Abstract
Natural herbal medicine (NHM) has been used to control infectious diseases for thousands of years. In view of the possible beneficial effect of NHM on SARS, we conducted this study to examine whether NHM is of any benefit as a supplementary treatment of SARS or SARS-like infectious disease. This was a randomized, double-blind, placebo-controlled trial. Twenty-eight patients fulfilled the WHO inclusion criteria and our exclusion criteria. All enrolled patients received routine western-medicine treatment. Patients were randomly allocated to one of the three supplementary treatment groups: NHM A (Group A, n = 9) NHM B (Group B, n = 9) or placebo (Group C, n = 10). Chest X-ray was done every 1 or 2 days for every patient. Reading radiologists use a standard 0–3 scoring system (0: no infiltration; 1: focal haziness or even small patchy lesion; 2: ground glass picture; 3: lobar consolidation) according to the severity of infiltration in each lung field (three lung fields in both right and left lungs). The main outcome measurements were the improving chest radiographic scores (IRS) and the duration (days) till improvement (DI). One patient from the placebo group passed away. Patients from NHM A took less days before showing improvement (6.7 ± 1.8) compared with placebo group (11.2 ± 4.9), which showed statistical significance (P = 0.04). The cases were too few to be conclusive, the initial observations seem to indicate NHM appears to be safe in non-criticallly ill patients and clinical trials are feasible in the setting of pandemic outbreaks.
Keywords: avian influenza, nature herbal medicine, SARS
Introduction
As of August 15, 2003, the cumulative SARS cases reported to the WHO from 29 countries were 8098, with 774 deaths (1,2). A novel coronavirus is the cause of this disease (3–5). Because the pathogenesis is still under study, there is no effective treatment as yet (6). Although many guidelines or protocols have been proposed (7,8), there are few well-designed studies. Natural herbal medicine (NHM) has been used to control infectious diseases for thousands of years. In view of the possible beneficial effect of NHM on SARS or SARS-like infectious diseases, we conducted this randomized, double-blind clinical trial with placebo-control to examine its effectiveness.
Methods
Setting and Patients
Our hospital (Taipei Hospital, Taiwan) was committed to take care of local SARS patients in the middle of March, 2003. A 52-bed ward was rebuilt as negative pressure seclusion rooms to admit SARS patients.
The subjects were recruited from April 25, 2003 to June 30, 2003. They were admitted via the out-patient department and emergency service of our hospital, or transferred from other hospitals. All the patients met the WHO inclusion criteria (9). We excluded aged patients over 55 years, patients younger than 18 years old, and those with severe clinical conditions such as endotracheal intubation or unstable vital signs from the beginning (Table 1, Fig. 1). The protocol was approved by the Human Ethics Committee of our hospital.
Table 1.
Inclusion (WHO criteria9) and exclusion criteria
| Inclusion criteria |
| Suspect case |
| A person presenting with history of high fever (>38°C) AND cough or breathing difficulty |
| And one or more of the following exposures during the 10 days prior to onset of symptoms: |
| - close contact with a person who is a suspect or probable case of SARS; |
| - history of travel to an area with recent local transmission of SARS |
| - residing in an area with recent local transmission of SARS |
| Probable case |
| (i) A suspect case with radiographic evidence of infiltrates consistent with pneumonia or respiratory distress syndrome (RDS) on chest X-ray (CXR). |
| (ii) A suspect case of SARS that is positive for SARS coronavirus by one or more assays. |
| Exclusion criteria |
| At the time of entry into the study, a case should be excluded: |
| (I) If an alternative diagnosis can fully explain the illness. |
| (II) Age <18 and >55 years |
| (III) More than 10 days from the date of onset of fever, on endotrachea tube, or in severe conditions with other multiple medical problems. |
| (IV) GOT, GPT > 100 U/l, serum creatinine >2.5 mg/dl |
| (V) Breastfeeding or pregnant women. |
| (VI) Taken any Chinese herbs in the last 3 months |
| (VII) Any other conditions not suitable for trial as evaluated by the physician on duty. |
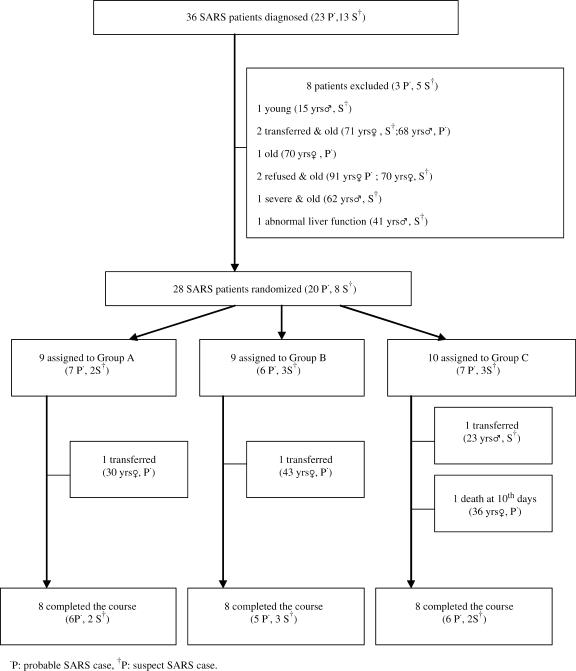
Figure 1.
Trial profile.
A clinical herb trial notification and a letter in which we explained how to take the NHM were given soon after the patient was admitted. Chinese herbal doctor would explain the details of the study and maintained close communication with the patient during the course of study. Informed consent was obtained from all the enrolled patients. The patients were free to withdraw from the study at any time.
Randomization and Blindness
Every subject was randomly assigned to one of the groups by computer. The SARS patients were isolated in the negative pressure seclusion wards having no contact with other patients. Thus, the patients were fully blind to the herb trial.
We provided two teams to care for the SARS patients, the western medical team and the Chinese medical team. The latter was responsible for Chinese-herb preparation and consultation. These two teams worked cooperatively but independently. The western medical doctors remained unaware of the herbal regimens and their effect.
To avoid bias from the radiologists who read the chest X-ray films, we substituted the chart number labeled on the X-ray films with a different number before submitting them for reading. Thus, our reading radiologists also remained unaware of the study procedures.
Herb Preparation and Treatment
NHM A was designed by our Chinese-herb consulting physician team according to the theory of Chinese medicine and our clinical experience. A total of 13 different kinds of herbs with 6–16 mg of each ingredient made up the formula (Table 2), which were popular be used in Taiwan and buy from the same Chinese medicine shop with certificate of standardized given. A sachet of NHM A weighed 120 gm, to which we added 600 ml water and simmered for 30 min until only 250 ml of decoction remained. NHM B was a health-care product popularly used in Taiwan, which was made from the elixir of Agaricus brazei Murill, Panax ginseng and Cordyceps sinensis (10–12). Every day, 250 ml of this elixir, diluted 1:200 with water, were taken by the subject. Placebo-control was 250 ml of 1:200 diluted brown sugar water.
Table 2.
Natural herbal medicine A composition
| Chinese name | Pharmaceutical name | Dose (gm) | Weight (%) |
|---|---|---|---|
| Shi Gao | Gypsum fibrosum | 16 | 13.3 |
| Chai Hu | Bupleurum chinenese | 12 | 10 |
| Zhi Zi | Gardeniae jasminoides | 12 | 10 |
| Fang Fong | Siler divaricatum | 10 | 8.3 |
| Huang Gin | Scutellaria baicalensis | 10 | 8.3 |
| Qiang Huo | Notopterygium incisium | 8 | 6.7 |
| Jing Jie | Schizonepeta tenuifolia | 8 | 6.7 |
| Fu Ling | Poria cocos | 8 | 6.7 |
| Shao Yao | Paeoniae lactiflorae | 8 | 6.7 |
| Ban Xia | Pinellia ternata | 8 | 6.7 |
| Hua Xiang | Agastache rugosa | 8 | 6.7 |
| Jie Geng | Platycodon grandiflorum | 6 | 5 |
| Mai Men Dong | Ophiopogon japonicus | 6 | 5 |
To maintain the quality of the NHM, the Chinese-medicine doctors who participated in the project had to undergo a training on NHM preparation. The first CHM or placebo was administered within 12 h after the patient was admitted. The treatment lasted throughout hospitalization.
Western Medical Treatment
The western medical doctors in charge of the SARS patients prescribed routinely the following.
Robatrol (Ribavirin, 200 mg) 10 tablets loading, then 2 tablets am and three tablets pm for 10–14 days.
Klaricid (Clarithromycin, 250 mg) two tablets, bid, for 10–14 days.
In case the respiratory symptoms aggravated, the patients were usually given extra steroid, such as methylprednisolone (Solu-Medrol, 40 mg) 1 vial intravenous injection every 8 hours for a 2–3 day course, and the dose then tapered. If acute respiratory distress syndrome developed, they administered intravenous immunoglobulin (IVIG in short, Octagam, Gamimune, 2.5 g in 50 ml) 20–30 vials intravenous drip for 6–8 hours per day for two consecutive days.
Main Outcome Assessment
The chest X-ray film was reported to be the standard assessment and compatible with the progression of SARS (7,10,11). Chest X-ray was done 1 or 2 days for every SARS patient. Our reading radiologists use a standard 0 to 3 scoring system (0: no infiltration; 1: focal haziness or even small patchy lesion; 2: ground glass picture; 3: lobar consolidation) according to the severity of infiltration in each lung field (three lung fields in both right and left lungs). The scores were then added up to a total of 0–18 scores for each film (7). Three radiologists gave the scores of the serial films independently. If the scores reached 12, denoting a poor condition, the patient would be transferred to the intensive care unit (ICU) and intubation was considered. The main outcome measurements were the IRS and the DI, which were defined as follows.
IRS = Maximal Score (MS) − Final Score (FS).
DI = Duration (days) between the MS day and FS day,where MS day is the day on which the most severe condition was read during hospitalization; and FS day is the day on which the patient's condition is improved or become stabilized.
Laboratory and Clinical Presentations
All nasopharyngeal washing specimens, deep throat gargles and blood of the patients were submitted to our Disease Control Bureau for SARS-CoV polymerase chain reaction (PCR) examination (13–17). We also sampled the patients’ serum during hospitalization and after discharge for SARS-CoV antibody (16–19). The antibody and conditions used in indirect immunofluorescence test (IIFT). The method employed for antibody test was IIFT. Antibody titers were reported according to the intensity of fluorescence obtained at different sample dilutions. A serum dilution of 1:10 or higher with weaker or stronger fluorescence in SARS-CoV-infected cell cytoplasm was evaluated as positive. Two sets of sample were sent to our Disease Control Bureau and our laboratory for double testing. We collected simultaneously the demographic data, initial symptoms, vital signs of the subjects during the in-patient period. The serial laboratory data taken every 1–3 days included white blood cell count, platelet count, lymphocyte count, alanine aminotransferase, creatine kinase, lactate dehydrogenase and creatinine.
Statistical Analysis
Outcome was measured with student t-test to evaluate the difference between the groups. The other data were evaluated with ANOVA or chi-square test according to the data characteristics. All P-values were two-tailed and the α-level of significance was set at 0.05.
Results
Demographic
Among the 56 patients admitted to the negative pressure seclusion wards of Taipei Hospital in Taiwan, 36 patients fulfilled the diagnostic criteria of either suspected or probable SARS (Fig. 1). Of the 36 subjects, 8 were excluded. We randomly assigned the patients into three groups. Three patients were transferred to other hospitals due to personal reason (transferred to the same hospital with their family). One patient of Group C died on the ninth day. In the end, 24 patients completed the study (Fig. 1). The demographic and clinical profiles of each group at the time of admission were indistinguishable from one another (Table 3).
Table 3.
Patients’ characteristics at entry
| Group A Mean (SD) (n = 8) | Group B Mean (SD) (n = 8) | Group C Mean (SD) (n = 8) | P | |
|---|---|---|---|---|
| Variables | ||||
| Age, years old | 34.8 (9.4) | 39.9 (13.2) | 39.5 (8.8) | 0.46 |
| Sex ratio, ♀: ♂ | 3:5 | 5:3 | 4:4 | 0.61 |
| Positive of PCR or antibody rate | 0.5 | 0.6 | 0.6 | 0.84 |
| Days after disease onset before enrolled in the trial | 3.3 (1.0) | 3.3 (2.0) | 3.5 (1.6) | 0.94 |
| Initial vital signs | ||||
| Body temperature, °C | 38.5 (0.8) | 38.1 (0.7) | 38.4 (0.8) | 0.56 |
| Systolic blood pressure; mmHg | 121.0 (16.1) | 121.1 (16.8) | 118.8 (16.1) | 0.65 |
| Diastolic blood pressure, mmHg | 77.6 (13.6) | 77.4 (15.0) | 69.8 (13.3) | 0.45 |
| Respiratory rate, times/min | 21.8 (1.2) | 21.1 (1.7) | 22.6 (4.4) | 0.57 |
| Heart rate, beats/min | 95.3 (13.6) | 99.8 (17.2) | 95.5 (19.3) | 0.84 |
| O2 saturation‡ (%) on room air | 97.5 (1.4) | 95.4 (4.0) | 97.6 (1.0) | 0.16 |
| Initial symptoms rate | ||||
| Fever (>38°C) | 1.0 | 1.0 | 1.0 | 1.0 |
| Cough | 0.6 | 0.9 | 0.9 | 0.36 |
| Diarrhea | 0.8 | 0.3 | 0.4 | 0.11 |
| Received drug rate | ||||
| Robatrol | 1.0 | 1.0 | 0.9 | 0.35 |
| Intravenous immunoglobulin | 0.1 | 0.0 | 0.1 | 0.58 |
| Steroid | 0.4 | 0.5 | 0.6 | 0.61 |
| Antibiotics | 1.0 | 1.0 | 1.0 | 1.00 |
| Initial data | ||||
| Radiographic scores | 2.1 (1.2) | 2.5 (1.6) | 3.0 (2.7) | 0.67 |
| Lymphocytes, 10−9/l | 1.4 (0.7) | 1.0 (0.7) | 1.3 (0.6) | 0.71 |
| Platelets, 10−9/l | 235.1 (150.1) | 186.8 (56.9) | 182.5 (74.6) | 0.66 |
| Creatine Kinase, U/l | 281.5 (360.1) | 188.3 (130.9) | 156.3 (92.6) | 0.53 |
| Lactate dehydrogenase, U/l | 268.8 (132.9) | 287.0 (120.8) | 194.5 (46.3) | 0.21 |
| Creatinine, mg/dl | 1.0 (0.4) | 1.0 (0.2) | 0.9 (0.3) | 0.93 |
Outcomes
The chest X-ray scores at initial (IS), maximal (MS), and final (FS) stages were compared (Table 4). No significant statistical difference in chest radiographic scores was found among the groups. Patients from group A took less days before showing improvement (6.7 ± 1.8) compared with Group C (11.2 ± 4.9), which showed statistical significance (P = 0.04).
Table 4.
Analysis of outcomes
| Group A (n = 8) | Group B (n = 8) | Group C (n = 8) | P–value Comparison between group | |||
|---|---|---|---|---|---|---|
| Outcomes | Mean (SD) | Mean (SD) | Mean (SD) | A, B | B, C | A, C |
| Chest radiographic scores | ||||||
| Initial (IS) | 2.1 (1.2) | 2.5 (1.6) | 3.0 (2.7) | 0.57 | 0.69 | 0.43 |
| Maximal (MS) | 5.9 (3.5) | 5.6 (3.4) | 7.9 (4.0) | 0.85 | 0.23 | 0.31 |
| Final (FS) | 0.7 (0.9) | 0.5 (0.6) | 2.7 (3.4) | 0.75 | 0.12 | 0.15 |
| IRSa | 5.3 (3.7) | 5.0 (3.5) | 5.6 (4.7) | 0.91 | 0.91 | 0.99 |
| Duration (days) till improvement | ||||||
| Worsening (DW)b | 5.5 (4.2) | 3.8 (2.8) | 7.8 (5.7) | 0.36 | 0.10 | 0.37 |
| Improvement (DI)c | 6.7 (1.8) | 9.2 (5.9) | 11.2 (4.9) | 0.28 | 0.47 | 0.04 |
| Course (DC)d | 12.2 (5.0) | 13.0 (7.8) | 19.0 (10.0) | 0.81 | 0.20 | 0.11 |
aIRS (Improving Radiographic Scores), MS – FS; bDW, Duration (days) between IS to MS; cDI, Duration (days) between MS to FS; dDC, DW+DI [Duration (days)].
Expired Case
A 36-year-old female patient in Group C passed away, her severe radiographic scores were 16 on the ninth day after onset of disease. Her condition deteriorated fast. After endotracheal intubation and being transferred to the ICU, she died the next day.
Adverse Effects
No patients withdrew from the study because of discomfort or adverse effects associated with the treatment. Three patients developed mild diarrhea and one patient had upper abdominal discomfort after taking NHM A. No major adverse effects were noticed.
Discussion
Large outbreaks of SARS occurred in China (5327 cases with 349 deaths) Hong Kong (1755 cases with 299 deaths) and Taiwan (346 cases with 37 deaths); accounting for 91.7% of cumulative cases and 88.5% of total death globally (2). According to Treatise on Febrile Disease (Shang Han Lun), a variety of herbal formulas have been employed to treat patients with infectious diseases for over 1800 years. When the outbreak began in Guandong, NHM had already been anecdotally used as a supplementary regimen. Therefore, we had conceived the trial strategy before the SARS outbreak that occurred at the end of April in several hospitals in Taiwan. NHM is generally well accepted among the Chinese population; 95% of our patients agreed to participate in our study. In fact, many anecdotal reports have been proposed on the use of NHM for SARS patients, unfortunately none was a controlled trial (20).
To our knowledge, this is the first double-blind and placebo-controlled clinical pilot study on supplementary treatment of SARS or SAR-like diseases. SARS patients were in strict seclusion and were cared for independently by the SARS care team. The NHM preparation was provided by another Chinese medical team. Therefore, neither patients nor doctors-in-charge knew to which herbal trial group each patient was assigned. The SARS care team held daily meeting at 5:00 pm to discuss the patients’ conditions, treatment strategy and SARS control measures in the hospital. The Chinese medical team did not interfere with the management decision on each patient.
Related papers showed that younger children seem to have a less aggressive clinical course (21). Our young case, a 5-year-old child with positive SARS-CoV PCR, also had mild symptoms (only cough, fever, diarrhea, chest X-ray score of 1, chest radiographs showing complete resolution within 5 days). Advanced age and the presence of underlying medical illness have been reported to be risky factors for severe course (6,22–26). All probable SARS patients in Canada who required intubation or died had either underlying medical illnesses or were older than 55 years of age (6,14,24). So we excluded patients of old (>55years) and young age (<18 years), those with complicated underlying medical illness, and those already intubated from the beginning of our study.
All probable SARS patients showed infiltration on chest radiography (7,13,14). The radiographic feature of pneumonia is also one of the SARS diagnostic criteria (9). Chest radiographic finding is compatible with the condition of disease (6,7,25,26). The duration of illness course and radiological improvement were also taken into consideration. So we took the change in chest radiographic scores and the duration till improvement as outcome measurements.
The outbreak of SARS posed great challenges. The medical profession had less experience of this unprecedented and deadly disease. Although NHM is only a supplementary treatment, these initial findings seem to indicate a possible favorable effect of NHM on SARS or SARS-like diseases in shortening the course of recovery. All subjects treated with NHM A and B survived. One patient from the placebo group expired. Although our cases were too few to be conclusive, the initial observations seem to indicate the possible benefit of NHM on SARS or SARS-like diseases. The findings need to be verified with a larger sample.
Discussion on mechanisms behind NHM treatment is inevitably speculative. However, there are gaps in our understanding of the immunopathogenesis of SARS (27). This study was designed only to detect changes in IRS and DI. Nevertheless, our literature review offers significant insight worthy of further exploration on SARS. Avendano and Booth reported that many SARS cases in Toronto presented with hypocalcemia within 3 days of admission (6,14). We checked serum calcium in one of our cases, which showed hypocalcemia on the fourth day after the onset of disease (the second day of admission). The major ingredient of our NHM A is a mineral, Gypsum fibrosum (16 gm, 13.3% of recipe by weight), which contains mainly calcium sulfate, popularly used for antipyretic and clearing the accumulated heat in the lung according to the traditional Chinese medical theory. We speculate that one of the effective mechanisms of NHM A is associated with correction of electrolyte imbalance that may play a very important role in enhancing immunity. Some natural products have been found to inhibit SARS-CoV 3C-like protease activity (28). The mechanism of anti-SARS effect in natural products merits further investigation.
Despite the encouraging results, our study still had limitations. First, the sample size was small since we had only eight cases of each group. However, within such a short span of time and in view of the unpredictability of the disease, enrollment was completely beyond our control. Second, the study subjects were 54% positive rate of SARS-CoV PCR or antibody. The SARS-CoV PCR and antibody were not the necessary WHO criteria of SARS during our study period. However, after randomization procedure, the rate of positive SARS-CoV PCR and antibody were balance among three groups. The results were also focused on SARS or SARS-like diseases.
Like SARS, the threat of a possible pandemic due to person-to-person transmission of avian influenza A (H5N1) has aroused much concern among health professionals (29). While different pathogens are involved in SARS, SARS-like diseases and avian influenza, all of them are severe infectious diseases with similar clinical presentations (29,30) and pose serious adverse impact on the respiratory system. We have learned from the experience of using NHM as supplementary treatment that it has potential benefits on SARS or SARS-like infectious diseases or other contagious respiratory diseases such as avian flu. Thus, it is desirable to conduct similar studies and clinical trials in case of an outbreak of avian influenza in the future.
Conclusion
The cases were too few to be conclusive, the initial observations seem to indicate (i) NHM appears to be safe in non-criticallly ill patients, (ii) clinical trials are feasible in the setting of pandemic outbreaks and (iii) the results of this study are encouraging but a large trial is required to asses the efficacy of treatment.
Acknowledgements
We thank the participants of the study; Kong-Yeng Tseng, Yen-Yi Ho, Chien-Chung Chen, Shu-Ling Hsieh, Cheng-Chin Ker, Lin-Chen Chien, Feng-Chi Hsieh, Weng-Kei Ma, Kuei-Feng Chen, Shin-Ming Chang, Shin-Yuan Chen, Pi-Kung Hsu, Yue-Lin Chang, Tzu-Lin Hsu, Mei-Chiao Lu, Shih-Hua Lin, Yu-Mei Lin, Chi-Jung Wang who were members of our SARS care team; Hao-Hung Liao, Tsui-Eo Chen, Chang-Feng Kuo and CDC of Taiwan for antibody and PCR analysis. We also thank Shian-chiou Lin and Yuen-Sen Association for providing the herbal sample, and all colleagues who worked in the SARS wards of Taipei Hospital, Taiwan. This study was supported by the Taipei Hospital.
References
- 1.World Health Organization. Update 96 - Taiwan, China SARS transmission interrupted in last outbreak area. [July 15, 2003]; Available at: http//www.who.int./csr/sarscountry/2003_07_05/en/
- 2.World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. [(last accessed September 30, 2003)]; Available at: http//www.who.int./csr/sarscountry/2003_09_26/en/
- 3.Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 4.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus association with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 5.Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–25. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:1–9. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 7.Ho W. Guidelines on management of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1313–5. doi: 10.1016/S0140-6736(03)13085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.So LKY, Lau ACW, Yam LYC, et al. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet. 2003;361:1615–7. doi: 10.1016/S0140-6736(03)13265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Case definitions for surveillance of severe acute respiratory syndrome (SARS). [(last accessed April 22, 2003)]; Available at: http//www.who.int./csr/casedefinition/en.
- 10.Sorimachi K, Ikehara Y, Maezato G, Okubo A, Yamazaki S, Akimoto K, et al. Inhibition by Agaricus brazei Murill fractions of cytopathic effect induced by western equine encephalitis virus on VERO cell in vitro Biosci, Biotechnoi Biochem. 2001;65:1645–7. doi: 10.1271/bbb.65.1645. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima A, Ishida T, Koga M, Takeuchi T, Mazda O, Takeuchi M, et al. Effect of hot water extract from Agaricus brazei Murill on antibody-production cell in mice. Int Immunopharmacol. 2002;2:1205–11. doi: 10.1016/s1567-5769(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Shao HJ, Su YB. Coimmunization of Agaricus brazei Murill extract with hepatitis B virus core protein through DNA vaccine enhances cellular and humoral immune response. Int Immunopharmacol. 2004;4:403–9. doi: 10.1016/j.intimp.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–94. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 14.Avendano M, Derkach P, Swan S. Clinical course and management in health care workers in Toronto: a case series. ACMAJ. 2003;168:1–13. [PMC free article] [PubMed] [Google Scholar]
- 15.Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–70. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.From the CDC. Updated Interim Surveillance Case Definition for Severe Respiratory Syndrome (SARS)-United States, April 29, 2003. JAMA. 2003;289:2367–9. [Google Scholar]
- 17.From the CDC. Updated Interim Surveillance Case Definition for Severe Respiratory Syndrome (SARS)-United States, June 11, 2003. JAMA. 2003;290:34. [Google Scholar]
- 18.Li G, Chen X, Xu A. Profile of specific antibody to the SARS-associated coronavirus. N Engl J Med. 2003;349:508–9. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- 19.Hoey J. Updated SARS case definition using laboratory criteria. CMAJ. 2003;168:1566–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Jia W, Gao W. Is traditional Chinese medicine useful in the treatment of SARS? Phytother Res. 2003;17:840–1. doi: 10.1002/ptr.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hon KL, Leung CW, Cheng WT, Chan PK, Chu WC, Kwan YW, et al. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361:1701–3. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masur H, Ezekiel E, Lane HC. Severe acute respiratory syndrome providing care in face of uncertainty. JAMA. 2003;289:10–2. doi: 10.1001/jama.289.21.JED30036. [DOI] [PubMed] [Google Scholar]
- 23.Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Cote S, et al. Virological features and clinical manifestations associated with human metalpneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–4. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 24.Poutanen SM, Low DE, Henry B, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 25.Tsang KW, Ho PL, Ool GC, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–85. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 26.Chan JW, Ng CK, Chan YH, Mok TY, Lee S, Chu SY, et al. Short-term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58:686–9. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontoyiannis DP, Pasqualini R, Arap W. Aminopeptidase N inhibitors and SARS. Lancet. 2003;361:1558. doi: 10.1016/S0140-6736(03)13186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CN, Lin CPC, Huang KK, Chen WC, Hsieh HP, Liang PH, Hsu JTA. Inhibition of SARS-CoV 3C-like Protease Activity by Theaflavin-3,3?-digallate (TF3) Evid Based Complement Altern Med. 2005;2:209–15. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, et al. Probable person-to-person transmission of avian influenza A(H5N1) N Engl J Med. 2005;352:338–41. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 30.Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]