Abstract
We determined the therapeutic efficacy of atractylenolide I (ATR), extracted from largehead atractylodes rhizome, in managing gastric cancer cachexia (GCC), and interpreted its probable pharmacological mechanism via investigating tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6) and proteolysis-inducing factor (PIF). This was a randomized but not-blinded pilot. The study group (n = 11) received 1.32 g per day of atractylenolide I (ATR) and the control group (n = 11) received 3.6 g per day of fish-oil-enriched nutritional supplementation (FOE) for 7 weeks. Conservative therapy was similar in both groups. Clinical [appetite, body weight, mid-arm muscle circumference (MAMC), Karnofsky performance status (KPS) status], biomarker (TNF-α, IL-1, IL-6 and PIF) were evaluated in the basal state, at the third and seventh weeks. To analyze changes of cytokines, an immumohistochemistry technique was adopted. Base line characteristics were similar in both groups. Effects on MAMC and body weight increase, TNF-α increase and IL-1 decreases of serum level were significant in both groups (P < 0.05). ATR was significantly more effective than FOE in improving appetite and KPS status, and decreasing PIF positive rate (P < 0.05). Slight nausea (3/11) and dry mouth (1/11) were shown in intervention groups but did not interrupt treatment. These preliminary findings suggest that ATR might be beneficial in alleviating symptoms, in modulating cytokine and in inhibiting PIF proteolysis of gastric cancer cachexia. Further research using a randomized controlled design is necessary to confirm these pilot study findings.
Keywords: cytokine, largehead atractylodes rhizome, proteolysis-inducing factor
Introduction
Cachexia, the most common manifestation of advanced malignant diseases, is responsible for 22% of all cancer patients’ deaths (1,2). Cachexia is characterized by progressive weight loss and depletion of lean and fat body mass (3), and is associated with shorter survival period and reduced quality of life (4,5). Patients with gastric cancer have a high incidence of cachexia. Currently, an optimized treatment of cancer is the best therapy of cachexia, however, no effective systemic antineoplastic therapy has been found, and the toxicity of conventional chemotherapy may do harm to a patient's nutritional status. Therefore, it is necessary to find a simple, inexpensive and non-toxic compound to improve appetite and ameliorate quality of life for patients with advanced gastric malignancy.
In China, largehead atractylodes rhizome [family: Compositae] has been used to ameliorate gastrointestinal tract symptoms, such as anorexia for 2000 years. Largehead atractylodes rhizome is used well clinically as tonic. It has been applied clinically for recuperation after surgery or chronic diseases with symptoms such as exhaustion, fatigue, pale face, diarrhea, loss of appetite, vomiting and anorexia, especially for the patients having digestive disorder (6). Largehead atractylodes rhizome is also used for the treatments of carcinoma (7). Modern pharmacological researchers have confirmed that largehead atractylodes rhizome is effective in promoting gastrointestinal motility and intestinal secretion (8–10). Atractylodes rhizome and especially constituents of it has multiple pharmacological actions which are favorable for treating cancer (11–13). Atractylenolide I (ATR), the main bioactive chemical compound of largehead atractylodes rhizome (14–16), has been reported to induce apoptosis and bring about cytotoxicity of human promyeloleukemic HL-60 cells (17). ATR and atractylon were the major cytotoxic principle constituents of atractylodes rhizome on leukemia cell lines, but atractylenolides II and III showed no significant inhibition effects on tumor cell's growth (15). Furthermore, atractylenolide I has an anti-inflammatory effect (16). All of the data above suggests that ATR might play a useful role in the palliation of the cancer cachexia syndrome. However, currently available data does not show whether ATR is effective in managing cancer cachexia, and whether the mechanism also needs to be elucidated. It is the aim of this study to document the effects of largehead atractylodes rhizome treatment in alleviating symptoms via modulating cytokine and in inhibiting of proteolysis-inducing factor (PIF) proteolysis of gastric cancer cachexia.
Materials and Methods
Study Approval, Participant Criteria and Recruitment
Between November 2002 and May 2005 (31 months), 22 in-patients (13 men and 9 women) out of 62 gastric cachexia cancer patients in the Traditional Chinese Medicine department of the First Affiliated Hospital of Xi’an Jiaotong University were recruited, and meet the following eligibility criteria: (i) diagnosis of advanced unresectable gastric cancer; (ii) diminished or absent appetite; (iii) ability to take drugs orally; (iv) life expectancy >2 months and (v) no indication for further chemo or radiotherapy. Patients with active infection, severe heart, renal, or hepatic disease, primary or metastasis brain tumor, diabetes mellitus and those receiving nutritional support were excluded. Patient characteristics at baseline are outlined in Table 1. No differences of baseline variables were found. Eleven healthy people (seven men/four women) between the ages of 45 and 75 years old (mean 59.5 years) were selected for the healthy control group. Healthy volunteers were recruited through placard in our hospital and network advertisements run for a period of 1 month. We conducted a physical examination to rule out underlying pathology. All subjects gave their informed consent. The ethics committee of Xi’an Jiaotong University approved this study.
Table 1.
Patients’ characteristics at baseline
| FOE | ATR | |
|---|---|---|
| Age (years) | 56 (49–75 years) | 58 (45–75 years) |
| Stage (n) | ||
| IIIB | 3 | 2 |
| IV | 8 | 9 |
| Karnofsky performance scale (n) | ||
| 90–100 | 0 | 0 |
| 70–80 | 2 | 2 |
| 50–60 | 7 | 8 |
| 30–40 | 2 | 1 |
| Rate of body weight loss (kg) | −0.10 to −0.07 | −0.12 to −0.10 |
| Proteolysis-inducing factor positive (n) | 9 (81.8%) | 10 (90.9%) |
Rate of body weight loss: (LWW − HSW)/HSW. HSW: Historical stable weight; LWW: last week body weight.
Study Design
We randomly divided 22 patients into two groups, and each group had 11 patients. First, each group had a separate randomization schedule. After recruiting five patients of each group, we planned to recruit about an equal number, age and state of an illness of participants in the two groups by adaptive randomization method. The control and treated herb was hid separately in a code box with the same appearance. After each patient got his/her randomized code from statistician, he/she came to the pharmacy to get the certain drug of his/her code. All of the participants completed the study after randomization.
It was clear for both the patients and the curer which patient belonged to which treatment arm. Each patient belonging to the fish-oil-enriched nutritional supplementation group (briefed as FOE) received eight gelatine capsules of fish oil (EPA + DHA315 mg) twice a day. Each capsule contained 0.45 g of fish oil. The second group, briefed as ATR, received 6 ml (containing atractylenolide I 0.11 g ml−1) twice a day, half an hour after meals. The total duration of the study was 7 weeks, which was divided into two treatment phases. Each treatment phase contained 3 weeks of treatment and 1 week of rest. Patients received supportive care such as non-opiate analgesic, antiemetics and laxatives during therapy.
Outcome Measures
Study endpoints assessed and analyzed for each of the participants consisted of both objective and subjective measures. Primary outcome measures consisted of subjective scores from two self-reported questionnaires including the state/trait appetite scale and the performance status (Karnofsky) score. Secondary objective measures included body weight, mid-arm muscle circumference (MAMC) serum level of tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6), and PIF of urine sample. Aspartate transaminase (AST), alanine transaminase (ALT) and urinary creatinine were assessed each week for patient safety and the potential for liver or kidney toxicity. All outcome measures were obtained at baseline, the third week and seventh week by the same researcher who was blind to group assignment.
Assessing Changes in Clinical Parameters
To evaluate patients’ appetite status, an Appetite Visual Analogue Scale (VAS) was constructed. This is a 10-point scale ranging from 0 (no appetite) to 10 (normal appetite) (18). Each patient did self-report measures with instructions, and was asked to return the assessments in an enclosed self-addressed envelope at the end of the study.
We determined the rate of change of patients’ Karnofsky performance status (RCKPS) and appetite scale (RCA) as a surrogate to assess symptom relief. We also calculated the rate of body-weight loss (RBWL) and rate of change of mid-arm muscle circumference (RCMAMC), and assessed the difference between the two therapies.
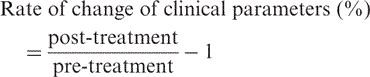 |
Biochemical Analysis
Eleven healthy individuals’ (seven men/four women) plasma sample were used as control values for cytokines. Blood samples were immediately centrifuged at 3800 g at 4°C for 10 min. The supernatant was then discarded and stored at −70°C. Analysis of the cytokines was conducted by a radio immunoassay technique using kits from Company of Fu-ri Biological Engineer (Beijing, China).
Purification of PIF from the Urine of Patients and Western Blot Analysis for PIF
24-h urine was treated with ammonium sulfate (80% saturation) and stored overnight at 4°C. After two rounds of 1 h centrifuge, the supernatant was removed. Samples of precipitate (5–10 μg) were resolved on 12% sodium dodecylsulphate, polyacrylamide gels (SDS/PAGE). They were transferred to 0.45 μm nitrocellulose membranes, which were blocked with 5% Marvell in Tris-buffered saline, pH 7.5, at 4°C overnight. 1:1000 dilution of PIF-specific antibodies and 1:1500 dilution of anti-rat IgG conjugated with horseradish peroxidase were incubated for 45 min at room temperature, and development was visualized by enhanced chemiluminescences. The resulting bands were compared to pre-stained molecular weight standards, which were run simultaneously to verify the appropriate molecular weight.
Plant Material and Preparation of Atractylenolide I
Largehead atractylodes rhizome was supplied and identified by the Taxonomist/Curator of the Department of Chinese Herbs of the University of Shaanxi Traditional Chinese Medicine. It was washed thoroughly with tap water, dried, cut into small pieces, and then was extracted by CO2 supercritical fluid extraction. Esquiterpenesolide extracts of largehead atractylodes rhizome were prepared with three stages as follows: flow rate of CO2 155–180 kg h−1, and first step: pressure 8 MPa, temperature 40°C, extraction time 2.5 h; second step: 10 MPa/40°C, extraction time 2 h; third step: 20 MPa/40°C, extraction time 2 h. The volatile oil was only collected at the third step, and the yield (%) of it was 2.42%. After the extract was placed in a water bath for 72 days at 41°C, it was further separated by four stage molecular distillation (MD) with separation process. Experiment parameters of wiped film distillation were as follow: feed temperature: 4°C; distillation temperature: 40–70°C; pressure: 10–15 Pa; rotating speed: 300–320 r min−1, feed rate: 3.0 ml h−1; and time 2 h. The yield of distilled oil was 28.6%. Chemical constituents of distilled oil were analyzed with GC–MS. The extracted compounds were separated on an HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm). The column oven temperature was programmed as follows: 50°C (2 min) to 120°C at 5°C min−1, then to 140°C at 3°C min−1, and maintained at 140°C for 5 min, then to 280°C at 10°C min−1, and maintained at 280°C for 30 min. The injection temperature and ion source temperature were 250°C and 230°C. Helium was used as the carrier gas with a flow rate of 1.0 ml min−1. The ionizing energy was 70 eV. All data were obtained by collecting the full-scan mass spectra within the scan range 40–350 amu. The four active compounds (atractylenolide I, atractylenolide II, actylone, 3-β-hydroxyatxactylone) in the essential oil were verified by their standards. The percentage of ATR was calculated via area normalization method. And the purity of it was 95.8%. ATR was dissolved with tweenum-80 and was taken 6 ml twice a day, half an hour after meals.
Statistical Analysis
Data were expressed as point estimates, with 95% confidence intervals. Analysis of serum levels IL-1, IL-6 and TNF-α and changes of clinical parameters were performed using a two-tailed and paired t-test. Comparisons of urine PIF between the two groups were made using exact probabilities in a 2 × 2 table. P < 0.05 was considered statistically significant.
Results
Clinical Parameters
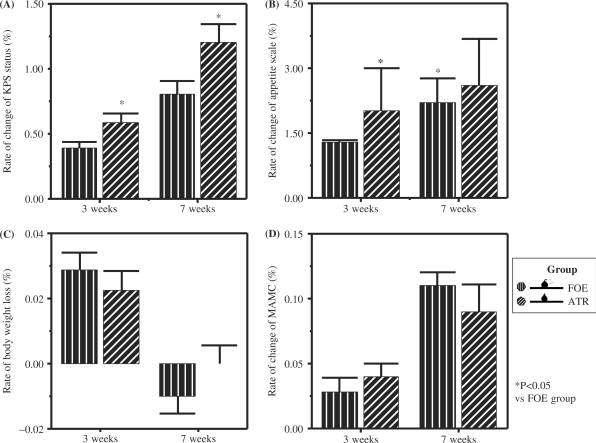
The Karnofsky Performance Score reflects the functional ability of patients. As shown in Table 2 and Fig. 2, ATR could improve appetite and KPS status, and was much more efficient than FOE (P1,2,3,4 < 0.05). FOE and ATR could both increase MAMC, and there was no statistical difference between them. All patients were losing weight before treatment (FOE: at a rate of −0.09 kg; ATR: at a rate of −0.11 kg, Table 1), whereas after 3 weeks therapy there was a median weight gain rate of 0.03 kg (FOE) and 0.02 kg (ATR). However, both ATR and FOE could not completely reverse weight loss. After 7 weeks of treatment, overall, 5 patients experienced weight gain, 10 had stable weights, and 7 continued to lose weight but at a reduced rate (FOE: median −0.09 kg before supplementation vs −0.11 kg after supplementation; ATR: median −0.11 kg before supplementation vs 0.00 kg after supplementation).
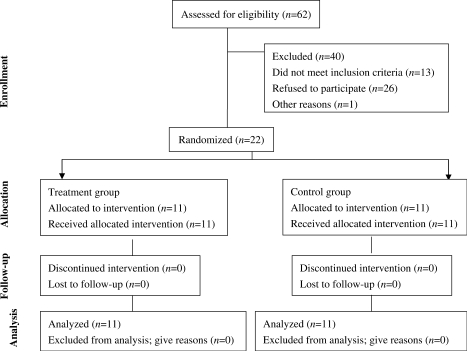
Figure 1.
Study design and flow of subjects.
Table 2.
Rate of change of clinical parameters of GCC after 3 and 7 weeks of treatment with ATR or FOE (%)
| 3 weeks Assessment |
7 weeks Assessment |
|||
|---|---|---|---|---|
| FOE | ATR | FOE | ATR | |
| RCKPS | 0.33–0.47 | 0.47–0.73*P1 | 0.60–1.00 | 0.93–1.47*P2 |
| RCA | 1.16–1.43 | 2.09–2.11*P3 | 2.13–2.27 | 2.46–2.73*P4 |
| RBWL | 0.02–0.04 | 0.01–0.03 | −0.02–0.00 | −0.01–0.01 |
| RCMAMC | 0.02–0.04 | 0.03–0.05 | 0.10–0.12 | 0.07–0.11 |
GCC: Gastric cancer cachexia; ATR: Atractylenolide I; FOE: Fish-oil-enriched nutritional supplementation group; RCKPS: rate of change of KPS status; RCA: rate of change of appetite scale; RBWL; rate of body weight loss; RCMAMC: rate of change of mid-arm muscle circumference. *P < 0.05 vs. fish-oil-enriched nutritional supplementation group. *P1 = 0.01; *P2 = 0.02; *P3 = 0.00; *P4 = 0.00.
Figure 2.
Comparison of effects of FOE and ATR on gastric cancer cachectic patients in change of clinical parameters after 3 weeks and 7 weeks treatment (A) rate of change of KPS status; (B) rate of change of appetite scale; (C) rate of body weight loss; (D) rate of change of mid-arm muscle circumference (MAMC). The values represent the means and SEM of serum cytokine levels. *P < 0.05 when compared with FOE group.
Serum Cytokine Levels
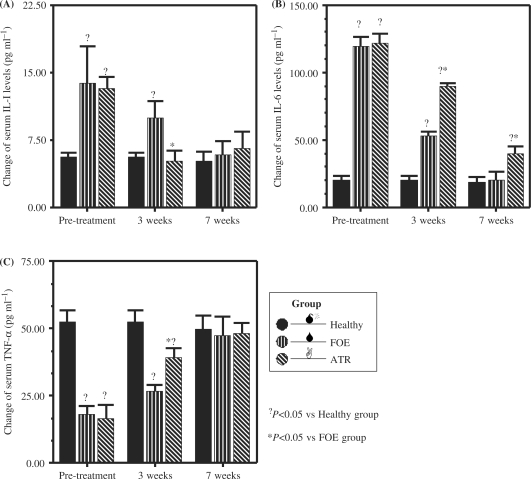
As shown in Tables 3 and 4 and Fig. 3, compared with that of healthy people, serum IL-6 and IL-1 levels increased and serum TNF-α levels decreased in gastric patients (P < 0.05). After 3 weeks of treatment, only ATR could statistically down-regulate the serum level of IL-1 towards normality. After 7 weeks of treatment, FOE could statistically modulate serum levels of IL-1, IL-6 and TNF-α towards normality, whereas ATR had no effect on IL-6.
Table 3.
Change of Serum Cytokine levels of GCC after 3 weeks of treatment with ATR or FOE (pg ml−1)
| FOE (n = 11) |
ATR (n = 11) |
Healthy | |||
|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||
| IL-1 | 5.39–22.31†P1 | 5.87–13.93†P2 | 10.44–15.96†P3 | 2.61–7.59*P12 | 4.59–6.61 |
| IL-6 | 113.11–126.29†P4 | 50.31–56.09†P5 | 113.64–128.96†P6 | 87.48–92.32†P7 *P13 | 17.14–23.06 |
| TNF | 15.11–21.29†P8 | 24.75–29.05†P9 | 10.99–22.01†P10 | 35.97–42.83 †P11 *P14 | 47.93–57.07 |
GCC: Gastric cancer cachexia; ATR: Atractylenolide I; FOE: Fish-oil-enriched nutritional supplementation group; †P < 0.05 vs Healthy group; P1 = 0.05; P2 = 0.01; P3 = 0.00; P4 = 0.00; P5 = 0.00; P6 = 0.00; P7 = 0.00; P8 = 0.00; P9 = 0.00; P10 = 0.00; P11 = 0.00; *P < 0.05 vs fish-oil-enriched nutritional supplementation group; P12 = 0.03; P13 = 0.00; P14 = 0.00.
Table 4.
Change of Serum Cytokine levels of GCC after 7 weeks of treatment with ATR or FOE (pg ml−1)
| FOE (n = 11) |
ATR (n = 11) |
Healthy | |||
|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||
| IL-1 | 5.39–22.31†P1 | 2.94–8.86 | 10.44–15.96†P2 | 2.77–10.43 | 3.12–7.28 |
| IL-6 | 113.11–126.29†P3 | 14.25–26.35 | 113.64–128.96†P4 | 34.36–45.24†P5 *P8 | 14.97–22.63 |
| TNF | 15.11–21.29†P6 | 40.28–54.92 | 10.99–22.01†P7 | 44.70–52.10 | 44.69–54.91 |
GCC: Gastric cancer cachexia; ATR: Atractylenolide I; FOE: Fish-oil-enriched nutritional supplementation group; †P < 0.05 vs Healthy group, P1 = 0.05; P2 = 0.00; P3 = 0.00; P4 = 0.00; P5 = 0.00; P6 = 0.00; P7 = 0.00; *P < 0.05 vs fish-oil-enriched nutritional supplementation group, P8 = 0.00.
Figure 3.
Comparison of effects of FOE and ATR on gastric cancer cachectic patients in change of serum cytokine levels after 3 weeks and 7 weeks treatment (A) change of serum IL-1 levels; (B) change of serum IL-6 levels; (C) change of serum TNF-α levels. The values represent the means and SEM of serum cytokine levels. ?P < 0.05 when compared with healthy group; *P < 0.05 when compared with FOE group.
Positive Rate of Urine PIF
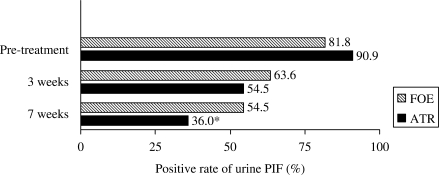
As shown in Table 5 and Fig. 4, there was no significant change of PIF positive rate after 3 weeks of treatment in both groups. Both therapies decreased PIF positive rate, but only ATR decreased PIF positive rate after 7 weeks treatment at a statistically significant rate (P < 0.05).
Table 5.
Positive rate of urine proteolysis-inducing factor of GCC after 3 and 7 weeks of treatment with ATR or FOE (n, %)
| Pre-treatment |
3 Weeks Assessment |
7 Weeks Assessment |
||||
|---|---|---|---|---|---|---|
| FOE | ATR | FOE | ATR | FOE | ATR | |
| Rate of PIF positive | 9 (48–98) | 10 (59–100) | 7 (31–89) | 6 (23–83) | 6 (23–83) | 4 (11–69)† |
| Rate of PIF negative | 2 (2–52) | 1 (0–41) | 4 (11–69) | 5 (17–77) | 5 (17–77) | 7 (31–89) |
GCC: Gastric cancer cachexia; ATR: atractylenolide I; FOE: Fish-oil-enriched nutritional supplementation group. †P < 0.05 vs Pre-treatment.
Figure 4.
Comparison of effects of FOE and ATR on gastric cancer cachectic patients in change of positive rate of urine PIF after 3 weeks and 7 weeks treatment. The bars represent the percent of urine positive rate of PIF. *P < 0.05 when compared with pre-treatment.
Adverse Events
Almost all patients complained the bad taste of ATR. The adverse events in treatment group that happened at days 7–12 visit included slight nausea (3/11) and dry mouth (1/11). But all of adverse events were needed not to be cured. Almost all patients (9/11) considered continuing use of ATR after termination of the study.
Discussion and Conclusion
Gastric cancer cachexia is a common condition with a dismal outlook, as pharmacological and nutritional therapy has led to rather disappointing results in the treatment of cancer cachexia. For instance, although different TNF synthesis inhibitors preserve the efficacy of antitumor treatment, they failed to improve the appetite or to increase the weight of cachectic patients (19). Progestational agents relieve anorexia and reverse weight loss in many patients; however, the weight gain is a result of fat rather than muscle accumulation (20).
As epidemiological and experimental studies have shown that fish oil (rich in n-3 polyunsaturated fatty acids) has the ability to chemo prevent and to chemo suppress tumor growth (21–24) via mediating the production of cytokines such as IL-6 and TNF, normalizing the metabolic response in patients with cancer cachexia, and improving a patient's nutritional status (25,26).
Anorexia, defined as the loss of appetite and early satiety, accounts for the malnutrition that is invariably associated with cancer cachexia. Host cytokines (TNF-α, IL-1, and IL-6) are thought to play a key role in cancer-induced anorexia and to be associated with weight loss (25–31). This study indicates that ATR might improve appetite, therefore might partially explain why largehead atractylodes rhizome can improve appetite in the light of TCM theory. What's more, ATR could down-regulate the serum IL-1 level, and up-regulate serum TNF level. This might partly explains why ATR has the ability to improve appetite, and reverse weight loss.
Both humoral (TNF and IL-6) and tumoral factors (PIF) have been shown to be able to activate the proteolysis mechanism of skeletal intracellular muscle, and thus, have a very important role in the induction of cachexia (31,34). Tumor-derived PIF, a 24-kDa proteoglycan (35), is present in a broad spectrum of cancer patients (i.e. carcinomas of the breast, lung, ovary, melanoma, gastro intestine). PIF produces direct proteolysis and a direct inhibitory effect on glucose consumption by skeletal muscle (36). Muscle protein waste is associated with patient immobility, respiratory dysfunction, lower immunity and poor performance. It was also proved that ATR was successful in improving KPS status, MAMC and decreasing PIF positive rate. These findings indicate that ATR might have some effects on muscle metabolism, therefore partially explains why physical capacity states are ameliorate.
A complex network of cytokines in combination with other factors may be responsible for the cachexia state of cancer. None of the mediators alone can fully explain all the facets of cachectic syndrome (37,38). Therefore, ATR can up-regulate TNF, down-regulate IL-1, decrease PIF positive rate and elevate appetite, but it cannot completely reverse weight loss.
One of the limitations of this study is that since this was a small unblinded pilot study, and a normal placebo-treated group was missing in term of ethical concern; there was potential bias in this study. Therefore it is necessary to carry out a standard double-blind randomized control trial in future to reassess the favorable effect of ATR on cancer cachexia and to test the possible side-effect. Another limitation of this study is that there was neither direct measurement on the concentration of ATR in gastric tissue nor on the change of gastric tissue to ethical concerns after ATR exposure. Instead, the effects of ATR on gastric cancer cachexia were measured by the change in biomarkers of serum cytokine levels such as TNF-α, IL-1, and IL-6 and change in biomarkers of urine PIF positive rate. Since no definite mediators of cancer cachexia have yet been identified (37,38) and the role of cytokines is still quite uncertain, the mechanism of action by which ATR exerts its beneficial effects is far from elucidation.
Taken together, our data provide evidence indicating that the ATR ameliorates the symptoms of gastric cancer cachexia, most likely through mediation of cytokine production, and inhibition of PIF proteolysis. These preliminary findings might aid the development of drugs to treat cancer cachexia to some extent.
Acknowledgements
The author is grateful to Dr Yimei T. for her assistance in the extraction of atractylenolide I; Mrs Sabrina K for her contributions on modifying the manuscript; and to Professor Chengzu Y for the supply of the plant material (largehead atractylodes rhizome) used in this study.
References
- 1.Argilés JM, Alvarez BF, López-Soriano J. The metabolic basis of cancer cachexia. Med Res. 1997;5:477–98. doi: 10.1002/(sici)1098-1128(199709)17:5<477::aid-med3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Harvey KB, Bothe A, Blackburn GL. Nutritional assessment and patient outcome during oncological therapy. Cancer. 1979;5:2065–9. doi: 10.1002/1097-0142(197905)43:5+<2065::aid-cncr2820430714>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Luis FBP, Costa R. Exercise as a time-conditioning effector in chronic disease: a complementary treatment strategy. Evid Based Complement Alternat Med. 2004;1:63–70. doi: 10.1093/ecam/neh018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inagaki J, Rodriguez V, Bodey GP. Causes of death in cancer patients. Cancer. 1974;33:568. doi: 10.1002/1097-0142(197402)33:2<568::aid-cncr2820330236>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.O’Gorman P, McMillan DC, McArdle CS. Impact of weight loss, appetite and the inflammatory response on quality of life in gastrointestinal cancer patients. Nutr Cancer. 1998;32:76. doi: 10.1080/01635589809514722. [DOI] [PubMed] [Google Scholar]
- 6.Li SZ. Ben-cao gang-mu, In: Jiangxi (ed). Vol. 2. Beijing: The People's Health Pub., House; 1977. pp. 880–5. [Google Scholar]
- 7.Lu Zh. Ben-jing feng-yuan. Beijing: The China Press of Traditional Medicine, House; 1996. p. 29. [Google Scholar]
- 8.Yamahara J, Matsuda H, Huang Q, Li Y, Fujimura H. Intestinal motility enhancing effect of Atractylodes lancea rhizome. J Ethnopharmacol. 1990;29:341–4. doi: 10.1016/0378-8741(90)90044-t. [DOI] [PubMed] [Google Scholar]
- 9.Nogami M, Moriura T, Niahimura M, Kubo M. Studies on the origin, processing and quality of crude drugs. III. Pharmacological evaluations of the Chinese drug “zhu” in experimental stomach ulcer (3) effect of extract of Atractylodes lancea var. chinensis on gastric secretion. Yakugaku Zasshi. 1985 doi: 10.1248/yakushi1947.105.10_973. [DOI] [PubMed] [Google Scholar]
- 10.Nakai Y, Kido T, Hashimoto K, Kase Y, Sakakibara I, Higuchi M, et al. Effect of the rhizomes of Atractylodes lancea and its constituents on the delay of gastric emptying. J Ethnopharmacol. 2003;84:51–5. doi: 10.1016/s0378-8741(02)00260-x. [DOI] [PubMed] [Google Scholar]
- 11.Mori H. Mechanisms of antitumor activity of aqueaes extracts from Chinese herbs: their immunopharmacological properties. Jan J Pharmacol. 1989;49:423. doi: 10.1254/jjp.49.423. [DOI] [PubMed] [Google Scholar]
- 12.Kimura I. Medical benefits of using natural compounds and their derivatives having multiple pharmacological actions. Yakugaku Zasshi. 2006;126:133–43. doi: 10.1248/yakushi.126.133. [DOI] [PubMed] [Google Scholar]
- 13.Tsuneki H, Ma EL, Kobayashi S, Sekizaki N, Maekawa K, Sasaoka T, et al. Antiangiogenic activity of beta-eudesmol in vitro and in vivo. Eur J Pharmacol. 2005;512:105–15. doi: 10.1016/j.ejphar.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 14.Nisikawa Y, Watanabe Y, Seto T, Yasuda I. Studies on the components of Atractylodes. I. New sesquiterpenoids in the rhizone of Atractylodes lancea De Candolle. Yakugaku Zasshi. 1976;96:1089–93. [PubMed] [Google Scholar]
- 15.Wang CC, Chen LG, Yang LL. Cytotoxic activity of sesquiterpenoids from Atractylodes ovata on leukemia cell lines. Planta Med. 2002;68:204–8. doi: 10.1055/s-2002-23144. [DOI] [PubMed] [Google Scholar]
- 16.Endo K, Toguchi T, Toguchi F, Hikino H, Yamahara J, Fujimura H. Antiinflammatory principles of Atractylodes rhizomes. Chem Pham Bull. 1979;27:2954–58. doi: 10.1248/cpb.27.2954. [DOI] [PubMed] [Google Scholar]
- 17.Ching-Chiung W, Shyr-Yi L, Huey-Chuan Ch, Wen-Chi H. Pro-oxidant and cytotoxic activities of atractylenolide I in human promyeloleukemic HL-60 cells. Food Chem Toxicol. 2006;44:1308–15. doi: 10.1016/j.fct.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Simons JP, Aaronson NK, Vansteenkiste JF, Velde GP, et al. Effects of medroxy progester one acetate on appetite, weight, and quality of life in advanced-stage non-hormone sensitive cancer: a placebo-controlled multicenter study. J Clin Oncol. 1996;14:1077–84. doi: 10.1200/JCO.1996.14.4.1077. [DOI] [PubMed] [Google Scholar]
- 19.Skudicky D. Beneficial effects of pentoxifylline in patients with idiopathic dilated cardiomyopathy treated with angiotensinconverting enzime inhibitors and carvedilol: results of a randomized study. Circulation. 2001;103:1083–88. doi: 10.1161/01.cir.103.8.1083. [DOI] [PubMed] [Google Scholar]
- 20.Loprinzi CL, Schaid DJ, Dose AM. Body-composition changes in patients who gain weight while receiving megestrol acetate. J Clin Oncol. 1993;11:152–4. doi: 10.1200/JCO.1993.11.1.152. [DOI] [PubMed] [Google Scholar]
- 21.Karmali RA. Historical perspective and potential use of n-3 fatty acids in therapy of cancer cachexia. Nutrition. 1996;12:S2–4. doi: 10.1016/0899-9007(96)90008-8. [DOI] [PubMed] [Google Scholar]
- 22.Sauer LA, Dauchy RT, Blask DE. Mechanism for the antitumor and anticachetic effects of n-3 fatty acids. Cancer Res. 2000;60:5289–95. [PubMed] [Google Scholar]
- 23.Sauer LA, Dauchy RT, Blask DE. Polyunsaturated fatty acids, melatonin,and cancer prevention. Biochem Pharmacol. 2001;61:1455–62. doi: 10.1016/s0006-2952(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 24.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83:217–44. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 25.Wigmore SJ, Fearon KCH, Maingay JP, Ross JA. Down-regulation of the acute-phase response in patients with pancreatic cancer cachexia receiving oral eicosapentaenoic acid is mediated via suppression of interleukin-6. Clin Sci. 1997;92:215–21. doi: 10.1042/cs0920215. [DOI] [PubMed] [Google Scholar]
- 26.Barber MD, McMillan DC, Preston T, Ross JA, Fearon KCH. The metabolic response to feeding in weight-losing pancreatic cancer patients and its modulation by a fish oil-enriched nutritional supplement. Clin Sci. 2000;98:389–99. [PubMed] [Google Scholar]
- 27.Nelson KA. The cancer anorexia-cachexia syndrome. Semin Oncol. 2000;1:64–8. [PubMed] [Google Scholar]
- 28.Davis MP, Dickerson D. Cachexia and anorexia: cancer's covert killer. Support Care Cancer. 2000;8:180–7. doi: 10.1007/s005200050282. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani G, Maccio A, Lai P. Cytokine involvement in cancer anorexia/cachexia: role of megestrol acetate and medroxy-progesterone acetate on cytokine down-regulation and improvements on clinical symptoms. Crit Rev Oncog. 1998;9:99–106. doi: 10.1615/critrevoncog.v9.i2.10. [DOI] [PubMed] [Google Scholar]
- 30.Scott HR, McMillan DC, Crilly A, McArdle CS, Milroy R. The relationship between weight loss and interleukin 6 in non–small-cell lung cancer. Br J Cancer. 1996;73:1560. doi: 10.1038/bjc.1996.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fearon KCH, McMillan DC, Preston T, et al. Elevated circulating interleukin-6 is associated with an acute-phase response but reduced fixed hepatic protein synthesis in patients with cancer. Ann Surg. 1991;213:26. doi: 10.1097/00000658-199101000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantovani G, Maccio A, Mura L. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J Mol Med. 2000;78:554–61. doi: 10.1007/s001090000137. [DOI] [PubMed] [Google Scholar]
- 33.Rubin H. Cancer cachexia: its correlations and causes. Proc Natl Acad Sci USA. 2003;100:5384–9. doi: 10.1073/pnas.0931260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani G, Maccio A, Mura L. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J Mol Med. 2000;78:554–61. doi: 10.1007/s001090000137. [DOI] [PubMed] [Google Scholar]
- 35.Todorov P, Cariuk P, McDevitt T, Coles B, Fearon K, Tisdale M. Characterization of a cancer cachectic factor. Nature. 1996;379:739–42. doi: 10.1038/379739a0. [DOI] [PubMed] [Google Scholar]
- 36.Lorite MJ, Thomspon MG, Drake JL, Carling GM, Tisdale J. Mechanism of muscle protein degradation induced by a cancer cachectic factor. Br J Cancer. 1998;78:850–6. doi: 10.1038/bjc.1998.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coodley GO, Loveless MO, Merrill J. The HIV wasting syndrome: a review. J Acquir Immune Defic Syndr. 1994;7:681–94. [PubMed] [Google Scholar]
- 38.Nelson KA, Walsh D, Sheehan FA. The cancer anorexia-cachexia syndrome. J Clin Oncol. 1994;12:213–25. doi: 10.1200/JCO.1994.12.1.213. [DOI] [PubMed] [Google Scholar]