Abstract
There is a need for effective nutraceuticals for osteoarthritis care. The fruit of Phyllanthus emblica is used as a powerful rejuvenator in Ayurvedic medicine. This study measured the chondroprotective potential of P. emblica (‘Amalaki’) fruits in vitro. We used aqueous extracts of unprocessed P. emblica fruit powder (powder A), and the powder obtained after hot water extraction and drying of powder A (powder B). Chondroprotection was measured in three different assay systems. First, we tested the effects of both fruit powders on the activities of the enzymes hyaluronidase and collagenase type 2. Second, an in vitro model of cartilage degradation was set-up with explant cultures of articular knee cartilage from osteoarthritis patients. Cartilage damage was assayed by measuring glycosaminoglycan release from explants treated with/without P. emblica fruit powders. Aqueous extracts of both fruit powders significantly inhibited the activities of hyaluronidase and collagenase type 2 in vitro. Third, in the explant model of cartilage matrix damage, extracts of glucosamine sulphate and powder B (0.05 mg/ml) exhibited statistically significant, long-term chondroprotective activity in cartilage explants from 50% of the patients tested. This result is important since glucosamine sulphate is the leading nutraceutical for osteoarthritis. Powder A induced a statistically significant, short-term chondroprotective activity in cartilage explants from all of the patients tested. This is the first study to identify and quantitate new chondroprotective activities of P. emblica fruits. These data provide pilot pre-clinical evidence for the use of P. emblica fruits as a chondroprotective agent in osteoarthritis therapy.
Keywords: collagenase, glycosaminoglycans, hyaluronidase, Phyllanthus emblica
Introduction
Osteoarthritis (OA) is a serious, degenerative disease. A systematic research of randomized, placebo-controlled clinical trials performed between 1980 and 2002, confirmed the efficacy of oral glucosamine sulphate (GS) on arthritis (1). However, controversies on therapeutic efficacy of glucosamine in OA prevail (2–4). Therefore, it is urgent and important to identify new chondroprotective nutraceuticals. Ayurvedic tradition has long recognized the medicinal properties of Phyllanthus emblica or Emblica officianlis fruits. Phyllanthus emblica fruits (‘Amalaki’), are primarily used for their anti-inflammatory activity and rejuvenating properties. According to Svoboda, the ancient Indian text Charakha Samhita; cites ‘Amalaki’ as the single most potent rejuvenating medicine (5).
Three in vitro assays were used to evaluate the hypothesis that crude aqueous extracts of P. emblica fruit powder exhibit chondroprotective activity. The matrix-metalloprotease enzymes (MMP's 1,3,8) express collagenase type 2 activity which can degrade cartilage matrix (6). Therefore, in the first assay, we tested effects of P. emblica fruit powder on the gelatinase activity of pure collagenase type 2. An earlier report suggested that P. emblica fruit juice inhibits activity of hyaluronidase (7), an enzyme which degrades cartilage matrix (8). Thus, in the second assay, we tested effects of P. emblica fruit powders on the activity of hyaluronidase in vitro.
Studies on chondroprotective drugs report that glycosaminoglycans (GAG) release by cartilage explants is a proven marker of cartilage matrix damage in vitro (9,10). Aggrecan is the major proteoglycan found in cartilage matrix. Human osteoarthritic explants treated with glucosamine-3-sulphate showed down regulated expression of aggrecan and aggrecanase mRNA, suggesting that glucosamine might reduce enzymatic breakdown of the extra-cellular matrix (11,12). Therefore in the third assay, we measured effects of P. emblica fruit powder extracts on GAG release from explant cultures of cartilage obtained from chronic OA patients during knee replacement surgery. GS was used as a positive control, to validate this experimental model.
This is the first report to identify and quantitate three novel chondroprotective activities of aqueous extracts of P. emblica fruit powder. First, P. emblica fruit extracts strongly inhibited activities of hyaluronidase and collagenase type 2 enzymes in vitro. Next, P emblica fruit powder B and GS caused a statistically significant, long-term decrease in levels of glycosaminoglycans released from human cartilage explants in a subset of OA patients tested, suggesting that both these drugs have similar chondroprotective effects on cartilage matrix in vitro. In contrast, P. emblica fruit powder A showed a significant short-term chondroprotective effect on cartilage explants from all of the patients tested.
Methods
Phyllanthus emblica Fruit Powders
Dried fruits of P. emblica (Indian Goooseberry) were collected from reliable sources and authenticated at National Institute of Science Communication and Information Resources (NISCAIR), New Delhi. (Standard herbarium specimen Acc. No. 3482).
Standardized, authenticated, P. emblica fruit powders were obtained from the Department of Health Sciences, Pune University, as part of the NMITLI-OA project sponsored by the Council for Scientific and Industrial Research, New Delhi, India. Powdered fruits were extracted with hot water. The extract was spray dried and standardized by HPLC (High Performance Liquid Chromatography) using gallic acid as a reference standard. The chromatogram revealed presence of gallic acid (retention time 4.3 min) in the sample at a wavelength of 270 Nm. HPLC Chromatograms can be provided if required.
We used unprocessed dry fruit powder (powder A), and the powder obtained after hot water extraction and spray drying of powder A (powder B). Powders A and B were solubilized in distilled water (10 mg/ml) by limited autoclaving (5 pounds pressure for 7 min); and filter sterilized (13 mm, 0.45 μm CN membrane), after addition to culture media.
Reagents and Tissue Culture Supplies
Growth media, electrophoresis reagents, and collagenase type 2 were from Life Technologies, Gibco, USA. Chondroitin sulphate was purchased from Calbiochem Corp, USA. GS capsules (‘Rejoint’) were from Nicholas Piramal, Ltd, India. Other reagents were from Qualigens Corp, India and Sigma Chemicals, (USA). Tissue culture plasticware was from Falcon Corporation, (USA).
Collagenase Type 2 Enzyme Assay
An electrophoretic assay was used to detect gelatinase activity of pure collagenase type 2 (13). Enzyme was freshly prepared (0.50% w/v solution in 50 mM Tris buffer pH 6.8) for each assay.
Hyaluronidase Enzyme Assay
Bovine testicular hyaluronidase (800 U/ml) and human umbilical cord hyaluronic acid (HA) (Potassium salt) (0.40 mg/ml.) were prepared in buffers specified by the supplier (Calbiochem Corpn, USA). Enzyme activity was assayed spectrophotometrically by monitoring digestion of HA substrate by the enzyme at 37°C for 1 h (14). Digestion of HA substrate was quantitated by measuring Absorbance415Nm (A415 Nm). Enzyme activity ranged from 75–95%, (i.e. enzyme digested 75–95% of the HA.) This method has been used to screen for hyaluronidase inhibitors (14). The following formulae were used for calculations.
A415 Nm value of undigested hyaluronic acid substrate is set at 100%
Enzyme activity = (100%) − {A415 Nm of HA + Enzyme/A415 Nm of HA × 100}
Percent enzyme activity in presence of herbal extract was calculated using the following ratio:
(Extract + HA + Enzyme) − (Extract alone)/
(Extract + HA) − (Extract alone).
To apply formula number 3, appropriate spectrophotometric data (A415 Nm values) are used for each of the terms shown within parentheses.
Explant Model of Cartilage Damage
Patient Profile
Donor cartilage was obtained from OA patients during knee replacement surgery. Patients of age 55–80 years had suffered chronic OA for 5–15 years. Only non-calcified, grade 1–2 cartilage (15) from the lateral femoral condyles was used.
Explant Cultures
Explant cultures of cartilage from OA patients (10–20 mg each) were set-up within 1.5 h of surgery, in 24 well tissue culture plates. Growth media contained a 1:1 mixture of DMEM: Ham's F12 basal media with 10% heat inactivated fetal bovine serum (FBS) + gentamycin 8 μg/ml. After 1 day in culture, explants in triplicate, were treated for 24 h with/without sterile aqueous extracts of powders A or B, (0.05 or 0.10 mg/ml). Next, explants were re-fed with media lacking herbal extract every 2 days for 8 days. Prior to each re-feeding, conditioned media (CM) samples from explants were collected and stored at −20°C. Thus, CM samples at four time points were available from each explant per patient (Day 2,4,6 and 8 days post 24 h treatment of explants with/without herbal extract). Time points of assay labeled as ‘1,2,3,4’ in Figs 2 and 3; correspond to GAG assays done on CM samples collected on Day 2,4,6 and 8, respectively. Explants were viable for the duration of the experiment.
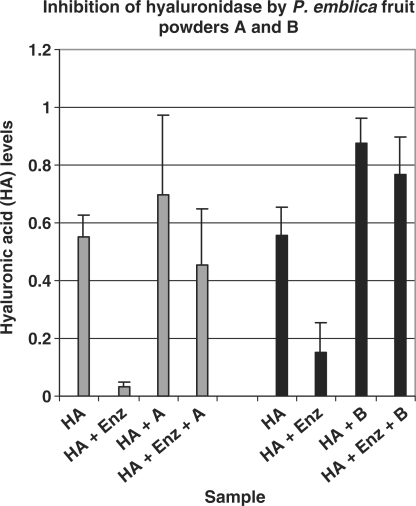
Figure 2.
Hyaluronidase inhibition by P. emblica fruit powders. The Y axis shows levels of HA measured by the spectrophotometer (A415Nm) in each sample. The X axis shows the contents of each sample. Powder A (gray columns): First column shows levels of HA alone. In presence of enzyme, HA levels decrease by 94% (second column), showing that hyaluronidase digested 94% of HA substrate. The third column shows high levels of HA incubated with powder A. The fourth column shows that addition of hyaluronidase to the mixture of HA + powder A, results in a 46% decrease of HA levels; indicative of decreased enzyme activity. Thus, aqueous extract of fruit powder A (0.30 mg/ml) caused 48% inhibition of enzyme activity (94–46%). Powder B (black columns): Columns 1 and 2 show that the enzyme digested 73% of HA substrate. In presence of powder B, enzyme activity was 18% (columns 3 and 4).Thus, aqueous extract of fruit powder B (0.30 mg/ml) caused 55% inhibition of the enzyme (73–18%). Each value represents Mean ± SD of six experiments for powders A and B. HA = Hyaluronic acid Enz = Enzyme (hyaluronidase) A = P. emblica fruit powder A B = P. emblica fruit powder B.
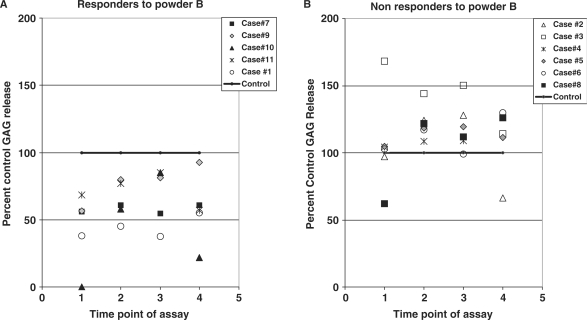
Figure 3.
(A) Responders to powder B in explant model of cartilage damage. Profile of GAG release in five OA cases (cases 1,7,9,10,11) which gave a long-term chondroprotective response to aqueous extract of powder B (0.05 mg/ml) is shown. In these five cases, powder B caused a significant decrease in the levels of GAG released relative to controls, at time points 1,2,3. Levels of GAG release by controls for all samples is set at 100%. (B) Non-responders to powder B in explant model of cartilage damage. This graph shows data for 6 of the 11 OA cases (cases 2,3,4,5,6,8) which did not give a chondroprotective response to aqueous extract of powder B. The levels of GAG release by controls for all samples is set at 100%.
DMMB Assay for Glycosaminoglycans
Total GAG content in each CM sample was measured by the dimethylmethylene blue dye binding assay using chondroitin sulphate (CS) as a standard (16). Data is expressed as GAG secreted in microgram equivalents of CS/mg of explant/ml of CM at each time point for each patient, in presence/absence of treatment with drugs.
Data Analysis
Raw data for GAG levels in CM samples released by explants treated with drugs, were compared with levels of GAG released by control (untreated) explants from the same patient at the same time point. Absolute differences between treated versus control values were tested for statistical significance using the Students 2 tailed t-test for paired samples. Data of statistical significance (P < 0.05%) were noted.
Presentation of Data
We calculated the effect of drug extracts on GAG present in each CM sample, at each time point, per patient sample; using the following ratio: GAG levels in CM from drug treated Explants/GAG levels in CM from control Explants. This ratio is graphically expressed as percent control GAG release for each powder for each subset of patients. GAG values in drug-treated samples, which showed a statistically significant decrease from GAG levels in the corresponding controls (P < 0.05) are shown as ‘Responders’. Data which lacked such statistical significance are shown as ‘Non-responders’ (Figs 2 and 3).
Results
Inhibition of Collagenase type 2 by P. Emblica Fruit Extracts
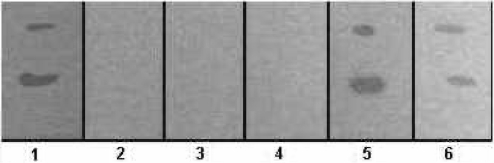
Figure 1 shows dose dependent inhibition of collagenase type 2 activity by aqueous extract of P. emblica fruit powder A. Figure 1 shows that the minimum inhibitory concentration (MIC) of fruit powder A required to inhibit the enzyme is 2.5 mg/ml (lane 4). Phyllanthus emblica fruit powder B also gave dose dependent inhibition of collagenase type 2 activity with a similar MIC value. As reported, collagenase type 2 was specifically inhibited by the zinc chelator, o-phenanthroline (17), with an MIC value of 2 mm (data not shown).
Figure 1.
Inhibition of gelatinase activity of collagenase type 2 by P. emblica fruit powder A. The gel shows gelatinase activity of collagenase type 2. The double bands indicate hydrolysis of gelatin on X-ray film by two forms of the enzyme. Lane 1 shows activity of enzyme alone (0.50% or 5 mg/ml). Lanes 2–6 show activity of the enzyme after incubation with a final concentration of 10, 5, 2.5, 1.25 and 0.625 mg/ml of extract of P. emblica fruit powder (A), respectively. The minimum inhibitory concentration (MIC) of fruit powder (A) required to inhibit the enzyme is 2.5 mg/ml (lane 4). This gel is representative of three experiments.
Dose dependent Inhibition of Hyaluronidase by P. emblica Fruit Powders Extracts
Aqueous extracts of P. emblica fruit powders A and B exhibited dose dependent inhibition of hyaluronidase. At a concentration of 0.60 mg/ml, both powders gave complete enzyme inhibition in four experiments. The IC50 value (concentration of powder required to give 50% enzyme inhibition) was 0.30 mg/ml for powders A and B (Fig. 2). Powder B (0.15 mg/ml) gave 19.43 ± 4.08% enzyme inhibition (n = 3). No detectable enzyme inhibition was observed with lower concentrations (0.03–0.10 mg/ml) of either fruit powder. These data are consistent with a patent report claiming that P. emblica fruit juice inhibits hyaluronidase (7).
Effects of P. emblica Fruit Powders in the Explant Model of OA Cartilage Damage
The inhibition of collagenase type 2 and hyaluronidase activities by extracts of P. emblica fruit powders, provided a rationale for testing the effects of these extracts on GAG release from cartilage explants of OA patients. GS was used as a positive control in this experimental model.
Long-term Chondroprotective Effects of P. Emblica Fruit Powder B and Glucosamine
Phyllanthus emblica Fruit Powder B
Figure 3A shows that extracts of P. emblica fruit powder B (0.05 mg/ml) induced a statistically significant long-term chondroprotective effect in 5/11 OA cases tested. In these five cases, the levels of PG release in response to fruit powder B extract were 54.67 ± 12.48%, 64.25 ± 14.27%, 68.57 ± 21.50% and 62.00 ± 26.70% of the control values in CM samples collected at time points 1,2, 3 and 4, respectively (P = 0.05 for time points 1,2,3). Thus, treatment with powder B extracts caused a statistically significant, (45–32%) reduction in GAG release from explants during the first 3 time points in culture. This chondroprotective effect of powder B was observed at a concentration of 0.05 mg/ml, but not at 0.10 mg/ml (data not shown).
Figure 3B shows GAG release profiles of cartilage explants from the six cases which did not give a chondroprotective response to powder B (non-responders). In these six cases, GAG release in response to powder B averaged 107.90 ± 33.30%, 121.12 ± 19.47%, 111.19 ± 22.18%, and 119.64 ± 37.18%, of corresponding controls at time points 1,2,3 and 4 respectively. These four GAG values were not significantly different from corresponding controls at these time points.
Glucosamine Sulphate (GS)
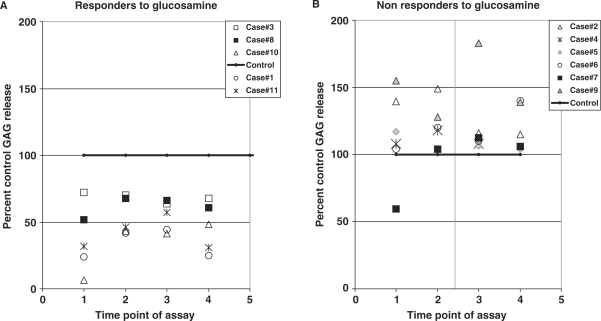
The patient cartilage samples tested with P. emblica fruit powder B (Fig. 2A), were tested in parallel with GS. Statistical analysis revealed that GS (0.05 mg/ml), caused a significant reduction in PG release from the explants for time points 2,3 and 4, in 5/11 patients. Figure 4A shows that in these five cases, the levels of PG release in response to GS were 37.04 ± 25.78%, 53.67 ± 13.75%, (P = 0.05), 54.60 ± 11.29% (P ≤ 0.001), and 51.15 ± 13.93% (P ≤ 0.001), of the control values from CM samples collected at time points 1,2,3 and 4, respectively (P = 0.05). Therefore, treatment with glucosamine also caused a significant 47–54% long-term decrease in GAG release from cartilage explants of these patients during the culture period.
Figure 4.
(A) Responders to glucosamine in explant model of cartilage damage. The graph shows profile of proteoglycan release in five OA cases (cases 1,3,8,10,11) which gave a long-term chondroprotective response to glucosamine (0.05 mg/ml). In these five cases, glucosamine caused a statistically significant decrease in the levels of GAG release relative to controls, at time points 2,3 and 4. Levels of GAG release by controls for all samples is set at 100%. (B) Non-responders to glucosamine in explant model of cartilage damage. This graph shows data for 6 of the 11 OA cases (cases 2,4,5,6,7,9) which did not give a chondroprotective response to aqueous extract of glucosamine. Levels of GAG release by controls for all samples is set at 100%.
Figure 4B shows GAG release profiles of cartilage explants from the six cases which did not respond to GS (non-responders). In these six cases, levels of GAG release in response to glucosamine averaged 120.91 ± 12.64%, 118.21 ± 29.81%, 121.67 ± 58.04% and 126.50 ± 34.85% of the corresponding controls at the time points 1,2,3 and 4, respectively. These four values were not statistically significantly different from corresponding controls at these 4 time points.
Phyllanthus emblica Fruit Powder A
We then tested effects of aqueous extracts of powder A on damage of cartilage matrix from OA patients. Use of powder A allowed testing bioactivity of unprocessed P. emblica fruits, and verification of the data on powder B. Due to insufficient cartilage available from each patient, explants from only 6 of the 11 OA cases were tested with Powder A. Therefore, cartilage explants from an additional three OA cases were also tested with this powder (cases 12,13,14); so that cartilage explants from a total of nine OA patients were tested with powder A (0.05 mg/ml and 0.10 mg/ml).
Results showed that explants from nine OA patients treated with powder A extract (0.10 mg/ml), released 65.05 ± 21.27% (P < 0.005) of GAG levels with respect to controls, at the first time point only. Therefore, P. emblica fruit powder A caused a statistically significant short-term, chondroprotection in explants from all nine OA patients. In these nine cases, levels of GAG release in response to powder A, were not significantly different from controls at subsequent time points (Values of GAG released in response to powder A were 108.36 ± 29.00%, 108.74 ± 28.60%, and 98.42 ± 32.15% of corresponding controls at time points 2,3 and 4 respectively). Notably, lower concentrations of powder A (0.05 mg/ml), lacked chondroprotective activity (data not shown).
Chondroprotective Effects of Glucosamine and P. Emblica Fruit Powders
Explants from 5/11 OA cases gave a long-term chondroprotective response to GS and P. emblica fruit powder B (Figs 3 and 4), whereas explants from the remaining six cases gave no significant response to either drug. Interestingly, three of five cases responded to GS and Powder B (cases 1,10,11). Similarly, four of six cases did not respond to either of these drugs (cases 2,4,5,6). Data showed that Powder A at the higher concentration (0.10 mg/ml) induced a short-term chondroprotective response in a total of nine cases, (6 of which belong to the group of 11 cases in which GS and powder B were tested).
In summary, aqueous extracts of P. emblica fruit powder B and clucosamine (0.05 mg/ml), induce a comparable long-term chondroprotective response in cartilage explants from 5/11 OA patients tested. In contrast, Powder A (0.10 mg/ml), induced a short-term chondroprotective response in cartilage explants from all nine OA patients in which it was tested
Potencies of Chondroprotective Effects of P. emblica Fruit Powders A and B
Powder B induced a long-term chondroprotective response at 0.05 mg/ml, whereas powder A induced a short-term chondroprotective response at 0.10 mg/ml. This data strongly suggests that water extraction of powder A results in a powder (powder B), with higher chondroprotective potency. However, powder A was superior to powder B in terms of the percentage of OA cartilage samples in which it induced a significant chondroprotective response. i.e. explants of cartilage of all nine OA patients tested, responded to powder A (0.10 mg/ml). In contrast, explants from only 5/11 OA patients, responded to powder B (0.05 mg/ml).
Drug Responders versus Non-Responders
One in vitro study also showed that chondrocytes from 40% of OA cases did not show increased aggrecan core protein levels, and decreased levels of MMP-protein in response to GS treatment (18). In this context, our data showing that explants from 5/11 of OA cases were ‘responders’ to the chondroprotective effects Glucosamine and P. emblica powder B (Figs 3 and 4); is not surprising. This is because diseased cartilage from OA patients (during knee replacement) may well give a partial/heterogenous response to such nutraceuticals.
Indeed, a partial response to glucosamine and chondroitin sulphate is reported in Glucosamine/chondroitin Arthritis Intervention Trial (GAIT), the most comprehensive clinical trial testing the effects of these nutraceuticals on pain (4). The GAIT study showed that both nutraceuticals caused a statistically significant decrease in pain in OA patients with moderate—severe pain (22% of the patients). However, in patients with mild pain (78% of the patients), these nutraceuticals did not cause a significant change in pain levels with respect to placebo (NCCAM 2006). Therefore, results of the GAIT study strongly suggest that even the best nutraceuticals only benefit a subset of OA patients. In this context, our pilot data showing that long-term chondroprotective activity of P. emblica fruit powder B mimics that of glucosamine, has pre-clinical value.
The phenomenon of responders and non-responders to drugs applies to ayurvedic medicine, wherein individual OA patients would be treated with different drugs according to body constitution, etc. Our data support this ayurvedic notion that only a subset of OA cases respond to P. emblica, because only a subset would have been prescribed this drug for OA.
Discussion
This is the first report showing chondroprotective activity of P. emblica fruit powders on human arthritic cartilage in vitro. Indeed, the long–term chondroprotective activity of P. emblica fruit powder B is comparable to that of GS in terms of magnitude and potency (Figs 3 and 4). Phyllanthus emblica fruit powder A is notable in inducing a strong short-term chondroprotective response in cartilage explants from all OA patients in which it was tested.
These results are physiologically relevant for three important reasons. First, we only used cartilage from chronic OA cases, since any chondroprotective drug must have a therapeutic benefit on arthritic cartilage. Second, to mimic Ayurvedic formulations, we used crude aqueous extracts of P. emblica fruit powders. Third, we only used standard serum containing growth media for cartilage cultures. For these reasons, the bioactivities of P. emblica fruit powders measured in this explant model of OA cartilage damage are likely to reflect their in vivo activity.
Thus far, the cytoprotective actions of P. emblica fruit in lymphocytes in vitro (19) and in rat models of ischemic injury (20) have been ascribed to its antioxidant activity. The mechanisms of chondroprotection by P. emblica fruit powder B (Fig. 3) and powder A, in human cartilage may in part be due to its hyaluronidase and gelatinase inhibitory activities (Figs 1 and 2). In this respect, polyphenols of blackberry fruit (21) and green tea (22), reportedly inhibit hyaluronidase and gelatinase activities, respectively.
None of the commercial chondroprotective drugs are reported to inhibit hyaluronidase; whereas the drugs nimusulide (23) and doxycycline (24) inhibit activities of collagenase and gelatinase in cartilage derived from osteoarthritis patients in vitro. In this context, the observed hyaluronidase and gelatinase inhibitory activities of P. emblica fruit powders is valuable pre-clinical evidence for an anti-arthritic role for P. emblica fruit. Future animal and human clinical trials will be required to extend and confirm these findings.
Acknowledgements
The authors are indebted to the orthopedic surgeons Dr S. Tapasvi, Dr A. Mapari; for providing cartilage samples from OA patients. We thank Ms T. Chinchwade for collagenase assays. We sincerely thank Dr S. Naranan for guidance on statistics, and Dr S. Gangal for valuable inputs.
References
- 1.Richy F, Bruyere O, Ethgen O, Cucherat M, Henerotin Y, Reginister JY. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis: a comprehensive meta-analysis. Arch Intern Med. 2003;163:1514–22. doi: 10.1001/archinte.163.13.1514. [DOI] [PubMed] [Google Scholar]
- 2.Cibere J, Kopec JA, Thorne A, Singer J, Canvin J, Robinson DB, et al. Randomized, double-blind, placebo-controlled glucosamine discontinuation trial in knee osteoarthritis. Arthritis Rheum. 2004;51:738–45. doi: 10.1002/art.20697. [DOI] [PubMed] [Google Scholar]
- 3.da Camara CC, Dowless GV. Glucosamine sulfate for osteoarthritis. Ann Pharmacother. 1998;32:580–7. doi: 10.1345/aph.17214. [DOI] [PubMed] [Google Scholar]
- 4.2006. NIH Glucosamine and Chondroitin Arthritis Intervention (GAIT) trial. [Google Scholar]
- 5.Svoboda RE. Ayurvedic Life Health and Longevity. Penguin Books India Ltd.; 1992. p. 161. [Google Scholar]
- 6.Milner JM, Elliot SF, Cawston TE. Activation of procollagenases is a key control point in cartilage collagen degradation: interaction of serine and metalloproteinase pathways. Arthritis Rheumatism. 2001;44:2084–96. doi: 10.1002/1529-0131(200109)44:9<2084::AID-ART359>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Patent filed by Takara Shuzo Co. Ltd. 1999. Pub No. JP 11-071290. [Google Scholar]
- 8.Sugimoto K, Iizawa T, Harada H, Yamada K, Katsumata M, Takahashi M. Cartilage degradation independent of MMP/aggrecanases. Osteoarthr Cartilage. 2004;12:1006–14. doi: 10.1016/j.joca.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Blot L, Marcelis A, Devogelaer J, Manicourt DH. Effects of diclofenac, aceclofenac and meloxicam on the metabolism of proteoglycans and hyaluronan in osteoarthritis human cartilage. Br J Pharmacol. 2000;131:1413–21. doi: 10.1038/sj.bjp.0703710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Squires GR, Okuneff S, Lonescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum. 2003;48:1261–70. doi: 10.1002/art.10976. [DOI] [PubMed] [Google Scholar]
- 11.Uitterlinden EJ, Jahr H, Koevoet JL, Jenniskens YM, Bierma-Zeinstra SM, Degroot J, et al. Glucosamine decreases expression of anabolic and catabolic genes in human osteoarthritic cartilage explants. Osteoarthr Cartilage. 2006;14:250–7. doi: 10.1016/j.joca.2005.10.001. . Epub November 18, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Patwari P, Kurz B, Sandy JD, Grodinsky AJ. Mannosamine inhibits aggrecanase-mediated changes in the physical properties and biochemical composition of articular cartilage. Arch Biochem Biophys. 2000;374:79–85. doi: 10.1006/abbi.1999.1538. [DOI] [PubMed] [Google Scholar]
- 13.Harsulkar AM, Giri AP, Gupta VS, Sainani MN, Deshpande VV, Patankar AG, et al. Characterization of Helicoverpa armigera gut proteinases and their interaction with proteinase inhibitors using gel X-ray film contact print technique. Electrophoresis. 1998;19:1397–402. doi: 10.1002/elps.1150190834. [DOI] [PubMed] [Google Scholar]
- 14.Tung JS, Mark GE, Hollis GF. A microplate assay for hyaluronidase and hyaluronidase inhibitors. Anal Biochem. 1994;223:149–52. doi: 10.1006/abio.1994.1560. [DOI] [PubMed] [Google Scholar]
- 15.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg. 1961;43-B:752–7. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 16.Hoemann CD, Sun J, Chrzanowski V, Bhushman MD. A Multivalent assay detect glycosaminoglycan, protein, collagen, RNA, and DNA content in milligram samples of cartilage or hydrogel-based repair cartilage. Anal Biochem. 2002;300:1–10. doi: 10.1006/abio.2001.5436. [DOI] [PubMed] [Google Scholar]
- 17.Springman EB, Nagase H, Birkedal-Hansen H, Van Wart HE. Zinc content and function in human fibroblast collagenase. Biochemistry. 1995;34:15713–20. doi: 10.1021/bi00048a016. [DOI] [PubMed] [Google Scholar]
- 18.Dodge GR, Jimenez SA. Glucosamine sulfate modulates the levels of the aggrecan and matrix metalloproteinase-3 synthesized by cultured human osteoarthritis articular chondrocytes. Osteoarthr Cartilage. 2003;11:424–32. doi: 10.1016/s1063-4584(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 19.Neetu D, Yogesh B, Anju B, Dipti P, Pauline T, Sharma SK, et al. Cyto-protective and immunomodulating properties of Amla (Emblica officinalis) on lymphocytes: an in vitro study. J Ethnopharmacol. 2002;81:5–10. doi: 10.1016/s0378-8741(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 20.Rajak S, Banerjee SK, Sood S, Dinda Ak, Gupta YK, Gupta SK, et al. Emblica officinalis causes myocardial adaptation and protects against oxidative stress in ischemic-reperfusion injury in rats. Phytother Res. 2004;18:54–60. doi: 10.1002/ptr.1367. [DOI] [PubMed] [Google Scholar]
- 21.Marquina MA, Coroa GM, Araujo L, Buitrago D, Sosa M. Hyaluronidase inhibitory activity from the polyphenols in the fruit of blackberry (Rubus fruticosus B.) Fitoterapia. 2002;73:727–9. doi: 10.1016/s0367-326x(02)00222-8. [DOI] [PubMed] [Google Scholar]
- 22.Demeule M, Brossard M, Page M, Gingras D, Beliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochem Biophys Acta. 2000;1478:51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier JP, Martel-Pelletier J. Effects of nimesulide and naproxen on the degradation and metalloprotease synthesis of human osteoarthritic cartilage. Drugs. 1993;46(Suppl 1):34–9. doi: 10.2165/00003495-199300461-00008. [DOI] [PubMed] [Google Scholar]
- 24.Smith GN, Jr, Yu LP, Jr, Brandt KD, Capello WN. Oral administration of doxycycline reduces collagenase and gelatinase activities in extracts of human osteoarthritic cartilage. J Rheumatol. 1998;25:532–5. [PubMed] [Google Scholar]