp P, Ashby J, Owens W. The OECD program to validate the rat uterotrophic bioassay to screen compounds for in vivo estrogenic responses: phase 1. Environ Health Perspect. 2001;109:785–94. doi: 10.1289/ehp.01109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noda S, Sawaki M, Shiraishi K, Yamasaki K, Yamaguchi R. Age-related changes of genital systems in the female Crj:CD (SD) IGS rats during sexual maturation. J Vet Med Sci. 2002;64:315–9. doi: 10.1292/jvms.64.315. [DOI] [PubMed] [Google Scholar]
- 19.Scarselli R, Donadio E, Giuffrida MG, Fortunato D, Conti A, Balestreri E, et al. Towards royal jelly proteome. Proteomics. 2005;5:769–76. doi: 10.1002/pmic.200401149. [DOI] [PubMed] [Google Scholar]
- 20.Svoboda JA, Herbert EW Jr, Thompson MJ. Sterols of organs involved in brood food production and of royal jelly in honey bees. Insect Biochem. 1986;16:479–82. [Google Scholar]
- 21.Lercker G, Capella P, Conte LS, Ruini F, Giordani G. Components of royal jelly II. The lipid fraction, hydrocarbons and sterols. J Apicult Res. 1982;21:178–84. [Google Scholar]
- 22.Blum MS, Novk AF, Taber S. 10-Hydroxy-delta 2-decenoic acid, an antibiotic found in royal jelly. Science. 1959;130:452–3. doi: 10.1126/science.130.3373.452. [DOI] [PubMed] [Google Scholar]
- 23.Townsend GF, Morgan JF, Hazlett B. Activity of 10-hydroxydecenoic acid from royal jelly against experimental leukaemia and ascetic tumours. Nature. 1959;183:1270–1. doi: 10.1038/1831270a0. [DOI] [PubMed] [Google Scholar]
- 24.Okuda H, Kameda K, Morimoto C, Matsuura Y, Chikaki M, Jiang M. Studies on insulin-like substances and inhibitory substances toward angiotensin-converting enzyme in royal jelly. Honeybee Sci. 1998;19:9–14. (in Japanese) [Google Scholar]
- 25.Koya-Miyata S, Okamoto I, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci Biotechnol Biochem. 2004;68:767–73. doi: 10.1271/bbb.68.767. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 27.Narita Y, Nomura J, Ohta S, Inoh Y, Suzuki KM, Araki Y, et al. Royal jelly stimulates bone formation: physiologic and nutrigenomic studies with mice and cell lines. Biosci Biotechnol Biochem. 2006;70:2508–14. doi: 10.1271/bbb.60240. [DOI] [PubMed] [Google Scholar]
- 28.Hidaka S, Okamoto Y, Uchiyama S, Nakatsuma A, Hashimoto K, Ohnishi ST, et al. Royal jelly prevents osteoporosis in rats: beneficial effects in ovariectomy model and in bone tissue culture model. Evid Based Complement Alternat Med. 2006;3:339–48. doi: 10.1093/ecam/nel019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 30.Vallette G, Christeff N, Bogard C, Benassayag C, Nunez E. Dynamic pattern of estradiol binding to uterine receptors of the rat. Inhibition and stimulation by unsaturated fatty acids. J Biol Chem. 1988;263:3639–45. [PubMed] [Google Scholar]
- 31.Borras M, Leclercq G. Modulatory effect of nonesterified fatty acids on structure and binding characteristics of estrogen receptor from MCF-7 human breast cancer cells. J Recept Res. 1992;12:463–84. doi: 10.3109/10799899209074807. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Burdette JE, Sun Y, Deng S, Schlecht SM, Zheng W, et al. Isolation of linoleic acid as an estrogenic compound from the fruits of Vitex agnus-castus L. (chaste-berry) Phytomedicine. 2004;11:18–23. doi: 10.1078/0944-7113-00331. [DOI] [PubMed] [Google Scholar]
- 33.Burns CP, Luttenegger DG, Dudley DT, Buettner GR, Spector AA. Effect of modification of plasma membrane fatty acid composition on fluidity and methotrexate transport in L1210 murine leukemia cells. Cancer Res. 1979;39:1726–32. [PubMed] [Google Scholar]