Abstract
Pharmacopuncture, the injection of subclinical doses of drugs into acupoints reduces drug undesirable side effects, residues in animal consumption products and treatment costs in large animals. Acepromazine (Acp) produces several undesirable effects, such as hypotension. Previous studies with the injection of 1/10 of Acp dose in dog acupoints showed its advantage for sedation, minimizing undesirable effects. Eight horses were randomly submitted to four different treatment protocols according to a Latin Square double-blind design: (i) 0.1 ml kg−1 of saline subcutaneously injected at the cervical region, (ii) 0.1 mg kg−1 of Acp injected subcutaneously at the cervical region, (iii) 0.01 ml kg−1 of saline injected into GV1 acupoint (aquapuncture) and (iv) 0.01 mg kg−1 of Acp injected into GV1 acupoint (pharmacopuncture). Heart rate, respiratory rate, head height and degree of sedation were measured before and at 30, 60 and 90 min after treatments. Signs of sedation were observed in all treated groups at 30 min and only in 1/10Acp-GV1 at 60 min after the treatments. Only the group treated with 0.1 mg kg−1 of Acp s.c. had significantly lower values of head height at 30 min. Respiratory rate tended to reduce in all groups but was significantly lower only in horses treated with 0.1 mg kg−1 of Acp s.c. Heart rate remained unchanged in all groups. Acp-pharmacopuncture on GV1 in horses produced a mild sedation when compared with the conventional dose of Acp. More investigations are necessary to determine the optimal dosage of Acp-pharmacopuncture for sedation in horses.
Keywords: acupoint, acupuncture, equine, injection, sedation
Introduction
Acupuncture (AP) is the insertion of needles in specific cutaneous locations of the body, known as acupoints, for the treatment or prevention of several diseases (1). Although this technique is widely used for the treatment of pain and other conditions, the rational basis underlying its use remains unclear (2).
Acupoint injection is one of the methods used for AP treatment. It is a good option for AP in large animals as it requires a shorter period of restraint and uses ordinary material like hypodermic needle. Aquapuncture or water puncture is the injection of saline or distillated water into acupoints. The goal is to provide a prolonged mechanical stimulus in the acupoint. Other substances may also be used for this purpose like glucose and vitamins. Autologous blood (hemopuncture) and bee venom (apipuncture) are injected mainly for anti-inflammatory purposes (3–7). Recently, pharmacopuncture, i.e. injection of subclinical doses of drugs in acupoints, has been adopted with successful results. Chinese authors claim that this technique enhances the mechanical stimulus period and produces similar effects as conventional doses (8–10).
Pharmacopuncture has been a useful tool in veterinary practice. In large animals, it reduces drug undesirable side effects, residues in animal consumption products and treatment costs (11). A subclinical dose of prostaglandin (0.5 mg of PGF2α, 1/10 of the conventional dose) injected in the Bai Hui acupoint was as effective as the conventional dose (5 mg kg−1) to produce luteolysis and reduce plasma progesterone concentration in mid-luteal phase mares (11,12). Similar results were observed with prostaglandin in cows and hCG in mares (11). A subclinical dose of somatotropin (50 mg) injected in the Bai Hui acupoint in adult cows was equally effective in increasing the weight gain of their 30- to 40-day-old lactating calves as the conventional dose (500 mg) injected intramuscularly (11). These results are promising for pharmacopuncture as an adjunct to hormonotherapy, reducing adverse effects of large doses of hormones, treatment costs, as well as less exposure of consumers to hormones.
According to Traditional Chinese Medicine, the acupoint Yin Tang, also known as Governing Vessel 24 (GV24), has a sedative effect in humans and animals (13,14). In dogs, a subclinical dose of acepromazine (Acp) (0.01 mg kg−1) injected in the Yin Tang reduced in 30% the dose of thiopentone necessary to induce loss of the interdigital reflex, against 50% reduction after administration of the conventional dose of Acp (0.1 mg kg−1 s.c.). These data suggest an improvement of the sedative effect produced by Acp when administrated at the Yin Tang acupoint (11). The Ho Hai acupoint, also named Chang Qiang (Governing Vessel 1—GV1), has also a sedative indication. However, GV1 is a lesser used sedation acupoint in humans due to its location, on the midpoint of the line connecting the end of the coccyx and anus, below the end of coccyx. On the other hand, in horses, GV1, located between the anus and tail, is an alternative point to the Yin Tang acupoint.
Acp is a phenothiazine tranquilizer commonly used in the equine species. It is regularly used to reduce stress during transportation and for clinical or surgical procedures. In horses, the side effects are protrusion of the penis and cardiorespiratory depression, leading to hypotension which is the main limitation for its use (15,16).
Few specific reports concerning the sedation effect of AP are available. However, clinical and experimental studies suggest that during AP session a certain degree of sedation and a transitory reduction in heart rate is observed (17,18). AP clinical observations are limited by methodological considerations such as placebo effects and lack of control groups (1,19,20). Therefore, research on the use of AP in sedation will bring new perspectives towards investigations and clinical use in this area. To the best of our knowledge, there are no reports about the use of subclinical doses of Acp injected in acupoints to induce sedation in horses.
The aim of this study was to investigate the sedative effect of both aquapuncture (i.e. saline injection, 0.01 ml kg−1) and Acp pharmacopuncture (i.e. a subclinical dose of Acp, 0.01 mg kg−1) in GV1 acupoint, compared to the conventional dose of a subcutaneous injection of Acp (0.1 mg kg−1) for sedation in horses.
Methods
Eight clinically healthy mixed breed horses weighing between 250 and 380 kg and age ranging from 4 to 15 years were used. Horses were maintained unrestrained in a paddock, during the experimental time and were randomly submitted to four different treatment protocols, at 1 week intervals according to a Latin Square double-blind design, as follows:
Negative Control Group (C, n = 8): 0.01 ml kg−1 subcutaneous saline injection in the cervical region.
Positive Control Group (Acp, n = 8): 0.1 mg kg−1 of Acp 1% injected subcutaneously in the cervical region.
Aquapuncture Group (Sal-GV1, n = 8): 0.01 ml kg−1 of saline injected into GV1 acupoint.
Pharmacopuncture Group (1/10Acp-GV1, n = 8): 0.01 mg kg−1 of Acp injected into GV1 acupoint.
In all cases the volume was 1 ml per 100 kg. Saline solution was used as vehicle where applicable.
The following measurements were performed before and at 30, 60 and 90 min after treatments: heart rate (with a stethoscope), respiratory rate (by observation of the thoracic movements), head height (distance from the floor to the animal's muzzle) and degree of sedation. To evaluate the degree of sedation, seven different parameters were graded as ‘0’ (no response), ‘1’ (reduced response) and ‘2’ (normal response). These scores were performed for labial ptosis, palpebral ptosis, response to touching at the coronary region of the hind limb, the ear pinnae and the shoulder with a pen tip and visual (by waving a piece of white cloth in front of the head) and auditory stimuli (by clapping hands beside the animal). The sum of the scores of all the imposed stimuli was used for data analysis.
Statistical analysis was performed using ANOVA for repeated measures to investigate differences in time in each group and between treatments. Statistical significance (*P < 0.01 and # P < 0.05) was determined by Newman–Keuls multiple comparison test.
Results
1/10Acp-GV1 Induced 60 min Sedation
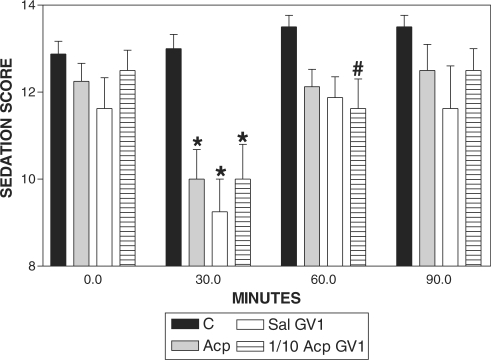
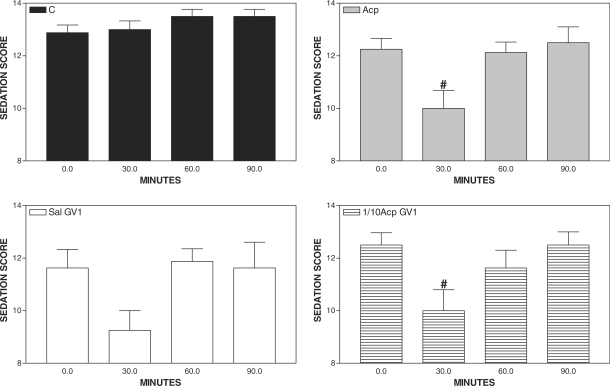
Sedation was observed both in Acp and 1/10Acp-GV1-treated horses at 30 min when compared with basal values. However, only the 1/10Acp-GV1 group showed sedation at 60 min after treatment, when compared with C (Fig. 1). Signs of sedation were not observed in C and Sal-GV1 when compared with the basal value (Fig. 2).
Figure 1.
Pharmacopuncture and sedation score in horses. Mean ± SEM of sedation score of horses treated with acepromazine (Acp, 0.1 mg kg−1 s.c., n = 8), aquapuncture (Sal-GV1, 0.01 ml kg−1 of saline in GV1, n = 8), pharmacopuncture (1/10 Acp-GV1, 0.01 mg kg−1 of Acp in GV1, n = 8) or saline (C, 0.01 ml kg−1 of saline s.c., n = 8). *P < 0.01 and #P < 0.05 compared with the control group (ANOVA, followed by Newman–Keuls multiple comparison test).
Figure 2.
Pharmacopuncture and sedation score in horses. Mean ± SEM of sedation score of horses treated with saline (C, 0.01 ml kg−1 of saline s.c., n = 8), acepromazine (Acp, 0.1 mg kg−1 of Acp s.c., n = 8), aquapuncture (Sal-GV1, 0.01 ml kg−1 of saline in GV1, n = 8) or pharmacopuncture (1/10 Acp-GV1, 0.01 mg kg−1 of Acp in GV1, n = 8). #P < 0.05 compared with the basal value (ANOVA, followed by Newman–Keuls multiple comparison test).
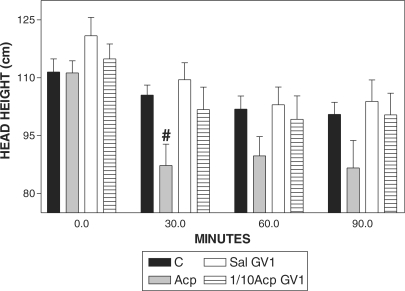
Only Acp Produced Lower Values of Head Height
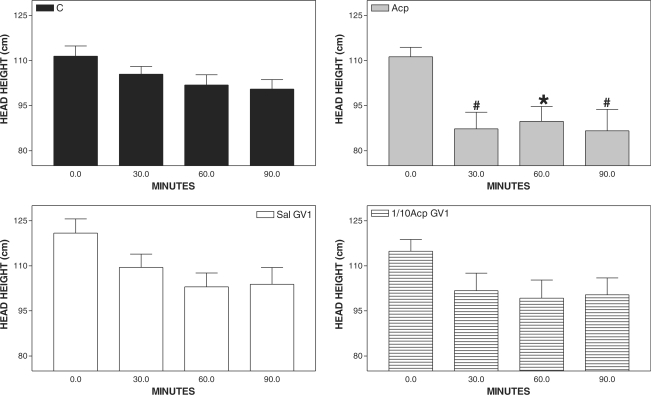
Head height was reduced significantly only 30 min after Acp, when compared with C (Fig. 3) and when compared with the basal value (Fig. 4).
Figure 3.
Pharmacopuncture and head height in horses. Mean ± SEM of head height (cm) of horses treated with acepromazine (Acp, 0.1 mg kg−1 of Acp s.c., n = 8), aquapuncture (Sal-GV1, 0.01 ml kg−1 of saline in GV1, n = 8), pharmacopuncture (1/10 Acp-GV1, 0.01 mg kg−1 of Acp in GV1, n = 8) andsaline (C, 0.01 ml kg−1 of saline s.c., n = 8). #P < 0.05 compared with control group (C, n = 8) (ANOVA, followed by Newman–Keuls multiple comparison test).
Figure 4.
Pharmacopuncture and head height in horses. Mean ± SEM of head height (cm) of horses treated with acepromazine (Acp, 0.1 mg kg−1 of Acp s.c., n=8), aquapuncture (Sal-GV1, 0.01 ml kg−1 of saline in GV1, n = 8), pharmacopuncture (1/10 Acp-GV1, 0.01 mg kg−1 of Acp in GV1, n = 8) and saline (C,0.01ml kg−1 of saline s.c., n = 8). *P < 0.01 and # P <0.05 compared with basal values (ANOVA, followed by Newman–Keuls multiple comparison test).
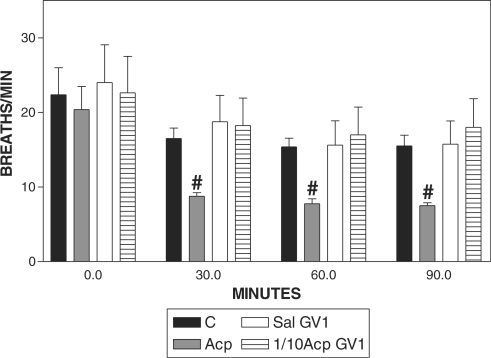
Acp Reduced the Respiratory Rate
Respiratory rate reduced from 30 to 90 min in Acp group when compared with C (Fig. 5) and when compared with the basal value (Fig. 6).
Figure 5.
Pharmacopuncture and respiratory rate in horses. Mean ± SEM of respiratory rate (breath min−1) of horses treated with acepromazine (Acp, 0.1 mg kg−1 of Acp s.c., n = 8), aquapuncture (Sal-GV1, 0.01 ml kg−1 of saline in GV1, n = 8), pharmacopuncture (1/10 Acp-GV1, 0.01 mg kg−1 of Acp in GV1, n = 8) and saline (C, 0.01 ml kg−1 of saline s.c., n = 8). #P < 0.05 compared with control group (C, n = 8) (ANOVA, followed by Newman–Keuls multiple comparison test).
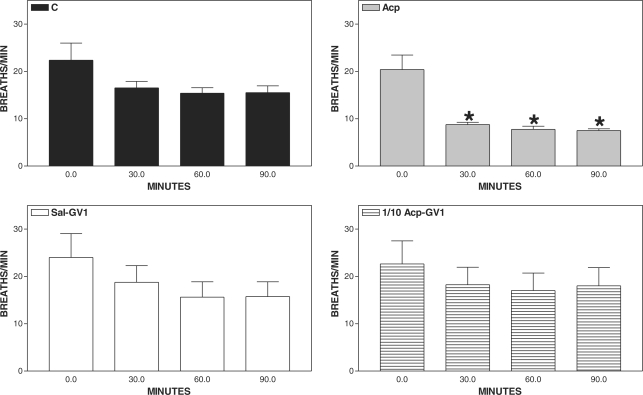
Figure 6.
Pharmacopuncture and respiratory rate in horses. Mean ± SEM of horses treated with acepromazine (Acp, 0.1 mg kg−1 of Acp s.c., n = 8), aquapuncture (Sal-GV1, 0.01 ml kg−1 of saline in GV1, n = 8), pharmacopuncture (1/10 Acp-GV1, 0.01 mg kg−1 of Acp in GV1, n = 8) and saline (C, 0.01 ml kg−1 of saline s.c., n = 8). *P < 0.01 compared with basal values (ANOVA, followed by Newman–Keuls multiple comparison test).
Heart Rate Did Not Change
The heart rate remained unchanged in all groups (data not shown).
Discussion
Acp produced typical signs of sedation in horses as previously reported (15,16). Aquapuncture (Sal-GV1) and pharmacopuncture (1/10Acp-GV1) induced signs of sedation 30 min after treatment, confirming the sedative effect of GV1 in horses (21). Only 1/10Acp-GV1 treatment was able to maintain a certain degree of sedation at 60 min, indicating a long-lasting effect of pharmacopuncture.
As expected, the reduction in respiratory rate was observed in horses treated with 0.1 mg kg−1 of Acp s.c., indicating that decreased respiratory rate was produced either by sedation or by a respiratory depression (15,16). Both aquapuncture (Sal-GV1) and pharmacopuncture (1/10Acp-GV1) produced signs of sedation without respiratory depression. This fact supports the effect of pharmacopuncture to enhance the effect of subclinical doses of drugs, with the advantage of reduction of undesirable side effects.
Head height is an external sign of sedation in horses, and it is used as a clinical parameter for evaluating the effectiveness of a sedative drug. External signs of sedation, showed by the association of reduced head height and sedation score, were observed only in the Acp group. All treated groups showed low sedation scores at 30 min after the onset of the study. Sedation is usually observed in animals by AP practitioners in most clinical cases. Several acupoints may be used for analgesia in surgical procedures and also for sedation (22–24). As low sedation scores occurred for the first 30 min in all treated groups and remained low for 60 min only in 1/10Acp-GV1, these results are in agreement with previous findings showing the potentiation effect of subclinical doses of drugs injected in an acupoint, i.e. pharmacopuncture (11). Previous reports in dogs showed that Acp-pharmacopuncture produced potentiation of thiopentone anaesthesia (11). These data demonstrate that more investigation is required to determine pharmacopuncture optimal dosage to reduce sedation score and head height as observed in horses treated with Acp.
The animals were randomly submitted to four different treatment protocols, at 1 week intervals according to a Latin Square double-blind design. According to that, any residual effect of Acp would be eliminated by the randomization. It would also be expected that the effect of Acp would be abated after 1 week of treatment (15,16).
The horses were maintained by the Veterinary School for educational purpose. They were used to the environment and easily handled. This may explain the tendency of reduction in head height and respiratory rate in all groups throughout the experimental time, demonstrating the adaptation to the environment. The uniform values of heart rate observed in the negative control group (C) during the study (data not shown) supports the fact that there were no environmental interferences in the animals' behavior. Although a transient reduction in heart rate induced by AP or by a somatosensory stimulus in humans and animals have already been documented (23–26), this was not the case in the present study.
In conclusion, although the conventional dose of Acp produced the lower head height, 1/10Acp-GV1 produced a longer sedative effect showing that, apparently, both regimens produced similar sedation in horses. The saline injection in GV-1 also produced sedation in horses.
Results indicate the potential application of pharmacopuncture in horses. Further studies could elucidate the best doses, drugs and acupoints to achieve an optimal effect, comparable to the conventional doses of drugs.
References
- 1.Habacher G, Pittler MH, Ernst E. Effectiveness of acupuncture in veterinary medicine: Systematic review. J Vet Intern Med. 2006;20:480–8. doi: 10.1892/0891-6640(2006)20[480:eoaivm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Ralt D. Intercellular communication, NO and the biology of Chinese medicine. Cell Commun Signal. 2005;3:1–6. doi: 10.1186/1478-811X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin JH, Wu LS, Wu YL, Lin CS, Yang NYJ. Aquapuncture therapy of repeat breeding in dairy cattle. Am J Chin Med. 2002;30:397–404. doi: 10.1142/S0192415X02000296. [DOI] [PubMed] [Google Scholar]
- 4.Lee JD, Park HJ, Chae Y, Lim S. An overview of bee venom acupuncture in the treatment of arthritis. Evid Based Complement Alternat Med. 2005;2:79–84. doi: 10.1093/ecam/neh070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek YH, Huh JE, Lee JD, Choi Y, Park DS. Antinociceptive effect and the mechanism of bee venom acupuncture (apipuncture) on inflammatory pain in the rat model of collagen-induced arthritis: mediation by alpha2-Adrenoceptors. Brain Res. 2006;1073/1074:305–10. doi: 10.1016/j.brainres.2005.12.086. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y. Acupuncture plus point-injection for 32 cases of obstinate urticaria. J Tradit Chin Med. 2006;26:22–3. [PubMed] [Google Scholar]
- 7.Martin BB, Jr, Klide AM. Use of acupuncture for the treatment of chronic back pain in horses: stimulation of acupuncture points with saline solution injections. J Am Vet Med Assoc. 1987;190:1177–80. [PubMed] [Google Scholar]
- 8.Zhang AL, Wu Y, Jiang XL. Analysis on therapeutic effect of acupoint-injection on chronic hepatitis B (Chinese) Zhongguo Zhen Jiu. 2005;25:25–6. [PubMed] [Google Scholar]
- 9.Zhu YH, Chen YH. On effects of acupoints and drugs in acupoint-injection treatment (Chinese) Zhongguo Zhen Jiu. 2005;25:46–8. [PubMed] [Google Scholar]
- 10.Jin JJ, Xu YL, Zheng Y. Effects of acupoint-injection of Kaixilai on TG, TC, MDA, SOD, AST and ALT in the patient of non-alcoholic fatty liver (Chinese) Zhongguo Zhen Jiu. 2006;26:100–2. [PubMed] [Google Scholar]
- 11.Wynn SG, Luna SPL, Liu H, Xie H, Nan TC, Chien CH. Global acupuncture research: previously untranslated studies. Studies from Brazil. In: Schoen AM, editor. Veterinary Acupuncture: Ancient Art to Modern Medicine. St Louis: Mosby; 2001. pp. 53–7. [Google Scholar]
- 12.Nie GJ, Goodin AN, Braden TD, Wenzel JG. Luteal and clinical response following administration of dinoprost tromethamine or cloprostenol at standard intramuscular sites or at the lumbosacral acupuncture point in mares. Am J Vet Res. 2001;62:1285–9. doi: 10.2460/ajvr.2001.62.1285. [DOI] [PubMed] [Google Scholar]
- 13.Dos Santos JG, Jr, Tabosa A, do Monte FH, Blanco MM, de Oliveira Freire A, Mello LE. Electroacupuncture prevents cognitive deficits in pilocarpine-epileptic rats. Neurosci Lett. 2005;384:234–8. doi: 10.1016/j.neulet.2005.04.079. [DOI] [PubMed] [Google Scholar]
- 14.Ovechkin A, Kim KS, Lee JW, Lee SM. Thermo-visual evaluation of the Yin-Tang acupuncture point for intracranial hypertension syndrome. Am J Chin Med. 2003;31:455–66. doi: 10.1142/S0192415X03001041. [DOI] [PubMed] [Google Scholar]
- 15.Chou CC, Chen LC, Rice BL, Colahan PT. Reduced resident time and pharmacodynamic effects of Acepromazine after subclinical multiple dosage in exercised thoroughbreds. J Vet Pharmacol Ther. 2002;25:379–82. doi: 10.1046/j.1365-2885.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 16.Miksa IR, Cummings MR, Poppenga RH. Determination of acepromazine, ketamine, medetomidine, and xylazine in serum: multi-residue screening by liquid chromatography-mass spectrometry. J Anal Toxicol. 2005;29:544–51. doi: 10.1093/jat/29.6.544. [DOI] [PubMed] [Google Scholar]
- 17.Kenjinji I, Hiroshi K. Comparison of transient heart rate reduction associated with acupuncture stimulation in supine and sitting subjects. Acupunct Med. 2003;21:133–7. doi: 10.1136/aim.21.4.133. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W, Fu LW, Tjen-A-Looi SC, Li P, Longhurst JC. Afferent mechanisms underlying stimulation modality-related modulation of acupuncture-related cardiovascular responses. J Appl Physiol. 2005;98:872–80. doi: 10.1152/japplphysiol.01079.2004. [DOI] [PubMed] [Google Scholar]
- 19.Kaptchuk TJ, Stason WB, Davis RB, Legedza AR, Schnyer RN, Kerr CE, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. Br Med J. 2006;332:391–7. doi: 10.1136/bmj.38726.603310.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarney RW, Lasserson TJ, Linde K, Brinkhaus B. An overview of two Cochrane systematic reviews of complementary treatments for chronic asthma: acupuncture and homeopathy. Respir Med. 2004;98:687. doi: 10.1016/j.rmed.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Fleming P. Transpositional equine atlas. In: Schoen AM, editor. Veterinary Acupuncture: Ancient Art to Modern Medicine. St Louis: Mosby; 2001. pp. 393–431. [Google Scholar]
- 22.Kim DH, Cho SH, Song KH, Lee SE, Lee SH, Kwon GO, et al. Electroacupuncture analgesia for surgery in cattle. Am J Chin Med. 2004;32:131–40. doi: 10.1142/S0192415X0400176X. [DOI] [PubMed] [Google Scholar]
- 23.Xie H, Ott EA, Harkins JD, Tobin T, Colahan PT, Johnson M. Influence of electro-acupuncture on pain threshold in horses and its mode of action. J Equine Vet Sci. 2001;27:591–600. [Google Scholar]
- 24.Wang JD, Kuo TB, Yang CC. An alternative method to enhance vagal activities and suppress sympathetic activities in humans. Auton Neurosci. 2002;100:90–5. doi: 10.1016/s1566-0702(02)00150-9. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Ma SX. Effects of l-arginine-derived nitric oxide synthesis on cardiovascular responses to stimulus-evoked somatosympathetic reflexes in the gracile nucleus. Brain Res. 2002;958:330–7. doi: 10.1016/s0006-8993(02)03664-8. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Ma SX. Nitric oxide in the gracile nucleus mediates depressor response to acupuncture (ST36) J Neurophysiol. 2003;90:780–5. doi: 10.1152/jn.00170.2003. [DOI] [PubMed] [Google Scholar]