Abstract
The protective action against oxidative stress of red cabbage (Brassica oleracea) extract was investigated. Diabetes was induced in male Wistar rats using streptozotocin (60 mg/kg body weight). Throughout the experimental period (60 days), diabetic rats exhibited many symptoms including loss of body weight, hyperglycemia, polyuria, polydipsia, renal enlargement and renal dysfunction. Significant increase in malondialdehyde, a lipid peroxidation marker, was observed in diabetic kidney. This was accompanied by a significant increase in reduced glutathione and superoxide dismutase activity and a decrease in catalase activity and in the total antioxidant capacity of the kidneys. Daily oral ingestion (1 g/kg body weight) of B. oleracea extract for 60 days reversed the adverse effect of diabetes in rats. B. oleracea extract lowered blood glucose levels and restored renal function and body weight loss. In addition, B. oleracea extract attenuated the adverse effect of diabetes on malondialdehyde, glutathione and superoxide dismutase activity as well as catalase activity and total antioxidant capacity of diabetic kidneys. In conclusion, the antioxidant and antihyperglycemic properties of B. oleracea extract may offer a potential therapeutic source for the treatment of diabetes.
Keywords: Brassica oleracea, diabetes, diabetic nephropathy, oxidative stress, red cabbage
Introduction
Microvascular complications of diabetes share a common pathophysiology that may be explained as a direct or indirect consequence of hyperglycemia-mediated overproduction of reactive oxygen species (ROS). Microvascular deterioration is preventable either by the inhibition of superoxide accumulation or by modulating the blood glucose levels, and among several microvascular disorders, nephropathy (1,2) can be improved by antioxidants (3,4). The antioxidant protection of natural plants is a promising therapeutic remedy for free radical pathologies (5). Among myriad natural plants, red cabbage (RC) (Brassica oleracea var capitata) and other Brassica vegetables, vegetables endemic to the Mediterranean region, have been found to have antioxidant, antihyperglycemic (6–8), anticancer (9–11) and hypocholesterolemic (12) properties. RC extract has also prevented oxidative stress induced in livers and brains of animals exposed to paraquate (13) and N-methyl-d-aspartate (14).
The principle constituents of RC are isothiocyanates (glucosinolate), vitamins A, B, C and anthocyanins (11,15,16). Anthocyanins, a group of phenolic natural pigments present in RC, were found to have the strongest antioxidizing power of 150 flavonoids (17). Our objective was to investigate RC polar extract for antioxidant scavenging enzymes and oxidative stress in the kidney of streptozotocin-induced diabetic rats.
Methods
Chemicals
Fresh RCs were purchased from a local market at Al Ain, UAE. Reagent kits for urea and creatinine were acquired from BioMerieux (RCS Lyon, France). Thiobarbituric acid, reduced glutathione, 5,5-dithiobis (2-nitrobenzoic acid), Folin's reagent, epinephrine, superoxide dismutase (SOD) enzyme, H2O2, Streptozotocin (STZ) and bovine albumin were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other standard chemicals came from common commercial suppliers.
Albino Rats
Male Wistar rats (130–180 g) were obtained from the Animal House at the Faculty of Medicine and Health Sciences, UAE-University, UAE. Rats were maintained on standard pellet diet and tap water ad libitum. They were kept in plastic cages under a 12 h light/dark cycle and room temperature 22–24°C and were acclimatized to the environment for 2 weeks prior to experimental use. Procedures for the care and use of research animals at UAE meet or exceed all applicable local, national and international laws and regulations.
Preparation of Red Cabbage Extract
RC leaves were sliced into small pieces and oven-dried at 50°C. Dried plants (800 g) were extracted in 8000 ml of 70% aqueous ethanol using ultrasonic treatment at an intensity of 70 W/cm2 and oscillation frequency at 20 kHz for 5 min. The use of an ultrasound extraction method has been shown to diminish the danger of thermal degradation (18). Ultrasound also provides a greater penetration of solvent into cellular materials, via cavitation, and improves the release of cell contents into the bulk medium (18). Ultrasonic irradiation was applied by means of a Branson 450 digital sonifier (20 kHz, 450 W) equipped with a cylindrical titanium alloy probe (12.70 mm in diameter). The use of dry plants can be effective to minimize enzymatic degradation of phonetic compounds inside plant tissues. After overnight maceration, the extract was filtered through gauze and ethanol was evaporated under reduced pressure at 50°C by using a rotary evaporator. The remaining water extract was dried using a freeze dry system under reduced pressure. The dried extract was dissolved in distilled water to a concentration of 1 g/ml before administration to normal and diabetic rats. The extraction yield for RC was about 15%.
Induction of Experimental Diabetes
Diabetes was induced by injection of a single intraperitoneal dose of STZ (freshly prepared in 0.01 M citrate buffer, pH 4.5) as described previously (19). The control rats were only injected with citrate buffer. Diabetes was identified by polydipsia, polyuria (visual observations) and measuring blood glucose concentration 72 h after injection of STZ. Rats with a fasting blood glucose level above 200 mg/dl were considered diabetic and were used in this study.
Experimental Groups
RC extract was dissolved in distilled water and given orally by gavage at a dose of 1 g/kg body weight and volume 5 ml/kg body weight. The daily dose of RC (1 g/kg body weight) was based on that previously shown to be effective in preventing the oxidative stress induced by another toxic agent in animals (14). Non-diabetic control rats as well as diabetic rats were each randomly assigned to two groups that ingested either vehicle or RC extracts at 1 g/kg body weight/day for 8 weeks, respectively. At the end of the experimental period, rats were anesthetized with diethyl ether after fasting for 12 h and blood and kidney samples were collected.
Sample Collection
Blood Sampling
For daily glucose analysis, a drop of blood was collected from the tail. Serum was extracted from blood collected from retro-orbital plexus (20) after rats were subject to light anesthesia. Serum urea and creatinine determined in fresh serum were indicators of kidney function.
Collection of Kidneys
At the end of the experimental period, rats were weighed and sacrificed. Kidneys were removed, weighed and kidney/body weight ratio was calculated. Data were expressed as relative organ weight of one kidney to 100 g of total body weight. The other kidney was excised and homogenized (1 g/10 ml ice-cold potassium chloride, 150 mM). The homogenate was then used for determination of the levels of reduced glutathione (GSH), lipid peroxidation (LP) product Malondialdehyde (MDA), total proteins and activities of SOD and catalase (CAT).
Biochemical Assays
Blood Glucose
The blood glucose level was determined by glucose oxidase method using a one touch basic plus glucometer (Lifescane Ltd., California, USA).
Reduced Glutathione
GSH content in the kidney homogenate was determined using the method described by Van Dooran et al. (21). The basis of the GSH determination method is the reaction of Ellman's reagent 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) with thiol group of GSH at pH 8.0 to give yellow color of 5-thiol-2-nitrobenzoate anion.
Malondialdehyde
MDA is the most abundant individual aldehyde resulting from LP breakdown in biological systems. It is used as an indirect index of LP. The determination of MDA in biological materials, as described in Uchiyama and Mihara (22), is based on its reaction with thiobarbituric acid (TBA) to form a pink complex with absorption maximum at 535 nm.
Catalase and Superoxide Dismutase Enzymes
The activity of SOD enzyme in kidney homogenate was determined according to the method described by Sun and Zigman (23). This method is based on the ability of SOD to inhibit the auto-oxidation of epinephrine at alkaline pH to adrenochrome and other derivatives, which are easily monitored in the near-UV region of the absorption spectrum. CAT activity was determined by measuring the exponential disappearance of H2O2 at 240 nm and expressed as units/mg of protein as described by Aebi (24).
Total Antioxidant Capacity
The total antioxidant capacity in the kidneys was evaluated using ferric reducing antioxidant power (FRAP) assay. The FRAP assay was determined as described by Benzie and Strain (25) and as developed in our laboratory. Briefly, the FRAP assay measures the change in absorbance at 593 nm due to the formation of a blue colored ferrous-tripyridyltriazine complex from colorless oxidized ferric form by the action of electron donating antioxidants.
Creatinine and Urea
Creatinine and urea levels were determined in serum according to the method described by Houot (26) and Fawcett and Scott (27), respectively. The assay was performed using BioMerieux reagent kits. The total protein content in kidney was determined according to the Lowry's method and as modified by Peterson (28). Absorbance was recorded using a Shimadzu recording spectrophotometer UV-160A.
Statistical Analysis
The statistical analysis was carried out using SPSS version 10 statistical programs (SPSS Inc., Chicago, IL, USA). Differences among treatments within an experiment were analyzed by one-way analysis of variance (ANOVA). Significant differences between treatment means were determined by Dunnett t-test. The results are presented as mean ± SEM of five independent experiments unless stated otherwise.
Results
Red Cabbage Ingestion Attenuated Symptoms of Diabetes
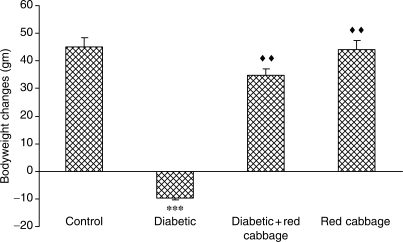
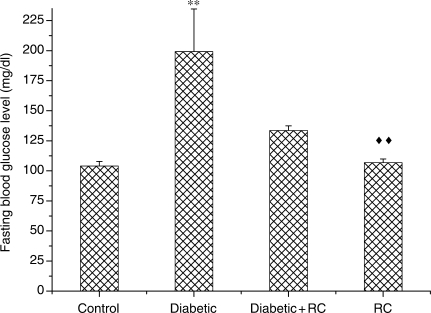
Intraperitoneal administration of STZ (60 mg/kg body weight) induced all characteristic symptoms of diabetes such as increased food and water intake (judged by the amount of food and water consumed per day), polyuria (data are not shown), failure to gain weight (Fig. 1). These symptoms were evident throughout the entire experimental period of 60 days. After 72 h of STZ injections, fasting blood glucose concentrations were increased as compared with non-diabetic control rats (Fig. 2). Blood glucose levels above 200 mg/dl were considered diabetic. Diabetic rats that daily ingested polar extract of RC (1 g/kg body weight) showed improvement in diabetic polyuria that almost disappeared by the end of the experimental period. Food and water consumption also declined when diabetic rats ingested RC extract. RC extract also abridged the weight gain loss in diabetic rats (Fig. 1) as well as blood glucose levels (Fig. 2) at the end of the experimental period. Rats that ingested RC extract showed no significant changes as compared with control rats in any of the parameters considered.
Figure 1.
Body weight changes in rats that did not ingest (Control) or ingested RC extract (RC), and STZ-induced diabetes in rats that did not ingest (Diabetic) or ingested RC extract (Diabetic+RC), ***P < 0.001 as compared with control, ↑↑P < 0.01 as compared with diabetic.
Figure 2.
Fasting blood glucose levels, **P < 0.01 as compared to control, ↑↑P < 0.01 as compared with diabetic.
Symptoms of Nephropathy Ameliorated in Diabetic Rats
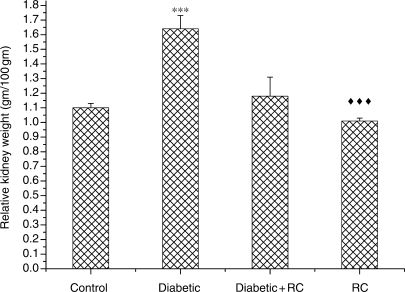
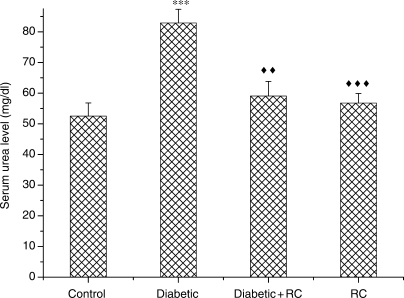
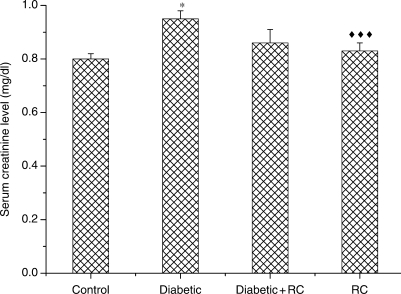
At the end of the experimental period (60 days), diabetic rats exhibited significant (P < 0.001) renal enlargement as compared with non-diabetic control rats (Fig. 3). In diabetic rats, elevation in serum urea (Fig. 4) and serum creatinine concentrations (Fig. 5) indicated impairment in kidney function. Kidney nephropathy was improved when RC extract was ingested. Fig. 3 shows that kidney weight in diabetic rats that ingested RC extract was not different from control rats (P > 0.05). In addition, serum urea (Fig. 4) as well as serum creatinine (Fig. 5) concentrations were ameliorated with ingestion of RC extract.
Figure 3.
Relative kidney weights. ***P < 0.001, as compared with control, ↑↑↑P < 0.001 as compared with diabetic.
Figure 4.
Serum urea concentrations. ***P < 0.001, as compared with control, ↑↑P < 0.01; ↑↑↑P < 0.001 as compared with diabetic.
Figure 5.
Serum creatinine concentrations, *P < 0.05, as compared with control, ↑↑↑P < 0.001 as compared with diabetic.
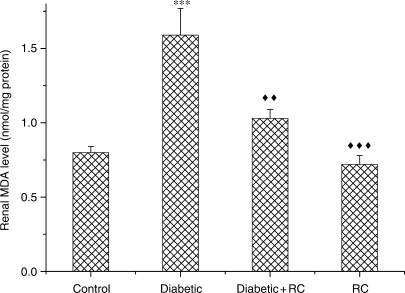
Increase in Renal Malondialdehyde Abolished in Diabetic Rats
Severe LP in diabetic kidneys implicated oxidative stress. Fig. 6 indicates an increase (P < 0.001) in MDA concentrations. This increase was abolished when diabetic rats ingested RC extract for 60 days.
Figure 6.
Renal level of MDA,***P < 0.001, as compared with control, ↑↑P < 0.01; ↑↑↑P < 0.001 as compared with diabetic.
Red Cabbage Modulated Change in Renal Antioxidants
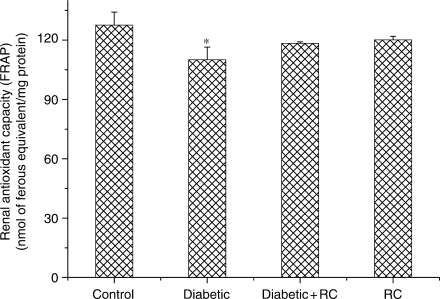
Total antioxidant capacity in kidney tissues was analyzed using FRAP assay. Kidneys of diabetic rats showed reduction (P < 0.05) in antioxidant capacity in comparison with control rats (Fig. 7).
Figure 7.
Kidney antioxidant capacity. *P < 0.05, as compared with control.
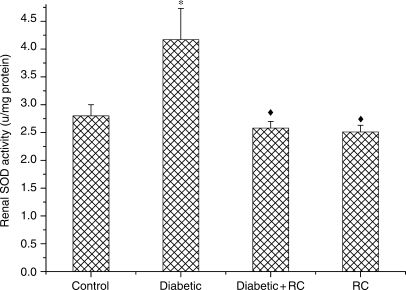
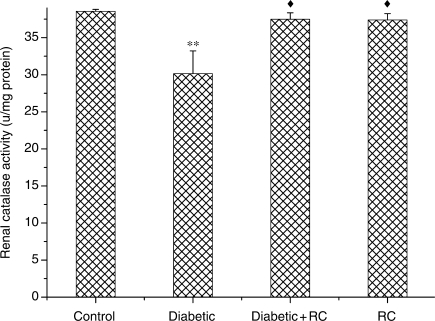
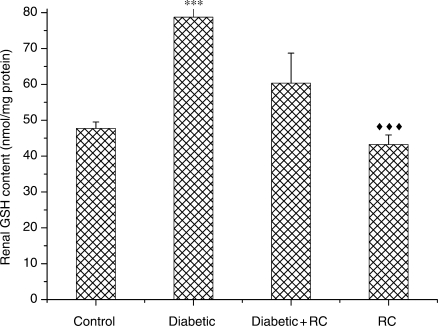
To understand the effect of RC extract on antioxidant eminence, we measured the activities of enzymic antioxidants SOD and CAT and non-enzymic antioxidant GSH. SOD activity showed an increase (P < 0.05) at the end of experimental period (Fig. 8). Meanwhile, a significant decline (P < 0.01) in CAT activity was observed in diabetic rats (Fig. 9). GSH activity increased (P < 0.001) in diabetic rats (Fig. 10). Both enzymic and non-enzymic antioxidants were ameliorated after ingesting RC extract.
Figure 8.
Kidney SOD activity.*P < 0.05, as compared with control, ↑P < 0.05, as compared with diabetic.
Figure 9.
Kidney CAT activity. **P < 0.01, as compared with control, ↑P < 0.05, as compared with diabetic.
Figure 10.
Kidney GSH concentrations. ***P < 0.001, as compared with control, ↑↑↑P < 0.001 as compared with diabetic.
Discussion
Tight control of blood glucose can reduce clinical complications in diabetic patients; however, alternative treatment strategies are needed to prevent oxidative stress complications and to optimize recovery (1). It is well documented that modulation of oxidative stress through treatment with antioxidants can effectively reduce diabetic snag. Oxidative stress induced by hyperglycemia in diabetics is a major cause for development and progression of diabetic microvascular complications such as nephropathy (1,2).
We investigated the therapeutic value of RC against diabetic nephropathy. These plants, members of the Cruciferae and genus Brassica such as RC, broccoli, cauliflower, kale and Brussel sprouts, contain anthocyanin pigments that are described as free-radical scavenging and antioxidant agents (11,15,16). Anthocyanin isolates and anthocyanin-rich mixtures of bioflavonoid provide protection against myriad physiological failures such as LP, decreasing capillary permeability and fragility and membrane strengthening (39). RC extract contains vitamins A, B and C (11,15,16), all of which have protective roles against oxidative damage.
Measuring FRAP in kidney tissues revealed that anti-oxidant potency of the STZ-diabetic kidneys was significantly reduced, indicating oxidative stress and production of ROS. Generation of ROS by diabetic hyperglycemia is evident through many biochemical pathways such as glucose autoxidation and protein glycation (1,29). This oxidative stress was further assessed by the increase in renal MDA as an LP marker (22). These data confirmed previously published reports of a state of oxidative stress in diabetics (30–32). Our results, renal MDA concentrations correlated with blood hyperglycemia, which is in agreement with Ha et al. (33) who found that elevated glucose level per se, could cause oxidative stress in isolated rat glomeruli in vitro.
STZ-diabetic rats showed symptoms of renal nephropathy such as kidney hypertrophy that were attributed to an increase in blood glucose and polyuria. In addition, STZ-diabetic rats showed elevated serum urea and creatinine, as in previous reports (7,19,34). RC polar extract prevented renal enlargement and attenuated polyuria. Meanwhile, elevated serum urea and creatinine in STZ-diabetic rats were also normalized to control values after 60 days with ingestion of RC polar extract. This was consistent with other reports using chemical antioxidants (35,36), and diet of natural antioxidant plants (4,7,34) to normalize nephropathy in STZ-diabetic rats. Although STZ-diabetic rats showed elevated serum urea and creatinine and kidney hypertrophy, the measurement of other indicators of nephropathy such as glomerular filtration rate and histopathology is needed to further investigate our diabetic model.
Diabetic rats had elevated levels of enzymic antioxidants SOD, reduced CAT and increased concentrations of non-enzymic antioxidant GSH. RC extract ameliorated these changes. These elevations in GSH level and SOD activity may be compensatory mechanisms for the chronic overproduction of free radicals and oxidative stress. The upregulation of SOD and GSH in diabetic rats is consistent with a number of reports (35,38,39). Others, however, reported a reduction in both SOD and GSH levels (36,37). These discrepancies may be due to the nature of the tissue used, experimental period, the STZ dose and the severity of diabetic state as well as oxidative stress. The protective qualities of RC are quite consistent with its ability to protect against oxidative damage induced by toxic agents in other tissues of animals (13,14).
Other investigations have shown that acylated anthocyanins isolated from RC protect against paraquat-induced oxidative stress in rats (13). Our data showed that acylated anthocyanins were effective antioxidants against hepatic LP elevation and CAT depletion induced by paraquat in rats. Several vegetable aqueous extracts including Brassica vegetables such as white cabbage, RC, green mustard leaf, red mustard leaf, white turnip and red turnip were examined for their neuroprotective action against oxidative stress induced by N-methyl-d-aspartate in brains of mice (14). Only RC showed a markedly restored glutathione levels and prevented LP induced by N-methyl-d-aspartate in the brains of mice at a dose of 1 gm/kg body weight. RC polar extract was more protective than white cabbage against amyloid protein induced neuronal damage (40). These data indicate that the neuronal cell protective capacities of RC might be due to high total phenolic contents and antioxidative activity. Among the three different cultivated forms of cabbage, RC has higher Vitamin C (24.38 mg/100 g), dl-α-tocopherol (0.261 mg/100 g) and phenolic content (101.30 mg/100 g) as compared with white and Savoy cabbage (16), all of which protect against oxidative damage.
RC and all other edible plants belonging to the family Cruciferae and genus Brassica (e.g. broccoli and cauliflower) contain substantial quantities of isothiocyanates (mostly in the form of their glucosinolate precursors) some of which (e.g. sulforaphane or 4-methylsulfinylbutyl isothiocyanate) are very potent antioxidants (11,15,16). Anthocyanin, which is a phenolic natural pigment present in RC, was demonstrated to have the antioxidizing power of 150 flavonoids (17). We report that RC polar extract has significant antihyperglycemic activity that may, at least in part, modulate the oxidative stress caused by hyperglycemia-induced generation free radicals. Natural health products of vegetable origin have shown promise for the prevention of chronic diseases (41).
Daily ingestion of RC polar extract (1 g/kg body weight) ameliorates oxidative stress and ameliorates diabetic nephropathy. The dose response of RC for the pharmacological benefits observed here is still to be determined and further study is needed to see whether a higher dose and different routes of administration can protect against diabetic nephropathy.
Acknowledgement
This work was financially supported by the Research Affairs at the UAE University under a contract no. 07-04-2-11/04. Our gratitude also extends to the Department of Biology, Faculty of Science for their help and support. We also thank Mr. Paul DeBaufer (University of Illinois at Chicago (UIC), USA) for proofreading the manuscript.
References
- 1.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 2.Kikkawa R, Koya D, Haneda M. Progression of diabetic nephropathy. Am J Kidney Dis. 2003;41:S19–21. doi: 10.1053/ajkd.2003.50077. [DOI] [PubMed] [Google Scholar]
- 3.Kedziora-Kornatowska K, Szram S, Kornatowski T, Szadujkis-Szadurski L, Kedziora J, Bartosz G. Effect of vitamin E and vitamin C supplementation on antioxidative state and renal glomerular basement membrane thickness in diabetic kidney. Nephron Exp Nephrol. 2003;95:E134–43. doi: 10.1159/000074840. [DOI] [PubMed] [Google Scholar]
- 4.Yildirim O, Buyukbingol Z. In vivo effect of vitamin C with cobalt on oxidative stress in experimental diabetic rat kidney. Diabetes Nutr Metab. 2003;16:208–13. [PubMed] [Google Scholar]
- 5.Halvorsen BL, Holte K, Myhrstad MC, Barikmo I, Hvattum E, Remberg SF, et al. A systematic screening of total antioxidants in dietary plants. J Nutr. 2002;132:461–71. doi: 10.1093/jn/132.3.461. [DOI] [PubMed] [Google Scholar]
- 6.Roman-Ramos R, Flores-Saenz JL, J A-AF. Anti-hyperglycemic effect of some edible plants. J Ethnopharmacol. 1995;48:25–32. doi: 10.1016/0378-8741(95)01279-m. [DOI] [PubMed] [Google Scholar]
- 7.Grover Jk, Yadav SP, Vats V. Effect of feeding Murraya koeingii and Brassica juncea diet kidney functions and glucose levels in streptozotocin diabetic mice. J Ethnopharmacol. 2003;85:1–5. doi: 10.1016/s0378-8741(02)00355-0. [DOI] [PubMed] [Google Scholar]
- 8.Yokozawa T, Kim HY, Cho EJ, Yamabe N, Choi JS. Protective effects of mustard leaf (Brassica juncea) against diabetic oxidative stress. J Nutr Sci Vitaminol. 2003;49:87–93. doi: 10.3177/jnsv.49.87. [DOI] [PubMed] [Google Scholar]
- 9.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–72. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komatsu W, Miura Y, Yagasaki K. Induction of tumor necrosis factor production and antitumor effect by cabbage extract. Nutr Cancer. 2002;43:82–9. doi: 10.1207/S15327914NC431_10. [DOI] [PubMed] [Google Scholar]
- 11.Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, et al. Urinary isothiocyanate level, Brassica, and human breast cancer. Cancer Res. 2003;63:3980–6. [PubMed] [Google Scholar]
- 12.Komatsu W, Miura Y, Yagasaki K. Suppression of hypercholesterolemia in hepatoma-bearing rats by cabbage extract and its component, S-methyl-L-cysteine sulfoxide. Lipids. 1998;33:499–503. doi: 10.1007/s11745-998-0233-7. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi K, Kimura Y, Takenaka A. Preventive effect of dietary cabbage acylated anthocyanins on paraquat-induced oxidative stress in rats. Biosci Biotechnol Biochem. 2000;64:1600–7. doi: 10.1271/bbb.64.1600. [DOI] [PubMed] [Google Scholar]
- 14.Lee KJ, Sok DE, Kim YB, Kim MR. Protective effect of vegetable extracts on oxidative stress in brain of mice administered with NMDA. Food Res Inter. 2002;35:55–63. [Google Scholar]
- 15.Repetto MG, Llesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res. 2002;35:523–34. doi: 10.1590/s0100-879x2002000500003. [DOI] [PubMed] [Google Scholar]
- 16.Jagdish Singh AK, Upadhyay A, Bahadur B, Singh B, Singh KP, Mathura Rai AK. Antioxidant phytochemicals in cabbage (Brassica oleracea L. var. capitata) Scientia Horticulturae. 2006;108:233–7. [Google Scholar]
- 17.Sterling M. Got anthocyanins. These plant pigments are more than coloring agents for fruit juices, wine and other beverages; they also contain an array of health-promoting benefits. NSN. 2000;5:231–4. [Google Scholar]
- 18.Kimbaris AC, Siatis NG, Daferera DJ, Tarantilis PA, Pappas CS, Polissiou MG. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum) Ultrason Sonochem. 2006;13:54–60. doi: 10.1016/j.ultsonch.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Yanardag R, Bolkent S, Karabulut-Bulan O, Tunali S. Effects of vanadyl sulfate on kidney in experimental diabetes. Biol Trace Elem Res. 2003;95:73–85. doi: 10.1385/BTER:95:1:73. [DOI] [PubMed] [Google Scholar]
- 20.Paget GE, Thomson R. Standard Operating Procedures in Toxicology. Lancaster: England: M T P Press limited; 1979. [Google Scholar]
- 21.Van Dooran R, Leijdekkers CM, Henderson PT. Synergistic effects of phorone on the hepatotoxicity of bromobenzene and paracetamol in mice. Toxicology. 1978;11:225–33. doi: 10.1016/s0300-483x(78)91389-6. [DOI] [PubMed] [Google Scholar]
- 22.Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 23.Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 1978;247:81–9. doi: 10.1016/0003-2697(78)90010-6. [DOI] [PubMed] [Google Scholar]
- 24.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–26. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 25.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of (antioxidant power): The FRAP assay. Anal Biochem. 1996;293:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 26.Houot O. In: Interpretation of Clinical Laboratory Tests. Siest G, Henny J, Schiele F, editors. [Google Scholar]
- 27.Fawcett JK, Scott JK. A rapid and precise method for the determination of urea. Brit J Clin Path. 1960;13:156–9. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson GL. A simplification of the protein assay method of Lowry et al, which is more generally applicable. Anal Biochem. 1977;83:346–56. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 29.Giugliano D, Ccriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:256–67. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 30.Greene DA, Obrosova I, Stevens MJ, Feldman EL. Pathways of glucose-mediated oxidative stress in diabetic neuropathy. In: Packer L, Rosen P, Tritschler HJ, King GL, Azzi A, editors. Antioxidants in Diabetes Management. New York: Marcel Dekker Inc.; 2000. pp. 111–9. [Google Scholar]
- 31.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 32.Stevens MJ, Obrosova I, Pop-Busui R, Greene DA, Feldman EL. Pathogenesis of diabetic neuropathy. In: Porte D, Sherwin Jr RS, Baron A, editors. Ellenberg and Rifkin's Diabetes Mellitus. New York: McGraw Hill; 2002. pp. 747–70. [Google Scholar]
- 33.Ha H, Yu MR, Kim KH. Melatonin and taurine reduced early glomerulopathy in diabetic rats. Free Rad Bio Med. 1999;26:944–50. doi: 10.1016/s0891-5849(98)00276-7. [DOI] [PubMed] [Google Scholar]
- 34.Anjaneyulu M, Chopra K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exper Pharmacol Physiol. 2004;31:244. doi: 10.1111/j.1440-1681.2004.03982.x. [DOI] [PubMed] [Google Scholar]
- 35.Aksoy N, Vural H, Sabuncu T, Aksoy S. Effect of melatonin on oxidative-antioxidative status of tissues in streptozotocin-induced diabetic rats. Cell Biochem Funct. 2003;21:121–5. doi: 10.1002/cbf.1006. [DOI] [PubMed] [Google Scholar]
- 36.Orrosova IG, Fathallah L, Liu E, Nourooz-Zadeh J. Early oxidative stress in the diabetic kidney effect of DL-lipoic acid. Free Rad Bio Med. 2003;34:186–95. doi: 10.1016/s0891-5849(02)01195-4. [DOI] [PubMed] [Google Scholar]
- 37.Punitha ISR, Rajendran K, Shirwaikar A, Shirwaikar A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin–nicotinamide induced diabetic rats evid based complement. Altern Med. 2005;2:375–81. doi: 10.1093/ecam/neh099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Limaye PV, Raghuram N, Sivakami S. Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin-induced diabetic rats. Mol cell Biochem. 2003;243:147–52. doi: 10.1023/a:1021620414979. [DOI] [PubMed] [Google Scholar]
- 39.Otsyula M, King MS, Ketcham TG, Sanders RA, Watkins JB. Oxidative stress in rats after 60 days of hypergalactosemia or hyperglycemia. Int J Toxicol. 2003;22:423–7. doi: 10.1177/109158180302200603. [DOI] [PubMed] [Google Scholar]
- 40.Heo HJ, Lee CY. Phenolic phytochemicals in cabbage inhibit amyloid. Protein-induced neurotoxicity. LWT - Food Science and Technology. 2006;39:331–7. [Google Scholar]
- 41.Haddad PS, Azar GA, Groom S, Boivin M. Natural health products, modulation of immune function and preventaion of chronic diseases. Evidence-based Complem Altern Med. 2005;2:513–20. doi: 10.1093/ecam/neh125. [DOI] [PMC free article] [PubMed] [Google Scholar]