Abstract
The chemical composition of ethanol extracts from samples of Brazilian propolis (EEPs) determined by HPLC and their activity against Trypanosoma cruzi, Staphylococcus aureus, Streptococcus pneumoniae, Klebisiella pneumoniae, Candida albicans, Sporothrix schenckii and Paracoccidioides brasiliensis were determined. Based on the predominant botanical origin in the region of samples' collection, the 10 extracts were separated into three groups: A (B. dracunculifolia + Auraucaria spp), B (B. dracunculifolia) and C (Araucaria spp). Analysis by the multiple regression of all the extracts together showed a positive correlation, higher concentrations leading to higher biological effect, of S. aureus with p-coumaric acid (PCUM) and 3-(4-hydroxy-3-(oxo-butenyl)-phenylacrylic acid (DHCA1) and of trypomastigotes of T. cruzi with 3,5-diprenyl-4-hydroxycinnamic acid derivative 4 (DHCA4) and 2,2-dimethyl-6-carboxyethenyl-2H-1-benzopyran (DCBEN). When the same approach was employed for each group, due to the small number of observations, the statistical test gave unreliable results. However, an overall analysis revealed for group A an association of S. aureus with caffeic acid (CAF) and dicaffeoylquinic acid 3 (CAFQ3), of S. pneumoniae with CAFQ3 and monocaffeoylquinic acid 2 (CAFQ2) and of T. cruzi also with CAFQ3. For group B, a higher activity against S. pneumoniae was associated DCBEN and for T. cruzi with CAF. For group C no association was observed between the anitmicrobial effect and any component of the extracts. The present study reinforces the relevance of PCUM and derivatives, especially prenylated ones and also of caffeolyquinic acids, on the biological activity of Brazilian propolis.
Keywords: bactericidal activity, chemical composition, fungicidal activity, propolis, statistical analysis, Trypanosoma cruzi
Introduction
Propolis presents a complex composition depending basically on the plant sources accessible to the bees, possessing a variety of biological and pharmacologic activities, attracting the interest of an increasing number of researchers (1). Brazillian samples present striking differences in their chemical composition when compared with samples from temperate zones (2). Besides, differences are also found among tropical samples depending on the local flora at the site of collection (3). As examples we found the Brazilian green or Alecrim propolis, originated from Baccharis dracunculifolia (Asteraceae) (4–6), the Cuban red propolis from Clusia nemorosa (Clusiaceae) (7) and more recently the red propolis collected in the North region of Brazil (8). While the microbicidal activity of European propolis has been associated with the presence of flavonoids and derivatives of caffeic acid (9,10), in the case of tropical samples, the main bioactive compounds are phenolic acids and prenylated derivatives (11).
Due to the increasing interest in the characteristics of Brazilian propolis, we undertook a study using samples collected in different regions, aiming to determine their effect against Trypanosoma cruzi and different species of bacteria and fungi. Trypanosoma cruzi is the etiologic agent of Chagas’ disease, an important Public Health problem in Latin America, the treatment of which is still inadequate since the available drug benznidazole, a nitroderivative, causes severe side effects and its efficacy for chronic patients is questionable (12,13). Streptococcus pneumoniae and Staphylococcus aureus were assayed since they can colonize the human nasopharynx and represent potential agents of several diseases (14,15). Klebisiella pneumoniae was also included in the present work. Candida albicans, Sporothrix schenckii and Paracoccidioides brasiliensis, was selected due to their importance as etiologic agents of mycosis in Brazil (16,17).
Materials and Methods
Propolis Extracts
Eleven samples were collected in different regions in Brazil and 30 g of each resin were triturated and extracted with 100 ml ethanol (Merck Darmstadt, Germany) for 20 days in the dark. Afterwards, the material was cooled for precipitation of waxes, which were removed by filtration and the extract was dried under reduced pressure at 60°C. Their yield varied between 40 and 60%. Stock solutions of the eleven extracts were prepared in dimethylsulfoxide (DMSO). Based on the predominant botanical origin in the region of samples collection they were separated as: group A (B. dracunculifolia plus Auraucaria spp, State of Paraná): EEP-01 to EEP-04; group B (B. dracunculifolia, State of Minas Gerais): EEP-05 to EEP-07 and, group C (Auraucaria spp, State of Paraná) EEP-08 to EEP-10. Another extract obtained from a red propolis collected in the State of Alagoas, EEP-11 (Schinus terebinthifolius, Diplotropis incexis and Manilkara huberi) was also included for the biological assays (8).
HPLC-MS Analysis
EEP-1 to EEP-10 were analyzed by HPLC (D-7000 Merck-Hitachi, Germany) equipped with a pump (model L-6200, Merck-Hitachi, Germany) and a diode array detector (L-3000, Merck-Hitachi, Germany) as previously described (18). Detection of the components was monitored at 280 and 340 nm and standard compounds were co-chromatographed with the extracts. 1H-NMR and 13C-NMR were recorded using a Varian Gemini 300 spectrophotometer and the mass spectra obtained in a Hewlett–Packard apparatus (model 5890 Series II Plus).
Fungicidal Activity
The agar cup method was used (19) to assay the effect of the EEPs against C. albicans (Ca IOC-3781), S. schenckii (Ss IOC-2832) and P. brasiliensis (Pb IOC-3698). Yeast cells of C. albicans were grown in Sabouraud medium (Difco Laboratories, Detroit, MI, USA) at 25°C and yeast-like cells of S. schenckii and P. brasiliensis were grown in brain heart infusion (BHI) (Difco) and peptone-yeast extract-glucose (PYG) (Difco) at 36°C. The fungal cells were washed in phosphate-buffered saline (PBS, pH 7.2) counted in a hemocytometer and the concentration adjusted to 3.4 × 107 cells ml−1. A plate of a suitable sterile agar, poured to a depth of 4 mm, was allowed to set and a single cup (15 mm diameter), cut from the centre of the plate. The cell suspensions (50 µl) were streaked radially from the cup to the edge of the plate on the suitable agar medium surface for each fungus and the cup filled with 200 µl of EEP (2–16 mg ml−1). The plates were incubated for 3 days at 25°C for C. albicans and for 6 days at 36°C for both S. schenckii and P. brasiliensis. The antifungal activity was measured as the diameter of the inhibitory zones. Controls were performed with DMSO, the solvent of the stock solutions of the extracts; no inhibition zone was observed. Diameters of less than 15 mm were considered as lack of activity.
Bactericidal Activity
The minimal inhibitory concentration (MIC) was determined by means of the broth microdilution method described by the National Committee for Clinical Laboratory Standards (20), followed by subculture. S. pneumoniae (ATCC 49619), S. aureus (ATCC 25923) and K. pneumoniae (ATCC 70603) were grown in Müeller-Hinton agar supplemented with 5% sheep blood and incubated for 18 h at 37°C. S. pneumoniae was incubated in a 5% CO2 atmosphere. Bacterium inoculum was prepared in Müeller-Hinton Broth (Oxoid Ltd Basingstone, Hampshire, England), adjusted to 0.5 McFarland turbidity standard (108 CFU ml−1) and then diluted 1:10. This suspension (100 µl) was added to equal volume of the EEP, previously prepared by 2-fold serial dilutions in 96-well plates. After 18 h, subculture (10 μl) from each well was made in the same conditions. MIC was considered the lowest concentration of the extract that yields negatives subcultures. The final concentration of DMSO in the assays did not interfere with proliferation of the bacteria.
Trypanocidal Activity
The Y strain of T. cruzi was used (21). Bloodstream trypomastigotes were obtained at the peak of parasitemia from infected albino mice, isolated by differential centrifugation and resuspended with Dulbecco's modified Eagle medium (DME) to a parasite concentration of 10 × 106 cells ml−1 in the presence of 10% blood. This suspension (100 µl) was added to the same volume of the EEP, previously prepared at twice the desired concentrations also in DME (0.025–4 mg ml−1) in 96-well plates and then incubated at 4°C. Trypomastigote concentration in the wells was 5 × 106 cells ml−1 containing 5% blood (22). Cell counts were performed after 24 h of incubation and the activity of the extracts was expressed as IC50 values, corresponding to the concentration that lysed 50% of the parasites. Experiments showed that in concentrations up to 0.2%, DMSO had no deleterious effect on the parasites.
Statistical Analysis
The correlation of the trypanocidal (IC50) or bactericidal (MIC) activity with the composition of each extract determined by HPLC, expressed in mg g−1 of dried extract (Table 1), was performed by the method of analysis of multiple regressions using the software SPSS for Windows 8.0. Positive correlation means that higher concentrations of a specific component are associated with higher antimicrobial activity, while negative correlation, means that at lower concentrations a higher activity was achieved. It is important to note that, for a given extract, lower values of the parameters IC50 and MIC indicate a higher activity. The comparison between the IC50 values for T. cruzi was performed by ANOVA followed by the Student-Newman–Keuls test (P < 0.05).
Table 1.
Chemical composition of ethanol extracts of Brazilian propolis samples (mg g−1 of dried extract)
| Group A |
Group B |
Group C |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
B. dracunculifolia + Auraucaria spp |
B. dracunculifolia |
Auraucaria spp |
|||||||||
| Compounds | EEP-01 | EEP-02 | EEP-03 | EEP-04 | EEP-05 | EEP-06 | EEP-07 | EEP-08 | EEP-09 | EEP-10 | |
| 3-Prenyl-4-hydroxycinnamic acid (PHCA) | 8.31 | 2.69 | 1.42 | 7.35 | 16.82 | 19.95 | 13.23 | 0.00 | 0.00 | 0.00 | |
| 2,2-Dimethyl-6-carboxyethenyl-2H- 1-benzopyran (DCBEN) | 10.02 | 3.48 | 1.54 | 7.62 | 0.00 | 4.80 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2,2-Dimethyl-8-prenyl-2H-1-benzopyran-6-propenoic acid (DPB) | 33.75 | 11.39 | 6.41 | 23.31 | 27.52 | 28.66 | 19.58 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid (DHCA) | 20.80 | 5.03 | 2.38 | 26.70 | 36.13 | 38.37 | 33.04 | 0.00 | 0.00 | 0.00 | |
| 3-(4-Hydroxy-3-(oxo-butenyl)-phenylacrylic acid (DHCA1) | 3.10 | 1.67 | 0.00 | 0.95 | 1.35 | 1.30 | 0.83 | 0.00 | 0.00 | 0.00 | |
| 3-Prenyl-4-(2-methoxypropionyloxy) cinnamic acid (DHCA2) | 2.97 | 1.29 | 0.00 | 2.05 | 0.06 | 0.75 | 2.08 | 0.00 | 0.00 | 0.00 | |
| 3,5-Dihydroxy-5-prenylcinnamic (DHCA3) | 3.75 | 1.58 | 0.00 | 1.62 | 0.48 | 0.08 | 1.41 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid derivative 4 (DHCA4) | 2.15 | 0.08 | 0.00 | 1.61 | 1.37 | 3.59 | 5.84 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid derivative 5 (DHCA5) | 2.96 | 1.18 | 0.00 | 2.43 | 1.48 | 0.46 | 2.69 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid derivative 6 (DHCA6) | 3.71 | 1.31 | 0.00 | 2.87 | 3.67 | 3.07 | 3.37 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid derivative 7 (DHCA7) | 0.00 | 0.00 | 0.00 | 0.00 | 2.43 | 0.06 | 2.28 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid derivative 8 (DHCA8) | 0.00 | 0.00 | 0.00 | 0.00 | 3.09 | 7.20 | 3.73 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid derivative 9 (DHCA9) | 0.00 | 0.00 | 0.00 | 0.00 | 9.25 | 3.75 | 2.64 | 0.00 | 0.00 | 0.00 | |
| 3-Hydroxy-2,2-dimethyl-8-prenyl-2H- 1-benzopyran-6-propenoic acid (DHCA10) | 0.00 | 0.00 | 0.00 | 0.00 | 4.95 | 5.81 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid derivative 11 (DHCA11) | 0.00 | 0.00 | 0.00 | 0.00 | 5.86 | 4.85 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid derivative 12 (DHCA12) | 0.00 | 0.00 | 0.00 | 0.00 | 5.24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid derivative 13 (DHCA13) | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 3,5-Diprenyl-4-hydroxycinnamic acid derivative 14 (DHCA14) | 0.00 | 0.00 | 0.00 | 0.00 | 3.47 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| p-Coumaric acid (PCUM) | 19.07 | 9.56 | 0.79 | 15.42 | 33.48 | 32.47 | 18.81 | 0.00 | 0.00 | 0.00 | |
| Caffeic acid (CAF) | 2.39 | 1.54 | 0.00 | 0.00 | 1.35 | 1.55 | 2.94 | 0.00 | 0.00 | 0.00 | |
| Cinnamic acid derivative 1 (CIN1) | 1.58 | 1.01 | 0.00 | 7.07 | 0.00 | 0.00 | 0.00 | 0.00 | 5.28 | 0.00 | |
| 3-Prenyl-4-dihydrocinnamoyl-oxy- cinnamic acid (CIN2) | 0.00 | 0.00 | 0.00 | 1.42 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Benzoic acid (BENZ) | 0.00 | 0.00 | 6.53 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 24.74 | 0.00 | |
| Monocaffeoylquinic acid 1 (CAFQ1) | 7.35 | 6.35 | 0.00 | 6.05 | 9.79 | 13.59 | 4.31 | 0.00 | 0.00 | 0.00 | |
| Monocaffeoylquinic acid 2 (CAFQ2) | 1.57 | 1.49 | 0.00 | 0.00 | 4.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Dicaffeoylquinic acid 1 (CAFQ3) | 2.79 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Dicaffeoylquinic acid 2 (CAFQ4) | 0.61 | 0.50 | 0.13 | 0.37 | 0.99 | 1.85 | 0.63 | 0.00 | 0.00 | 0.00 | |
| Pinobanksin (PK) | 0.00 | 0.00 | 0.00 | 0.00 | 45.61 | 34.92 | 26.00 | 0.00 | 0.00 | 0.00 | |
| Kaempferol (KAEMP) | 0.00 | 0.00 | 0.00 | 3.91 | 0.00 | 0.00 | 0.00 | 0.00 | 1.60 | 0.00 | |
| Kaempferide (KAEMP1) | 20.80 | 0.00 | 0.00 | 11.76 | 37.05 | 34.08 | 33.04 | 0.00 | 0.00 | 0.00 | |
| Crysine (CRYS) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 6.24 | 0.00 | |
| 3-Methoxy-4-hydroxy-benzaldehyde (VAN) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.48 | 8.76 | 22.82 | |
| 3-Methoxy-4-hydroxycinnamaldehyde (G2) | 0.00 | 0.00 | 1.01 | 5.09 | 0.00 | 0.00 | 0.00 | 0.72 | 0.32 | 2.95 | |
| 2-[1-Hydroxymethyl]vinyl-6-acetyl-5-hidroxycumarane (I) | 0.00 | 0.00 | 2.31 | 0.00 | 0.00 | 0.00 | 0.00 | 3.86 | 0.00 | 6.19 | |
| Galangin (GAL) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 11.50 | 0.00 | |
| Compound E (E) | 48.52 | 15.48 | 0.00 | 43.46 | 78.00 | 77.81 | 56.22 | 0.00 | 0.00 | 0.00 | |
| Compound H (H) | 0.00 | 0.00 | 20.60 | 0.00 | 0.00 | 0.00 | 0.00 | 11.61 | 97.38 | 17.68 | |
| Compound L2 (L2) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.67 | 0.00 | 3.61 | |
Results
Chemical Composition and Fungicidal Activity of the Extracts
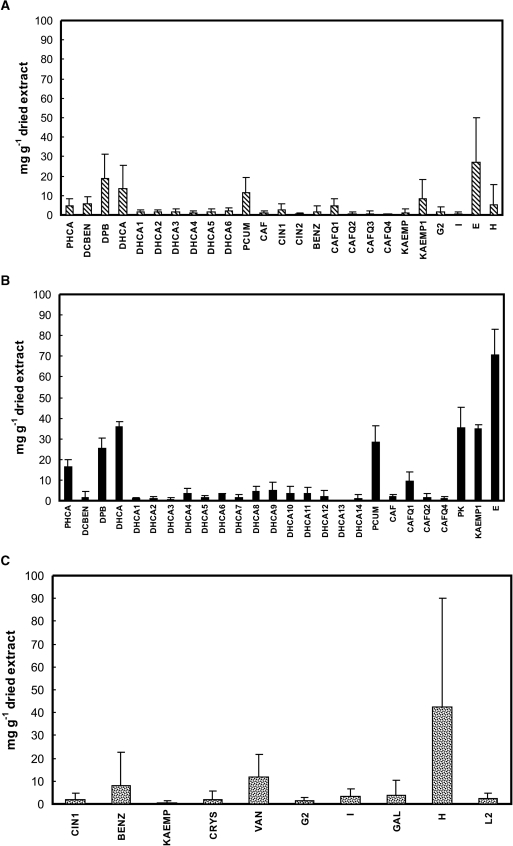
The chemical composition of each EEP quantified by HPLC is presented in Table 1. In order to better visualize the distribution of the components in groups A, B and C based on the predominant vegetation the concentration of each component was included in each group expressed as mean ± standard deviation (Fig. 1).
Figure 1.
Concentration expressed in mg g−1 dried extract (mean ± standard deviation) of individual components of the ethanol extracts of Brazilian propolis samples separated by botanical origin: (A) group A; (B) group B; (C) group C.
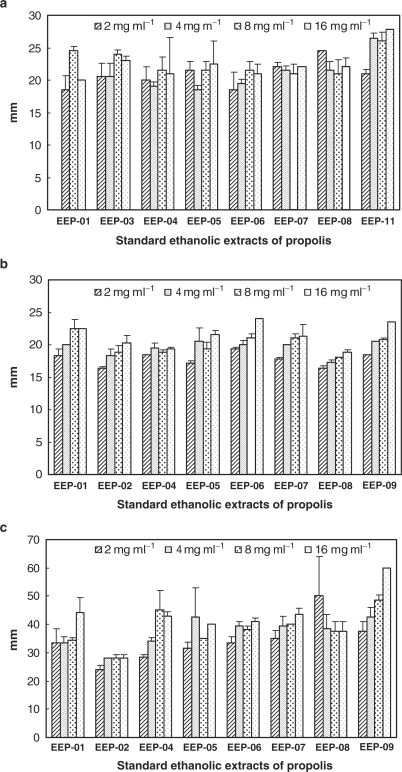
The effect of the extracts against C. albicans, S. schenckii and P. brasiliensis is presented in Fig. 2, being the latter species the most susceptible to propolis. No statistical correlation was performed; the values were expressed in diameter of inhibition zone expressed in mm, for four different concentrations of each extract.
Figure 2.
Fungicidal activity of Brazilian propolis extracts (2, 4, 8 and 16 mg ml−1) expressed as diameter of inhibition in mm: (a) Candida albicans; (b) Sporothrix schenckii; (c) Paracoccidiodes brasiliensis.
Bactericidal Activity and Correlation With the Chemical Composition
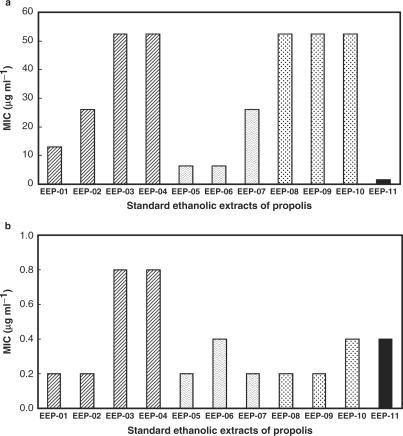
The MIC range for S. pneumoniae was 0.2–0.8 μg ml−1 and for S. aureus, 1.6–52.4 μg ml−1 (Fig. 3). All the extracts were inactive against K. pneumoniae. Analysis by the method of multiple regression of all the extracts together showed for S. aureus a positive correlation for p-coumaric acid (PCUM) (P = 0.0002) and 3-(4-hydroxy-3-(oxo-butenyl)-phenylacrylic acid (DHCA1) (P = 0.006), meaning higher concentrations of each compound led to higher bactericidal effect and a negative one for 3-methoxy-4-hydroxycinnamaldehyde (G2) (P = 0.001) and vanillin (VAN) (P = 0.026). While for S. pneumoniae, a negative correlation between the activity and the concentration of G2 (P = 0.024) was observed. When the same approach was employed for each group, since the number of extracts was four, three and three, for groups A, B and C, respectively, the multiple regression analysis gave unreliable results. However, an overall analysis revealed for group A (B. dracunculifolia + Auraucaria spp) there was an association of concentrations of caffeic acid (CAF) and dicaffeoylquinic acid 3 (CAFQ3) with the activity against S. aureus, while for S. pneumoniae such association occurred and between CAFQ3 and monocaffeoylquinic acid 2 (CAFQ2). In group B (B. dracunculifolia) higher activity against S. pneumoniae was associated with 2,2-dimethyl-6-carboxyethenyl-2H-1-benzopyran (DCBEN). For group C (Araucaria spp) no association was observed between the bactericidal effect and any component of the extracts.
Figure 3.
Bactericidal activity of Brazilian propolis extracts expressed as MIC values in µg ml−1: (a) Streptococcus pneumoniae; (b) Staphylococcus aureus.
Trypanocidal Activity and Correlation With the Chemical Composition
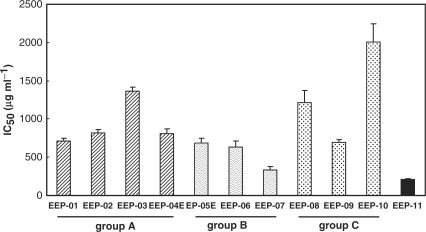
The activity of the extracts against bloodstream trypomastigote forms of T. cruzi is shown in Fig. 4. For each group the order of decreasing activity (P < 0.05) was: EEP-01> EEP-02, EEP-04 > EEP-03, for group A; EEP-07 > EEP-05, EEP-06, for group B and EEP-9 > EEP-8 > EEP-10, for group C.
Figure 4.
Effect of the Brazilian propolis extracts against bloodstream trypomastigotes of Trypanosoma cruzi after 1 day of treatment at 4°C. The bars represent the standard deviation of at least three independent experiments. The activity of EEP-01 was higher than that of EEP-02, EEP-04 and EEP-03 (P < 0.05); the activity of EEP-07 was higher than that of EEP-052, EEP-06 (P < 0.05); the activity of EEP-9 was higher than that of EEP-8, which has higher than that of EEP-10 (P < 0.05).
The statistical analysis of all the extracts together and the activity against T. cruzi showed a positive correlation with compounds 3,5-diprenyl-4-hydroxycinnamic acid derivative 4 (DHCA4) (P = 0.004) and DCBEN (P = 0.039) and a negative one for 2-[1-hydroxymethyl]vinyl-6-acetyl-5-hydroxycumarane (I) (P = 0.00008) and L2 (P = 0.006), a still non-identified compound. As stated earlier the multiple regression analysis could not be applied for each separated group of extracts, but there was a positive association of the trypanocidal activity with dicaffeoylquinic acid 3 (CAFQ3) for group A and with CAF for group B. For group C (Araucaria spp) no association was observed between the effect against trypomastigote forms and any component of the extracts.
Discussion
Propolis samples from tropical zones, such as Brazil with its vast biodiversity, have become a subject of increasing scientific and economic attention. From Brazilian propolis, several bioactive derivatives of hydroxycinnamic, caffeoylquinic and diterpenic acids besides benzofuranes and benzopyranes have already been characterized (18,23–25).
As typical of Brazilian propolis (2), most of the compounds present in the EEPs were phenolics, being present in high content hydroxycinnamic acids, such as PCUM and CAF and their prenylated derivatives. For group A (B. dracunculifolia + Araucaria spp), the extracts are typified as BRP(PR) based on the main bioactive components DHCA, DCBEN, 3-prenyl-4-hydroxycinnamic acid (PHCA) and 2,2-dimethyl-8-prenyl-2H-1-benzopyran-6-propenoic acid (DPB) (26). For group B (Araucaria spp), they are typified as BRP-1 (26) with the main components being DHCA, PHCA, DPB, PCUM and CAF, containing also caffeoylquinic acid derivatives. The extracts originated from Auraucaria spp (group C) are characterized as BRG, rich in coniferaldehyde compounds (VAN, G2 and I) (26). In relation to flavonoids, PK (26.0–45.6 mg g−1 of dried extract) and kaempferide (KAEMP1) (33.0–37.1 mg g−1) were detected only in the three extracts of group B. KAEMP1 was also detected in two out of the four EEPs of group A.
From EEP-11, the most active extract, a red propolis collected in the State of Alagoas, has been already isolated: simple phenolics, triterepenoids, isoflavonoids, prenylated benzophenones and naphtoquinone epoxide (8). Red colored propolis has been previously reported as typical in Cuba (7) and in Venezuela (11).
The fungicidal activity of Brazilian propolis has already been reported (27,28). All the extracts assayed presented similar activities considering a given fungus species and an overall analysis shows that P. brasiliensis was more susceptible to the extracts than C. albicans and S. schenckii.
As observed with propolis from temperate regions, Brazilian samples also present a higher activity against Gram-positive bacteria than Gram-negative ones (29,30), as observed in the present work using K. pneumoniae. The relationship of propolis origin, chemical composition and activity against S. aureus, as observed in the present study, was in accordance with the reports of other investigators (31,32). The statistical analysis of all the extracts together revealed that higher concentrations of PCUM and DHCA1 are correlated with higher activity against S. aureus.
The IC50 for the activity against T. cruzi was in the range of 200–2000 μg ml−1, while for the standard drug, crystal violet, in the same experimental conditions, the value was 187.0 ± 21.0 μg ml−1 (33). Statistical analysis of all the extracts showed that higher levels of DHCA4 and DCBEN were associated with higher trypanocidal effect.
The separation of the extracts in groups A, B and C, decreasing the number of observations in each group, prevented the application of multiple regression analysis. However, for S. aureus, S. pneumoniae and T. cruzi, we found, always a positive association of the biological activity with derivatives of PCUM and of caffeolyquinic acids, which correspond to caffeic acids esterified with sugar residues.
For both S. aureus and T. cruzi higher levels of 4-hydroxy cinnamic acid and derivatives were associated with a stronger biological activity, while no correlation was found between such activities and the content of flavonoids. DHCA and DCBEN, previously characterized both in Brazilian propolis and in its main plant source, B. dracunculifolia, were active against T. cruzi and S. aureus (18,34) and are useful markers to typify different samples (26).
The association of the chemical composition of propolis from different geographic regions with biological activities lead to the identification of active principles, a fundamental tool to achieve standardization of this bee product. The present study reinforces the relevance of PCUM and derivatives, especially prenylated ones and also of caffeolyquinic acids, on the biological activity of Brazilian propolis.
Acknowledgements
This work was supported by grants from the FAPESP (00/10031-0), CNPq and Papes/FIOCRUZ. Dr Alexandra C.H.F.Sawaya is also acknowledged for discussions about classification of propolis using Mass-fingerprint.
References
- 1.De Castro SL. Propolis: biological and pharmacological activities. Therapeutic uses of this bee-product. Ann Rev Biomed Sci. 2001;3:49–83. [Google Scholar]
- 2.Marcucci MC, Bankova VS. Chemical composition, plant origin and biological activity of Brazilian propolis. Curr Top Phytochem. 1999;2:115–23. [Google Scholar]
- 3.Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem. 2002;50:2502–6. doi: 10.1021/jf011432b. [DOI] [PubMed] [Google Scholar]
- 4.Park YK, Paredes-Guzman JF, Aguiar CL, Alencar SM, Fujiwara FY. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of southeastern Brazilian propolis. J Agric Food Chem. 2004;52:1100–3. doi: 10.1021/jf021060m. [DOI] [PubMed] [Google Scholar]
- 5.Salatino A, Teixeira EW, Negri G, Message D. Origin and chemical variation of Brazilian propolis. Evid Based Complement Alternat Med. 2005;2:33–8. doi: 10.1093/ecam/neh060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teixeira EW, Negri G, Meira RM, Message D, Salatino A. Plant origin of green propolis: bee behavior, plant anatomy and chemistry. Evid Based Complement Alternat Med. 2005;2:85–92. doi: 10.1093/ecam/neh055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez IM, Fernandez MC, Cuesta-Rubio O, Piccinelli AL, Rastrelli L. Polyprenylated benzophenone derivatives from Cuban propolis. J Nat Prod. 2005;68:931–4. doi: 10.1021/np0495884. [DOI] [PubMed] [Google Scholar]
- 8.Trusheva B, Popova M, Bankova VS, Simova S, Marcucci MC, Miorin PL, et al. Bioactive constituents of Brazilian red propolis. Evid Based Complement Alternat Med. 2006;3:249–54. doi: 10.1093/ecam/nel006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegazi AG, Abd-El-Hady FK, Abd-Allah FA. Chemical composition and antimicrobial activity of European propolis. Z Naturforsch. 2000;55C:70–5. doi: 10.1515/znc-2000-1-214. [DOI] [PubMed] [Google Scholar]
- 10.Prytzyk E, Dantas AP, Salomão K, Pereira AS, Bankova VS, De Castro SL, et al. Flavonoids and trypanocidal activity of Bulgarian propolis. J Ethnopharmacol. 2003;88:189–93. doi: 10.1016/s0378-8741(03)00210-1. [DOI] [PubMed] [Google Scholar]
- 11.Trusheva B, Popova M, Naydenski H, Tsvetkova I, Rodriguez JG, Bankova VS. New polyisoprenylated benzophenones from Venezuelan propolis. Fitoterapia. 2004;75:683–9. doi: 10.1016/j.fitote.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Chagas disease Thirteenth Programme Report UNDP/WB/TDR. Geneve. 1997;00:112–23. [Google Scholar]
- 13.Coura JR, De Castro SL. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz. 2002;91:3. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs MR. Streptococcus pneumoniae: epidemiology and patterns of resistance. Am J Med. 2004;117:3S–15S. doi: 10.1016/j.amjmed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Todd JK. Staphylococcal infections. Ped Rev. 2005;26:444–50. [PubMed] [Google Scholar]
- 16.Barros MBL, Schubach TMP, Galhardo MCG, Schubach AO, Monteiro PCF, Reis RS, et al. Sporotrichosis: An emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777–9. doi: 10.1590/s0074-02762001000600006. [DOI] [PubMed] [Google Scholar]
- 17.Borges-Walmsley MI, Chen D, Shu X, Walmsley AR. The pathobiology of Paracoccidioides brasiliensis. Trends Microbiol. 2002;10:80–7. doi: 10.1016/s0966-842x(01)02292-2. [DOI] [PubMed] [Google Scholar]
- 18.Marcucci MC, Ferreres F, Garcia-Viguera C, Bankova VS, De Castro SL, Dantas AP, et al. Phenolic compounds from Brazilian propolis with pharmacological activities. J Ethnopharmacol. 2001;74:105–12. doi: 10.1016/s0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 19.Spooner FD, Sykes G. Laboratory assessment of antibacterial activity. In: Norris E, Ribbons DN, editors. Methods in Microbiology. London: Academic Press; 1972. pp. 45–9. [Google Scholar]
- 20.NCCLS. Technical Report M07-A6. 6th. PA: Wayne; 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. [Google Scholar]
- 21.Silva LHP, Nussenszweig V. Sobre uma cepa de Trypanosoma cruzi virulenta para o camundongo branco. Folia Clin Biol. 1953;20:191–207. [Google Scholar]
- 22.Cunha IBS, Salomão K, Shimizu M, Bankova VS, Custodio AR, Castro SL, et al. Antitrypanosomal activity of Brazilian propolis from Apis mellifera. Chem Pharm Bull. 2004;52:602–4. doi: 10.1248/cpb.52.602. [DOI] [PubMed] [Google Scholar]
- 23.Bankova VS, Marcucci MC, Simova S, Nikolova N, Kujumgiev A, Popov S. Antibacterial diterpenic acids from Brazilian propolis. Z Naturforsch. 1996;51C:277–80. doi: 10.1515/znc-1996-5-602. [DOI] [PubMed] [Google Scholar]
- 24.Basnet P, Matsushige K, Hase K, Kadota S, Namba T. Potent antihepatotoxic activity of dicaffeoyl quinic acids from propolis. Biol Pharm Bull. 1996;19:655–7. doi: 10.1248/bpb.19.655. [DOI] [PubMed] [Google Scholar]
- 25.Hirota M, Matsuno T, Fujiwara T, Sugiyama H, Mineshita S. Enhanced cytotoxicity in a Z-photoisomer of a benzopyran derivative of propolis. J Nat Prod. 2000;63:366–70. doi: 10.1021/np990463m. [DOI] [PubMed] [Google Scholar]
- 26.Marcucci MC. 2000. Process to typing natural products. Patente requerida Instituto Nacional de Propriedade Intelectual. INPI n°. PI 0105471-6, December 22. [Google Scholar]
- 27.Sforcin JM, Fernandes A, Jr, Lopes CAM, Funari SRC, Bankova VS. Seasonal effect of Brazilian propolis on Candida albicans and Candida tropicalis. J Venom Anim Toxins. 2001;7:139–44. [Google Scholar]
- 28.Salomão K, Dantas AP, Borba CM, Campos LC, Machado DG, Aquino Neto FR, et al. Chemical composition and microbicidal activity of extracts from Brazilian and Bulgarian propolis. Lett Appl Microbiol. 2004;38:87–92. doi: 10.1111/j.1472-765x.2003.01458.x. [DOI] [PubMed] [Google Scholar]
- 29.Sforcin JM, Fernandes A, Jr, Lopes CAM, Bankova VS, Funari SRC. Seasonal effect on Brazilian propolis antibacterial activity. J Ethnopharmacol. 2000;73:243–9. doi: 10.1016/s0378-8741(00)00320-2. [DOI] [PubMed] [Google Scholar]
- 30.Pinto MS, Faria JE, Message D, Cassini STA, Pereira CS, Gioso MM. Efeito de extratos de própolis verde sobre bactérias patogênicas isoladas do leite de vacas com mastite. Braz J Vet Res Anim Sci. 2001;38:278–83. [Google Scholar]
- 31.Nieva Moreno MI, Isla MI, Cudmani NG, Vattuone MA, Sampietro AR. Screening of antibacterial activity of Amaicha del Valle (Tucuman, Argentina) propolis. J Ethnopharmacol. 1999;15:97–102. doi: 10.1016/s0378-8741(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 32.Lu LC, Chen YW, Chou CC. Antibacterial activity of propolis against Staphylococcus aureus. Int J Food Microbiol. 2005;15:213–20. doi: 10.1016/j.ijfoodmicro.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 33.De Castro SL, Pinto MCFR, Pinto AV. Screening of natural and synthetic drugs against Trypanosoma cruzi: I- Establishing a structure/activity relationship. Microbios. 1994;78:83–90. [PubMed] [Google Scholar]
- 34.Aga H, Shibuya T, Sugimoto T, Kurimoto M, Nakajima SH. Isolation and identification of antimicrobial compounds in Brazilian propolis. Bios Biotech Biochem. 1994;58:945–6. [Google Scholar]